- 1Department of Oncological Surgery, North China University of Science and Technology Affiliated Hospital, Tangshan, Hebei, China
- 2College of Nursing and Rehabilitation, North China University of Science and Technology, Tangshan, Hebei, China
Background: The omission of axillary lymph node dissection (ALND) or axillary radiation (AxRT) remains controversial in patients with clinical node-negative early breast cancer and a positive sentinel lymph node.
Methods: We conducted a comprehensive review by searching PubMed, Embase, Web of Science, and Cochrane databases (up to November 2023). Our primary outcomes were overall survival (OS), disease-free survival (DFS), locoregional recurrence (LRR), and axillary recurrence (AR).
Results: We included 26 studies encompassing 145,548 women with clinical node-negative early breast cancer and positive sentinel lymph node. Pooled data revealed no significant differences between ALND and sentinel lymph node biopsy (SLNB) alone in terms of OS (hazard ratio [HR]0.99, 95% confidence interval [CI] 0.91-1.08, p=0.84), DFS (HR 1.04, 95% CI 0.90-1.19, p=0.61), LRR (HR 0.76, 95% CI 0.45-1.20, p=0.31), and AR (HR 1.01, 95% CI 0.99-1.03, p=0.35). Similarly, no significant differences were observed between AxRT and SLNB alone for OS (HR 0.57, 95% CI 0.32-1.02, p=0.06) and DFS (HR 0.52, 95% CI 0.26-1.05, p=0.07). When comparing AxRT and ALND, a trend towards higher OS was observed the AxRT group (HR 0.08, 95% CI 0.67-1.15), but the difference did not reach statistical significance (p=0.35, I2 = 0%). Additionally, no significant differences significance observed for DFS or AR (p=0.13 and p=0.73, respectively) between the AxRT and ALND groups.
Conclusion: Our findings suggest that survival and recurrence rates are not inferior in patients with clinical node-negative early breast cancer and a positive sentinel lymph node who receive SLNB alone compared to those undergoing ALND or AxRT.
Introduction
Axillary lymph node dissection (ALND) has been the standard therapeutic approach for breast cancer patients with positive sentinel lymph nodes. However, ALND is associated with various complications, including lymphedema, paresthesia, infections, axillary seromas, and other significant morbidities (1). Currently, sentinel lymph node biopsy (SLNB) is recommended for assessing axillary nodal lymph node status in early breast cancer patients who are clinically node-negative. The National Comprehensive Cancer Network (NCCN) suggests that ALND is not required for breast cancer patients with a negative sentinel node. The role of ALND for early-stage breast cancer patients with a limited number of metastatic sentinel lymph nodes remains controversial. According to the American Society of Clinical Oncology Clinical Practice Guideline, ALND should be considered for women with early breast cancer and one to two positive sentinel lymph nodes who are planning to undergo mastectomy (2). Pepels et al. indicated that ALND was recommended in patients with sentinel micrometastases and unfavorable tumor characteristics (3), while no ALND for patients with sentinel lymph nodes micrometastases resulted in a higher five -year regional recurrence rate compared to ALND (4).
However, Galimberti et al. suggest that ALND may be overtreatment for early-stage breast cancer patients, particularly when the tumor burden in the sentinel lymph nodes is minimal or moderate (5). NCCN also suggests that patients who have T1/T2 tumors, one to two positive sentinel lymph nodes, and plan to undergo whole-breast radiotherapy (RT) following breast-conserving therapy are not recommended for ALND (6). The ACOSOG Z0011 (American College of Surgeons Oncology Group) trial demonstrated that patients with clinical T1/T2 tumors and fewer than three positive sentinel lymph nodes undergoing lumpectomy and whole-breast radiation therapy could avoid ALND without negatively impacting local recurrence, disease-free survival, and overall survival (7).Additionally, the IBCSG 23-01 trial, designed to compare outcomes in patients with one or more sentinel micrometastases (≤2 mm) treated with ALND versus no ALND, showed no significant differences in five-year overall survival and five-year disease-free survival (8). A previous retrospective study also indicated that ALND did not improve either post-mastectomy overall survival or disease-free survival among breast cancer patients with one to three positive sentinel lymph nodes (9).
The AMAROS trial aimed to evaluate whether axillary radiation (AxRT) achieved better regional control and fewer side effects compared to ALND. Finding demonstrated that AxRT offered similar axillary control for patients with T1/T2 breast cancer and positive sentinel lymph nodes, while significantly reducing. the occurrence of lymphedema (10). A retrospective cohort study comparing patients with T1/T2 and less than two macrometastases (>2 mm) who underwent either AxRT or non-AxRT also observed similar overall and disease-free survival rates. There was no statistically significant difference in the five-year outcomes between the two groups (11). Motivated by these finding, we conducted a systematic review to compare outcomes in clinical node-negative early breast cancer patients with sentinel lymph node metastasis who underwent mastectomy or breast-conserving surgery. This meta-analysis aims to assess overall survival, disease-free survival, locoregional recurrence and axillary recurrence according to the type of axillary management(SLNB alone, ALND, or AxRT).
Materials and methods
Study selection
We conducted a systematic search of English literature in PubMed, Embase and Cochrane Library databases up to November 2023. Our search encompassed published data only. Search terms included keywords and MeSH terms such as “breast cancer/breast carcinoma”, “sentinel lymph node biopsy,” “axillary lymph node dissection”, and “axillary radiation,” Two authors (C.Z.L and P.Z) independently reviewed the available literature based on the inclusion criteria. Subsequently, potentially relevant references with sufficient information in their titles and abstracts were retrieved full-text article assessment. If the included studies were based on the same data, we selected the latest published version. Any disagreements were resolved through discussion and consensus among the authors. The study selection process adhered to the PRISMA guidelines.
Study inclusion and exclusion criteria
The inclusion criterial were as follows: (1) Design: randomized controlled trials (RCTs) and retrospective studies. (2) Patient eligibility: Studies enrolling patients with clinical node-negative early breast cancer and positive sentinel lymph node (3) Comparative interventions: SLNB alone versus ALND, ALND versus AxRT, and SLNB alone versus AxRT. (4) Outcomes: Studies reporting on overall survival, disease-free survival, axillary recurrence, and locoregional recurrence. The exclusion criterial included abstracts, reviews, case reports, and articles deemed irrelevant or containing missing data.
Data extraction and management
Following the Cochrane Handbook guidelines, two authors independently extracted data from the included studies. Recorded information included the authors’ names, publication year, number of participants, study design, intervention type, tumor stage, micometastasis or macrometastsis count, adjuvant radiation therapy, follow-up duration (years), outcomes, and the quality of evidence in each study. Any discrepancies were addressed and resolved through discussion or with the assistance of a third author.
Quality assessment in individual studies
The risk of bias in all included studies was evaluated using guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (https://training.cochrane.org/handbooks) (12). Two authors independently assessed the potential risk of bias, including selection bias, performance bias, detection bias, attrition bias, reporting bias, and confounding bias, and other sources of bias. For RCTs, the GRADEpro GDT (Grading of Recommendation Assessment Development and Evaluation Profiler Guide line Development Tool)was used to assess evidence quality. This online too, available at https://www.gradepro.org, evaluates five factors: risk of bias, inconsistency, indirectness, imprecision, and other considerations. Based on these factors, evidence quality is classified into four levels: high (⊕⊕⊕⊕), moderate (⊕⊕⊕⊖), low (⊕⊕⊖⊖) or very low (⊕⊖⊖⊖). For non-RCTs, the Newcastle-Ottawa Scale (NOS) served as the quality assessment tool (13). The NOS awards stars based on three domains: quality of patient selection (up to four stars), comparability between cases and controls (up to two stars), and adequate ascertainment of exposure (up to three stars). Studies with more than seven stars were considered to have a high level of evidence. Two authors independently assessed the quality of evidence in the included studies, With any disagreements resolved through discussion.
Statistical analysis
We used the Review Manager software (version 5.4), update by the Cochrane Library for Systematic Review, to perform the analysis. The summary statistic of generic inverse variance (overall survival, disease-free survival, axillary recurrence, and locoregional recurrence) was assessed by hazard ratios (HRs). 95% confidence intervals (CIs) were calculated using the fixed-effect model. The statistical heterogeneity of the included studies was quantified and examined using the I2 statistics. An I2 value of 0% to 25% indicates low heterogeneity, 25% to50% indicates moderate heterogeneity, 50% to75% indicates large heterogeneity, and 75% to100% indicates huge heterogeneity (14). When heterogeneity was observed, we employed the random-effects model. We conducted the subgroup analysis based on the type of axillary management (SLNB alone, ALND, and AxRT). Sensitivity analysis was performed to identify sources of heterogeneity. A funnel plot was used to assess publication bias in the included studies. A p value less than 0.05 was considered statistically significant.
Results
Study selection
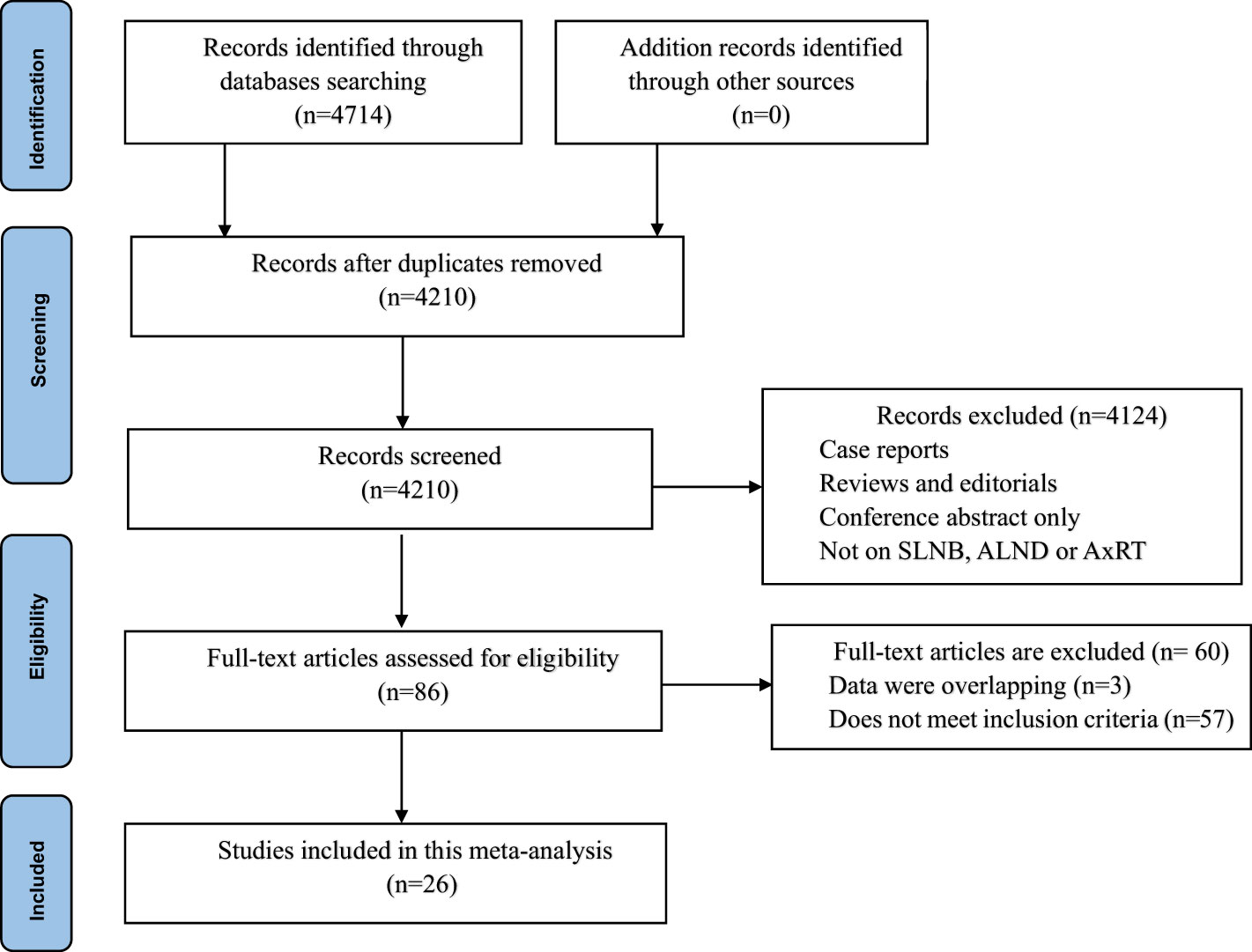
Our initial database search yielded a total of 4,714 studies. After removing 504 duplicate studies, we screened the titles and abstracts of 4,210remaining studies. A total of 4,124 studies were excluded due to irrelevance (non-related studies, review articles, case reports, meta-analysis, or lack of data). At the full-text level, 86 potentially eligible studies were assessed, t of which 60 were ultimately excluded after a thorough review. This left 26 studies involving 145,548 patients for inclusion in the meta-analysis (5, 7, 11, 15–36) The PRISMA flowchart in Figure 1 illustrates this selection process.
Study characteristics
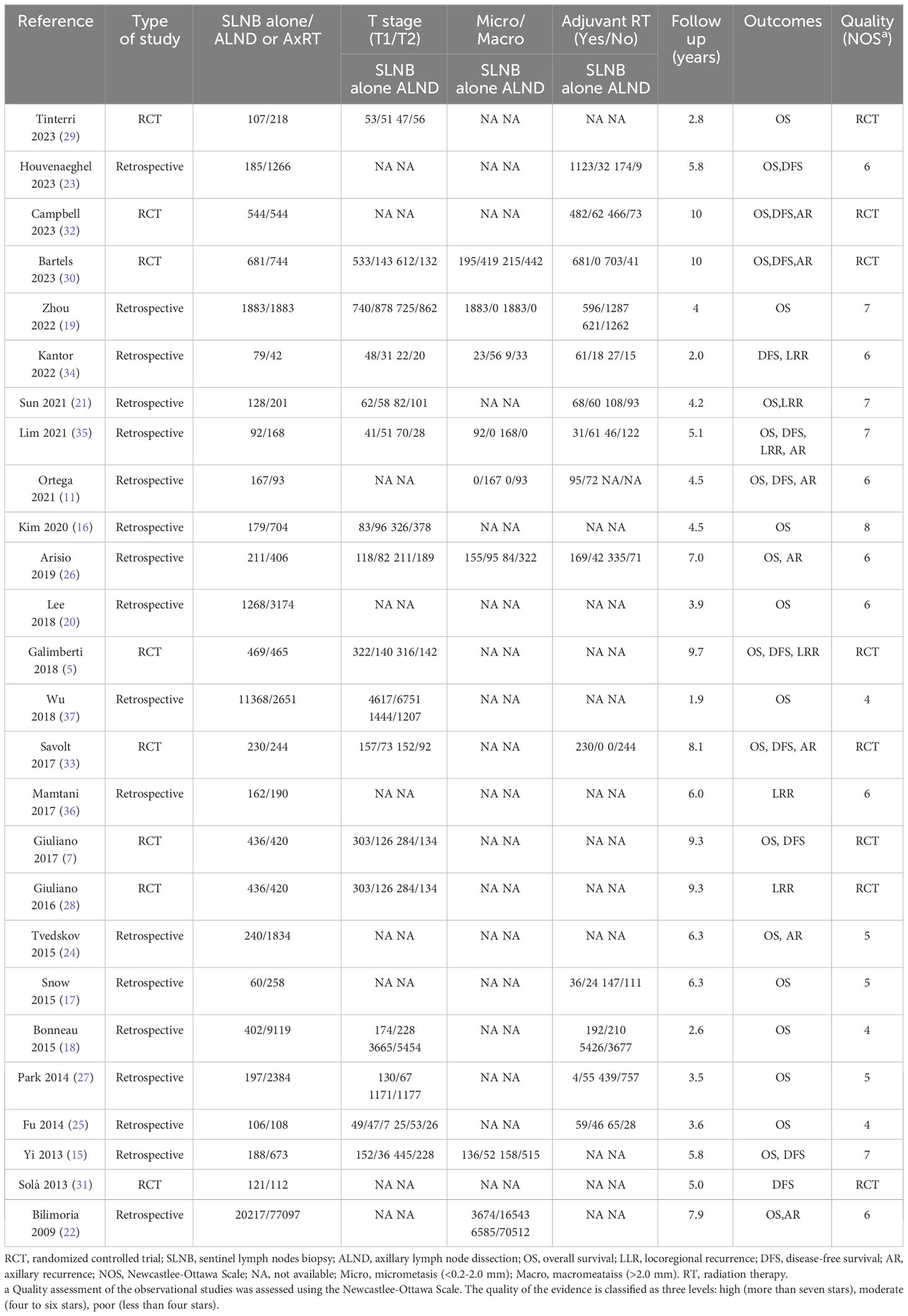
Table 1 outlines the characteristics of the included studies. Eighteen studies were retrospective cohort studies (11, 18–27, 34–36), while the remaining eight were RCTs (5, 7, 28–33). The studies were published between April 2009 to July 2023.Sample sizes range from 121 to 97,314 patients, with a total of 145,548 patients analyzed. Among these patients, 40,156 received SLNB alone, while 105,418 underwent either ALND or AxRT. All included studies were published in English. Twenty-two studies reported overall survival data (5, 7, 11, 15–27, 29, 30, 32, 33, 35). Eleven studies reported disease-free survival data (5, 7, 11, 15, 23, 30–35). Six studies analyzed locoregional recurrence (21, 26, 28, 35, 36), and eight studies reported axillary recurrence (11, 22, 24, 26, 30, 32, 33, 35).
Quality assessment
The NOS was used to assess the quality of evidence in non-RCT trials. Five studies received a rating of seven or more stars, indicating high quality (15, 16, 19, 21, 35).Seven studies received a rating of six stars (11, 20, 22, 23, 26, 34, 36), and six studies were assessed as having four to five stars (See Table 1). The GRADEpro GDT tool was used to classify the evidence of RCTs comparing ALND to SLNB alone for patients with clinical node-negative early-stage breast cancer and positive sentinel lymph nodes. The quality of evidence was high for disease-free survival and locoregional recurrence, and moderate in overall survival (See Table 2).
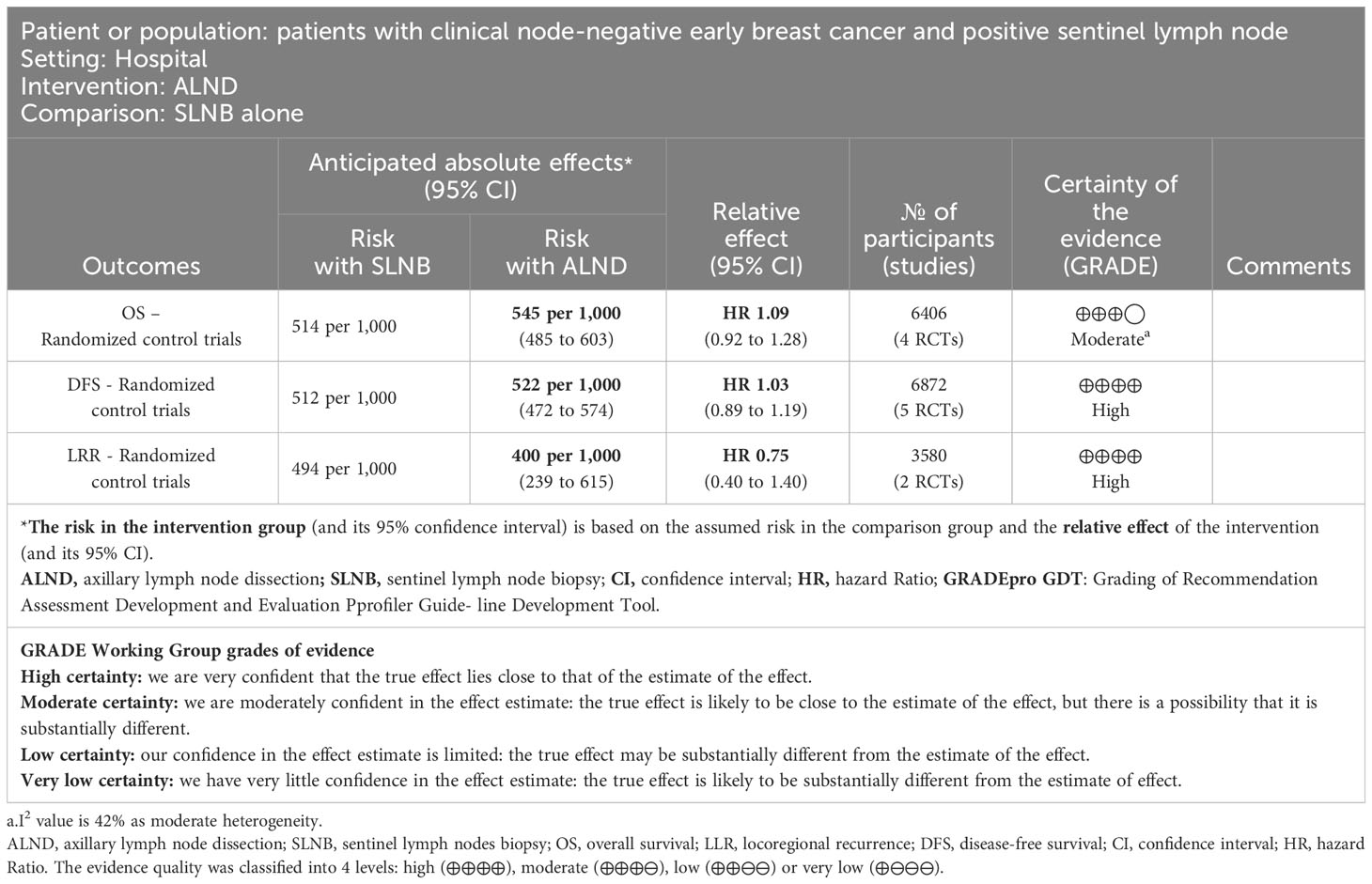
Table 2 Evaluating the quality of evidence in randomized controlled trials by GRADEpro GDT ALND compared to SLNB alone for patients with clinical node-negative early breast cancer and positive sentinel lymph node.
Effect of ALND versus SLNB alone
Eighteen studies (5, 7, 15–27, 29, 32, 35) reported the overall survival data for patients with SLNB alone versus ALND. The pooled results revealed no statistically significant difference between the groups (HR0.99, 95% CI0.91-1.08; p=0.84), and no significant heterogeneity was observed (I2 = 30%, p=0.11) (Figure 2A). A potential publication bias was suggested by the the asymmetry of the funnel plot (Figure 3A), Subgroup analysis showed no statistically significant difference in overall survival between SLNB alone and ALND in either RCTs (HR 1.09, 95% CI:0.92-1.28; p=0.33) or retrospective studies (HR 0.96, 95% CI:0.86-1.06; p=0.40). Data pooled from the eight studies (5, 7, 15, 20, 29, 31, 32, 35) demonstrated no substantial difference in disease-free survival between the SLNB alone group and ALND groups (HR 1.04, 95%CI:0.90-1.19; p=0.61). Additionally, the studies showed no significant heterogeneity (I2 = 0%, p=0.73) (Figure 2B). The funnel plot suggested the presence of publication bias (Figure 3B). Subgroup analysis revealed no significant difference in disease-free survival between RCTs (HR 1.03, 95%CI:0.89-1.19; p=0.72) or retrospective studies (HR 1.09, 95%CI:0.77-1.54; p=0.64).
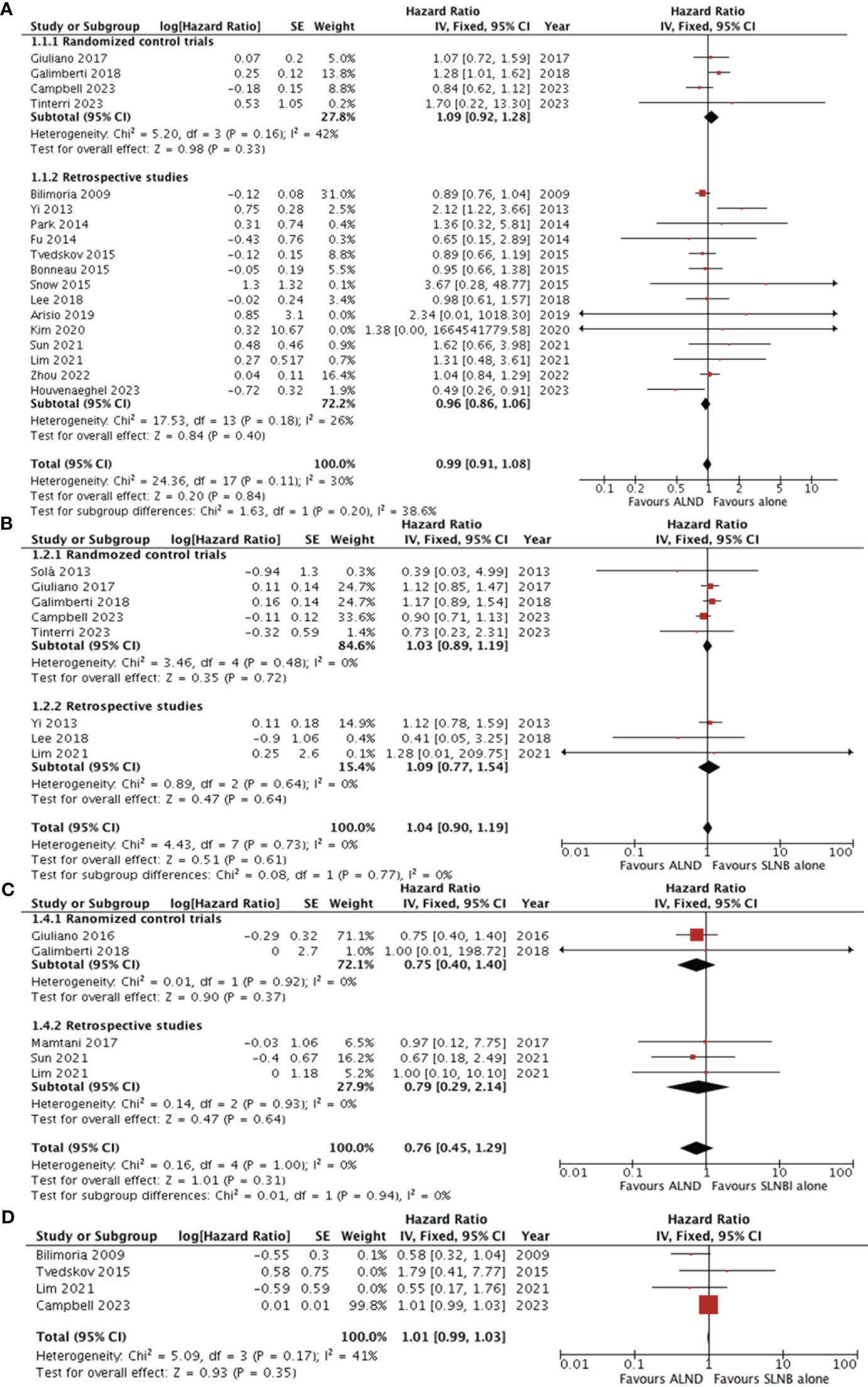
Figure 2 Forest plot of ALND versus SLNB alone (A) overall survival (B) disease-free survival (C) locoregional recurrence (D) axillary recurrence. ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; CI, confidence interval; SE, standard error; IV, Inverse Variance.
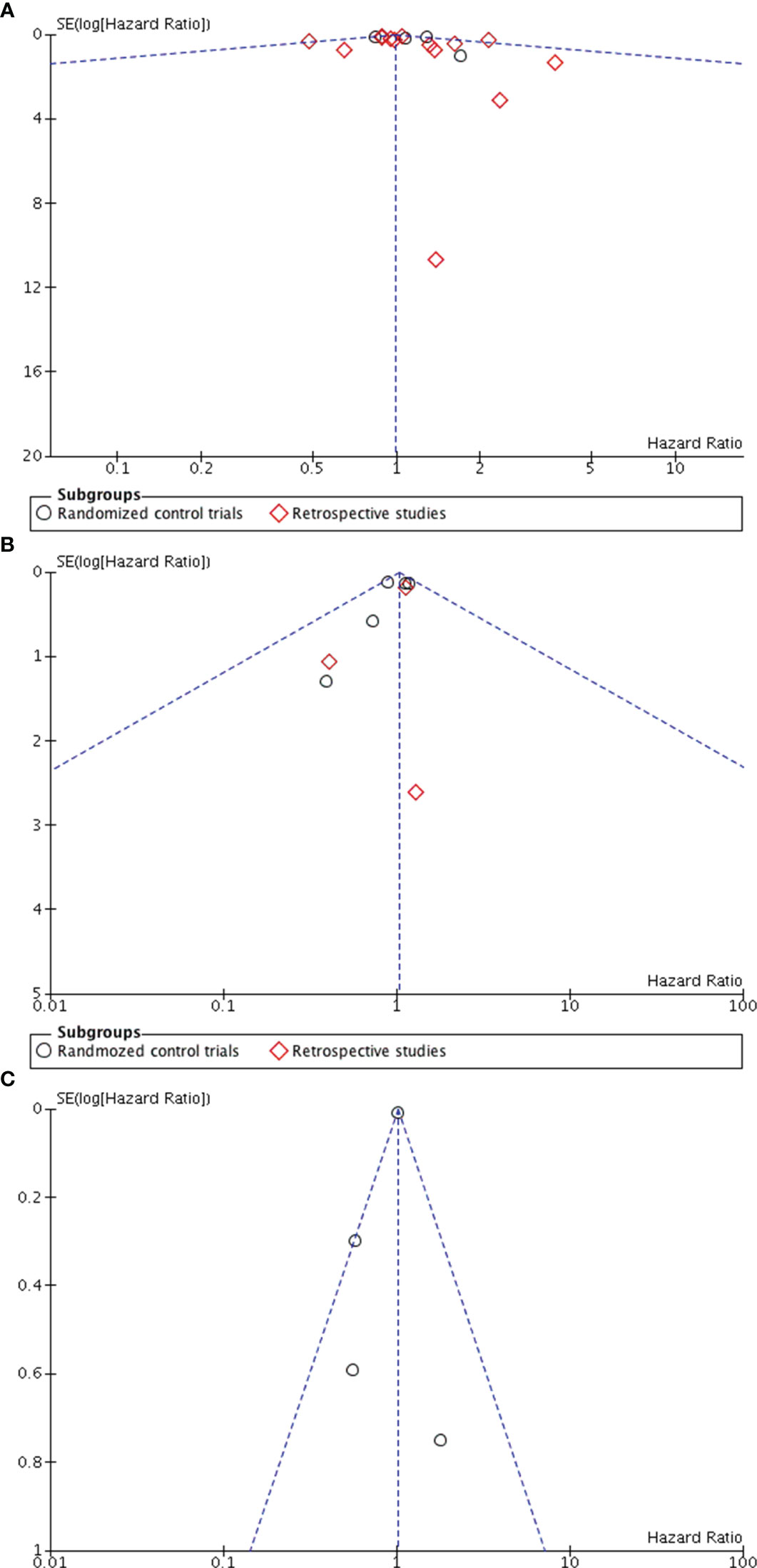
Figure 3 Funnel plot in ALND versus SLNB alone (A) Funnel plot for overall survival (B) Funnel plot for disease-free survival (C) Funnel plot for locoregional recurrence.
Five studies evaluated locoregional recurrence (5, 21, 28, 35, 36). Pooled data indicated no statistically significant difference between patients who received SLNB alone and those who underwent ALND (HR 0.76, 95%CI 0.45-1.29; p = 0.31) (Figure 2C).However, the asymmetry of the funnel plot (Figure 3C) suggests potential publication bias.
Four studies reported the five-year cumulative incidence of axillary recurrence (22, 24, 32, 35). Although the axillary recurrence rate was higher in the SLNB alone group compared to the ALND group, but this difference was not statistically significant (HR1.01, 95% CI 0.99-1.03; p = 0.35) (Figure 2D).Additionally, the studies showed no significant heterogeneity (I2 = 41%, p = 0.17). However, only one study reported the 10-year axillary recurrence outcome (32). This study found that axillary recurrence was more frequent among those who received SLNB alone compared to ALND (HR 5.47, 95% CI 1.21-24.63; p=0.013).
Effect of ALND versus AxRT
Four studies (25, 30, 33, 37) compared overall survival between ALND and AxRT. Pooled data analysis revealed no significant difference between the two groups (HR 0.88, 95% CI: 0.67-1.15; p=0.35) (Figure 4A). Additionally, pooling data from three studies (30, 33, 34) assessing disease-free survival also showed no significant difference between ALND and AxRT (HR 0.85, 95% CI: 0.68-1.05; p=0.13). Furthermore, these studies exhibited no significant heterogeneity(I2 = 0%, p=0.71) (Figure 4B). Two studies (30, 33) reported axillary recurrence. While the axillary recurrence rate was higher in the AxRT group compared to the ALND group, the difference was not statistically significant (HR 0.94, 95% CI: 0.68-1.31; p=0.73) (Figure 4C). There was also no significant between-study heterogeneity(I2 = 21%, p=0.26).
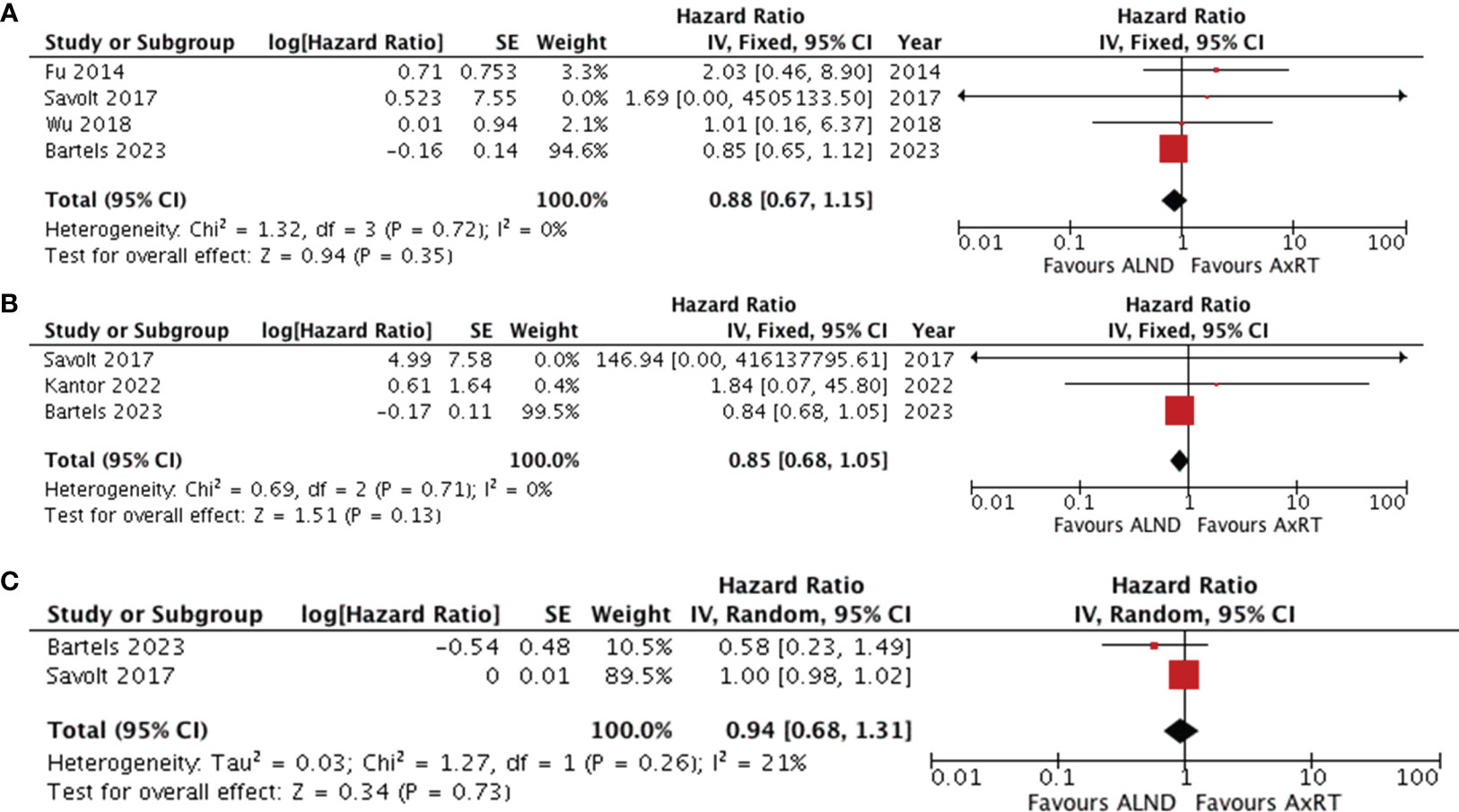
Figure 4 Forest plot of ALND versus AxRT (A) overall survival (B) disease-free survival (C) axillary recurrence. ALND, axillary lymph node dissection; AxRT, axillary radiation; CI, confidence interval; SE, standard error; IV, Inverse Variance.
Effect of AxRT versus SLNB alone
Four studies (11, 25, 35, 37) assessed overall survival by comparing the AxRT group to the SLNB alone group. The results revealed no statistical difference in overall survival between the groups (HR 0.57, 95% CI: 0.32-1.02; p=0.25), with moderate heterogeneity between studies (χ2 = 4.12, I2 = 27%, p=0.27) (Figure 5A). The asymmetry of the funnel plot suggests publication bias.
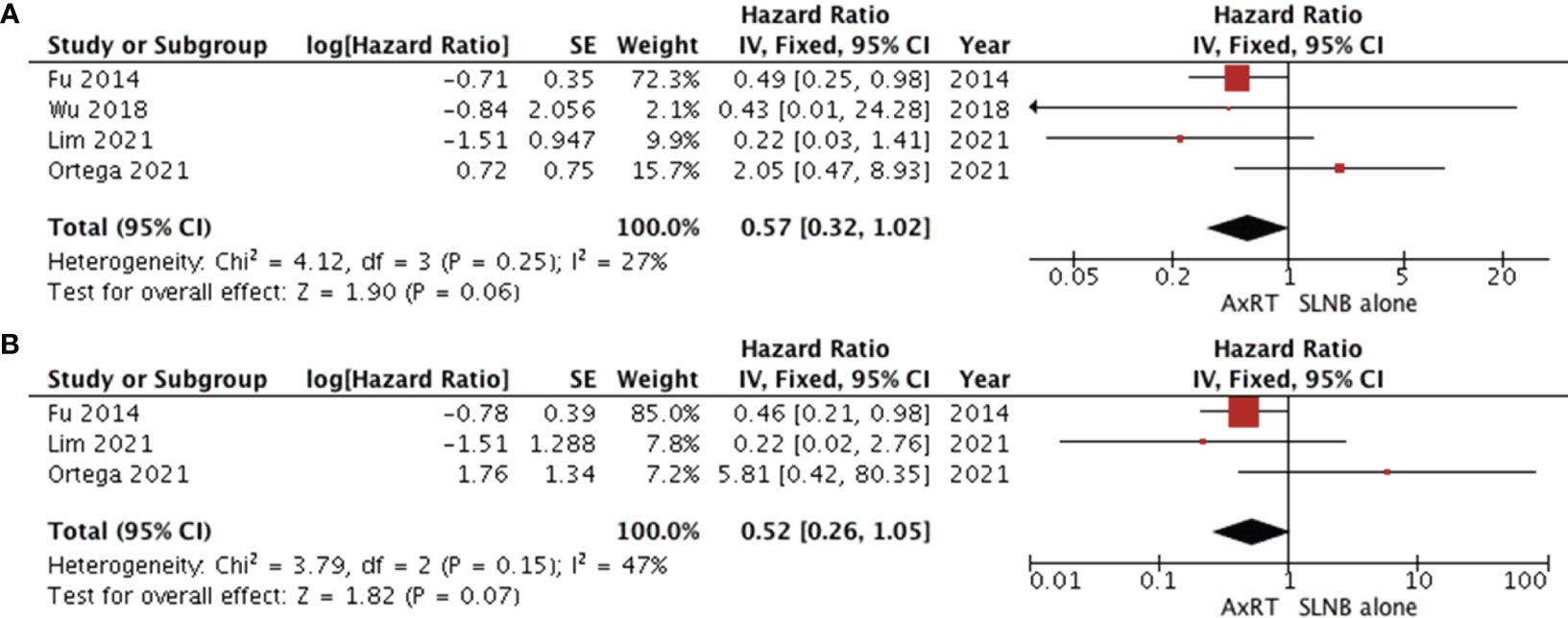
Figure 5 Forest plot of AxRT versus SLNB lone (A) overall survival (B) disease-free survival. AxRT, axillary radiation; SLNB, sentinel lymph node biopsy; CI, confidence interval; SE, standard error; IV,Inverse Variance.
Three studies (11, 25, 35) reported disease-free survival. Patients who received AxRT had a higher rate of disease-free survival than those who underwent SLNB alone (HR 0.52, 95% CI: 0.26-1.05).However, no statistically significant difference was observed between groups (p=0.07). There was moderate heterogeneity among studies (χ2 = 3.79, I2 = 47%, p=0.15) as shown in Figure 5B.
Discussion
This meta-analysis aimed to compare the effects of SLNB alone, ALND, and AxRT on various outcomes, including overall survival, disease-free survival, locoregional recurrence, and axillary recurrence, in 145,548 patients with early-stage breast cancer, clinical negative axillary lymph nodes, and positive sentinel lymph nodes. The collected data revealed no significant differences in overall survival, disease-free survival, locoregional recurrence, and axillary recurrence between the SLNB alone group and the ALND or AxRT groups. While the AxRT group showed a higher overall survival rate compared to the ALND group, this difference was not statistically significant. Additionally, no significant disparities were observed in terms of overall survival and disease-free survival between patients who received AxRT and those who received SLNB alone.
Several meta-analyses (38–47) have been conducted to compare the differences in overall survival, disease-free survival, and recurrence rates between ALND and SLNB alone in early-stage breast cancer patients with positive sentinel lymph nodes. However, the impact of ALND remains controversial. Peristeri et al. (38) performed a meta-analysis comparing the effects of SLNB/RT and ALND in five RCTs. Their pooled data showed that the SLNB/RT group had better overall and disease-free survival than the ALND group, with a statistically significant difference in axillary recurrence favoring the ALND group. However, our previous meta-analysis comparing the two approaches in early-stage breast cancer with sentinel lymph node metastasis (43), found no significant differences in overall survival, disease-free survival and locoregional recurrence between the SLNB alone and the ALND group. Similarly, a meta-analysis of Real-World Evidence in the Post-ACOSOG Z0011 trial (39), which included one RCT and six retrospective studies with 8,864 early-stage breast cancer patients with one or two SLN metastases, found no differences between SLNB alone and ALND groups in overall survival, disease-free survival, and recurrence rate. However, the incidence rate of lymphedema was significantly lower in SLNB alone group Our systematic review and meta-analysis included eight RCTs and eighteen retrospective cohort studies. The results indicated no statistically significant difference in disease-free survival, overall survival, and locoregional recurrence between ALND and SLNB alone in clinical node-negative early breast cancer patients with positive sentinel lymph nodes. Three studies reported the five-year cumulative incidence of axillary recurrence. The rate was higher in the SLNB alone group compared to the ALND group, but this difference was not statistically significant (p = 0.36). However, when the follow-up period was extended to 10 years, Campbell et al. (32) found that that axillary recurrence was more frequent in the SLNB alone group compared to ALND group. Therefore, longer follow-up times are required to definitively compare the axillary recurrence rates between the two groups in this patients population. After ten year of follow-up, the Randomized Controlled EORTC AMAROS trials (30) showed that both AxRT and ALND groups achieved excellent locoregional control and survival in cT1-T2 breast cancer patients with positive sentinel lymph nodes. Additionally, the AxRT group had a lower rate of lymphedema and no difference in quality of life compared to the ALND group. This meta-analysis also confirms no significant differences in disease-free survival, overall survival, and axillary recurrence between patients treated with ALND versus AxRT.
Our review diverges from previous studies in several key aspects. First, by incorporating eight RCTs and 18 retrospective studies involving 145,548 patients, our meta-analysis significantly increase the sample size, leading to more precise and reliable results. Second, we employed the GRADEpro GTD tool to assess evidence quality within RCTs, revealing high quality for disease-free survival and locoregional recurrence, and moderate quality for overall survival. Furthermore, subgroup analyses based on study type (RCT vs. retrospective) demonstrated that the pooled analysis results remained unchanged, indicating the stability of our finding. Third, and uniquely, our review evaluated the effects of AxRT and SLNB alone in patients with clinical node-negative early breast cancer and positive sentinel lymph nodes, an aspect lacking in prior meta-analysis. However, limitations exist within our meta-analysis. First, our inclusion criteria restricted us to English studies only, potentially introducing publication bias by excluding unpublished data. Second, the NOS tool revealed six non-RCT studies with a, four to five-star rating, including lower-quality evidence. Third, both overall and disease-free survival analyses showed evidence of publication bias. Fourth, moderate heterogeneity was observed among studies regarding disease-free and overall survival when comparing the AxRT and SLNB alone groups. A study by Ortega et al. (11) identified potential sources of this heterogeneity. While removing their study resulted in a significant decrease in heterogeneity for both outcomes, the overall and disease-free survival data also changed substantially(Supplementary Figures 1, 2). Therefore, further careful evaluation of the effect of AxRT versus SLNB alone is required, and these results necessitate confirmation through well-designed prospective studies. Finally, because the lack of sufficient information within the included studies precluded further subgroup analyses based on factors like the T1/T2 stage, number of positive sentinel lymph nodes, micrometastasis or macrometastasis, molecular subtype, and age.
Conclusion
This study demonstrates that patients with early-stage breast cancer and positive sentinel lymph nodes who undergo SLNB alone achieve comparable locoregional control and survival to those who receive ALND or AxRT. Our findings suggest that omitting ALND or AxRT may be safe for these patients, although further verification is needed through rigorously designed prospective studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
PZ: Data curation, Formal Analysis, Methodology, Writing – review & editing. CL: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. JL: Data curation, Investigation, Project administration, Writing – review & editing. WD: Formal Analysis, Methodology, Writing – review & editing. BH: Investigation, Methodology, Project administration, Software, Supervision, Writing – review & editing. JZ: Data curation, Investigation, Methodology, Writing – review & editing. HZ: Investigation, Methodology, Project administration, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Hebei Province University Basic Research Business Fee Project (Project number: JYG2020003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1320867/full#supplementary-material
Supplementary Figure 1 | Forest plot of overall survival after heterogeneity analysis in AxRT versus SLNB lone.
Supplementary Figure 2 | Forest plot of disease-free survival after heterogeneity analysis in AxRT versus SLNB lone.
References
1. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol (2007) 25:3657–63. doi: 10.1200/JCO.2006.07.4062
2. Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: american society of clinical oncology clinical practice guideline update. J Clin Oncol (2017) 35:561–4. doi: 10.1200/JCO.2016.71.0947
3. Pepels MJ, de Boer M, Bult P, van Dijck JA, van Deurzen CH, Menke-Pluymers MB, et al. Regional recurrence in breast cancer patients with sentinel node micrometastases and isolated tumor cells. Ann Surg (2012) 255:116–21. doi: 10.1097/SLA.0b013e31823dc616
4. Pepels MJ, Vestjens JH, de Boer M, Smidt M, van Diest PJ, Borm GF, et al. Safety of avoiding routine use of axillary dissection in early stage breast cancer: a systematic review. Breast Cancer Res Treat (2011) 125:301–13. doi: 10.1007/s10549-010-1210-7
5. Galimberti V, Cole BF, Viale G, Veronesi P, Vicini E, Intra M, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol (2018) 19:1385–93. doi: 10.1016/S1470-2045(18)30380-2
6. National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology: breast. (2021). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed on 20 December 2021.
7. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA (2017) 318:918–26. doi: 10.1001/jama.2017.11470
8. Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol (2013) 14:297–305. doi: 10.1016/S1470-2045(13)70035-4
9. Joo JH, Kim SS, Son BH, Ahn SD, Jung JH, Choi EK, et al. Axillary lymph node dissection does not improve post-mastectomy overall or disease-free survival among breast cancer patients with 1-3 positive nodes. Cancer Res Treat (2019) 51:1011–21. doi: 10.4143/crt.2018.438
10. Donker M, van Tienhoven G, Straver ME, Meijnen P, van de Velde CJ, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol (2014) 15:1303–10. doi: 10.1016/S1470-2045(14)70460-7
11. Ortega Expósito C, Falo C, Pernas S, Pérez Carton S, Gil Gil M, Ortega R, et al. The effect of omitting axillary dissection and the impact of radiotherapy on patients with breast cancer sentinel node macrometastases: a cohort study following the ACOSOG Z0011 and AMAROS trials. Breast Cancer Res Treat (2021) 189:111–20. doi: 10.1007/s10549-021-06274-9
12. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. Wells GA SB OCD, Peterson J, Welch V, Peterson J, Welch V, Losos M, et al. The NewcastleOttawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
14. Hatala R, Keitz S, Wyer P, Guyatt G. Tips for learners of evidence-based medicine: 4. Assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. CMAJ (2005) 172:661–5. doi: 10.1503/cmaj.1031920
15. Yi M, Kuerer HM, Mittendorf EA, Hwang RF, Caudle AS, Bedrosian I, et al. Impact of the american college of surgeons oncology group Z0011 criteria applied to a contemporary patient population. J Am Coll Surg (2013) 216:105–13. doi: 10.1016/j.jamcollsurg.2012.09.005
16. Kim BK, Park BW, Hur MH, Lee HB, Park MH, Jeong J, et al. Omission of axillary lymph node dissection in patients who underwent total mastectomy with 1 or 2 metastatic lymph nodes. Ann Surg Treat Res (2020) 98:283–90. doi: 10.4174/astr.2020.98.6.283
17. Snow R, Reyna C, Johns C, Lee MC, Sun W, Fulp WJ, et al. Outcomes with and without axillary node dissection for node-positive lumpectomy and mastectomy patients. Am J Surg (2015) 210:685–93. doi: 10.1016/j.amjsurg.2015.05.004
18. Bonneau C, Hequet D, Estevez JP, Pouget N, Rouzier R. Impact of axillary dissection in women with invasive breast cancer who do not fit the Z0011 ACOSOG trial because of three or more metastatic sentinel lymph nodes. Eur J Surg Oncol (2015) 41:998–1004. doi: 10.1016/j.ejso.2015.04.003
19. Zhou Y, Pu S, Jiang S, Li D, Li S, Liu Y, et al. The prognostic significance of further axillary dissection for sentinel lymph node micrometastases in female breast cancer: A competing risk analysis using the SEER database. Front Oncol (2022) 12:1012646. doi: 10.3389/fonc.2022.1012646
20. Lee J, Choi JE, Kim SJ, Lee SB, Seong MK, Jeong J, et al. Comparative study between sentinel lymph node biopsy and axillary dissection in patients with one or two lymph node metastases. J Breast Cancer (2018) 21:306–14. doi: 10.4048/jbc.2018.21.e44
21. Sun J, Mathias BJ, Laronga C, Sun W, Zhou JM, Fulp WJ, et al. Impact of axillary dissection among patients with sentinel node-positive breast cancer undergoing mastectomy. J Natl Compr Canc Netw (2021) 19:40–7. doi: 10.6004/jnccn.2020.7597
22. Bilimoria KY, Bentrem DJ, Hansen NM, Bethke KP, Rademaker AW, Ko CY, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol (2009) 27:2946–53. doi: 10.1200/JCO.2008.19.5750
23. Houvenaeghel G, de Nonneville A, Chopin N, Classe JM, Mazouni C, Chauvet M, et al. The need to tailor the omission of axillary lymph node dissection to patients with good prognosis and sentinel node micro-metastases. Cancer Med (2023) 12:4023–32. doi: 10.1002/cam4.5257
24. Tvedskov TF, Jensen MB, Ejlertsen B, Christiansen P, Balslev E, Kroman N. Prognostic significance of axillary dissection in breast cancer patients with micrometastases or isolated tumor cells in sentinel nodes: a nationwide study. Breast Cancer Res Treat (2015) 153:599–606. doi: 10.1007/s10549-015-3560-7
25. Fu Y, Chung D, Cao MA, Apple S, Chang H. Is axillary lymph node dissection necessary after sentinel lymph node biopsy in patients with mastectomy and pathological N1 breast cancer? Ann Surg Oncol (2014) 21:4109–23. doi: 10.1245/s10434-014-3814-3
26. Arisio R, Borella F, Porpiglia M, Durando A, Bellino R, Bau MG, et al. Axillary Dissection vs. no Axillary Dissection in Breast Cancer Patients With Positive Sentinel Lymph Node: A Single Institution Experience. In Vivo (2019) 33:1941–7. doi: 10.21873/invivo.11689
27. Park HS, Chae BJ, Song BJ, Jung SS, Han W, Nam SJ, et al. Effect of axillary lymph node dissection after sentinel lymph node biopsy on overall survival in patients with T1 or T2 node-positive breast cancer: Report from the Korean Breast Cancer Society. Ann Surg Oncol (2014) 21:1231–6. doi: 10.1245/s10434-013-3350-6
28. Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the american college of surgeons oncology group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg (2016) 264:413–20. doi: 10.1097/SLA.0000000000001863
29. Tinterri C, Canavese G, Gatzemeier W, Barbieri E, Bottini A, Sagona A, et al. Sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer patients undergoing mastectomy with one to two metastatic sentinel lymph nodes: sub-analysis of the SINODAR-ONE multicentre randomized clinical trial and reopening of enrolment. Br J Surg (2023) 110:1143–52. doi: 10.1093/bjs/znad215
30. Bartels SAL, Donker M, Poncet C, Sauve N, Straver ME, van de Velde CJH, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS trial. J Clin Oncol (2023) 41:2159–65. doi: 10.1200/JCO.22.01565
31. Solá M, Alberro JA, Fraile M, Santesteban P, Ramos M, Fabregas R, et al. Complete axillary lymph node dissection versus clinical follow-up in breast cancer patients with sentinel node micrometastasis: Final results from the multicenter clinical trial AATRM 048/13/2000. Ann Surg Oncol (2013) 20:120–7. doi: 10.1245/s10434-012-2569-y
32. Campbell I, Wetzig N, Ung O, Espinoza D, Farshid G, Collins J, et al. 10-Year axillary recurrence in the RACS SNAC1 randomised trial of sentinel lymph node-based management versus routine axillary lymph node dissection. Breast (2023) 70:70–5. doi: 10.1016/j.breast.2023.06.009
33. Savolt A, Peley G, Polgar C, Udvarhelyi N, Rubovszky G, Kovacs E, et al. Eight-year follow up result of the OTOASOR trial: The Optimal Treatment Of the Axilla - Surgery Or Radiotherapy after positive sentinel lymph node biopsy in early-stage breast cancer: A randomized, single centre, phase III, non-inferiority trial. Eur J Surg Oncol (2017) 43:672–9. doi: 10.1016/j.ejso.2016.12.011
34. Kantor O, Means J, Grossmith S, Dey T, Bellon JR, Mittendorf EA, et al. Optimizing axillary management in clinical T1-2N0 mastectomy patients with positive sentinel lymph nodes. Ann Surg Oncol (2022) 29:972–80. doi: 10.1245/s10434-021-10726-3
35. Lim SZ, Kusumawidjaja G, Mohd Ishak HM, Tan BKT, Tan SY, Hamzah JL, et al. Outcomes of stage I and II breast cancer with nodal micrometastases treated with mastectomy without axillary therapy. Breast Cancer Res Treat (2021) 189:837–43. doi: 10.1007/s10549-021-06341-1
36. Mamtani A, Patil S, Stempel M, Morrow M. Axillary micrometastases and isolated tumor cells are not an indication for post-mastectomy radiotherapy in stage 1 and 2 breast cancer. Ann Surg Oncol (2017) 24:2182–8. doi: 10.1245/s10434-017-5866-7
37. Wu SP, Tam M, Shaikh F, Lee A, Chun J, Schnabel F, et al. Post-mastectomy radiation therapy in breast cancer patients with nodal micrometastases. Ann Surg Oncol (2018) 25:2620–31. doi: 10.1245/s10434-018-6632-1
38. Peristeri DV, Harissis HV. Axillary lymph node dissection vs sentinel biopsy only among women with early-stage breast cancer and sentinel node metastasis: A systematic review and meta-analysis. Breast J (2021) 27:158–64. doi: 10.1111/tbj.14140
39. Huang TW, Su CM, Tam KW. Axillary management in women with early breast cancer and limited sentinel node metastasis: A systematic review and metaanalysis of real-world evidence in the post-ACOSOG Z0011 era. Ann Surg Oncol (2021) 28:920–9. doi: 10.1245/s10434-020-08923-7
40. Cao S, Liu X, Cui J, Liu X, Zhong J, Yang Z, et al. Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: An up-to-date meta-analysis of 3,578 patients. Breast (2021) 59:256–69. doi: 10.1016/j.breast.2021.07.015
41. Liang S, Hallet J, Simpson JS, Tricco AC, Scheer AS. Omission of axillary staging in elderly patients with early stage breast cancer impacts regional control but not survival: A systematic review and meta-analysis. J Geriatr Oncol (2017) 8:140–7. doi: 10.1016/j.jgo.2016.12.003
42. Huang TW, Kuo KN, Chen KH, Chen C, Hou WH, Lee WH, et al. Recommendation for axillary lymph node dissection in women with early breast cancer and sentinel node metastasis: A systematic review and meta-analysis of randomized controlled trials using the GRADE system. Int J Surg (2016) 34:73–80. doi: 10.1016/j.ijsu.2016.08.022
43. Li CZ, Zhang P, Li RW, Wu CT, Zhang XP, Zhu HC. Axillary lymph node dissection versus sentinel lymph node biopsy alone for early breast cancer with sentinel node metastasis: A meta-analysis. Eur J Surg Oncol (2015) 41:958–66. doi: 10.1016/j.ejso.2015.05.007
44. Joyce DP, Manning A, Carter M, Hill AD, Kell MR, Barry M. Meta-analysis to determine the clinical impact of axillary lymph node dissection in the treatment of invasive breast cancer. Breast Cancer Res Treat (2015) 153:235–40. doi: 10.1007/s10549-015-3549-2
45. Glechner A, Wöckel A, Gartlehner G, Thaler K, Strobelberger M, Griebler U, et al. Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: a systematic review and meta-analysis. Eur J Cancer (2013) 49:812–25. doi: 10.1016/j.ejca.2012.09.010
46. Wang Z, Wu LC, Chen JQ. Sentinel lymph node biopsy compared with axillary lymph node dissection in early breast cancer: a meta-analysis. Breast Cancer Res Treat (2011) 129:675–89. doi: 10.1007/s10549-011-1665-1
Keywords: breast cancer, sentinel lymph node biopsy, axillary lymph node dissection, axillary radiation, axillary management
Citation: Li C, Zhang P, Lv J, Dong W, Hu B, Zhang J and Zhu H (2024) Axillary management in patients with clinical node-negative early breast cancer and positive sentinel lymph node: a systematic review and meta-analysis. Front. Oncol. 13:1320867. doi: 10.3389/fonc.2023.1320867
Received: 13 October 2023; Accepted: 13 December 2023;
Published: 08 January 2024.
Edited by:
Kevin Ni, St George Hospital Cancer Care Centre, AustraliaReviewed by:
Laurence Gluch, The Strathfield Breast Centre, AustraliaIbrahim Mohamed Elzayat, Aswan University, Egypt
Copyright © 2024 Li, Zhang, Lv, Dong, Hu, Zhang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan Zhang, emhhbmdwYW4wOTAxQDE2My5jb20=
 Changzai Li1
Changzai Li1 Pan Zhang
Pan Zhang