
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Oncol., 14 December 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1335730
This article is part of the Research TopicReassessing Corticosteroid Use in Neuro-Oncology PracticesView all articles
Dexamethasone has been commonly given to patients with a presumed new GBM in relatively large doses (6-16 mg daily for 1-2 weeks) since the 1960s without any rigorous evidence. This treatment with dexamethasone before the diagnosis and adjuvant therapy makes GBM patients unique compared to other newly diagnosed cancer patients. While dexamethasone may be beneficial, recent studies suggest that this potent immunosuppressant with pleiotropic effects is harmful in the long term. This perspective article summarizes the disadvantages of perioperative dexamethasone from multiple facets. It concludes that these growing data mandate rigorously testing the benefits of using perioperative dexamethasone.
Glioblastoma (GBM) is the most common, malignant, and therapy-resistant brain tumor (1). The median overall survival of GBM patients is stagnated around 15 months (2–4). Although there have been small studies showing benefit like lomustine-temozolomide combination for GBMs with methylated MGMT promoter (5) (a larger trial is underway, NCT05095376) and immunotherapy for GBMs with high mutational burden (6), besides surgical resection, only three GBM therapies have rigorous practice-changing data: radiation [1980 (7)], temozolomide chemotherapy [2005 (3)], and alternating electric fields [2017 (8)]. Countless other therapies with groundbreaking success in other cancers have failed to improve the survival of GBM patients. The recent failure of the revolutionary immune checkpoint inhibitors (2, 9, 10) now poses an urgent question: Is therapy failure iatrogenic? To answer, we must reexamine the common GBM treatment, which begins with a diagnostic surgery, then after about 3 weeks, adjuvant treatment with the above three therapies. Intriguingly, before and after the surgery (i.e., perioperatively), dexamethasone is commonly given to patients with a presumed new GBM in relatively large doses (6-16 mg daily for 1-2 weeks) (11, 12). This treatment with dexamethasone before the diagnosis and adjuvant therapy makes GBM patients unique compared to other newly diagnosed cancer patients. Dexamethasone is a potent immunosuppressive steroid with pleiotropic effects (13).
Perioperative dexamethasone decreases tumor- and surgery-associated symptoms. Symptoms in GBM patients arise from the brain directly injured or replaced by the tumor and the surrounding ‘normal’ brain that is compressed by the tumor and becomes edematous in response, contributing to the increased intracranial pressure. Dexamethasone mitigates symptoms by controlling this edema. Mechanistically, it is a long-acting synthetic corticosteroid that decreases microvascular permeability by reducing vascular response to and expression of tumor-derived permeability factors like vascular endothelial growth factor (VEGF) (14–16). Additionally, preoperative dexamethasone treatment is believed to decrease brain swelling during GBM surgery (17, 18). Postoperatively, dexamethasone is commonly continued as a high-dose, 2-week taper because of its anti-inflammatory and psychiatric effects. It lowers the normal inflammation caused by surgery and mitigates symptoms such as pain and nausea. Therefore, despite having a major operation, patients feel vigorous, are more active, leave the hospital earlier (19), and show better performance status at the start of adjuvant therapy (20). Because dexamethasone is very potent, these effects can be readily observed. Thus, since the 1960s (21), it has been used with no rigorous evidence (22–24).
Perioperative dexamethasone may be beneficial but concerns for its long-term harm are growing (Figure 1).
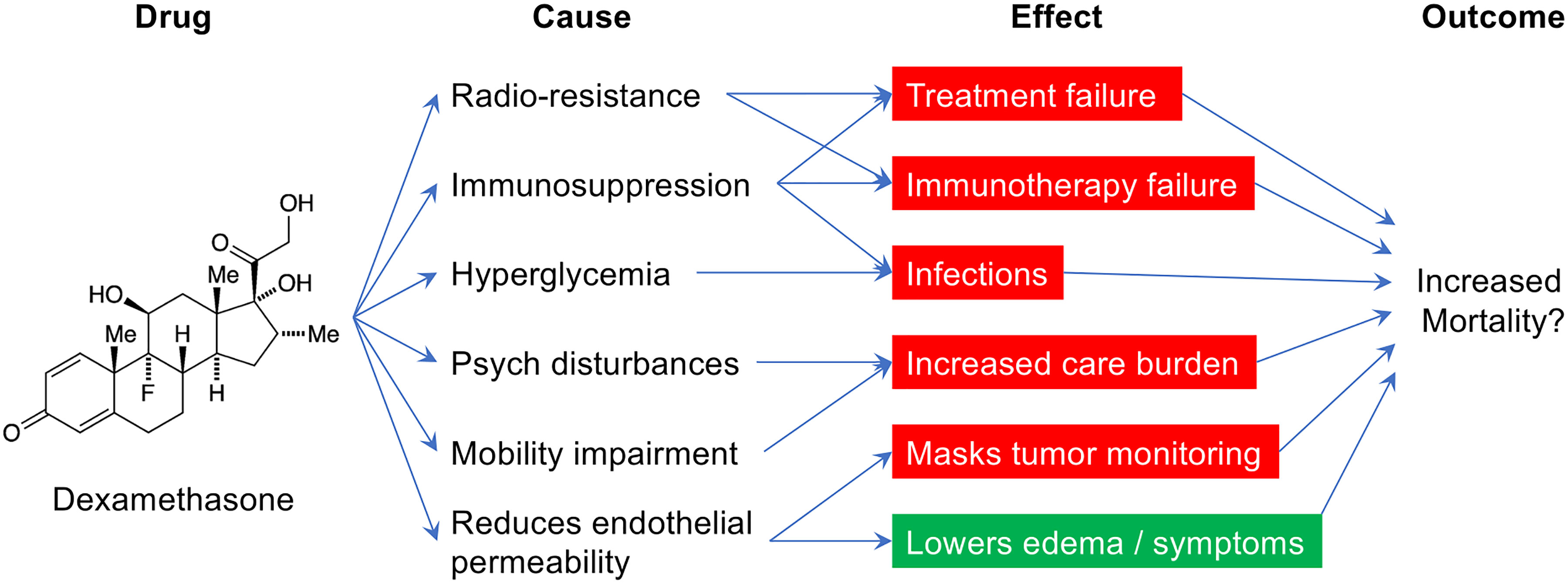
Figure 1 The various causes and potential effects of perioperative dexamethasone in glioblastoma patients.
Dexamethasone’s psychiatric and cognitive effects are deleterious (25). It increases the risk of postoperative insomnia, irritability, mania, and delirium, which can lead to early mortality (13, 26–28). A large, multicenter, phase 3 randomized controlled trial (RCT) showed significantly greater psychiatric disorders within 30 days in subdural hematoma neurosurgery patients randomized to a 2-week course of dexamethasone (29). Long-term use of dexamethasone leads to cognitive deficits. Recent sub-analysis of phase 3 RCT data shows poorer high-order neurological functions in dexamethasone-treated patients with recurrent GBM (30). Dexamethasone also leads to significant mobility impairment in GBM patients (31).
Perioperative dexamethasone use leads to its dependence during adjuvant therapy, which lowers survival. Retrospective analyses of large cohorts and post hoc analysis of two large, multicenter, phase 3 RCTs show that GBM patients treated with dexamethasone during chemoradiation have lower survival (32–35). Thus, the use of dexamethasone during chemoradiation has declined (36). However, GBM patients are often prescribed huge amounts of dexamethasone (>160 mg over 3 weeks) perioperatively before adjuvant chemoradiation (11, 12). And, our data show greater perioperative dexamethasone use leads to greater use of dexamethasone during chemoradiation, even in patients with a gross total GBM resection (11).
Perioperative dexamethasone increases GBM malignancy and resistance before adjuvant therapy. Dexamethasone enhances the proliferative and migrative capacities of GBM (37, 38) and makes it resistant to chemoradiation (39–43). For example, in a murine GBM model, dexamethasone administered daily for 3 days before radiation significantly lowered the survival (33).
Perioperative dexamethasone weakens the systemic immunity of GBM patients before adjuvant therapy. dexamethasone is 30x more potent an immunosuppressant than endogenous steroids (13, 44). Routine preoperative treatment with dexamethasone (commonly 4 mg three times a day for 7 days) significantly depletes B cells, T cells, and NK cells in the blood of newly diagnosed GBM patients before surgery (45, 46). Up to 30% of GBM patients treated with dexamethasone have blood CD4 T cell counts < 200/mm (3) before surgery (i.e., they meet AIDS criteria)! (45, 47). Postoperatively, GBM patients receive even greater doses of dexamethasone (6-16 mg daily for 1-2 weeks)! which further worsens lymphopenia before starting chemoradiation (11, 12). Lymphopenia at the start or during chemoradiation predicts a lower survival in GBM patients (11, 32, 48–50). Not surprisingly, higher doses of perioperative dexamethasone lead to more infections in GBM patients in the 90 days after surgery (11, 51, 52). Infections cause treatment delays, are a direct cause of death (53, 54), and are an independent predictor of survival in GBM patients (11). Perioperative dexamethasone is a well-established cause of infections in other neurosurgery patients (55, 56). A large, multicenter, phase 3 RCT showed significantly greater infections in subdural hematoma neurosurgery patients randomized to a 2-week course of dexamethasone (29). A similar 2-week dexamethasone course is commonly prescribed postoperatively to GBM patients, who unlike other neurosurgery patients become further immunosuppressed by chemoradiation.
Perioperative dexamethasone may deplete GBM-infiltrated immune cells before adjuvant therapy (57). Among cancers, GBM is uniquely depleted in lymphocytes (58). Although it is accepted as GBM’s natural state due to the brain’s immune privilege (59), this idea is likely confounded by dexamethasone. GBM patients are the only cancer patients who receive dexamethasone before surgery, which causes lymphocyte apoptosis (60). Data of recurrent GBMs from dexamethasone-treated patients support this hypothesis (61). Higher GBM-infiltrated T cells directly correlate with longer survival in GBM patients (62).
Perioperative dexamethasone may attenuate immunotherapy. Cancer immunotherapies activate immunity against cancer cells resulting in durable objective responses (63, 64). Dexamethasone hinders them (65). In murine GBM models, it eradicates the survival benefit of immune checkpoint inhibitors and oncolytic viral therapy by depleting T cells and blocking potent intratumoral and global immune responses (66, 67). Retrospective results in GBM patients are similar (66). Moreover, recent large, multicenter, multinational, phase 3 RCTs of immune checkpoint inhibitors in GBM patients were not efficacious (2, 9, 10). However, subgroup analysis of one trial showed efficacy in those who did not receive dexamethasone! (10) In another phase 2 RCT that failed to show the efficacy of a checkpoint inhibitor, 90% of the efficacious responses occurred in GBM patients who did not receive dexamethasone (68). In a neo-antigen vaccine trial in GBM patients, dexamethasone prevented favorable neo-antigen-specific T cell responses and infiltration of T cells in the tumor (69).
Last, dexamethasone clouds the response assessment for high-grade gliomas (RANO-HGG). Dexamethasone affects GBM’s radiographic visibility with contrast and its use is a criterion in clinically determining GBM progression (36, 70, 71). How dexamethasone affects RANO-HGG is unclear. For example, it is unknown if perioperative dexamethasone use leads to an increase in progression-free survival (i.e., “pseudoresponse”), as seen with drugs that block the cerebral vasculature, like anti-VEGF drugs, to decrease tumor visibility on radiocontrast-based imaging (70). Dexamethasone use is also a criterion in the response assessment for high-grade gliomas (RANO-HGG) (36, 70, 71). On the other hand, we have shown that higher perioperative Dex doses lead to dexamethasone use during adjuvant therapy (11). Thus, it could also be likely that patients treated with high doses of dexamethasone may be judged to have shorter progression-free survival.
Perioperative dexamethasone use remains high despite rigorous evidence and emerging concerns for its harm (23). Our retrospective study of 360 GBM patients showed that greater perioperative dexamethasone independently correlates with lower survival (11). This result is independent of tumor size and has been replicated at other major cancer centers (72, 73). It is paradoxical to expect a survival benefit in trials of new adjuvant therapies, especially immunotherapy, while pre-treating patients with high doses of a potent therapy-nullifying steroidal immuno-suppressant. Despite emerging reports of its harm, a 2022 worldwide survey showed that 80% of neurosurgeons use dexamethasone perioperatively, and 45% use it liberally irrespective of patient symptoms (74). A group of multidisciplinary experts have recently questioned the perioperative use of high doses of dexamethasone (75). To best drive a practice change, data from a well-designed, rigorous study are needed.
To generate rigorous evidence that informs the practice of perioperative dexamethasone, prospective comparative studies are necessary. The comparator to the regular 2-week high-dose dexamethasone taper can be no dexamethasone use, limited use of dexamethasone [justified here (75)], or an alternative to dexamethasone [for example, RAGE inhibitors (76)]. Given the harms of dexamethasone, some providers may think there is equipoise in not giving dexamethasone perioperatively altogether, especially considering that dexamethasone is not a proven treatment to reduce seizures, intracranial pressure in a similar manner to mannitol or hypertonic saline, and disability caused by hemorrhage or post-operative stroke (in fact, there is data that argue it is harmful in stroke). In the perioperative setting, dexamethasone is only believed to mitigate (not eradicate) symptoms from the compressed and edematous peri-tumoral brain—not the tumor-harboring brain. GBM resection provides equal or greater symptom control by lowering brain pressures. Neurosurgeons have surgically treated GBM patients without perioperative dexamethasone (72, 74), and a trial of not using perioperative dexamethasone in GBM patients is currently underway to rigorously test its safety (NCT04266977).
Dexamethasone has been used as part of the treatment for brain tumor patients since the 1960s including using it perioperatively (21). It is used with no rigorous evidence (22–24). While dexamethasone offers recognizable short-term benefits to the patients, the literature is increasingly pointing to it as a source of perioperative complications (52, 77) and long-term harms as well as an impediment to treatments and their response assessments. Collectively, the growing data mandate rigorous testing of the benefits of using perioperative dexamethasone.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
AM: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol (2021) 23(12 Suppl 2):iii1–iii105. doi: 10.1093/neuonc/noab200
2. Omuro A, Brandes AA, Carpentier AF, Idbaih A, Reardon DA, Cloughesy T, et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase 3 trial. Neuro Oncol (2023) 25(1):123–34. doi: 10.1093/neuonc/noac099
3. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med Mar 10 (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
4. Ostrom QT, Shoaf ML, Cioffi G, Waite K, Kruchko C, Wen PY, et al. National-level overall survival patterns for molecularly-defined diffuse glioma types in the United States. Neuro Oncol (2023) 25(4):799–807. doi: 10.1093/neuonc/noac198
5. Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet Feb 16 (2019) 393(10172):678–88. doi: 10.1016/S0140-6736(18)31791-4
6. Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol Jul 1 (2016) 34(19):2206–11. doi: 10.1200/JCO.2016.66.6552
7. Walker MD, Green SB, Byar DP, Alexander E Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of Malignant glioma after surgery. N Engl J Med Dec 4 (1980) 303(23):1323–9. doi: 10.1056/NEJM198012043032303
8. Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA (2017) 318(23):2306–16. doi: 10.1001/jama.2017.18718
9. Lim M, Weller M, Idbaih A, Steinbach J, Finocchiaro G, Raval RR, et al. Phase 3 trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol (2022) 24(11:1935–49. doi: 10.1093/neuonc/noac116
10. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkMate 143 phase 3 randomized clinical trial. JAMA Oncol (2020) 6(7):1003–10. doi: 10.1001/jamaoncol.2020.1024
11. Mistry AM, Jonathan SV, Monsour MA, Mobley BC, Clark SW, Moots PL. Impact of postoperative dexamethasone on survival, steroid dependency, and infections in newly diagnosed glioblastoma patients. Neurooncol Pract (2021) 8(5):589–600. doi: 10.1093/nop/npab039
12. Wiencke JK, Molinaro AM, Warrier G, Rice T, Clarke J, Taylor JW, et al. DNA methylation as a pharmacodynamic marker of glucocorticoid response and glioma survival. Nat Commun (2022) 13(1):5505. doi: 10.1038/s41467-022-33215-x
13. Schimmer BP, Funder JW. Adrenocorticotropic hormone, adrenal steroids, and the adrenal cortex. In: Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e. New York, NY: McGraw-Hill Education (2017).
14. Wong ET, Swanson KD. Dexamethasone-friend or foe for patients with glioblastoma? JAMA Neurol (2019) 76(3):247–8. doi: 10.1001/jamaneurol.2018.4530
15. Heiss JD, Papavassiliou E, Merrill MJ, Nieman L, Knightly JJ, Walbridge S, et al. Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest. (1996) 98(6):1400–8. doi: 10.1172/JCI118927
16. Fan Z, Sehm T, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan NE. Dexamethasone alleviates tumor-associated brain damage and angiogenesis. PloS One (2014) 9(4):e93264. doi: 10.1371/journal.pone.0093264
17. Perez de Arriba N, Antuna Ramos A, Martin Fernandez V, Rodriguez Sanchez MDC, Gonzalez Alarcon JR, Alvarez Vega MA. Risk factors associated with inadequate brain relaxation in craniotomy for surgery of supratentorial tumors. Cureus (2022) 14(5):e25544. doi: 10.7759/cureus.25544
18. Rasmussen M, Bundgaard H, Cold GE. Craniotomy for supratentorial brain tumors: risk factors for brain swelling after opening the dura mater. J Neurosurg (2004) 101(4):621–6. doi: 10.3171/jns.2004.101.4.0621
19. Alan N, Seicean A, Seicean S, Neuhauser D, Benzel EC, Weil RJ. Preoperative steroid use and the incidence of perioperative complications in patients undergoing craniotomy for definitive resection of a Malignant brain tumor. J Clin Neurosci (2015) 22(9):1413–9. doi: 10.1016/j.jocn.2015.03.009
20. Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol. (2015) 121(2):359–64. doi: 10.1007/s11060-014-1640-x
21. Galicich JH, French LA, Melby JC. Use of dexamethasone in treatment of cerebral edema associated with brain tumors. J Lancet (1961) 81:46–53.
22. Ly KI, Wen PY. Clinical relevance of steroid use in neuro-oncology. Curr Neurol Neurosci Rep (2017) 17(1):5. doi: 10.1007/s11910-017-0713-6
23. Jessurun CAC, Hulsbergen AFC, Cho LD, Aglio LS, Nandoe Tewarie RDS, Broekman MLD. Evidence-based dexamethasone dosing in Malignant brain tumors: what do we really know? J Neurooncol. (2019) 144(2):249–64. doi: 10.1007/s11060-019-03238-4
24. Phillips KA, Fadul CE, Schiff D. Neurologic and medical management of brain tumors. Neurol Clin (2018) 36(3):449–66. doi: 10.1016/j.ncl.2018.04.004
25. Kettunen S, Lappalainen OP, Palotie T, Furuholm J, Auro K, Snall J. Psychiatric morbidity is common in orthognathic surgery patients-a retrospective study. Oral Surg Oral Med Oral Pathol Oral Radiol (2023) 135(6):716–23.. doi: 10.1016/j.oooo.2022.09.009
26. Koning A, Satoer DD, Vinkers CH, Zamanipoor Najafabadi AH, Biermasz NR, Nandoe Tewarie RDS, et al. The DEXA-CORT trial: study protocol of a randomised placebo-controlled trial of hydrocortisone in patients with brain tumour on the prevention of neuropsychiatric adverse effects caused by perioperative dexamethasone. BMJ Open (2021) 11(12):e054405. doi: 10.1136/bmjopen-2021-054405
27. Wu Z, Li H, Liao K, Wang Y. Association between dexamethasone and delirium in critically ill patients: A retrospective cohort study of a large clinical database. J Surg Res (2021) 263:89–101. doi: 10.1016/j.jss.2021.01.027
28. Flanigan PM, Jahangiri A, Weinstein D, Dayani F, Chandra A, Kanungo I, et al. Postoperative delirium in glioblastoma patients: risk factors and prognostic implications. Neurosurgery (2018) 83(6):1161–72. doi: 10.1093/neuros/nyx606
29. Hutchinson PJ, Edlmann E, Bulters D, Zolnourian A, Holton P, Suttner N, et al. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med (2020) 383(27):2616–27. doi: 10.1056/NEJMoa2020473
30. Caramanna I, de Kort JM, Brandes AA, Taal W, Platten M, Idbaih A, et al. Corticosteroids use and neurocognitive functioning in patients with recurrent glioblastoma: Evidence from European Organization for Research and Treatment of Cancer (EORTC) trial 26101. Neurooncol Pract (2022) 9(4):310–6. doi: 10.1093/nop/npac022
31. Rogers JL, de la Cruz Minyety J, Vera E, Acquaye AA, Payen SS, Weinberg JS, et al. Assessing mobility in primary brain tumor patients: A descriptive feasibility study using two established mobility tests. Neurooncol Pract (2022) 9(3):219–28. doi: 10.1093/nop/npac013
32. Wong ET, Lok E, Gautam S, Swanson KD. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer. (2015) 113(2):232–41. doi: 10.1038/bjc.2015.238
33. Pitter KL, Tamagno I, Alikhanyan K, Hosni-Ahmed A, Pattwell SS, Donnola S, et al. Corticosteroids compromise survival in glioblastoma. Brain (2016) 139(Pt 5):1458–71. doi: 10.1093/brain/aww046
34. Zhou L, Shen Y, Huang T, Sun Y, Alolga RN, Zhang G, et al. The prognostic effect of dexamethasone on patients with glioblastoma: A systematic review and meta-analysis. Systematic Review. Front Pharmacol (2021) 12:727707(2318). doi: 10.3389/fphar.2021.727707
35. Petrelli F, De Stefani A, Ghidini A, Bruschieri L, Riboldi V, Dottorini L, et al. Steroids use and survival in patients with glioblastoma multiforme: a pooled analysis. J Neurol (2021) 268(2):440–7. doi: 10.1007/s00415-020-09731-5
36. Arvold ND, Armstrong TS, Warren KE, Chang SM, DeAngelis LM, Blakeley J, et al. Corticosteroid use endpoints in neuro-oncology: Response Assessment in Neuro-Oncology Working Group. Neuro Oncol (2018) 20(7):897–906. doi: 10.1093/neuonc/noy056
37. Luedi MM, Singh SK, Mosley JC, Hassan ISA, Hatami M, Gumin J, et al. Dexamethasone-mediated oncogenicity in vitro and in an animal model of glioblastoma. J Neurosurg (2018) 129(6):1446–55. doi: 10.3171/2017.7.JNS17668
38. Cenciarini M, Valentino M, Belia S, Sforna L, Rosa P, Ronchetti S, et al. Dexamethasone in glioblastoma multiforme therapy: mechanisms and controversies. Front Mol Neurosci (2019) 12:65. doi: 10.3389/fnmol.2019.00065
39. Kostopoulou ON, Mohammad AA, Bartek J Jr., Winter J, Jung M, Stragliotto G, et al. Glucocorticoids promote a glioma stem cell-like phenotype and resistance to chemotherapy in human glioblastoma primary cells: Biological and prognostic significance. Int J Cancer. (2018) 142(6):1266–76. doi: 10.1002/ijc.31132
40. Sur P, Sribnick EA, Patel SJ, Ray SK, Banik NL. Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia (2005) 50(2):160–7. doi: 10.1002/glia.20168
41. Glaser T, Wagenknecht B, Weller M. Identification of p21 as a target of cycloheximide-mediated facilitation of CD95-mediated apoptosis in human Malignant glioma cells. Oncogene (2001) 20(35):4757–67. doi: 10.1038/sj.onc.1204498
42. Kokunai T, Tamaki N. Relationship between expression of p21WAF1/CIP1 and radioresistance in human gliomas. Jpn J Cancer Res (1999) 90(6):638–46. doi: 10.1111/j.1349-7006.1999.tb00795.x
43. Das A, Banik NL, Patel SJ, Ray SK. Dexamethasone protected human glioblastoma U87MG cells from temozolomide induced apoptosis by maintaining Bax : Bcl-2 ratio and preventing proteolytic activities. Mol Cancer. (2004) 3(1):36. doi: 10.1186/1476-4598-3-36
44. Hedley-Whyte ET, Hsu DW. Effect of dexamethasone on blood-brain barrier in the normal mouse. Ann Neurol (1986) 19(4):373–7. doi: 10.1002/ana.410190411
45. Bracci PM, Rice T, Hansen HM, Francis SS, Lee S, McCoy LS, et al. Pre-surgery immune profiles of adult glioma patients. J Neurooncol. (2022) 159(1):103–15. doi: 10.1007/s11060-022-04047-y
46. Chitadze G, Fluh C, Quabius ES, Freitag-Wolf S, Peters C, Lettau M, et al. In-depth immunophenotyping of patients with glioblastoma multiforme: Impact of steroid treatment. Oncoimmunology (2017) 6(11):e1358839. doi: 10.1080/2162402X.2017.1358839
47. Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med (2018) 24(9):1459–68. doi: 10.1038/s41591-018-0135-2
48. Song AJ, Ding K, Alnahhas I, Laperriere NJ, Perry J, Mason WP, et al. Impact of lymphopenia on survival for elderly patients with glioblastoma: A secondary analysis of the CCTG CE.6 (EORTC 26062-22061, TROG03.01) randomized clinical trial. Neurooncol Adv (2021) 3(1):vdab153. doi: 10.1093/noajnl/vdab153
49. Lee C, Ahn S, Park JS, Song JH, Hong YK, Jeun SS. Effect of cumulative dexamethasone dose during concomitant chemoradiation on lymphopenia in patients with newly diagnosed glioblastoma. Brain Tumor Res Treat (2020) 8(2):71–6. doi: 10.14791/btrt.2020.8.e12
50. Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res (2011) 17(16):5473–80. doi: 10.1158/1078-0432.CCR-11-0774
51. Nair SK, Botros D, Chakravarti S, Mao Y, Wu E, Lu B, et al. Predictors of surgical site infection in glioblastoma patients undergoing craniotomy for tumor resection. J Neurosurg (2023) 138(5):1227–34. doi: 10.3171/2022.8.JNS212799
52. Jatana S, Mohammad AH, Al-Saadi TD, Carias M, Guevara-Moriones N, Ruiz-Barrera MA, et al. Characterization of perioperative glycemic status and dexamethasone use with associated postoperative complications in glioblastoma patients. Acta Neurochir (Wien). (2023) 165(4):1031–40. doi: 10.1007/s00701-023-05541-6
53. Best B, Nguyen HS, Doan NB, Gelsomino M, Shabani S, Ahmadi Jazi G, et al. Causes of death in glioblastoma: insights from the SEER database. J Neurosurg Sci (2019) 63(2):121–6. doi: 10.23736/S0390-5616.18.04599-X
54. Sizoo EM, Braam L, Postma TJ, Pasman HR, Heimans JJ, Klein M, et al. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro Oncol (2010) 12(11):1162–6. doi: 10.1093/neuonc/nop045
55. Lieber BA, Appelboom G, Taylor BE, Lowy FD, Bruce EM, Sonabend AM, et al. Preoperative chemotherapy and corticosteroids: independent predictors of cranial surgical-site infections. J Neurosurg (2016) 125(1):187–95. doi: 10.3171/2015.4.JNS142719
56. Merkler AE, Saini V, Kamel H, Stieg PE. Preoperative steroid use and the risk of infectious complications after neurosurgery. Neurohospitalist (2014) 4(2):80–5. doi: 10.1177/1941874413510920
57. Swildens KX, Sillevis Smitt PAE, van den Bent MJ, French PJ, Geurts M. The effect of dexamethasone on the microenvironment and efficacy of checkpoint inhibitors in glioblastoma: a systematic review. Neurooncol Adv (2022) 4(1):vdac087. doi: 10.1093/noajnl/vdac087
58. Combes AJ, Samad B, Tsui J, Chew NW, Yan P, Reeder GC, et al. Discovering dominant tumor immune archetypes in a pan-cancer census. Cell (2022) 185(1):184–203 e19. doi: 10.1016/j.cell.2021.12.004
59. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. (2020) 20(1):12–25. doi: 10.1038/s41568-019-0224-7
60. Cari L, De Rosa F, Nocentini G, Riccardi C. Context-dependent effect of glucocorticoids on the proliferation, differentiation, and apoptosis of regulatory T cells: A review of the empirical evidence and clinical applications. Int J Mol Sci (2019) 20(5):1142. doi: 10.3390/ijms20051142
61. Mathewson ND, Ashenberg O, Tirosh I, Gritsch S, Perez EM, Marx S, et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell (2021) 184(5):1281–1298.e26. doi: 10.1016/j.cell.2021.01.022
62. Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res (2011) 17(13):4296–308. doi: 10.1158/1078-0432.CCR-10-2557
63. Yost KE, Chang HY, Satpathy AT. Recruiting T cells in cancer immunotherapy. Science (2021) 372(6538):130–1. doi: 10.1126/science.abd1329
64. Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. (2021) 21(6):345–59. doi: 10.1038/s41568-021-00347-z
65. Giles AJ, Hutchinson MND, Sonnemann HM, Jung J, Fecci PE, Ratnam NM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer. (2018) 6(1):51. doi: 10.1186/s40425-018-0371-5
66. Iorgulescu JB, Gokhale PC, Speranza MC, Eschle BK, Poitras MJ, Wilkens MK, et al. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin Cancer Res (2021) 27(1):276–87. doi: 10.1158/1078-0432.CCR-20-2291
67. Koch MS, Zdioruk M, Nowicki MO, Griffith AM, Aguilar E, Aguilar LK, et al. Systemic high-dose dexamethasone treatment may modulate the efficacy of intratumoral viral oncolytic immunotherapy in glioblastoma models. J Immunother Cancer. (2022) 10(1):e003368. doi: 10.1136/jitc-2021-003368
68. Nayak L, Molinaro AM, Peters K, Clarke JL, Jordan JT, de Groot J, et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin Cancer Res (2021) 27(4):1048–57. doi: 10.1158/1078-0432.CCR-20-2500
69. Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature (2019) 565(7738):234–9. doi: 10.1038/s41586-018-0792-9
70. Chukwueke UN, Wen PY. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol (2019) 8(1):CNS28. doi: 10.2217/cns-2018-0007
71. Wen PY, van den Bent M, Youssef G, Cloughesy TF, Ellingson BM, Weller M, et al. RANO 2.0: update to the response assessment in neuro-oncology criteria for high- and low-grade gliomas in adults. J Clin Oncol Nov 20 (2023) 41(33):5187–99. doi: 10.1200/JCO.23.01059
72. Medikonda R, Patel K, Jackson C, Saleh L, Srivastava S, Feghali J, et al. The safety and efficacy of dexamethasone in the perioperative management of glioma patients. J Neurosurg (2022) 136(4):1062–9. doi: 10.3171/2021.4.JNS204127
73. Bhavsar S, Hagan K, Arunkumar R, Potylchansky Y, Grasu R, Dang A, et al. Preoperative statin use is not associated with improvement in survival after glioblastoma surgery. J Clin Neurosci (2016) 31:176–80. doi: 10.1016/j.jocn.2016.03.010
74. Jessurun CAC, Hulsbergen AFC, Lamba N, Nandoe Tewarie RDS, Smith TR, Broekman MLD. Practice variation in perioperative steroid dosing for brain tumor patients: an international survey. World Neurosurg (2022) 159:e431–41. doi: 10.1016/j.wneu.2021.12.067
75. Lim-Fat MJ, Bi WL, Lo J, Lee EQ, Ahluwalia MS, Batchelor TT, et al. Letter: when less is more: dexamethasone dosing for brain tumors. Neurosurgery (2019) 85(3):E607–8. doi: 10.1093/neuros/nyz186
76. Liu S, Song Y, Zhang IY, Zhang L, Gao H, Su Y, et al. RAGE inhibitors as alternatives to dexamethasone for managing cerebral edema following brain tumor surgery. Neurotherapeutics (2022) 19(2):635–48. doi: 10.1007/s13311-022-01207-w
77. Lehmann F, Potthoff AL, Borger V, Heimann M, Ehrentraut SF, Schaub C, et al. Unplanned intensive care unit readmission after surgical treatment in patients with newly diagnosed glioblastoma - forfeiture of surgically achieved advantages? Neurosurg Rev (2023) 46(1):30. doi: 10.1007/s10143-022-01938-6
Keywords: dexamethasone, glioblastoma, glioma, survival, immunosuppression
Citation: Mistry AM (2023) Perioperative dexamethasone in high-grade gliomas: the short-term benefits and long-term harms. Front. Oncol. 13:1335730. doi: 10.3389/fonc.2023.1335730
Received: 09 November 2023; Accepted: 04 December 2023;
Published: 14 December 2023.
Edited by:
Analiz Rodriguez, University of Arkansas for Medical Sciences, United StatesReviewed by:
L. Nicolas Gonzalez Castro, Harvard Medical School, United StatesCopyright © 2023 Mistry. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akshitkumar M. Mistry, YXhpdGFtbUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.