- 1Department of Medical Oncology, Georges Francois Leclerc Cancer Centre, Dijon, France
- 2Department of Biostatistics Georges Francois Leclerc Cancer Centre, Dijon, France
- 3Cancer Biology Research Platform, Centre Georges Francois Leclerc, Dijon, France
- 4Department of Bio-pathology, Georges Francois Leclerc Cancer Centre, Dijon, France
- 5Surgery Department Georges Francois Leclerc Cancer Centre, Dijon, France
- 6Department of Medical Oncology, University Hospital Mohammed VI, Tangier, Morocco
Background: The persistence of residual tumour after neoadjuvant chemotherapy (NAC) in localised triple-negative breast cancer (TNBC) is known to have a negative prognostic value. However, different degrees of expression of some immunohistochemical markers may correlate with different prognoses.
Methods: The expression of biomarkers with a known prognostic value, i.e., cytokeratin 5/6 (CK5/6), androgen receptor (AR), epidermal growth factor receptor (EGFR) proliferation-related nuclear antigen Ki-67, human epidermal growth factor receptor 2 (HER2), protein 53 (p53), forkhead box protein 3 (FOXP3), and cluster differentiation 8 (CD8), was analysed by immunohistochemistry in 111 samples after NAC in non-metastatic TNBC patients addressed to Georges-François Leclerc Cancer Centre Dijon, France. Clinical and pathological variables were retrospectively collected. Cox regression was used to identify immunohistochemical (IHC) and clinicopathological predictors of event-free survival (EFS) (relapse or death).
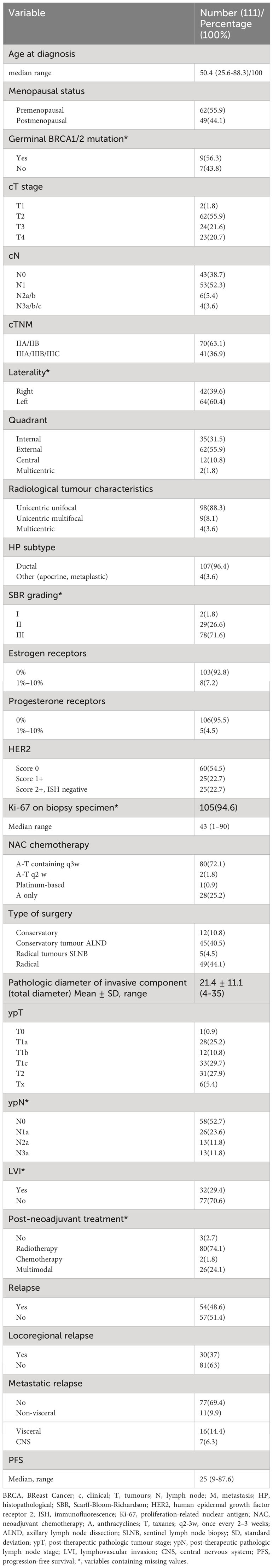
Results: Median age was 50.4 years (range 25.6–88.3), 55.9% (n = 62) were non-menopausal, 70 (63.1%) had stage IIA–IIB disease. NAC was mostly sequential anthracycline-taxanes (72.1%), and surgical intervention was principally conservative (51.3%). We found 65.7% ypT1, 47.2% lymph node involvement (ypN+), and 29.4% lymphovascular invasion (LVI). Most residual tumours were EGFR >110 (H-score) (60.5%, n = 66), AR ≥4% (53.2%, n = 58), p53-positive mutated (52.7%, n = 58), CD8 ≥26 (58.1%, n = 61), FOXP3 ≥7 (51.4%, n = 54), more than half in the stroma, and 52.3% (n = 58) HER2 score 0. After a median follow-up of 80.8 months, 48.6% had relapsed. Median EFS was 62.3 months (95% CI, 37.2–not reached (NR)). Factors independently associated with poor EFS were AR-low (p = 0.002), ypN+ (p < 0.001), and LVI (p = 0.001). Factors associated with lower overall survival (OS) were EGFR-low (p = 0.041), Ki-67 high (p = 0.024), and ypN+ (p < 0.001).
Conclusion: Post-NAC residual disease in TNBC showed biomarkers specific to a basal-like subtype and markers of lymphocyte infiltration mostly present in the stroma. Prognostic markers for EFS were AR, LVI, and ypN and warrant further validation in a prognostic model.
Introduction
Triple-negative breast cancer (TNBC) is defined by the absence of immunohistochemical expression of hormone receptors (estrogen, progesterone) and absent or very low expression of human epidermal growth factor receptor 2 (HER2) (1, 2). TNBC represents approximately 15% of breast cancers and remains the most aggressive phenotype (3). Residual disease (RD) after neoadjuvant chemotherapy (NAC) in localised TNBC is a negative prognostic factor in terms of relapse rate and disease-free survival (DFS) (4–6). For this reason, in the absence of pathologic complete response (pCR), breast cancer guidelines recommend systemic treatments post-surgery (7, 8).
Since the last positive trial proposing capecitabine in case of invasive RD in aggressive localised breast cancer after NAC (9), a panoply of post-neoadjuvant treatments have emerged, such as olaparib in germinal breast cancer (gBRCA) gene mutated cases or pembrolizumab (10, 11), to target more specifically the remaining tumour, as a demonstration of the need to reduce the risk of recurrence in these cases.
Localised TNBC is widely analysed by gene sequencing in chemo-naive primary tumour or in RD, and several prognostic scores have been proposed, but this approach is too expensive to be feasible in everyday clinical practice (12–14).
Several biomarkers assessable by immunohistochemistry have been analysed by other teams for their prognostic role in TNBC, regardless of whether the tumour was primary or residual. These biomarkers include epidermal growth factor receptor (EGFR), cytokeratin 5/6 (CK5/6), or proliferation-related nuclear antigen Ki-67 (15), as well as immunological markers, such as cluster differentiation 8 (CD8) and forkhead box protein 3 (FOXP3) expression, which are specifics to cytotoxic and regulatory T lymphocytes, respectively (16).
We selected some classical immunohistochemical biomarkers, with a view to analysing their prognostic role for event-free survival (EFS) (relapse or all-cause death) in residual tumour after NAC in localised TNBC.
Materials and methods
Data source and study
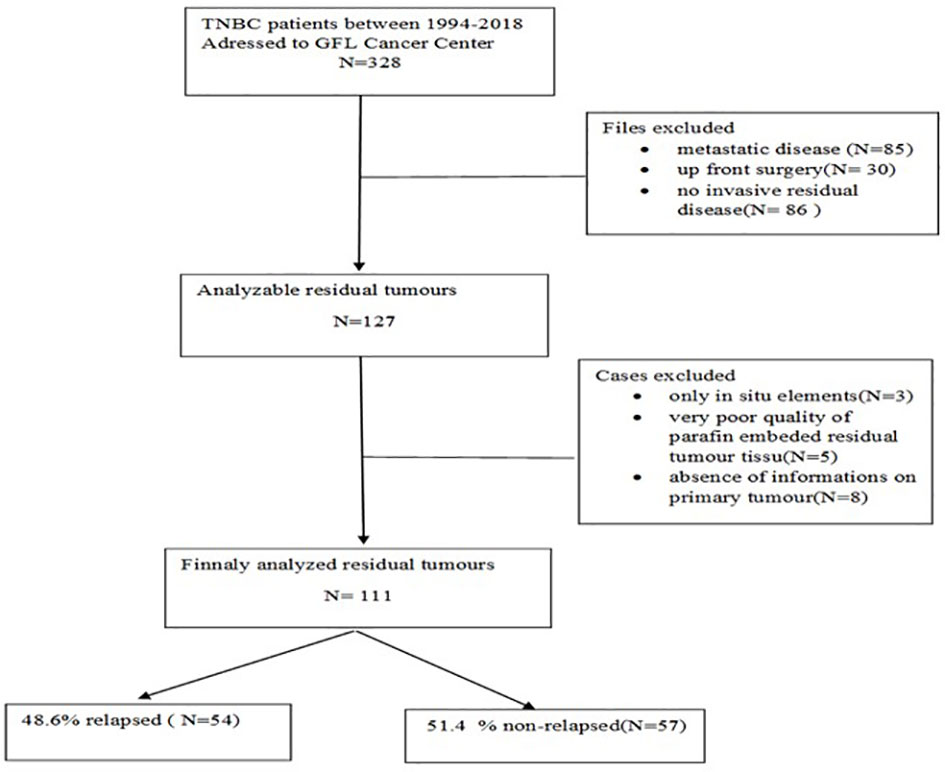
All patients with TNBC treated by NAC followed by surgery between 1994 and 2018 in the Georges-François Leclerc Cancer Center in Dijon, France, who displayed RD were included in this study (see CONSORT diagram in Figure 1). The study was declared on ClinicalTrials.gov under the identifier NCT04031612 and was carried out in accordance with the Helsinki Declaration and approved by CNIL (French National Commission for Data Privacy).
The inclusion criteria were as follows: adult women with unilateral localised TNBC according to the AJCC 8th edition (17) with a threshold of <10% for estrogen receptor (ER) and progesterone receptor (PgR) staining according to the definition in France (18) and according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) methods (2) and without HER2 overexpression, according to the CAP/ASCO definition from 2007 to 2018 if applicable, and otherwise according to local practice (19, 20).
We retrospectively analysed pathological parameters of RD, namely, the number and diameter of foci of invasive carcinoma, the number and type of lymph node involvement, the presence of in situ components, and the presence of lymphovascular or perineural involvement.
Immunohistochemistry
Tumour grade was classed according to the Scarff-Bloom-Richardson (SBR) grading system (21). CK5/6, EGFR, androgen receptor (AR), Ki-67, protein 53 (p53), FOXP3, CD8, and HER2 expression was assessed by immunohistochemistry on the residual paraffin-embedded surgical specimen following the REMARK guidelines (22). Briefly, 4–5-µm sections were stained with hematoxylin–eosin solution to identify and characterise residual invasive carcinoma, and IHC staining was subsequently performed. The antibody clones used and the immunohistochemistry protocol are indicated in the Supplementary Material for each biomarker (Supplementary Material 1).
CK5/6 was assessed as the percentage of cytoplasmic staining, whatever the intensity (23). EGFR expression was quantified by the H score, which comprises the percentage of positive membranous staining from negative to slightly positive (1+), moderately positive (2+), and strongly positive (3+), yielding values from 0 to 300 (all cells strongly positive) (24). AR was quantified as the percentage of nuclear staining, whatever the intensity (25).
For p53 positivity, we established two categories, namely, “very strong positive,” corresponding to missense mutation, and “moderate positive,” corresponding to non-mutated status, with normal p53 synthesis and no stain corresponding to a deletion mutation (26).
CD8 and FOXP3 nuclear staining were counted as absolute numbers in adjacent stromal and intratumoural compartments. We calculated the median value of five areas (27).
Cell proliferation was assessed by nuclear staining in at least 500 tumour cells in at least three representative fields using the corresponding antibody for Ki-67 and using the online software recommended by the French Association of Quality Assurance in Pathologic Anatomy and Cytology (AFAQAP): https://www.afaqap.fr/index-ki67/.
HER2 was assessed and quantified by IHC according to the ASCO/CAP 2018 guidelines (28).
IHC analysis was performed in our in-house laboratory and evaluated by two physicians. In case of disagreement, the fields were reexamined by high-power ×40 lens until a consensus was reached.
Statistical analysis
Categorical variables are described as number and percentage and continuous variables as mean ± standard deviation (SD) or median and interquartile range (IQR). Continuous variables were compared between groups using the Student’s t-test in case of normally distributed variables or the Wilcoxon test in case of non-normal distribution. The Shapiro–Wilk test was used to check the normality of the distribution. Categorical variables were compared using the chi-square or Fisher’s exact test, as appropriate. Tests were two-sided, and the threshold of significance was fixed at 5%. The median follow-up was calculated according to the reverse Kaplan–Meier (KM) method. Survival rates and median survival times with their associated 95% confidence interval (CI) were determined using the KM method. EFS was defined as the time in months between the date of breast cancer diagnosis and the first recurrence of either locoregional or distant metastasis or death. Overall survival (OS) was defined as the time in months between diagnosis and death from any cause or last follow-up. Survival curves were compared using the log-rank test. Univariate and multivariate Cox regression analyses were performed to determine independent predictive factors of survival. All variables with a p-value <0.20 and with <20% missing data were included in the multivariable model, which was adjusted for age. Correlations between eligible variables were tested. The threshold for retention in the final model was p < 0.05.
To determine the appropriate threshold for each biomarker, we used Cut Off finder web application (https://molpathoheidelberg.shinyapps.io/CutoffFinder_v1/). This method fits Cox proportional hazard models to the dichotomised variable and the survival variable. The optimal cutoff is defined as the point with the most significant split (log-rank test) (29). If no threshold was significant at 5% for a biomarker (or if the groups were too different in size), we used the median as the threshold. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients and treatment characteristics
Among 111 cases analysed (Figure 1), the median age was 50.4 years (25.6–88.3), 55.9% (n = 62) were non-menopausal, the majority (63.1%, n = 70) had stage IIA–IIB disease, mostly involving the left breast 60.4% (n = 64) and external quadrant 55.9% (n = 62). Radiologically, the tumour was principally unicentric and unifocal (88.3%, n = 98), and the main histopathological type on biopsy was ductal carcinoma (n = 107, 96.4%). A total of 71.6% (n = 78) were grade III and mostly ER-negative (92.8%, n = 103). HER2 expression was scored 0 in 54.5% (n = 60). Mean Ki-67 in the biopsy specimen was 42% ± 27.6%.
NAC was mostly sequential anthracycline-taxanes (72.1%, n = 80), and the surgical intervention was mainly conservative (51.3%, n = 57).
Forty-three patients (40.2%) had a family history of cancer, but germinal BRCA1/2 mutation was found in only 9 cases (56.3%) of the 16 in whom this analysis was performed.
Regarding the pathologic characteristics of RD, the mean diameter of the invasive component was 21.4 ± 11.1 mm, 66.7% (n = 74) were unifocal, the tumour pathological stage was predominantly ypT1c (29.7%, n = 33). Postoperative lymph node involvement (ypN+) was found in 52 cases (47.2%), including ypN3a in 11.8% (n = 13) and 29.4% had lymphovascular invasion (LVI) (n = 31).
A total of 108 patients (97.3%) received post-neoadjuvant treatment, of which 74.1% (n = 80) was radiotherapy.
After a median follow-up of 80.8 months (9.3–216.6), relapse was observed in 48.6% (n = 54), of whom 29.6% (n = 16) had visceral metastasis; the median time to progression was 25 months (9–87.6), and the death rate was 40.5% (n = 45). More than half (63.5%, n = 40) of the relapsed patients received second-line systemic treatment (Table 1).
Frequency of expression of biomarkers and their individual prognostic significance
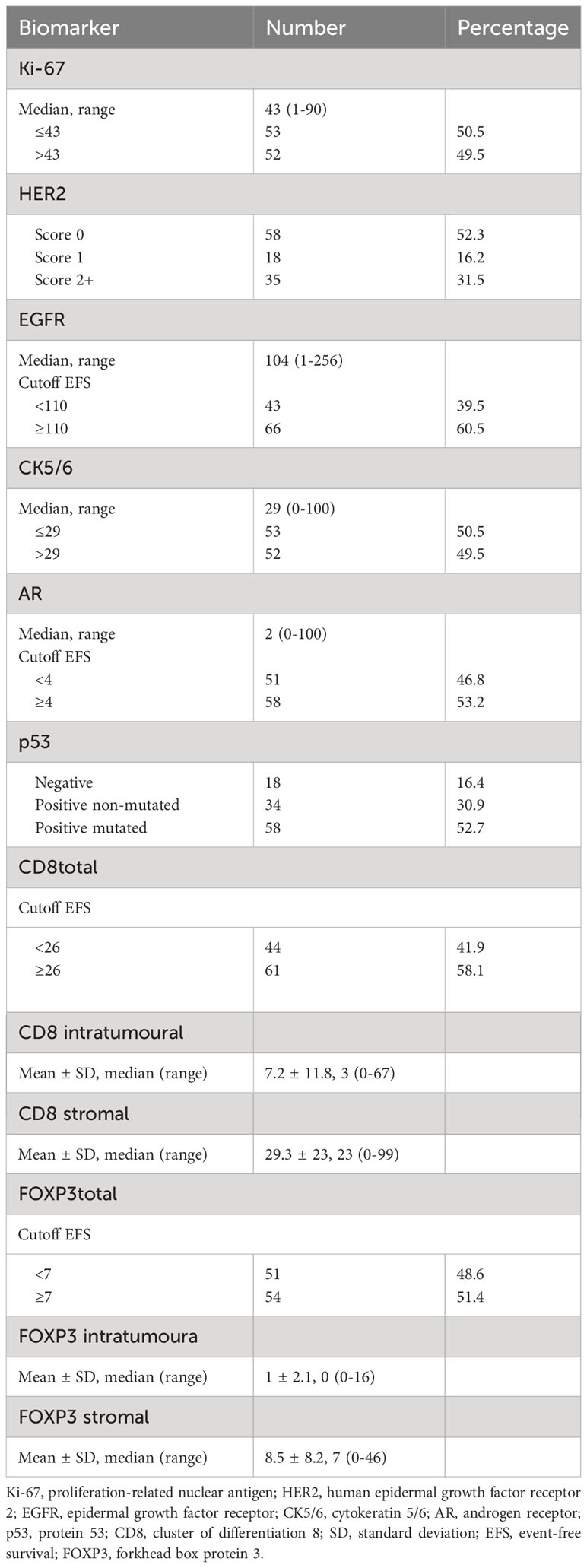
RD was mostly HER2 score 0 (52.3%); Ki-67 low, <43% (50.5%); CK5/6 low, ≤29% (50.5%); EGFR high, ≥100 H score (60.5%); AR high, ≥4% (53.2%); CD8 high, ≥26 (60%); and FOXP3 high, ≥7 (56.2%). CD8 was found mainly in the stroma, median 23 (0–99), while in the intratumoural compartment, representation was lower, median 3 (0–67). Similarly, for FOXP3, representation was higher in the stroma, median 7 (0–46), and very low in the tumour residue, median 0 (0–16). p53-positive strong intensity (by convention, corresponding to missense mutated TP53 status) was observed in 52.7% (n = 58) and negative (by convention, corresponding to deletion mutated TP53 status) in 16.4% (n = 18).
The frequency of expression of each biomarker in RD is displayed in Table 2.
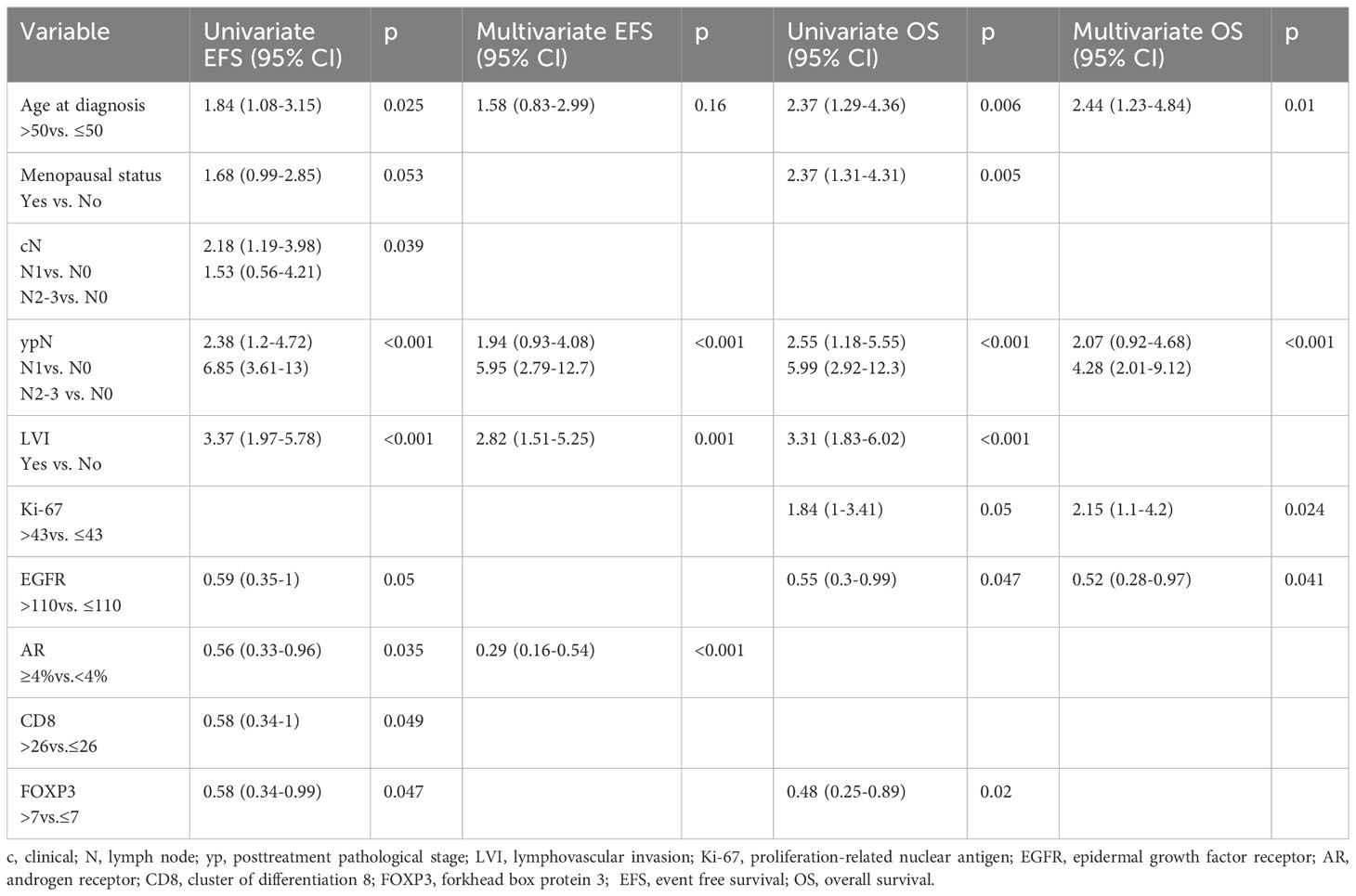
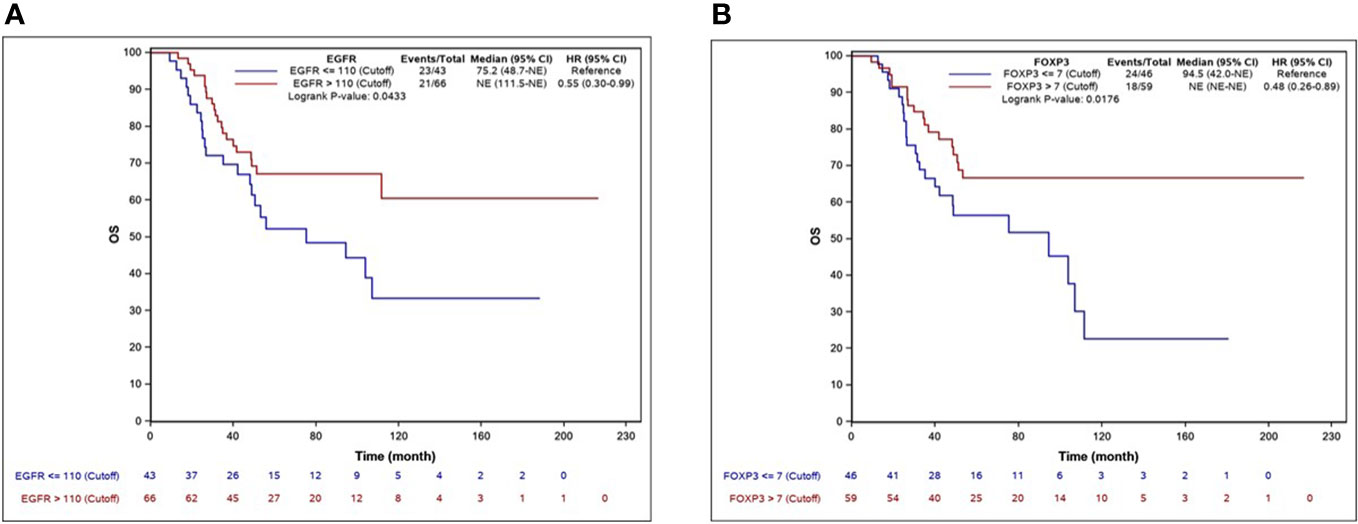
The median EFS was 62.3 months (95% CI, 37.2–NR), and the median OS was 111.5 months (95% CI 94.5–NR). By univariate Cox regression analysis, the biomarkers significantly associated with longer EFS were EGFR, AR, CD8, and FOXP3. The factors associated with OS were EGFR and FOXP3. Ki-67 >43% was significantly associated with shorter OS. The independent predictive factors for EFS identified by multivariate analysis are shown in Table 3. KM EFS curves showed better outcomes associated with CD8+ [hazard ratio (HR) 0.58, 95% CI 0.34–1, p = 0.0461], FOXP3 (HR 0.58, 95% CI 0.34–0.99, p = 0.044), EGFR >110 (HR 0.59, 95% CI 0.35–1, p = 0.0473), and AR ≥4 (HR 0.56, 95% CI 0.33–0.96, p = 0.0328). Regarding OS, the biomarkers for which a high level of expression was associated with improved survival were EGFR >110 (HR 0.55, 95% CI 0.3–0.99, p = 0.043) and FOXP3 >7 (HR 0.48, 95% CI 0.26–0.89, p = 0.0176). The KM curves for EFS and OS for each biomarker are illustrated in Figures 2, 3.
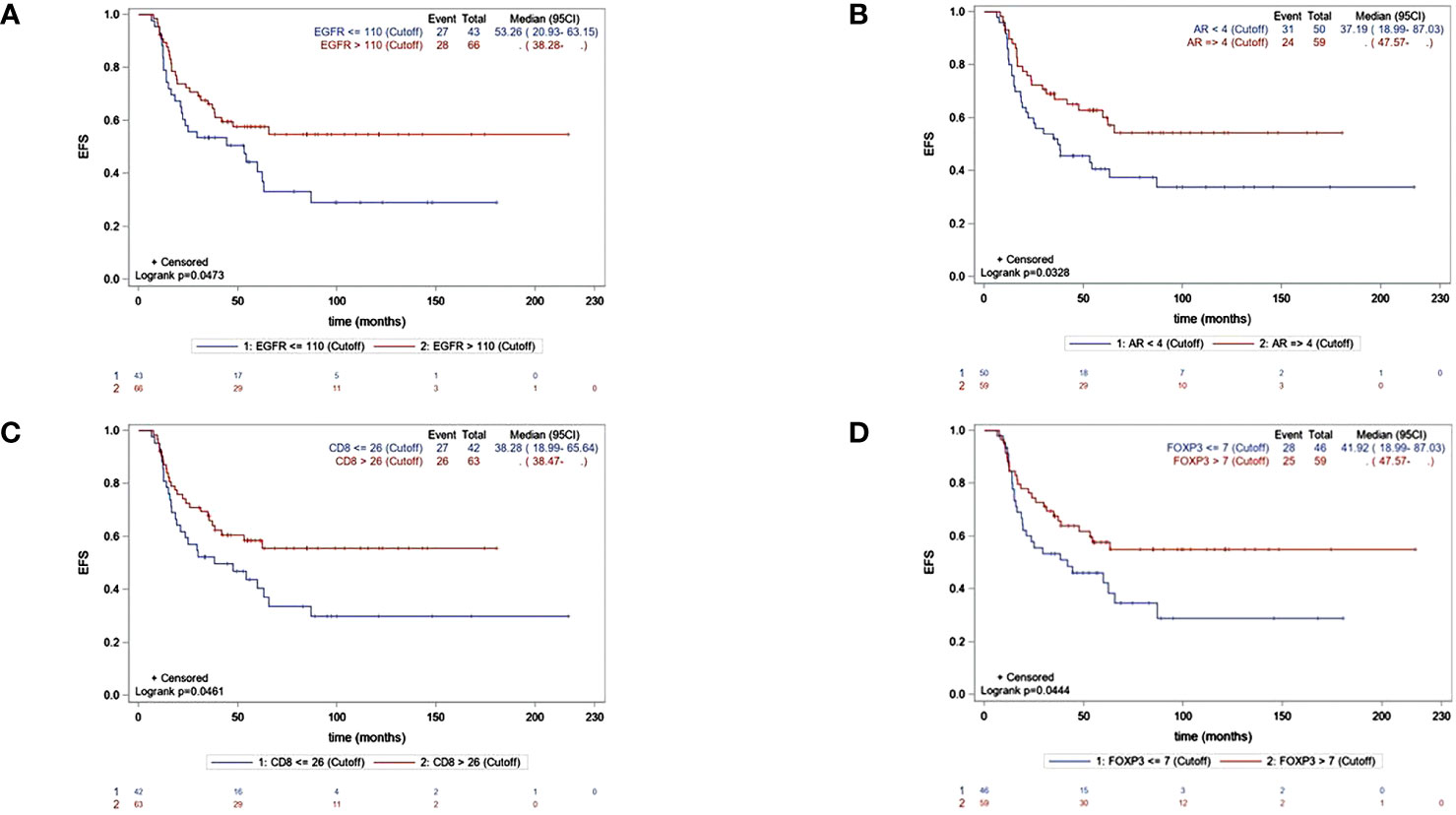
Figure 2 KM curves for EFS accordingly significant biomakers expression :EGFR (A), AR (B), CD8 (C), FOXP3 (D).
Discussion
In the present study, we investigated the prognostic role of a panel of immunohistochemical biomarkers whose clinical significance in breast cancer, especially the TNBC phenotype, has previously been studied. We sought to identify candidates that, in a subsequent analysis, extended to a larger number of cases, could be evaluated to create a multivariate prognostic score for RD.
Residual disease and biomarker expression
In our immunohistochemical analysis, the tumour residue was rich in EGFR and intensely p53-positive, the last one corresponding to TP53 missense mutation status. HER2 was mainly scored 0, corresponding to the initial status, while the Ki-67 value was intermediate for a triple-negative phenotype, CK5/6 was mainly low ≤29% and AR was high ≥4%. Representative photomicrographs for biomarker expression (×20 magnification) are shown in Figures 4A, B.
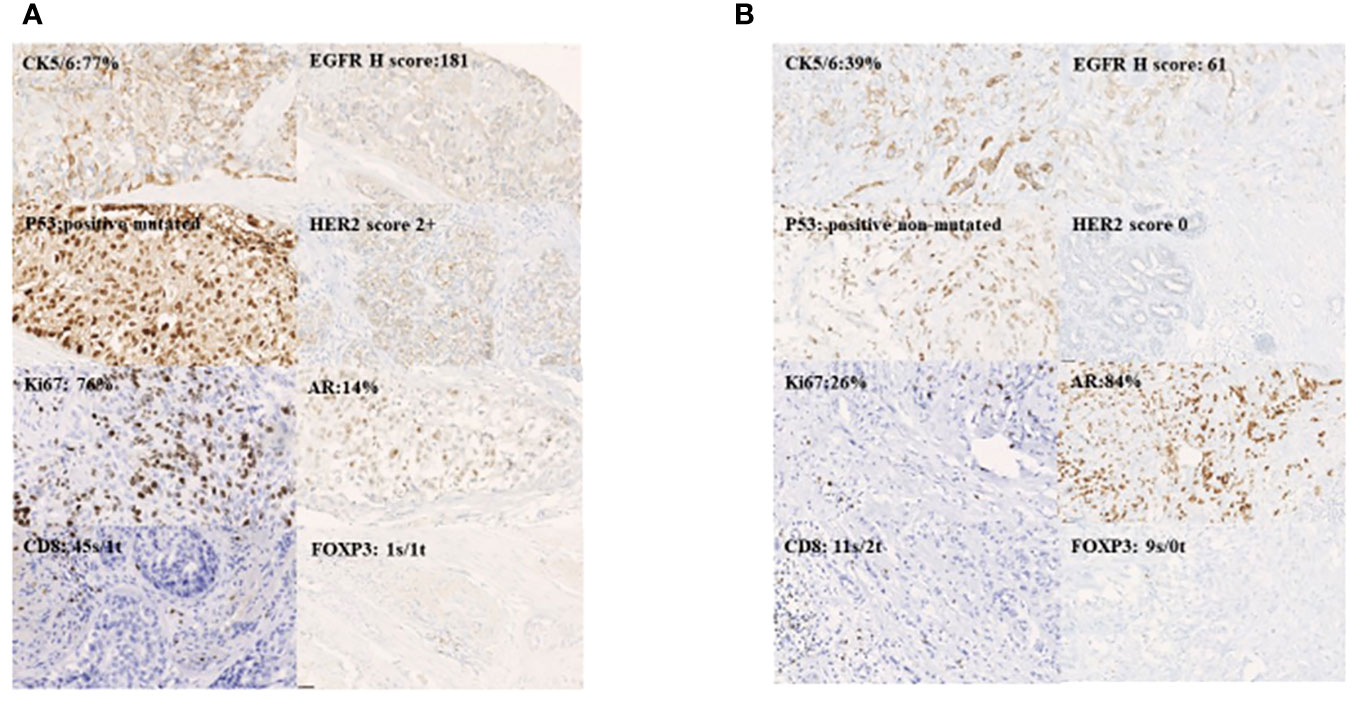
Figure 4 Representative photomicrographs for biomakers expression magnification 20X:basal-like (A) and luminal AR+ (B).
Our cutoff for Ki-67 of 43% is consistent with the results in the literature (30). In a recent meta-analysis that included mainly Asian studies (35 studies totalling 7,716 patients), and in which assessments were mainly performed in the chemo-naive TNBC tumour specimen, the cutoff of Ki-67 that was significant for DFS (HR 2.3, 95% CI 1.54–3.44, p < 0.001) and for OS (HR 2.95, 95% CI 1.67–5.19, p < 0.001) was 40% (31).
HER2 score 0 was found in 52.3% of the cases analysed, approaching the values found in the literature in triple-negative nonmetastatic breast cancer in general (32, 33), less evaluated in a targeted manner in the residual tumour (34).
EGFR overexpression is found in 50% of TNBC patients and in up to 90% in the basal-like subtype (35) for which it is also used as a surrogate (36).
CK5/6 is known as a marker of basal-like sub-phenotype and the percentages of positivity reported in triple-negative cancers are approximately 50% consistent with our result even though most analyses considered positive any identified staining while we set the cutoff of positivity according to the median percentage of positive cells (29%).
In our analysis, p53 was found positive in 83.6% (n = 92) of which 52.7% were positive-mutated. In the literature, p53 expression is found in more than half of the TNBC cases, regardless of the method of assessment, and correlates with EGFR overexpression (37). It is always associated with poor prognosis (38, 39). Some studies have reported that positivity could mean the presence of a poor-quality protein in the cytoplasm and thus correlates with an aggressive phenotype (38) or produced in excess as a compensatory mechanism of tumour DNA repair and to be a good prognostic factor (40).
Regarding the scoring system and cutoff for AR, in a 2020 meta-analysis, it was found that the majority of studies evaluated the expression of AR by the percentage of positive cells, of which 44.44% (12 studies) used a low cutoff value (0% or 1% or 7.5%), 37.04% (10 studies) used a higher cutoff value (10 or 45%), and only five studies (18.25%) used other methods such as Allred, which took into account the intensity and the percentage of positive cells (41).
A profile combining Ki-67 low, CK5/6 low, and AR positive status, mainly found in RD in our cases, could indicate selection by NAC of a luminal androgen receptor (LAR) phenotype containing cells that are less chemosensitive (13).
We choose CD8 and FOXP3 as surrogate markers for tumour-infiltrating lymphocytes (TILs) whose prognostic role is already known (42), since the lymphocyte infiltrate is heterogeneous, containing both helper and cytotoxic lymphocytes and regulatory-inhibitory lymphocytes (43), and as recent studies have shown direct correlations between TILs, programmed death-1 ligand (PD-L1), CD8, and FOXP3 in early TNBC (44).
In more than 50% of cases, the lymphocytic infiltrate was predominantly found in the stroma and was balanced in terms of CD8+ and FOXP3+ representation. CD8 and FOXP3 high were found to be associated with favourable prognosis for EFS, albeit only by univariate analysis.
Abundant CD8+ lymphocytic infiltrate is known to be associated with positive prognosis in ER-negative breast cancer (45). Other authors have reported that both peritumoural CD8+ and FOXP3+ lymphocytic infiltrates are associated with a good outcome (16), mainly in receptor-negative early breast cancer (46). In a recent study that aimed to determine the prognostic role of CD8, FOXP3, and PD-L1 expression in early-stage TNBC, the recognised marker of regulatory T lymphocytes (Tregs), FOXP3 (47) was found to correlate with better survival (HR 0.48, 95% CI 0.28–0.80, p = 0.004) for a cutoff of 57 (44). As our CD8/FOXP3 ratio was in favour of CD8, it is concordant with the results of another study published in 2015 and reporting that a high ratio predicts a good prognosis (48).
Individual prognostic role
In our analysis, the only biomarker whose expression had a protective effect against relapse was overexpression of androgenic receptors. AR expression has a controversial prognostic value in early TNBC. Several large retrospectives studies and meta-analyses have shown an association with improved DFS and OS (49–51), while others have reported an increase in mortality (52, 53). Few studies to date have evaluated the prognostic role of AR in residual tumour. In one study that evaluated the change in AR from primary to residual tumour after NAC, in 71 cases of localised TNBC, the authors found that AR loss was associated with a favourable prognosis, notably in terms of 5-year distant free survival rate (61.6%, 95% CI 44.26–79.14) vs. 25% (95% CI 3.94–87.21) (p = 0.01) in the groups with vs. without AR loss, respectively (54).
Among the pathological factors, LVI and residual lymph node involvement were found to be predictors of relapse. These are classically recognised as negative prognostic factors in localised breast cancer and are also found in specific analyses in the triple-negative phenotype. Accordingly, in a study by Kennedy et al. (55), in 108 patients with triple-negative residual tumour who had received NAC, the factors that were independent predictors of metastatic DFS were node positivity (HR 3.08, 95% CI 1.54–6.14, p = 0.001) and LVI (HR 1.91, 95% CI 1.07–3.43, p = 0.30). By summing negative prognostic factors including the presence of residual tumour with the addition of initial multifocal status, a prognostic model was developed based on the impact on survival, as follows: 0 factor, 5-year freedom from distant metastasis (FFDM) rate of 76%, and 4 factors, 0% of FFDM (55).
For OS, the biomarkers directly associated with the risk of death were the classic Ki-67 high and, interestingly, EGFR low status. Also, residual lymph node involvement emerged once again as a negative prognostic factor.
As regard to EGFR, in our study, we found that, according to our cutoff, receptor’s overexpression was an independent positive prognostic factor for OS. In the literature, results are conflicting regarding EGFR overexpression, with meta-analysis and retrospective analysis showing it to be an adverse prognostic factor (36, 56), while other reports failed to replicate this (57). Accordingly, in one study of a representative triple-negative European population, EGFR >50% assessed in the primary tumour (n = 284) was associated with a 2-fold increase in the risk of recurrence and death (HR for 4-year DFS 2.39, 95% CI 1.32–4.34, p = 0.004 and for OS, HR 2.34, 95% CI 1.2–4.9) (58). In an Asian study (n = 287), for EGFR >10%, 5-year DFS was significantly lower (69% vs. 83.8%, p = 0.011) as was OS (79.5% vs. 88.9%) in patients with EGFR <10% (59). In another study in 198 localised TNBC, improved survival was observed when at least one of three classic basal-like phenotype biomarkers (including EGFR) was positive; the proposed explanation was that this was probably due to a better response to NAC (60).
Our findings are likely related to the method of scoring, while other studies were mostly binary, considering only the percentage of positivity and not the intensity of staining.
In most studies evaluating the prognostic impact of EGFR in localised breast cancer, the antibody used was Dako or Zymed, whereas we used Roche in our analysis, and positivity was considered for between 1% and 10% of stained cells, independent of intensity (56), while we used the H-score and set the cutoff according to EFS (24).
Other studies have used other scoring systems, such as one European study that analysed 52 biopsy specimens from localised triple-negative tumours before any treatment. In that study, no association was found between EGFR+ and EFS (HR 1.114, 95% CI 0.977–1.269, p = 0.106) (61). The scoring system was semiquantitative, comprising a combination of staining intensity and the percentage of positive tumour cells. Intensity was scored as 0 (no membrane staining), 1 (low), 2 (moderate), and 3 (high), and the percentage of positive cells was scored as 1 (<10%), 2 (11%–50%), 3 (51%–80%), and 4 (>80%). A final EGFR score (0–12) was then calculated by combining these two parameters (61).
Another explanation could be that in our study, the analysis was performed in the post-neoadjuvant surgical specimen, where there is significant tissue heterogeneity, and where the intensity of EGFR expression may be different from that in the primary tumour. It seems that EGFR expression decreases after NAC, and that it is not the level of expression that plays a prognostic role but rather the difference between initial expression and expression in the residual tumour (62).
In terms of Ki-67, values above 30% in the residual tumour was found to be prognostic for lower DFS (HR 3.86, 95% CI 1.19–9.21, p = 0.008) in patients receiving NAC for localised hormone receptor (HR)-HER2-negative or -positive breast cancer (63). In a larger analysis performed on residual tumours from patients previously included in the GeparTrio study (n = 1,151), of whom 58% had no pCR (n = 667) and 5.4% (n = 36) were HR-negative, Ki-67 >35% was associated with 1.73-fold increase in the risk of recurrence (95% CI 0.87–3.42) in HR-negative tumours versus Ki-67 ≤35% (64).
Study limitations
The main limitation of this study is that due to the small number of events, we could not create a multivariate score. There is a persisting need to analyse a larger number of cases with a view to developing a prognostic score. Second, the results were interpreted in light of previous knowledge available in the literature and the prognostic value of the markers, mainly at the primary tumour level. A method for quantifying biomarker expression specific to residual cells should be developed.
Conclusion
In this study, RD in patients with TNBC after NAC harboured mainly markers of low aggressiveness, i.e., moderate expression of basal-like biomarkers, high ARs, and low Ki-67, while markers of lymphocyte infiltration were predominantly present in the stroma. Biomarkers found to be associated with outcome and which we propose for the development of a multivariate histopathological and immunohistochemical prognostic model for EFS are AR, lymphovascular invasion (LVI), and ypN. These will be validated in a larger cohort in a subsequent study. Future research should focus on how to evaluate the evolution between primary tumour and RD by immunohistochemistry.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Centre Georges Francois Leclerc, Dijon, France. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SI: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NB: Data curation, Formal analysis, Software, Writing – original draft. GC: Data curation, Formal analysis, Software, Writing – original draft. AI: Data curation, Investigation, Resources, Visualization, Writing – original draft. FB: Investigation, Supervision, Writing – original draft. SL: Conceptualization, Resources, Writing – original draft. ID: Resources, Writing – original draft. AH: Resources, Writing – original draft. AB: Data curation, Formal analysis, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CC: Funding acquisition, Resources, Writing – original draft. SC: Resources, Writing – original draft. NG: Formal analysis, Investigation, Resources, Writing – original draft. BC: Resources, Writing – original draft. LA: Formal analysis, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to Ms. Fiona Ecarnot, PhD, University Hospital Besancon France for providing the medical writing and editorial assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors–and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1309890/full#supplementary-material
References
1. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch Pathol Lab Med (2018) 142(11):1364–82. doi: 10.5858/arpa.2018-0902-SA
2. Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med (2010) 134(7):e48–72. doi: 10.5858/134.7.e48
3. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med (2010) 363(20):1938–48. doi: 10.1056/NEJMra1001389
4. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol (2012) 30(15):1796–804. doi: 10.1200/JCO.2011.38.8595
5. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (2014) 384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8
6. Kern P, Von Minckwitz G, Puetter C, Pavlidou S, Flach A, Kimmig R, et al. Prognostic impact of residual disease after neoadjuvant chemotherapy in 648 patients with triple-negative breast cancer. Anticancer Res (2015) 35(10):5479–84.
7. NCCN clinical practice guidelines in oncology for breast cancer V.4.2022 . Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
8. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2022) 30(8):1194–220. doi: 10.1093/annonc/mdz173
9. Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med (2017) 376(22):2147–59. doi: 10.1056/NEJMoa1612645
10. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med (2021) 384(25):2394–405. doi: 10.1056/NEJMoa2105215
11. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549
12. Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol (2014) 232(2):142–50. doi: 10.1002/path.4280
13. Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SAW, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res (2015) 21(7):1688–98. doi: 10.1158/1078-0432.CCR-14-0432
14. Jézéquel P, Loussouarn D, Guérin-Charbonnel C, Campion L, Vanier A, Gouraud W, et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res (2015) 17:43. doi: 10.1186/s13058-015-0550-y
15. da Silva JL, Cardoso Nunes NC, Izetti P, de Mesquita GG, de Melo AC. Triple negative breast cancer: A thorough review of biomarkers. Crit Rev Oncol Hematol (2020) 145:102855. doi: 10.1016/j.critrevonc.2019.102855
16. Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8+ cytotoxic T cell and FOXP3+ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat (2011) 130(2):645–55. doi: 10.1007/s10549-011-1647-3
17. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th edition. Vol. 1024. New York: Springer International Publishing (2017).
18. Institut National Du Cancer. Available at: https://www.e-cancer.fr/Professionnels-de-sante/Recomandations-et-outils-d-aide-a-la-pratique/Cancer-du-sein.
19. Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol (2007) 25(1):118–45. doi: 10.1200/JCO.2006.09.2775
20. Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol (2013) 31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984
21. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology (1991) 19(5):403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x
22. McShane LM, Altman DG, Sauerbrei W, Taube W, Gion M, Clark GM, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat (2006) 100(2):229–35. doi: 10.1007/s10549-006-9242-8
23. Banerjee S, Reis-Filho JS, Ashley S, Steele D, Ashworth A, Lakhani SR, et al. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol (2006) 59(7):729–35. doi: 10.1136/jcp.2005.033043
24. Pirker R, Pereira JR, von Pawel J, Krzakowski M, Ramlau R, Park K, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol (2012) 13(1):33–42. doi: 10.1016/S1470-2045(11)70318-7
25. Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol (2011) 24(7):924–31. doi: 10.1038/modpathol.2011.54
26. Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih IM, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol (2011) 24(9):1248–53. doi: 10.1038/modpathol.2011.85
27. Ladoire S, Arnould L, Mignot G, Coudert B, Rébé C, Chalmin F, et al. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat (2011) 125(1):65–72. doi: 10.1007/s10549-010-0831-1
28. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. J Clin Oncol (2018) 36(20):2105–22. doi: 10.1200/JCO.2018.77.8738
29. Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PloS One (2012) 7(12):e51862. doi: 10.1371/journal.pone.0051862
30. Wang W, Wu J, Zhang P, Fei X, Zong Y, Chen X, et al. Prognostic and predictive value of Ki-67 in triple-negative breast cancer. Oncotarget (2016) 7(21):31079–87. doi: 10.18632/oncotarget.9075
31. Wu Q, Ma G, Deng Y, Luo W, Zhao Y, Li W, et al. Prognostic value of ki-67 in patients with resected triple-negative breast cancer: A meta-analysis. Front Oncol (2019) 9:1068. doi: 10.3389/fonc.2019.01068
32. Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer (2021) 7(1):1. doi: 10.1038/s41523-020-00208-2
33. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol (2020) 38(17):1951–62. doi: 10.1200/JCO.19.02488
34. Jacot W, Maran-Gonzalez A, Massol O, Sorbs C, Mollevi C, Guiu S, et al. Prognostic value of HER2-low expression in non-metastatic triple-negative breast cancer and correlation with other biomarkers. Cancers (Basel) (2021) 13(23):6059. doi: 10.3390/cancers13236059
35. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res (2004) 10(16):5367–74. doi: 10.1158/1078-0432.CCR-04-0220
36. Abdelrahman AE, Rashed HE, Abdelgawad M, Abdelhamid MI. Prognostic impact of EGFR and cytokeratin 5/6 immunohistochemical expression in triple-negative breast cancer. Ann Diagn Pathol (2017) 28:43–53. doi: 10.1016/j.anndiagpath.2017.01.009
37. Tzaida O, Gogas H, Dafni U, Kyroudi A, Papaspyrou I, Kyriakou V, et al. Evaluation of the prognostic and predictive value of HER-1/EGFR in breast cancer patients participating in a randomized study with dose-dense sequential adjuvant chemotherapy. Oncology (2007) 72(5–6):388–96. doi: 10.1159/000113148
38. Li JP, Zhang XM, Zhang Z, Zheng LH, Jindal S, Liu YJ. Association of p53 expression with poor prognosis in patients with triple-negative breast invasive ductal carcinoma. Med (Baltimore) (2019) 98(18):e15449. doi: 10.1097/MD.0000000000015449
39. Mitri ZI, Abuhadra N, Goodyear SM, Hobbs EA, Kaempf A, Thompson AM, et al. Impact of TP53 mutations in triple negative breast cancer. NPJ Precis Oncol (2022) 6(1):64. doi: 10.1038/s41698-022-00303-6
40. Jin MS, Park IA, Kim JY, Chung YR, Im SA, Lee KH, et al. New insight on the biological role of p53 protein as a tumor suppressor: re-evaluation of its clinical significance in triple-negative breast cancer. Tumour Biol (2016) 37(8):11017–24. doi: 10.1007/s13277-016-4990-5
41. Xu M, Yuan Y, Yan P, Jiang J, Ma P, Niu X, et al. Prognostic significance of androgen receptor expression in triple negative breast cancer: A systematic review and meta-analysis. Clin Breast Cancer (2020) 20(4):e385–96. doi: 10.1016/j.clbc.2020.01.002
42. Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol (2014) 32(27):2959–66. doi: 10.1200/JCO.2013.55.0491
43. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol (2013) 14(10):1014–22. doi: 10.1038/ni.2703
44. Dieci MV, Tsvetkova V, Griguolo G, Miglietta F, Tasca G, Giorgi CA, et al. Integration of tumour infiltrating lymphocytes, programmed cell-death ligand-1, CD8 and FOXP3 in prognostic models for triple-negative breast cancer: Analysis of 244 stage I-III patients treated with standard therapy. Eur J Cancer (2020) 136:7–15. doi: 10.1016/j.ejca.2020.05.014
45. Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology (2011) 58(7):1107–16. doi: 10.1111/j.1365-2559.2011.03846.x
46. West NR, Kost SE, Martin SD, Milne K, Deleeuw RJ, Nelson BH, et al. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer (2013) 108(1):155–62. doi: 10.1038/bjc.2012.524
47. Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity (2005) 22(3):329–41. doi: 10.1016/j.immuni.2005.01.016
48. Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res (2015) 17(1):124. doi: 10.1200/jco.2015.33.15_suppl.510
49. Loibl S, Müller BM, von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat (2011) 130(2):477–87. doi: 10.1007/s10549-011-1715-8
50. Lyalkin SA, Verevkina NO, Alekseyenko OO, Syvak LA. Prognostic role of androgen receptor expression in patients with metastatic triple negative breast cancer. Exp Oncol (2020) 42(2):140–3. doi: 10.32471/exp-oncology.2312-8852.vol-42-no-2.14579
51. Bozovic-Spasojevic I, Zardavas D, Brohée S, Ameye L, Fumagalli D, Ades F, et al. The prognostic role of androgen receptor in patients with early-stage breast cancer: A meta-analysis of clinical and gene expression data. Clin Cancer Res (2017) 23(11):2702–12. doi: 10.1158/1078-0432.CCR-16-0979
52. Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res (2011) 17(7):1867–74. doi: 10.1158/1078-0432.CCR-10-2021
53. Jiang HS, Kuang XY, Sun WL, Xu Y, Zheng YZ, Liu YR, et al. Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget (2016) 7(27):41285–93. doi: 10.18632/oncotarget.9778
54. Jongen L, Floris G, Wildiers H, Claessens F, Richard F, Laenen A, et al. Tumor characteristics and outcome by androgen receptor expression in triple-negative breast cancer patients treated with neo-adjuvant chemotherapy. Breast Cancer Res Treat (2019) 176(3):699–708. doi: 10.1007/s10549-019-05252-6
55. Kennedy WR, Tricarico C, Gabani P, Weiner AA, Altman MB, Ochoa LL, et al. Predictors of distant metastases in triple-negative breast cancer without pathologic complete response after neoadjuvant chemotherapy. J Natl Compr Canc Netw (2020) 18(3):288–96. doi: 10.6004/jnccn.2019.7366
56. Gonzalez-Conchas GA, Rodriguez-Romo L, Hernandez-Barajas D, Gonzalez-Guerrero JF, Rodriguez-Fernandez IA, Verdines-Perez A, et al. Epidermal growth factor receptor overexpression and outcomes in early breast cancer: A systematic review and a meta-analysis. Cancer Treat Rev (2018) 62:1–8. doi: 10.1016/j.ctrv.2017.10.008
57. Nakajima H, Ishikawa Y, Furuya M, Sano T, Ohno Y, Horiguchi J, et al. Protein expression, gene amplification, and mutational analysis of EGFR in triple-negative breast cancer. Breast Cancer (2014) 21(1):66–74. doi: 10.1007/s12282-012-0354-1
58. Viale G, Rotmensz N, Maisonneuve P, Bottiglieri L, Montagna E, Luini A, et al. Invasive ductal carcinoma of the breast with the ‘triple-negative’ phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat (2009) 116(2):317–28. doi: 10.1007/s10549-008-0206-z
59. Liu D, He J, Yuan Z, Wang S, Peng R, Shi Y, et al. EGFR expression correlates with decreased disease-free survival in triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol (2012) 29(2):401–5. doi: 10.1007/s12032-011-9827-x
60. Schulmeyer CE, Fasching PA, Häberle L, Meyer J, Schneider M, Wachter D, et al. Expression of the immunohistochemical markers CK5, CD117, and EGFR in molecular subtypes of breast cancer correlated with prognosis. Diagnostics (Basel) (2023) 13(3):372. doi: 10.3390/diagnostics13030372
61. Sobande F, Dušek L, Matějková A, Rozkoš T, Laco J, Ryška A. EGFR in triple negative breast carcinoma: significance of protein expression and high gene copy number. Cesk Patol. (2015) 51(2):80–6.
62. van Reesema LLS, Zheleva V, Winston JS, Jansen RJ, O’Connor CF, Isbell AJ, et al. SIAH and EGFR, two RAS pathway biomarkers, are highly prognostic in locally advanced and metastatic breast cancer. EBioMedicine (2016) 11:183–98. doi: 10.1016/j.ebiom.2016.08.014
63. Tan QX, Qin QH, Yang WP, Mo QG, Wei CY. Prognostic value of Ki67 expression in HR-negative breast cancer before and after neoadjuvant chemotherapy. Int J Clin Exp Pathol (2014) 7(10):6862–70.
Keywords: neoadjuvant chemotherapy, residual disease, triple-negative breast cancer, prognostic biomarkers, immunohistochemical marker
Citation: Ilie SM, Briot N, Constatin G, Ilie A, Beltjens F, Ladoire S, Desmoulins I, Hennequin A, Bertaut A, Coutant C, Causeret S, Ghozali N, Coudert B and Arnould L (2024) Pathologic and immunohistochemical prognostic markers in residual triple-negative breast cancer after neoadjuvant chemotherapy. Front. Oncol. 13:1309890. doi: 10.3389/fonc.2023.1309890
Received: 08 October 2023; Accepted: 04 December 2023;
Published: 10 January 2024.
Edited by:
Ming Yi, Zhejiang University, ChinaReviewed by:
Bruna Cerbelli, Sapienza University of Rome, ItalyMiguel J. Gil Gil, Catalan Institute of Oncology, Spain
Copyright © 2024 Ilie, Briot, Constatin, Ilie, Beltjens, Ladoire, Desmoulins, Hennequin, Bertaut, Coutant, Causeret, Ghozali, Coudert and Arnould. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Mihaela Ilie, U0lsaWVAY2dmbC5mcg==
†These authors have contributed equally to this work
 Silvia Mihaela Ilie
Silvia Mihaela Ilie Nathalie Briot2†
Nathalie Briot2† Sylvain Ladoire
Sylvain Ladoire Niama Ghozali
Niama Ghozali