
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 21 December 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1310253
Africa is the continent most affected by esophageal cancer in the world. Alcoholic beverages are controversially blamed, as esophageal cancer is a rare disease in several other countries ranked in the top 10 for consumption of alcoholic beverages. This study aims to conduct a comprehensive systematic review of published literature, statistically summarizing the strength of the association between drinking patterns and types, and the risk of esophageal cancer in Africa. A computerized search of reputable databases such as Medline/PubMed, EMBASE, Web of Science, and African Journals Online was performed to identify relevant studies published up to September 2023. The quality of the studies was evaluated using the Newcastle-Ottawa scale for case-control studies and the Agency for Healthcare Research and Quality tool for cross-sectional studies. A funnel plot and Egger test were utilized to assess potential publication bias. Meta-analyses were conducted using random-effects models with RevMan 5.3 and Stata software to estimate summary effects. The systematic review identified a total of 758,203 studies, primarily from Eastern and Southern Africa. The pooled samples across all studies comprised 29,026 individuals, including 11,237 individuals with cancer and 17,789 individuals without cancer. Meta-analysis revealed a significant association between alcohol consumption and the risk of esophageal cancer (odds ratio [OR] = 1.81; 95% confidence interval [CI], 1.50-2.19). Further analysis based on the frequency of alcoholic beverage consumption indicated a stronger association with daily (OR = 2.38; 95% CI, 1.81-3.13) and weekly (OR = 1.94; 95% CI, 1.32-2.84) drinkers in contrast to occasional drinkers (OR = 1.02; 95% CI, 0.81-1.29). Additionally, consumption of traditional alcoholic beverages was significantly associated with the risk of esophageal cancer in African populations (OR = 2.00; 95% CI, 1.42-2.82). However, no relationship has been established between the exclusive consumption of non-traditional drinks and the risk of esophageal cancer. In conclusion, the results of this study confirm the hypothesis that daily and weekly drinking patterns, significantly increase the risk of esophageal cancer in Africa, while occasional consumption does not show a significant association. Additionally, the consumption of traditional alcoholic beverages is notably linked to the risk of esophageal cancer in African populations.
Alcoholic beverages hold a significant societal role, fostering social connections and engagement, especially within specific social and religious contexts and for pleasure (1). Consumption of these beverages has witnessed a notable increase in various countries in recent years, notably since the onset of the Covid-19 pandemic (2). Particularly in Africa, alcohol consumption has surged rapidly, rising from 8% in 2018 to 15% in 2023 (3). However, excessive alcohol intake is closely linked to health risks, including mental and behavioral disorders, alcohol dependence, as well as serious noncommunicable diseases such as cirrhosis of the liver, cardiovascular diseases, and certain cancers (4). According to the World Health Organization (WHO), harmful alcohol consumption is responsible for approximately 5.3% of all global annual deaths, marking it as a significant societal issue (4). Despite governmental regulations to mitigate the adverse effects of alcoholic beverages on both the body and behavior, they remain a pivotal risk factor for numerous types of cancers, particularly esophageal cancer (5, 6).
Esophageal cancer (EC) is the seventh most common type of cancer worldwide and the sixth most common cause of cancer death (7), with an incidence rate of 3.1%. It is generally asymptomatic during the early stages of the disease. As the disease progresses, dysphagia with or without weight loss becomes apparent (8). In 2020, around 604100 new cases and 544076 deaths were recorded worldwide, of which around 40% lived along the East African corridor stretching from Ethiopia to South Africa (7). In the absence of action, 739,666 new cases and 723,466 deaths will be recorded in 2030, and 987,723 new cases and 914,304 deaths in 2040 (7). In the high-risk regions of Africa, Esophageal Squamous Cell Carcinomas are the most common type (9). The average age at diagnosis is 55 years old, and men are more affected by the disease than women (10). This disease persists as a significant obstacle for health authorities in Africa countries, especially in East African corridor.
Various researchers have independently explored the association between alcohol consumption and EC, particularly within the regions of highest incidence in Africa. However, the potential link between alcohol consumption and cancer risk in these populations remains a topic of ongoing debate, lacking a clear consensus. Studies such as those by Segal et al. (11), Middleton et al. (10), and Musukume et al. (12) have reported notably high risks (3.77 ≤ OR ≤ 5.09) of developing esophageal cancer due to alcohol consumption. Conversely, other research works like those by Leon et al. (13) and Deybasso et al. (14) have indicated lower risks (OR < 1) of esophageal cancer development among individuals who consume alcoholic beverages compared to those who abstain. While a few global meta-analyses have examined the alcohol-esophageal cancer association (6), there hasn’t been a systematic analysis focusing on Africa. Moreover, no meta-analysis to date has established the relationship between the frequency of consumption of alcoholic beverages, or the types of alcoholic beverages consumed, and EC in Africa. Above all, the rich diversity of African cultures has given rise to a multitude of traditional alcoholic beverages, the precise composition of which often remains unknown. This diversity further complicates efforts to discern the link between alcohol consumption patterns and esophageal cancer. To comprehensively understand the prevalence of esophageal cancer in Africa, we conducted a qualitative and quantitative review of the literature examining the relationship between drinking habits and this disease in these regions.
This systematic review and meta-analysis were conducted based on the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The review protocol is registered at the International Prospective Register of Systematic Reviews (PROSPERO) under number CRD42023463704.
The following eligibility criteria were used to identify the studies. Inclusion criteria: (1) Observational studies with Alcohol consumption as the exposure and esophageal cancer (EC) risk as the outcome. (2) All studies must contain available data reporting the relationship between alcohol drinking and EC. (3) Studies must have been conducted on the African continent (Eastern and Southern Africa region) and involve human participants. Studies were excluded according to the following exclusion criteria: (1) Unpublished articles; nonhuman research; anonymous reports; editorials, letters, commentaries, and reviews will be excluded. (2) Studies that do not provide estimates of effect in the form of odds ratios, rate ratios, risk ratios, or relative risks, or that do not allow these values to be calculated, will also be excluded. (3) Studies whose data are inaccessible, even after request to their authors, will also be excluded. (4) No sample size restrictions will be considered.
Electronic searches of databases and manual searches of other resources were conducted by two researchers (GTK and EJN) to identify published studies for review. The Medline/PubMed, EMBASE, Web of Science, and African Journals Online databases were searched for studies published up to September 2023. These searches included a mix of free text and index terms to maximize the retrieval of potentially relevant articles. The keyword combinations used by the researchers were as follows: “Alcohol” OR “Alcohol Beverage” OR “Alcoholic Beverage” OR “Alcohol Drinking” OR “Alcohol Consumption” OR “Alcohol Intake” OR “Drink Beer” OR “Drink Wine” OR “Drink Spirit” OR “Local produced Alcohol Drinking” OR “Traditional Beer” OR “Drink Kachasu” OR “Drink Busaa” OR “Drink Chang’aa” OR “Drink Gongo” OR “Risk factor” OR “Risks factors” AND “Esophageal Neoplasm” OR “Esophagus Neoplasm” OR “Esophagus Neoplasms” OR “Cancer of Esophagus” OR “Esophagus Cancer” OR “Esophagus Cancers” OR “Esophageal Cancer” OR “Esophageal Cancers” OR “Esophageal Squamous Cell Carcinoma” OR “Esophageal Neoplasms” OR “ESCC”. Searches will then be adjusted according to the requirements of each specific database (i.e. the use of operators and symbols). A manual cross-search of the references cited in the studies and the bibliographies of the documents retrieved was then carried out. No limits were established in terms of date of publication or language of publication.
The authors initiated the selection process by independently evaluating the titles and abstracts of previously identified studies. Subsequently, a second independent selection was conducted by carefully examining the full text of articles that met the initial eligibility criteria, identifying those where eligibility remained unclear. Finally, the two authors rigorously and jointly assessed the eligibility of each study, particularly those with uncertain eligibility, to determine their inclusion in the systematic review and meta-analysis. The inclusion criteria encompassed observational studies involving residents of Africa, where alcohol consumption served as either a primary or secondary risk factor for EC. At each stage of study selection, the authors worked independently, and any disparities were addressed through a consensus-seeking discussion before progressing to the next stage.
For each study that met our eligibility criteria, comprehensive data were collected, including title, country, first author, publication date, number of cases, number of controls, participant recruitment methods, collection period, data collection methods, study population, alcoholic status, type of alcoholic beverage consumption, relative risk, and 95% confidence interval (CI), or odds ratio (OR) and 95% CI. In instances where comparative data were not available in the literature, they were calculated using appropriate statistical software. Study participants were categorized into two groups: those who had never consumed alcohol and those who had consumed alcohol. Studies presenting outcomes by race, type of beverage, or quantity smoked were aggregated before inclusion. Studies conducted across multiple countries (10) were disaggregated by country, with the author’s name duplicated and followed by the country’s initials. The authors proceeded to independently assess the quality of the studies using the Newcastle-Ottawa Scale (NOS) (15) for case-control studies and the Agency for Healthcare Research and Quality (AHRQ) tool (16) for cross-sectional studies. Any disagreements were resolved through consensus.
In this study, participants who drink alcoholic beverages daily, weekly, or occasionally, regardless of the type are considered drinkers. Individuals who drink less than once a month are considered occasional drinkers. People who drink every weekend is classified as weekly drinker, while someone who can’t refrain from drinking every day is classified as a daily drinker. As for the types of alcoholic beverage, we have grouped alcoholic beverages into manufactured beverages (beer, whisky, and red wine) that are respected during production because of their quality standards, and into traditional beverages (busaa; Chang’aa, gongo, kachasu; Amgba; Sha’a; Tchapalo; Matango; Meloucre), that include all beverages produced by the local populations that do not meet quality standards and have no known composition. Non-drinkers were defined as individuals who abstained from alcoholic beverage consumption. For the qualitative analysis, GTK and EJN meticulously extracted qualitative data from a variety of studies and subjected them to a systematic analysis. The summarized outcomes of these analyses are presented in Table 1. For quantitative synthesis, statistical analyses were conducted using the RevMan 5.3 (Cochrane, USA) software for Windows. Dichotomous data relating to the association between alcoholic beverage consumption and EC were represented as odds ratios (OR) with corresponding 95% Confidence Intervals (95% CI) in a forest plot. Drinking status was utilized for stratifying the data into subgroups, and random-effects meta-analyses were performed to account for inherent differences in the study populations. Heterogeneity among the included studies was assessed using the I2 statistic, with significance set at P < 0.05, as described by Higgins and Thompson (43). An I2 value between 75% and 100% denoted substantial heterogeneity. Subgroup analysis considered the frequency of alcohol consumption by the populations to identify those demonstrating a lower risk of esophageal cancer. Differences between subgroups were evaluated through visual inspection of confidence intervals and P values. The odds ratio was employed as a measure of risk for both the subgroup and the overall association between alcohol consumption and EC. The potential small-study effects and publication bias were graphically evaluated by the funnel plot. We also conducted Egger’s test for asymmetry, where P value < 0.1 was considered significant using Stata software (Version 17.0; StataCorp). P < 0.05 (two-sided) were considered as significance level.
The electronic and manual searches yielded a total of 758,203 studies. After eliminating duplicates (46,955 studies), a thorough review was conducted on 711,248 titles/abstracts. Following this review, 207 studies were selected for full-text examination. Subsequently, 175 studies were excluded for reasons such as non-alignment with the geographical focus of the study, being comments, abstracts from conferences, studies presenting only the frequency of cancer in alcoholics, and inadequate data even after a request to the corresponding author. Finally, 32 studies that fully met our inclusion criteria were selected for both qualitative and quantitative analysis (refer to Figure 1; Table 1).

Figure 1 Schematical flow diagram for the selection of study included in the systematic review and meta-analysis.
The 32 included studies, comprising 31 case-control studies and 1 cross-sectional study, encompassed a combined sample of 29,026 individuals, consisting of 11,237 cases and 17,789 controls or non-cancer individuals. These participants were sourced from five Southern African countries (South Africa, Malawi, Mozambique, Zambia, and Zimbabwe) and four Eastern African countries (Ethiopia, Kenya, Tanzania, and Uganda) (refer to Table 1). The cases comprised patients diagnosed endoscopically and confirmed either histologically, through CT scans, or imaging (barium swallow) for esophageal cancer or those meeting clinical criteria for EC. The control group comprised healthy volunteers recruited from the hospital setting with no family history or affiliation with any form of cancer. The key parameters addressed in these studies included alcohol status, frequency of consumption, and the type of alcoholic beverages consumed. Data across these studies were primarily collected through questionnaires.
The forest plot presented in Figure 2 illustrates the association between the consumption of alcoholic beverages and esophageal cancer in East and Southern Africa. Analysis of this figure unveils a pooled odds ratio of 1.81 (95% CI, 1.50-2.19, P < 0.00001) and a substantial degree of heterogeneity (I2 = 91%). These findings strongly suggest a significant association between alcohol consumption and esophageal cancer.
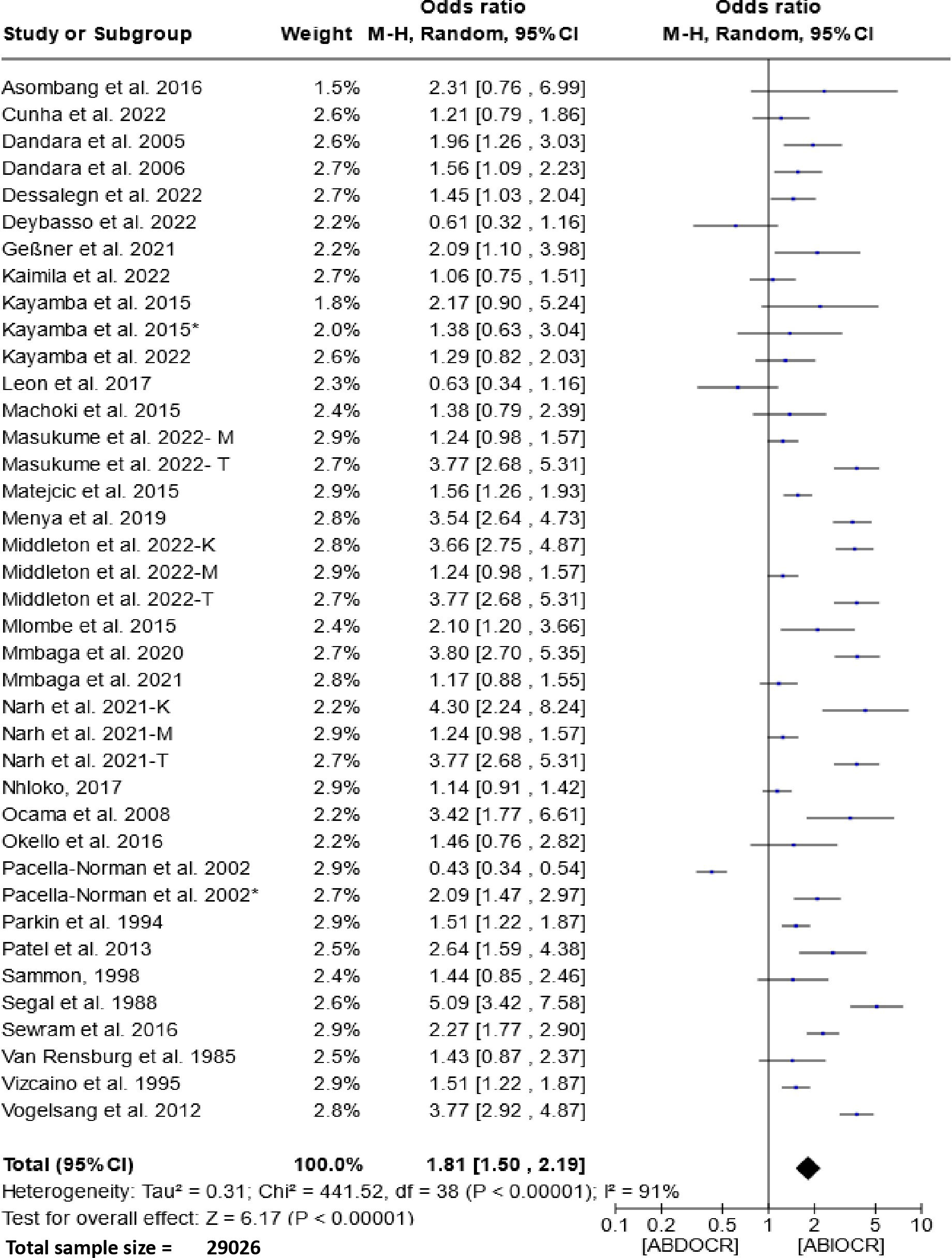
Figure 2 Forest plots for the association between drinking alcohol and esophageal cancer risks. Data is presented as Odds Ratio with 95% Confidence Intervals (CI) utilizing a random-effects model. Heterogeneity among the studies was assessed using the l2 statistic with a significance level of P < 0.05. ABDOCR, Alcoholic beverages decrease esophageal cancer risk; ABIOCR, Alcoholic beverages increase esophageal cancer risk.
The potential for publication bias was assessed using a funnel plot illustrated in Figure 3. Visual inspection of the funnel plot did not provide evidence for asymmetry. The Egger regression test also did not detect a potential publication bias (P-value = 0.7870). likewise, it is noteworthy that the sensitivity analysis, excluding these studies individually did not alter the significance of the overall outcome.
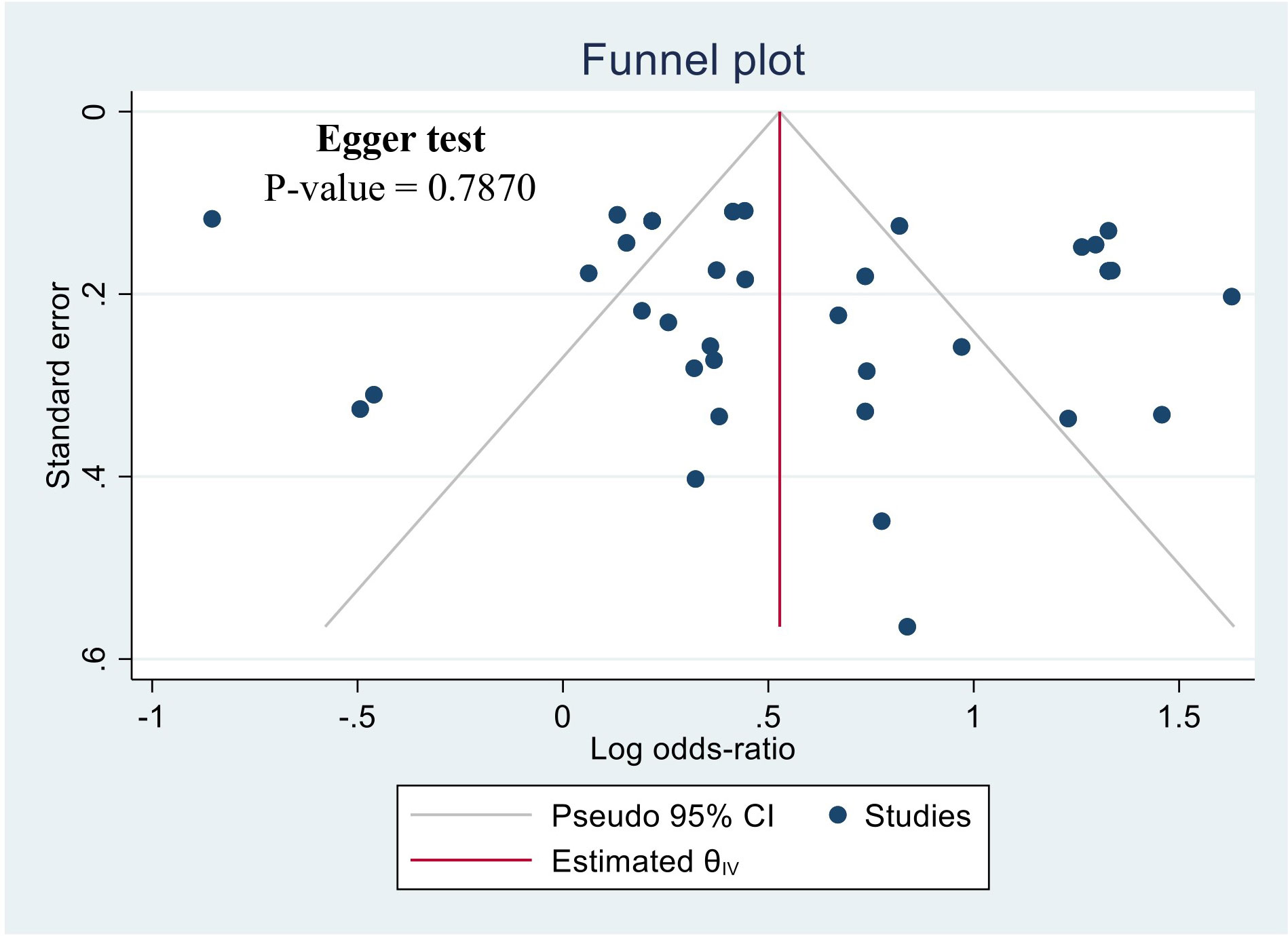
Figure 3 Funnel plot of the studies based on the association between drunk alcoholic beverages and the risk of esophageal cancer.
The meta-analysis of data concerning the influence of the frequency of alcoholic beverage consumption on the etiology of esophageal cancer is depicted in Figure 4. Analysis of this figure revealed that both daily (Figure 4A) and weekly (Figure 4B) alcohol consumption significantly increased the risk of esophageal cancer. The risk was notably higher in daily drinkers [OR = 2.38 (95% CI, 1.81-3.13); I2 = 72%, and P < 0.00001] than in weekly drinkers [OR = 1.94 (95% CI, 1.32-2.84); I2 = 90%, and P=0.00007]. However, no statistical significance was observed in the occasional drinker subgroup, with an OR of 1.02 (95% CI, 0.81-1.29), P=0.84, and a minimal degree of heterogeneity (I2 = 9%) (Figure 4C).

Figure 4 Forest plots for the association between Frequency of drinking alcohol and esophageal cancer risks. Data is presented as Odds Ratio with 95% Confidence Intervals (CI) utilizing a random-effects model. Heterogeneity among the studies was assessed using the I2 statistic with a significance level of P < 0.05. (A) Daialy alcohol drinker; (B) Weekly alcohol drinker; (C) Occasionally alcohol drinker; DDDOCR, daily drink decreases esophageal cancer risk; DDIOCR, daily drink increases esophageal cancer risk; WDDOCR, weekly drink decreases esophageal cancer risk; WDIOCR, weekly drink increases esophageal cancer risk; ODDOCR, Occasionally drink decrease esophageal cancer risk; ODIOCR, Occasionally drink increase esophageal cancer risk.
The impact of traditional alcoholic beverage consumption on the etiology of esophageal cancer is depicted in Figure 5. Analysis of this figure demonstrates a significant association between the consumption of these beverages and the risk of esophageal cancer within the populations of Southern and Eastern Africa. The Odds Ratio (OR) for this association is 2.00 (95% CI, 1.42-2.82), with a heterogeneity of 87% and P < 0.00001.
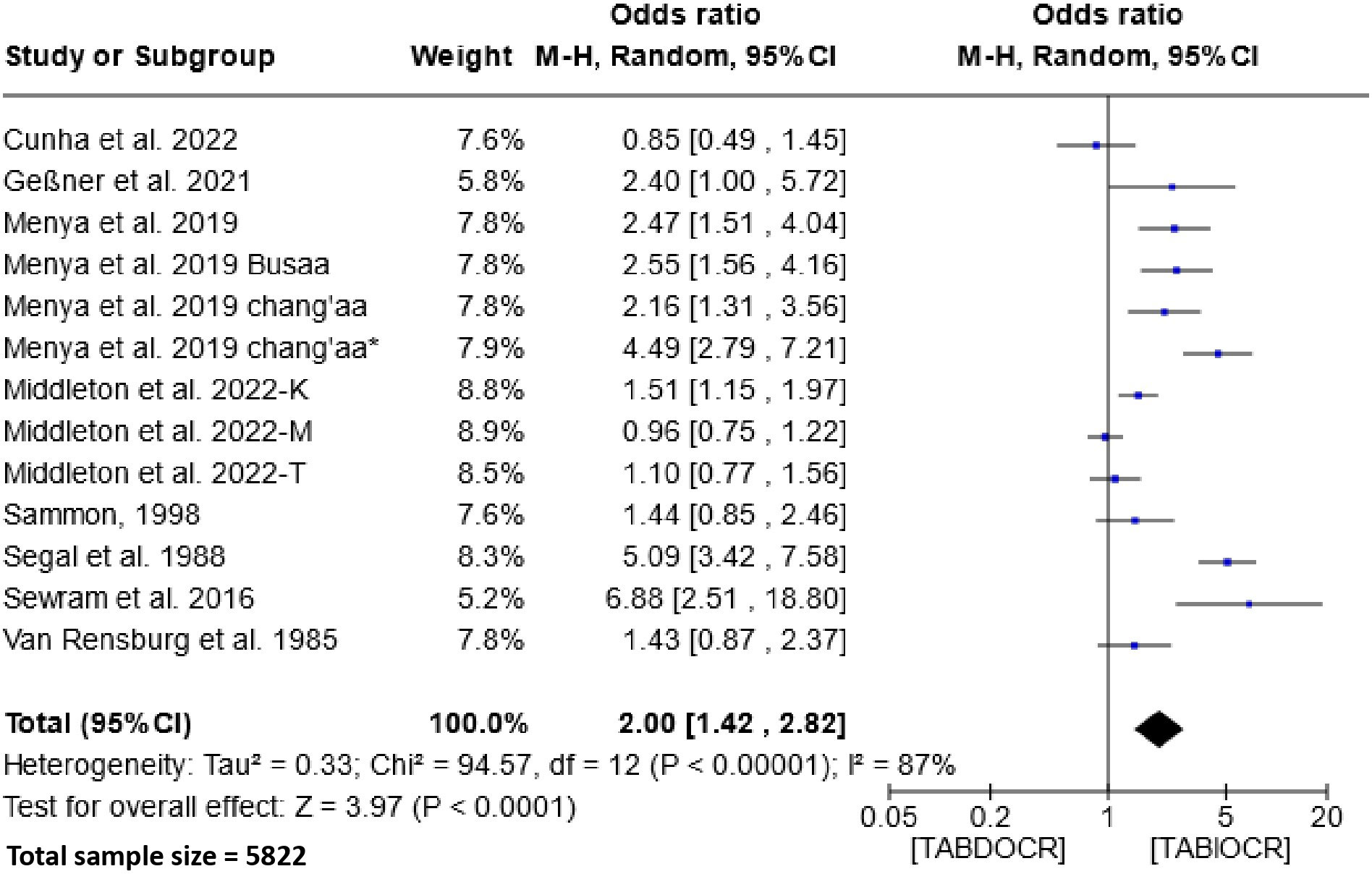
Figure 5 Forest plots for the association between traditional alcoholic beverages and esophageal cancer risks. Data is presented as Odds Ratio with 95% Confidence Intervals (CI) utilizing a random-effects model. Heterogeneity among the studies was assessed using the I2 statistic with a significance level of P < 0.05. TABDOCR, Traditional alcoholic beverage decreases esophageal cancer risk; TABIOCR, Traditional alcoholic beverages increase esophageal cancer risk.
The impact of non-traditional alcoholic beverage consumption on the etiology of EC is depicted in Figure 6. Analysis of this figure demonstrates a non-significant association between the consumption of these beverages and the risk of esophageal cancer within the populations. The Odds Ratio (OR) for this association is 1.20 (95% CI, 0.78-1.86), with a heterogeneity of 92% and P =0.82. Subgroup analysis showed no significant association between exclusive consumption of non-traditional beers [1.18 (95% CI, 0.54-2.59); P=0.68], wines [1.60 (95% CI, 0.62-4.10); P=0.33] and spirits [1.08 (95% CI, 0.45-2.55); P=0.87] and the risk of esophageal cancer.
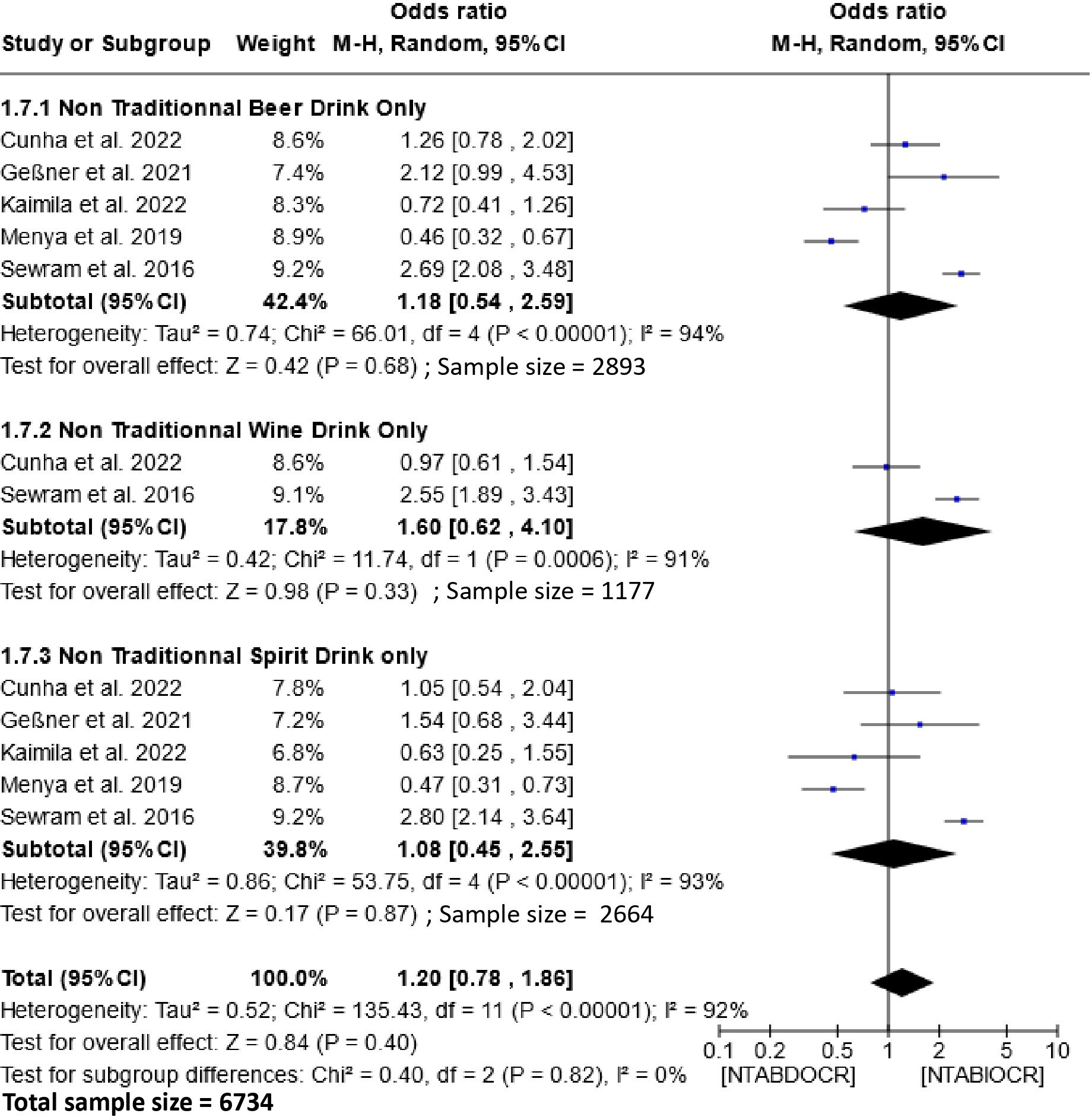
Figure 6 Forest plots for the association between non-traditional alcoholic beverages and esophageal cancer risks. Data is presented as Odds Ratio with 95% Confidence Intervals (CI) utilizing a random-effects model. Heterogeneity among the studies was assessed using the l2 statistic with a significance level of P < 0.05. NTABDOCR, Non-traditional alcoholic beverage decreases esophageal cancer risk; NTABIOCR, Non-traditional alcoholic beverages increase esophageal cancer risk.
Alcohol consumption is deeply ingrained in the social fabric of African communities, both rural and urban, predating colonization (44). Celebratory events involve the consumption of alcoholic beverages, often pursued for pleasure (1, 44, 45). However, this widespread practice is a major contributor to global morbidity and mortality, notably linked to chronic health issues such as esophageal cancer (46–48).
This systematic review and meta-analysis focused on evaluating the relationship between alcoholic beverage consumption and the risk of esophageal cancer in Africa. Despite ethanol, the primary component of alcoholic drinks, not being inherently carcinogenic (6), our meta-analysis revealed a significant association between alcoholic beverage consumption and esophageal cancer risk (OR = 1.81; 95% CI, 1.50-2.19) across 32 studies solely from Southern and Eastern Africa.
The robust link between alcoholic beverage consumption and esophageal cancer risk is primarily attributed to the first metabolite, acetaldehyde, formed during ethanol oxidation by Acetaldehyde dehydrogenases. Acetaldehyde, with its genotoxic effects on cellular processes, can bind to DNA or react with various cellular residues, causing DNA oxidation and lipid peroxidation (49–51). Genetic polymorphisms in ethanol metabolism enzymes and a mismatch in alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) activities also contribute to alcohol-induced neoplasms (52, 53).
Our meta-analysis further highlighted a significant association between frequent alcohol consumption (daily [OR = 2.38 (95% CI, 1.81-3.13)] and weekly [OR =1.94 (95% CI, 1.32-2.84)] and increased EC risk, while occasional consumption [OR =1.02 (95% CI, 0.81-1.29)] showed no significant association. This suggests that repeated alcohol intake may exacerbate carcinogenic effects on the esophagus. The accelerated division of esophageal stem cells due to cytotoxic ethanol concentrations, as seen in regular alcohol consumption, may play a role in maintaining cellular homeostasis challenged by carcinogens from ethanol (54, 55).
Moreover, our study demonstrated a substantial association (OR = 2.00; 95% CI, 1.42-2.82) between traditional alcoholic beverage consumption and EC risk in Southern and Eastern African populations. This association is potentially influenced by the uncontrolled ethanol content and the presence of carcinogenic substances like methanol, formaldehyde, and formic acid in traditional beverages (56–59). Additionally, these beverages might contain other carcinogens like aflatoxin, lead, and nitrosamines (28). However, the non-significance observed with non-traditional alcoholic beverages reflects the strict control of the various ingredients contained in the products and above all the absence of methanol. Potential toxins are destroyed in the beverages during pasteurization (60). Hence, EC in these people may be due to other risk factors.
In the present study, global comparisons and certain analyses of subgroups revealed a strong heterogeneity among studies. We have observed that the frequency of consumption (daily and weekly) and types of alcoholic beverages drinks could significantly influence the heterogeneity among the included studies. Obviously, variations in alcohol concentrations and other potential substances contained in different beverages can affect the results. Additionally, the high heterogeneity could be attributed to variation in population characteristics, like comorbidities, cancer stage, lifestyle (smoking, dietary habits, and other), and socioeconomic status (61). Furthermore, variation can be attributed to study characteristics, such as outcome measurement and study design (62).
Considering these findings, it is imperative that Southern and Eastern African governments formulate and implement consistent public education policies on the adverse health effects of frequent alcoholic beverage consumption, especially traditional ones. Urgent measures are needed to regulate the sale of questionable origin drinks and those failing to comply with international standards, containing numerous carcinogenic substances like methanol.
This study underscores the pivotal role of alcoholic beverage consumption, especially at regular intervals, in the incidence of EC in major African regions. It advocates for immediate governmental action to educate the public and enact regulations to mitigate these significant public health concerns.
This study is subject to several limitations. Firstly, observational studies inherently carry a risk of confounding and bias, and the potential for recall and selection biases was notable in this meta-analysis. Secondly, available data focused on Eastern and Southern African countries, data availability was then limited, with only nine countries contributing studies, and not all regions within these countries were adequately covered, affecting the comprehensiveness of the assessment. The lack of data on the quantity of alcoholic beverages and ethanol consumed hindered a deeper understanding of the association between consumption levels and EC risk. Another major limitation of this work is the absence of data in the included literature that could be used to verify whether age plays an important role in this association between alcoholic beverage consumption and EC risk. Addressing these limitations through future studies would reinforce the robustness of the association between alcohol consumption and EC risk.
In summary, this systematic review and meta-analysis establish a significant link between alcoholic beverage consumption and the heightened risk of EC in Africa. The risk amplifies with increased frequency of consumption, highlighting the urgency for targeted public health interventions. Additionally, traditional alcoholic beverages emerge as significant contributors to EC risk. Regions facing elevated risks should formulate and enact comprehensive strategies to educate the populace about the perils of alcohol misuse. A concerted subregional effort is imperative to guide policymakers in countering the proliferation of substandard and counterfeit beverages and to mitigate the health hazards posed by alcohol. Future research endeavors should prioritize determining ethanol concentrations associated with esophageal cancer risk in the African context to further inform prevention and intervention strategies.
GK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. EJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors extend their gratitude to the diverse authors whose contributions enabled the execution of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ritchie H, Roser M. Alcohol Consumption. OurWorldInData.org, Oxford, United Kingdom. (2018). Available at: https://ourworldindata.org/alcohol-consumption.
2. Grossman ER, Benjamin-Neelon SE, Sonnenschein S. Alcohol consumption during the COVID-19 pandemic: A cross-sectional survey of US adults. Int J Environ Res Public Health (2020) 17(24):9189. doi: 10.3390/ijerph17249189
3. IWSR. Is Africa the next big beverage alcohol market? Nutmeg House, 60 Gainsford St, London, SE1 2NY, United Kingdom: IWSR (2018). Available at: https://www.theiwsr.com/news-and-comment-is-africa-the-next-big-beverage-alcohol-market/.
4. World Health Organization (WHO). Alcohol. Geneva, Switzerland: World Health Organization (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/alcohol.
5. Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol (2006) 7(2):149–56. doi: 10.1016/S1470-2045(06)70577-0
6. Li Y, Yang H, Cao J. Association between alcohol consumption and cancers in the Chinese population–a systematic review and meta-analysis. PloS One (2011) 6(4):e18776. doi: 10.1371/journal.pone.0018776
7. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
8. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
9. Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi (2019) 41:19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005
10. Middleton DRS, Mmbaga BT, Menya D, Dzamalala C, Nyakunga-Maro G, Finch P, et al. Alcohol consumption and oesophageal squamous cell cancer risk in east Africa: findings from the large multicentre ESCCAPE case-control study in Kenya, Tanzania, and Malawi. Lancet Glob Health (2022) 10:e236–45. doi: 10.1016/S2214-109X(21)00506-4
11. Segal I, Reinach SG, de Beer M. Factors associated with oesophageal cancer in Soweto, South Africa. Br J Cancer (1988) 58(5):681–96. doi: 10.1038/bjc.1988.286
12. Masukume G, Mmbaga BT, Dzamalala CP, Mlombe YB, Finch P, Nyakunga-Maro G, et al. A very-hot food and beverage thermal exposure index and esophageal cancer risk in Malawi and Tanzania: findings from the ESCCAPE case–control studies. Br J Cancer (2022) 127:1106–15. doi: 10.1038/s41416-022-01890-8
13. Leon ME, Assefa M, Kassa E, Bane A, Gemechu T, Tilahun Y, et al. Qat use and esophageal cancer in Ethiopia: A pilot case-control study. PloS One (2017) 12(6):e0178911. doi: 10.1371/journal.pone.0178911
14. Deybasso HA, Roba KT, Nega B, Belachew T. Dietary and environmental determinants of oesophageal cancer in Arsi Zone, Oromia, Central Ethiopia: A case-control study. Cancer Manag Res (2021) 13:2071–82. doi: 10.2147/CMAR.S298892
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med (2015) 8:9. doi: 10.1111/jebm.12141
17. Asombang AW, Kayamba V, Lisulo MM, Trinkaus K, Mudenda V, Sinkala E, et al. Esophageal squamous cell cancer in a highly endemic region. World J Gastroenterol (2016) 22(9):2811–7. doi: 10.3748/wjg.v22.i9.2811
18. Cunha L, Fontes F, Come J, Lobo V, Santos LL, Lunet N, et al. Risk factors for oesophageal squamous cell carcinoma in Mozambique. Ecancer (2022) 16:1437. doi: 10.3332/ecancer.2022.1437
19. Dandara C, Ballo R, Parker MI. CYP3A5 genotypes and risk of oesophageal cancer in two South African populations. Cancer Lett (2005) 225(2):275–82. doi: 10.1016/j.canlet.2004.11.004
20. Dandara C, Li D-P, Walther G, Parker MI. Gene-environment interaction: the role of SULT1A1 and CYP3A5 polymorphisms as risk modifiers for squamous cell carcinoma of the oesophagus. Carcinogenesis (2006) 27(4):791–7. doi: 10.1093/carcin/bgi257
21. Dessalegn B, Enqueselassie F, Kaba M, Assefa M, Addissie A. Risk factors of oesophageal cancer at health facilities in Addis Ababa, Ethiopia: Unmatched case control study. Front Oncol (2022) 12:997158. doi: 10.3389/fonc.2022.997158
22. Geßner AL, Borkowetz A, Wilhelm TJ, Ludzu E, Baier M, Mastala Y, et al. Risk factors for esophageal cancer in a high-incidence area of Malawi. Cancer Causes Control (2021) 32(12):1347–54. doi: 10.1007/s10552-021-01482-6
23. Kaimila B, Mulima G, Kajombo C, Salima A, Nietschke P, Pritchett N, et al. Tobacco and other risk factors for esophageal squamous cell carcinoma in Lilongwe Malawi: Results from the Lilongwe esophageal cancer case control study. PloS Glob Public Health (2022) 2(6):e0000135. doi: 10.1371/journal.pgph.0000135
24. Kayamba V, Bateman AC, Asombang AW, Shibemba A, Zyambo K, Banda T, et al. HIV infection and domestic smoke exposure, but not human papillomavirus, are risk factors for esophageal squamous cell carcinoma in Zambia: a case-control study. Cancer Med (2015) 4(4):588–95. doi: 10.1002/cam4.434
25. Kayamba V, Mulenga C, Mubbunu M, Kazhila L, Hodges P, Kelly P. Association between oesophageal cancer and biomass smoke exposure: a case-control study. Ecancer (2022) 16:1422. doi: 10.3332/ecancer.2022.1422
26. Machoki M, Saidi H, Raja A, Ndonga A, Njue A, Biomdo I, et al. Risk factors for esophageal squamous cell carcinoma in a Kenyan population. Ann Afr Surg (2015) 12(1):38–43.
27. Matejcic M, Vogelsang M, Wang Y, Iqbal-Parker M. NAT1 and NAT2 genetic polymorphisms and environmental exposure as risk factors for oesophageal squamous cell carcinoma: a case-control study. BMC Cancer (2015) 15:150. doi: 10.1186/s12885-015-1105-4
28. Menya D, Kigen N, Oduor M, Maina SK, Some F, Chumba D, et al. Traditional and commercial alcohols and esophageal cancer risk in Kenya. Int J Cancer (2019) 144(3):459–69. doi: 10.1002/ijc.31804
29. Mlombe YB, Rosenberg NE, Wolf LL, Dzamalala CP, Chalulu K, Chisi J, et al. Environmental risk factors for oesophageal cancer in Malawi: A case-control study. Malawi Med J (2015) 27(3):88–92. doi: 10.4314/mmj.v27i3.3
30. Mmbaga BT, Mwasamwaja A, Mushi G, Mremi A, Nyakunga G, Kiwelu I, et al. Missing and decayed teeth, oral hygiene and dental staining in relation to esophageal cancer risk: ESCCAPE case-control study in Kilimanjaro, Tanzania. Int J Cancer (2020) 148(10):2416–28. doi: 10.1002/ijc.33433
31. Mmbaga EJ, Mushi BP, Deardorff K, Mgisha W, Akoko LO, Paciorek A, et al. A case-control study to evaluate environmental and lifestyle risk factors for esophageal cancer in Tanzania. Cancer Epidemiol Biomarkers Prev (2021) 30:305–16. doi: 10.1158/1055-9965.EPI-20-0660
32. Nhleko ML. Effects of smoking and alcohol use on oesophageal cancer amongst Black South Africans in Johannesburg from 1999 – 2009. Johannesburg, South Africa: University of the Witwatersrand (2017). MSc thesis, Epidemiology and Biostatistics.
33. Ocama P, Kagimu MM, Odida M, Wabinga H, Opio CK, Colebunders B, et al. Factors associated with carcinoma of the oesophagus at Mulago Hospital, Uganda. Afr Health Sci (2008) 8(2):80–4.
34. Okello S, Churchill C, Owori R, Nasasira B, Tumuhimbise C, Abonga CL, et al. Population attributable fraction of Esophageal squamous cell carcinoma due to smoking and alcohol in Uganda. BMC Cancer (2016) 16:446. doi: 10.1186/s12885-016-2492-x
35. Pacella-Norman R, Urban MI, Sitas F, Carrara H, Sur R, Hale M, et al. Risk factors for oesophageal, lung, oral and laryngeal cancers in black South Africans. Br J Cancer (2002) 86(11):1751–6. doi: 10.1038/sj.bjc.6600338
36. Parkin D, Vizcaino A, Skinner M, Ndhlovu A. Cancer patterns and risk factors in the African population of southwestern Zimbabwe, 1963–1977. Cancer Epidemiol Biomarkers Prev (1994) 3(7):537–47.
37. Patel K, Wakhisi J, Mining S, Mwangi A, Patel R. Esophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. Int Sch Res Notices (2013) 2013:503249. doi: 10.1155/2013/503249
38. Sammon AM. Protease inhibitors and carcinoma of the esophagus. Cancer (1998) 83(3):405–8. doi: 10.1002/(SICI)1097-0142(19980801)83:3<405::AID-CNCR6>3.0.CO;2-N
39. Sewram V, Sitas F, O’Connell D, Myers J. Tobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South Africa. Cancer Epidemiol (2016) 41:113–21. doi: 10.1016/j.canep.2016.02.001
40. Van Rensburg SJ, Bradshaw ES, Bradshaw D, Rose EF. Oesophageal cancer in Zulu men, South Africa: a case-control study. Br J Cancer (1985) 51(3):399–405. doi: 10.1038/bjc.1985.54
41. Vizcaino AP, Parkin DM, Skinner ME. Risk factors associated with oesophageal cancer in Bulawayo, Zimbabwe. Br J Cancer (1995) 72(3):769–73. doi: 10.1038/bjc.1995.408
42. Vogelsang M, Wang Y, Veber N, Mwapagha LM, Parker MI. The cumulative effects of polymorphisms in the DNA mismatch repair genes and tobacco smoking in oesophageal cancer risk. PloS One (2012) 7(5):e36962. doi: 10.1371/journal.pone.0036962
43. Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21(11):1539–58. doi: 10.1002/sim.1186
44. Nwagu EN, Dibia SIC, Odo AN. Socio-cultural norms and roles in the use and abuse of alcohol among members of a rural community in Southeast Nigeria. Health Educ Res (2017) 32(5):423–36. doi: 10.1093/her/cyx058
45. Matejcic M, Gunter MJ, Ferrari P. Alcohol metabolism and oesophageal cancer: a systematic review of the evidence. Carcinogenesis (2017) 38(9):859–72. doi: 10.1093/carcin/bgx067
46. Rehm J, Baliunas D, Borges GLG, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease—an overview. Addiction (2010) 105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x
47. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380:2224–60. doi: 10.1016/S0140-6736(12)61766-8
48. World Health Organization (WHO). Global Status Report on Alcohol and Health 2014. Geneva, Switzerland: World Health Organization (2014).
49. Yu HS, Oyama T, Isse T, Kitagawa K, Pham TT, Tanaka M, et al. Formation of acetaldehyde-derived DNA adducts due to alcohol exposure. Chem Biol Interact (2010) 188(3):367–75. doi: 10.1016/j.cbi.2010.08.005
50. Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med (2008) 45:1542–50. doi: 10.1016/j.freeradbiomed.2008.08.030
51. Ratna A, Mandrekar P. Alcohol and cancer: mechanisms and therapies. Biomolecules (2017) 7(3):E61. doi: 10.3390/biom7030061
52. Jelski W, Kozlowski M, Laudanski J, Niklinski J, Szmitkowski M. Alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase activity in the sera of patients with esophageal cancer. Clin Exp Med (2009) 9:131–7. doi: 10.1007/s10238-008-0028-7
53. Orywal K, Szmitkowski M. Alcohol dehydrogenase and aldehyde dehydrogenase in Malignant neoplasms. Clin Exp Med (2017) 17:131–9. doi: 10.1007/s10238-016-0408-3
54. López-Lázaro M. A local mechanism by which alcohol consumption causes cancer. Oral Oncol (2016) 62:149–52. doi: 10.1016/j.oraloncology.2016.10.001
55. Yoo JE, Shin DW, Han K, Kim D, Jeong SM, Koo HY, et al. Association of the frequency and quantity of alcohol consumption with gastrointestinal cancer. JAMA Netw Open (2021) 4(8):e2120382. doi: 10.1001/jamanetworkopen.2021.20382
56. Vioque J, Barber X, Bolumar F, Porta M, Santibáñez M, de la Hera MG, et al. Esophageal cancer risk by type of alcohol drinking and smoking: a case-control study in Spain. BMC Cancer (2008) 8:221. doi: 10.1186/1471-2407-8-221
57. Bindra P, Hazra A. Dielectric sensor system using tiO2Nanotubes for real-time detection of methanol contamination in alcoholic beverages. IEEE Trans Instrum Meas (2020) 69(9):6621–9. doi: 10.1109/TIM.2020.2971328
58. Botelho G, Anjos O, Estevinho LM, Caldeira I. Methanol in grape derived, fruit and honey spirits: A critical review on source, quality control, and legal limits. Processes (2020) 8(12):1–21. doi: 10.3390/pr8121609
59. Van Den Broek J, Bischof D, Derron N, Abegg S, Gerber PA, Güntner AT, et al. Screening methanol poisoning with a portable breath detector. Anal Chem (2021) 93(2):1170–8. doi: 10.1021/acs.analchem.0c04230
60. Ciont C, Epuran A, Kerezsi AD, Coldea TE, Mudura E, Pasqualone A, et al. Beer safety: new challenges and future trends within craft and large-scale production. Foods (2022) 11(17):2693. doi: 10.3390/foods11172693
61. Ayaz A, Arshad A, Malik H, Ali H, Hussain E, Jamil B. Risk factors for intensive care unit admission and mortality in hospitalized COVID-19 patients. Acute Crit Care (2020) 35(4):249–54. doi: 10.4266/acc.2020.00381
Keywords: Africa, esophageal cancer, drinking patterns, alcoholic beverage types, meta-analysis
Citation: Ndebia EJ and Kamsu GT (2023) Drinking patterns, alcoholic beverage types, and esophageal cancer risk in Africa: a comprehensive systematic review and meta-analysis. Front. Oncol. 13:1310253. doi: 10.3389/fonc.2023.1310253
Received: 09 October 2023; Accepted: 04 December 2023;
Published: 21 December 2023.
Edited by:
Xiang Shu, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Guochong Jia, Vanderbilt University, United StatesCopyright © 2023 Ndebia and Kamsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriel Tchuente Kamsu, Z2thbXN1LXRjaHVlbnRlQHdzdS5hYy56YQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.