
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 01 December 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1309699
Background and aims: Palliative primary tumor resection (pPTR) can benefit colorectal cancer patients with liver metastasis. Whether pPTR benefiting gastric cancer (GC) patients with liver metastasis is still controversial.
Methods: Data on patients with metastatic GC diagnosed between 2010 to 2019 was extracted from SEER database. Propensity score analysis with 1:1 matching was performed. The univariable and multivariable Cox proportional hazards regression models were used to explore prognostic factors. Kaplan–Meier method was used to analyze survival outcomes.
Results: Of 5691 GC patients with liver metastasis, 468 were included in the matched cohorts. The results showed that the median survival time was 6 months in the non-surgery groups and 14.5 months in the surgery groups (p < 0.001). Multivariable analysis showed that surgery was a protective prognostic factor for overall survival [hazard ratio (HR) = 0.416] as well as cancer-specific survival (HR = 0.417). Also, pPTR was only recommended for GC patients with isolated liver metastasis. Moreover, pPTR combined with chemotherapy brought the greatest therapeutic effect.
Conclusion: pPTR benefits GC patients with isolated liver metastasis, and GC patients with liver metastasis receiving pPTR combined with chemotherapy had the best survival outcomes than any other therapeutic model.
Gastric cancer is one of the most common types of cancer worldwide. Although its incidence has decreased recently, gastric cancer ranks fifth and fourth in incidence and cancer-related deaths worldwide (1–3). Gastric cancer is often diagnosed at a metastatic stage and is considered incurable (4). Traditionally, systemic therapy is advocated for patients with advanced gastric cancer (5–9). Surgery is performed only when patients show symptoms such as obstruction, tumor bleeding, or perforation; otherwise, surgery is not recommended according to the current guidelines (10). Liver metastasis often occurs from gastrointestinal tumors (11–13), and many studies have explored the function of primary tumor surgery in colorectal cancer patients with metastasis to liver at the same time (14–16). The outcomes showed that primary tumor surgery grants survival benefits to advanced colorectal cancer patients with, although the guidelines do not list it as an option.
Much debate remains on whether gastrectomy is suitable for patients with gastric cancer and liver metastasis. Although the REGATTA trial was a randomized controlled trial, it was soon terminated due to futility (17, 18). Therefore, uncovering the effect of gastrectomy on the survival of gastric cancer patients with liver metastasis is necessary. Our study utilized the SEER database to identify the clinical outcomes of these patients. In order to control the selection bias, 1:1 propensity score matching (PSM) analysis was adopted.
We acquired patient data from the SEER Research Plus Data, 17 Registries, Nov 2021 Sub (2000-2019) incidence database using the SEER*Stat software (V 8.4.0). After careful screening, 5,691 patients were included in this study; PSM matched 468 patients. The inclusion standards were (1) diagnosis of gastric cancer and only one primary tumor; (2) tumor stage M1; (3) survival time >1 month; and (4) the patient did not undergo metastasectomy. The detailed procedure is illustrated in Figure 1.
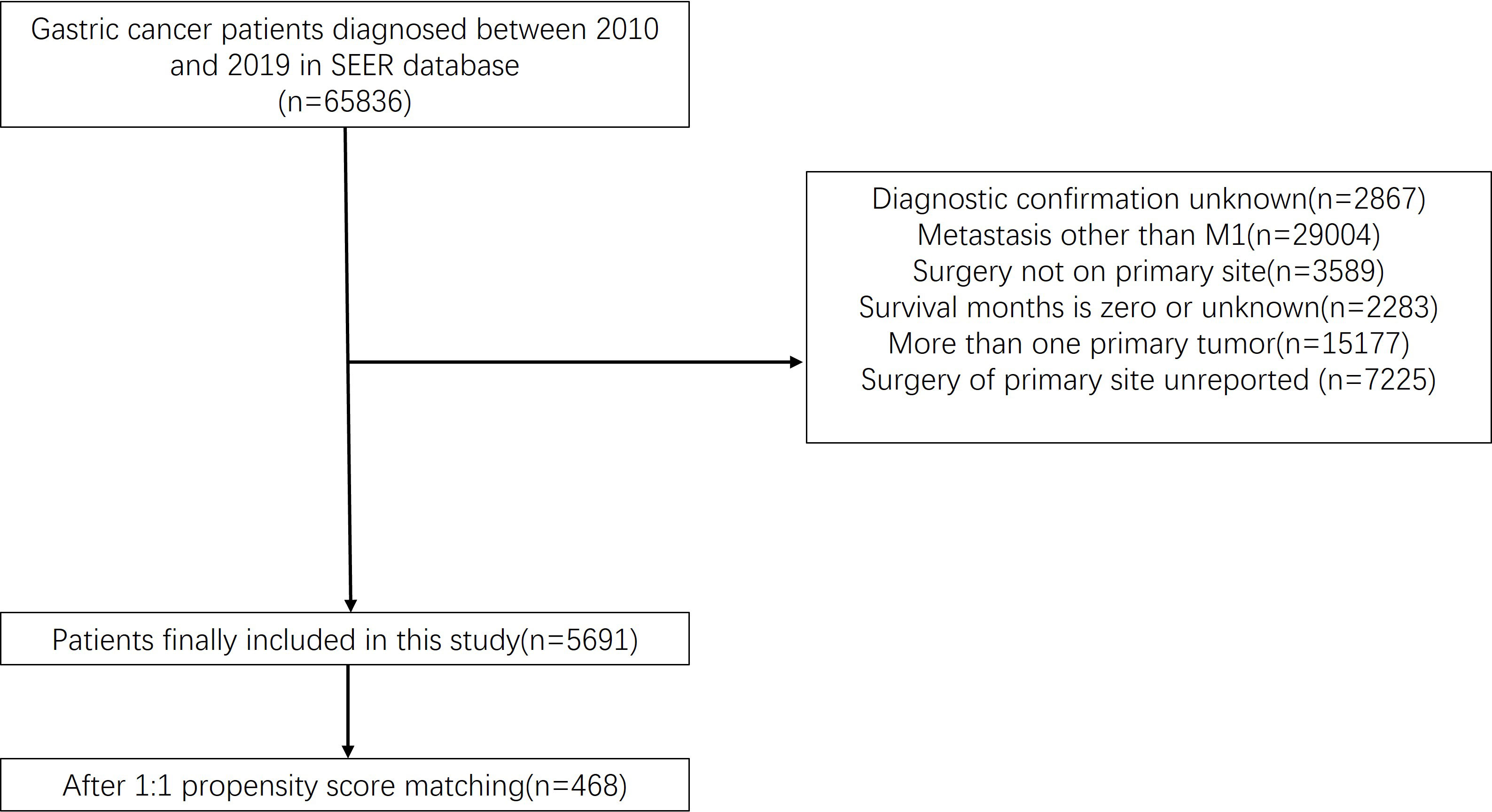
Figure 1 Flow chart illustrating patient inclusion and exclusion. A total of 65,836 patients were extracted from the database. There were 5,691 people left after we ruled out data that didn’t fit our criteria. A total of 468 patients were enrolled in the study after a propensity matched 1:1 test.
PSM was used to control the selection bias. Patients were propensity-matched 1:1 in the surgery and non-surgery groups using the nearest-neighbor method. The matched variables were marital status, age, sex, race, grade, T stage, N stage, primary site, adenocarcinoma, year of diagnosis, metastatic pattern, chemotherapy, and radiation therapy. The standardized mean differences before and after matching are shown in Figure 2.
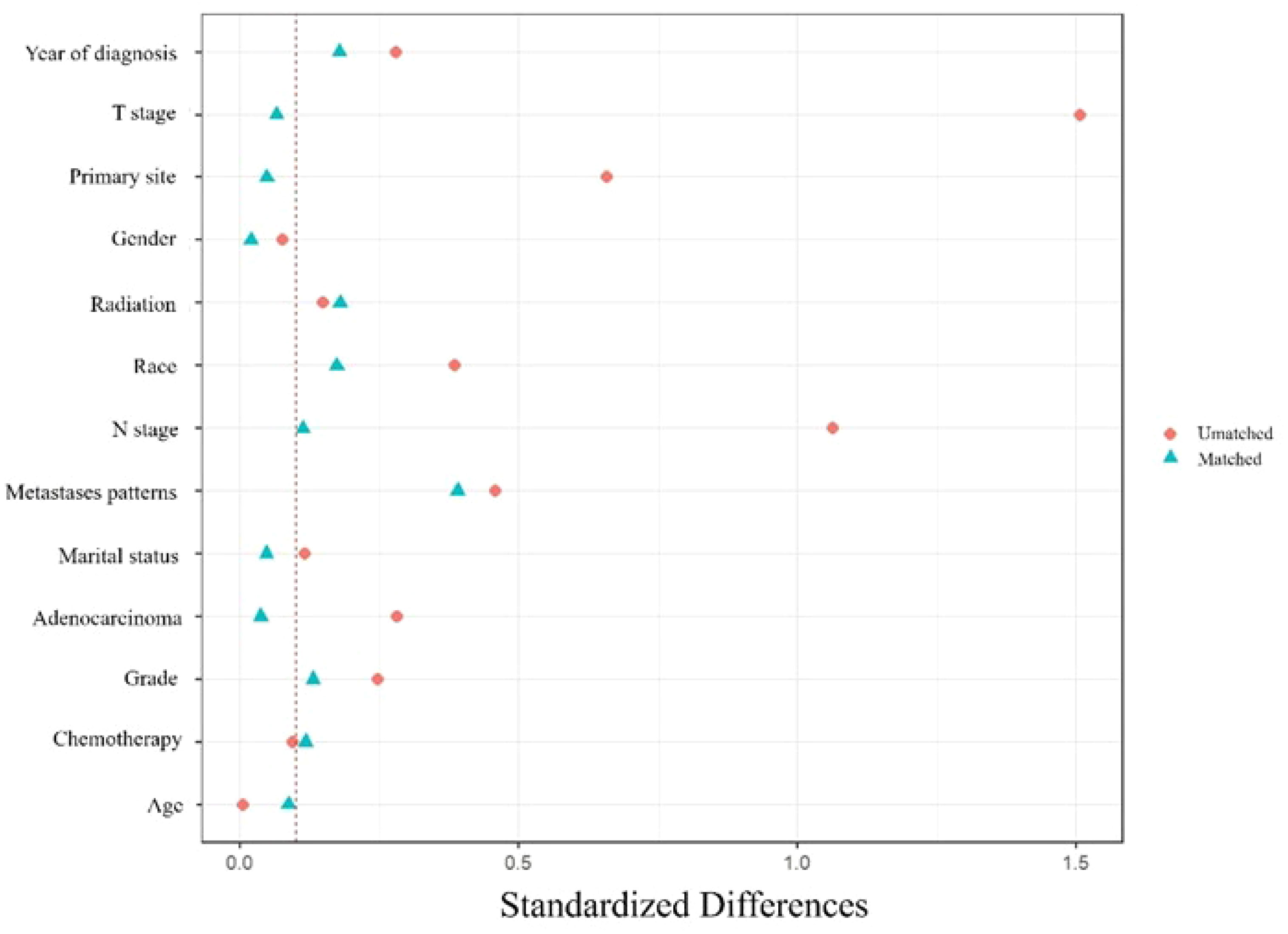
Figure 2 Standardized differences before and after PSM. Marital status, age, sex, race, grade, T stage, N stage, primary site, adenocarcinoma, year of diagnosis, metastatic pattern, chemotherapy, and radiation therapy were used to match.
All analyses were performed using SPSS (version 24.0) and R (version 4.1.2) software. Statistical significance was set at p<0.05. A 1:1 PSM analysis was adopted to reduce possible randomization. The χ2 test was utilized to compare the baseline characteristics of the patients in two groups: the matched and unmatched cohorts. Cox proportional hazards models were utilized to evaluate the hazard ratio (HR) and 95% confidence interval (CI) and to reveal the independent prognostic factors for gastric cancer patients. The endpoints were set according to overall survival (OS) and cancer-specific survival (CSS). Kaplan–Meier analysis and log-rank tests were used to compare survival between the patients who underwent surgery and the patients who did not choose in the matched population stratified by metastatic patterns, primary site, and treatments.
In this study, 5,691 of 65,836 patients with diagnosis of gastric cancer between 2010 and 2019 satisfied our inclusion and exclusion criteria for further study. Among these patients, 312 (5.6%) underwent a gastrectomy, and 5,379 (94.4%) did not choose the surgery (Table 1). PSM analysis was carried out later matching 468 patients 1:1 to form surgery and non-surgery cohorts (Table 2).
The Kaplan–Meier method was used to compute the survival outcomes of the matched cohort. A significant difference was observed (p<0.001) between the patients who underwent gastrectomy and those who did not (Figure 3A). Patients undergoing gastrectomy could have a median OS of 14.5 months, while those abandoning surgery only had 6 months. Univariate Cox proportional hazard regression analysis performed on the matched cohort found that OS was linked to age, surgery, primary site, adenocarcinoma, N stage, grade, chemotherapy, and synchronous metastasis patterns. Multivariate Cox analysis included these variables and showed that surgery was a strong protective factor for OS (HR 0.416, 95% CI: 0.330-0.525; p<0.001) (Table 3). Moreover, adenocarcinoma, N stage, tumor grade, chemotherapy, and synchronous metastatic patterns were confirmed to be independent prognostic factors.
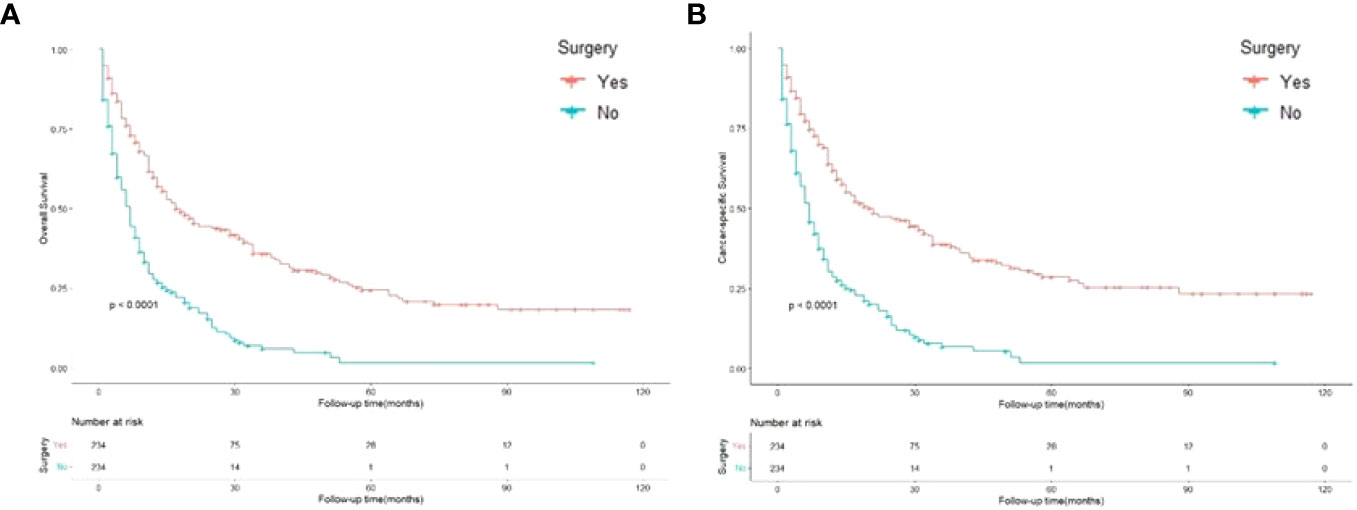
Figure 3 Kaplan-Meier curves for overall and cancer-specific survival in patients with and without gastrectomy. Life tables for patients at risk are given below each plot. (A) Overall survival of patients with and without gastrectomy. (B) Cancer-specific survival of patients with and without gastrectomy.
Figure 3B shows that the cancer-specific survival outcomes of patients who underwent surgery were better than those of patients who did not (p<0.001). Univariate Cox proportional hazard regression analysis revealed that CSS was linked to age, surgery, primary site, adenocarcinoma, N stage, grade, chemotherapy, and synchronous metastasis patterns. The Multivariable analysis, which showed that gastrectomy was an obvious protective factor for CSS (HR 0.417, 95% CI: 0.328–0.530; p<0.001) (Table 4). Furthermore, adenocarcinoma, grade, chemotherapy, and synchronous metastasis patterns had predictive significance of CSS.
Figure 4 shows the survival outcomes of patients with different primary sites. In the subgroup analyses, the cardia and fundus of the stomach and other sites presented more prominent OS outcomes than the gastric antrum and body of the stomach. In addition, the cardia and fundus of the stomach, the body of the stomach, and other sites showed better CSS than the gastric antrum.
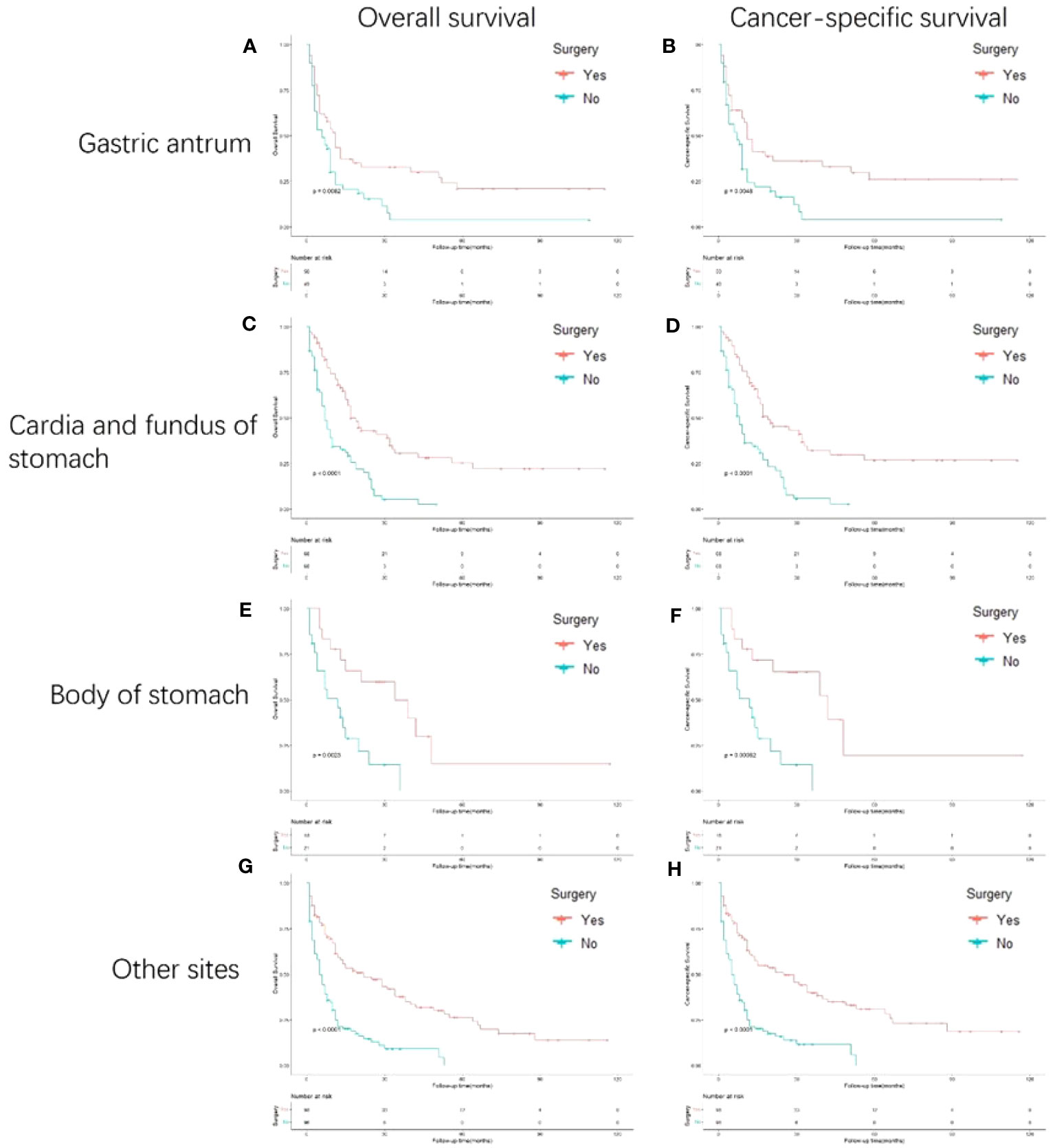
Figure 4 Kaplan-Meier curves for overall and cancer-specific survival in patients with and without gastrectomy stratified based on primary site. Life tables for patients at risk are given below each plot. (A) OS of patients whose primary site is gastric antrum patients with and without gastrectomy. (B) CSS of patients whose primary site is gastric antrum patients with and without gastrectomy. (C) OS of patients whose primary site is cardia and fundus of stomach with and without gastrectomy. (D) CSS of patients whose primary site is cardia and fundus of stomach with and without gastrectomy. (E) OS of patients whose primary site is body of stomach of stomach with and without gastrectomy. (F) CSS of patients whose primary site is body of stomach of stomach with and without gastrectomy. (G) OS of patients whose primary site is other sites of stomach with and without gastrectomy. (H) CSS of patients whose primary site is other sites of stomach with and without gastrectomy.
In the subgroup analysis stratified by synchronous metastasis patterns (Figure 5), the survival benefit of gastrectomy was observed only in patients with gastric cancer with isolated liver metastasis (p<0.001). However, no survival benefit of gastrectomy has been observed in gastric cancer patients with liver plus other metastases.
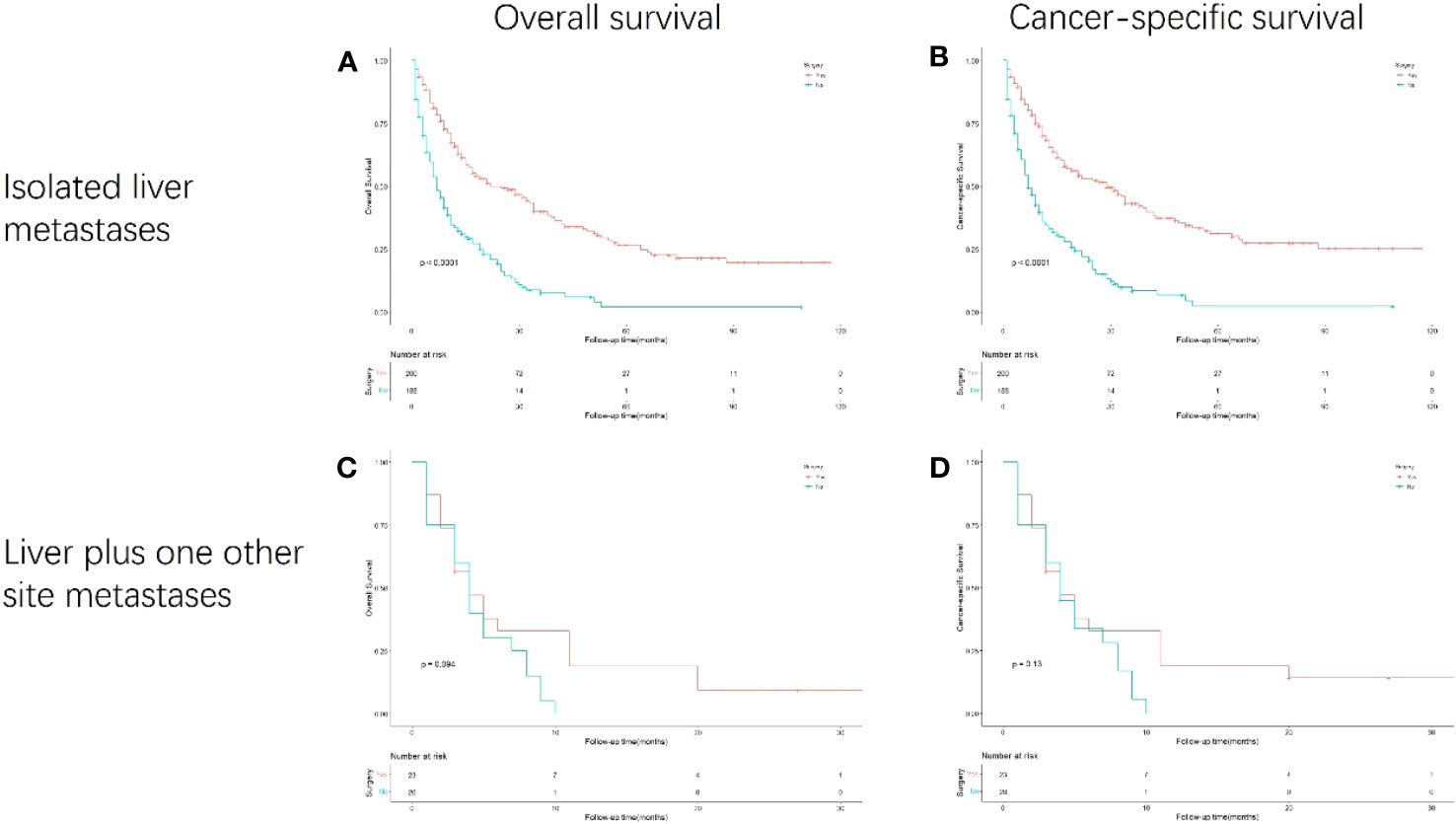
Figure 5 Kaplan-Meier curves for overall and cancer-specific survival in patients with and without gastrectomy stratified based on synchronous metastasis patterns. Life tables for patients at risk are given below each plot. (A) OS of isolated liver metastasis patients with and without gastrectomy. (B) CSS of isolated liver metastasis patients with and without gastrectomy. (C) OS of liver plus one other site metastases patients with and without gastrectomy. (D) CSS of liver plus one other site metastases patients with and without gastrectomy.
Based on the different therapies received, the patients were divided into subgroups (Figure 6). Surgery combined with chemotherapy yielded the best OS and CSS, followed by surgery and trimodal treatment (surgery + chemotherapy + radiation); radiation alone resulted in the worst survival (p<0.001). GC patients with liver metastasis receiving surgery combined with chemotherapy had the best survival outcomes than any other therapeutic model (HR: 0.156, 95% CI: 0.111- 0.219; p<0.001) (Table 5).
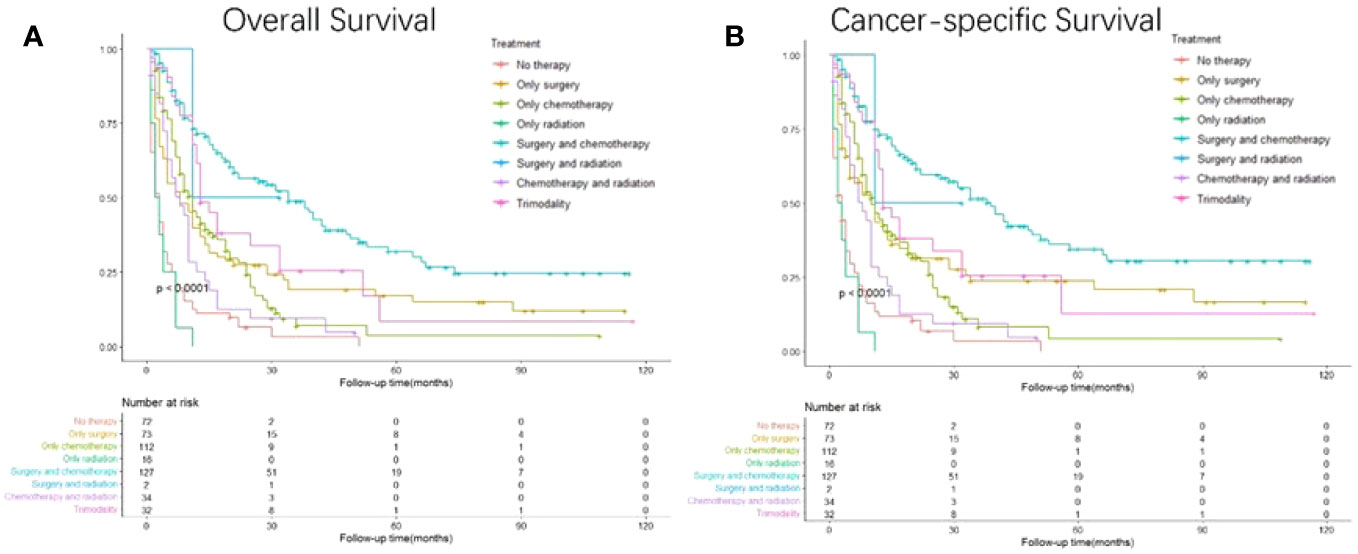
Figure 6 Kaplan-Meier curves for overall and cancer-specific survival in patients with and without gastrectomy stratified by treatment. Life tables for patients at risk are given below each plot. (A) Overall survival of patients receiving no therapy, only surgery, only chemotherapy, only radiation, surgery plus chemotherapy, surgery plus radiation, chemotherapy plus radiation and trimodality. (B) Cancer-specific survival of patients receiving no therapy, only surgery, only chemotherapy, only radiation, surgery plus chemotherapy, surgery plus radiation, chemotherapy plus radiation and trimodality.
Because of the low early diagnosis rate, most patients with gastric cancer present with unresectable distant synchronous metastases (19, 20). However, the treatment of advanced gastric cancer remains controversial, and many questions remain unresolved (21–24). Surgery is usually not recommended for gastric cancer patients with liver metastasis unless they show specific symptoms such as obstruction, tumor bleeding, or perforation. In addition, a previous study indicated that surgery showed no survival benefits in the treatment of gastric cancer patients with a single liver metastasis (25). However, other studies have considered surgery valuable for improving the prognoses of patients with metastatic gastric cancer patients (26–31). Therefore, much debate remains regarding the effect of surgery on patients with metastatic gastric cancer. REGATTA, a randomized controlled trial, attempted to solve this problem but failed in the end (17, 18). The limitations of the REGATTA trial included that the study analyzed patients only from Asia and the chemotherapy dose was inappropriate. Our study showed longer OS and CSS after gastrectomy in gastric cancer patients with isolated liver metastasis. These results indicated a gastrectomy is beneficial for patients with advanced gastric cancer.
Many previous studies have reported that surgery combined with chemotherapy improves the survival time of patients with metastatic gastric cancer patients (32–38). In metastatic tumors, today, with neoadjuvant therapies we can attempt a conversion to see if the patient recovers the operability criteria (39). Our study also showed that combining surgery and chemotherapy was more beneficial than surgery or chemotherapy alone. Chemotherapy is considered the best treatment for metastatic gastric cancer. Surgery can also reduce tumor burden and promote immune system recovery (40). Therefore, surgery combined with chemotherapy should be advocated in the future. Radiation therapy is generally considered a tool for cancer treatment (41, 42). Unexpectedly, we found that radiation therapy had the poorest survival benefit. We speculate that radiation therapy alone is not recommended for patients with metastatic gastric cancer; therefore, the sample size was limited. This limitation may have influenced the results.
In the multivariate analysis, tumor histology was associated with OS and CSS. Patients with adenocarcinoma had a higher hazard ratio. Adenocarcinomas are usually associated with poor survival. Moreover, surgery granted patients survival benefits for metastatic patterns when they had only liver metastasis or liver metastasis combined with metastasis at another site, in accord with a published study (43).
Our study has several advantages compared to other studies. First, this study focused on liver metastasis; therefore, the issues in gastric cancer patients with liver metastasis can be deeply understood. Second, our study comprehensively analyzed the treatment modalities. Three common treatments and their combinations were analyzed. In addition, we analyzed the survival outcomes of patients with gastric cancer according to the primary site.
Limitations cannot be neglected in our study. The SEER database does not provide specific patient information, including family history, chemotherapy cycles, and postoperative quality of life. For chemotherapy, it is very valuable to discuss the specific chemotherapy regimen for the patient’s prognosis. But we are unable to obtain the specific chemotherapy regimens and doses in SEER database. Moreover, the SEER database consists mainly of Americans; therefore, it does not represent all human beings. In addition, although PSM was conducted to minimize selection bias, unobserved confounders not addressed in the PSM remained. Therefore, randomized controlled trials are required to verify our findings. Also, the spread of sequencing technology for biopsies has helped doctors provide precision treatment for gastric cancer patients, but we cannot acquire such data from the SEER database to make our study more specific. Last, different pathologic types affect the prognosis of patients. In the adenocarcinoma data used in this article, there are insufficient numbers of specific pathological types of adenocarcinoma. This makes it impossible to make a more precise analysis based on the type of pathology.
In conclusion, using PSM to minimize selection bias, our study demonstrated the survival benefits of GC patients with liver metastasis undergoing pPTR. pPTR benefits GC patients with isolated liver metastasis, and GC patients with liver metastasis receiving pPTR combined with chemotherapy had the best survival outcomes than any other therapeutic model.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
We analyzed data from SEER database, after signing a data agreement (11187-Nov2021); moreover, our study was exempted from ethical review.
BR: Data curation, Formal Analysis, Writing – original draft. YY: Methodology, Writing – original draft. YL: Supervision, Writing – review & editing. KL: Conceptualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Key R&D Project of Shaanxi Province (No. 2020GXLH-Z-001), the Scientific Development Funding of the First Affiliated Hospital of Xi’an Jiaotong University (No. 2022QN-17) and the Natural Science Basic Research Program of Shaanxi Province (No.2023-JC-QN-0879).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. (2011) 14(4):301–16. doi: 10.1007/s10120-011-0085-6
2. Malvezzi M, Bonifazi M, Bertuccio P, Levi F, La Vecchia C, Decarli A, et al. An age-period-cohort analysis of gastric cancer mortality from 1950 to 2007 in Europe. Ann Epidemiol. (2010) 20(12):898–905. doi: 10.1016/j.annepidem.2010.08.013
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
4. Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol (2014) 20(38):13842–62. doi: 10.3748/wjg.v20.i38.13842
5. Aguiar PN Jr., Muniz TP, Miranda RR, Tadokoro H, Forones NM, Monteiro ID, et al. Current advances in targeted therapies for metastatic gastric cancer: improving patient care. Future Oncol (2016) 12(6):839–54. doi: 10.2217/fon.15.348
6. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(2):167–92. doi: 10.6004/jnccn.2022.0008
7. Kim WH, Gomez-Izquierdo L, Vilardell F, Chu KM, Soucy G, Dos Santos LV, et al. HER2 status in gastric and gastroesophageal junction cancer: results of the large, multinational HER-EAGLE study. Appl Immunohistochem Mol Morphol. (2018) 26(4):239–45. doi: 10.1097/PAI.0000000000000423
8. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol (2022) 33(10):1005–20. doi: 10.1016/j.annonc.2022.07.004
9. Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev (2017) 8(8):Cd004064. doi: 10.1002/14651858.CD004064.pub4
10. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer (2017) 20(1):1–19. doi: 10.1007/s10120-016-0622-4
11. Sirody J, Kaji AH, Hari DM, Chen KT. Patterns of gastric cancer metastasis in the United States. Am J Surg (2022) 224(1 Pt B):445–8. doi: 10.1016/j.amjsurg.2022.01.024
12. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. (2018) 18(1):78. doi: 10.1186/s12885-017-3925-x
13. Robinson JR, Newcomb PA, Hardikar S, Cohen SA, Phipps AI. Stage IV colorectal cancer primary site and patterns of distant metastasis. Cancer Epidemiol. (2017) 48:92–5. doi: 10.1016/j.canep.2017.04.003
14. Chen X, Hu W, Huang C, Liang W, Zhang J, Wu D, et al. Survival outcome of palliative primary tumor resection for colorectal cancer patients with synchronous liver and/or lung metastases: A retrospective cohort study in the SEER database by propensity score matching analysis. Int J Surg (2020) 80:135–52. doi: 10.1016/j.ijsu.2020.06.024
15. Xu Z, Becerra AZ, Fleming FJ, Aquina CT, Dolan JG, Monson JR, et al. Treatments for stage IV colon cancer and overall survival. J Surg Res (2019) 242:47–54. doi: 10.1016/j.jss.2019.04.034
16. Simillis C, Kalakouti E, Afxentiou T, Kontovounisios C, Smith JJ, Cunningham D, et al. Primary tumor resection in patients with incurable localized or metastatic colorectal cancer: A systematic review and meta-analysis. World J Surg (2019) 43(7):1829–40. doi: 10.1007/s00268-019-04984-2
17. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol (2016) 17(3):309–18. doi: 10.1016/S1470-2045(15)00553-7
18. D'Ugo D, Cananzi FCM, Persiani R, Agnes A, Biondi A. REGATTA trial: a call for the USA and Europe. Lancet Oncol (2016) 17(3):261–2. doi: 10.1016/S1470-2045(15)00619-1
19. Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol (2013) 107(3):230–6. doi: 10.1002/jso.23262
20. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (2020) 396(10251):635–48. doi: 10.1016/S0140-6736(20)31288-5
21. Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol (2016) 22(8):2403–14. doi: 10.3748/wjg.v22.i8.2403
22. Kole C, Charalampakis N, Tsakatikas S, Kouris NI, Papaxoinis G, Karamouzis MV, et al. Immunotherapy for gastric cancer: a 2021 update. Immunotherapy (2022) 14(1):41–64. doi: 10.2217/imt-2021-0103
23. Patel TH, Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol (2020) 21(9):70. doi: 10.1007/s11940-020-0616-8
24. Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer. (2021) 1876(2):188615. doi: 10.1016/j.bbcan.2021.188615
25. Li C, Yan M, Chen J, Xiang M, Zhu ZG, Yin HR, et al. Survival benefit of non-curative gastrectomy for gastric cancer patients with synchronous distant metastasis. J Gastrointest Surg (2010) 14(2):282–8. doi: 10.1007/s11605-009-1095-0
26. Yang K, Liu K, Zhang WH, Lu ZH, Chen XZ, Chen XL, et al. The value of palliative gastrectomy for gastric cancer patients with intraoperatively proven peritoneal seeding. Med (Baltimore). (2015) 94(27):e1051. doi: 10.1097/MD.0000000000001051
27. Ouchi K, Sugawara T, Ono H, Fujiya T, Kamiyama Y, Kakugawa Y, et al. Therapeutic significance of palliative operations for gastric cancer for survival and quality of life. J Surg Oncol (1998) 69(1):41–4. doi: 10.1002/(SICI)1096-9098(199809)69:1<41::AID-JSO8>3.0.CO;2-K
28. Hartgrink HH, Putter H, Klein Kranenbarg E, Bonenkamp JJ, van de Velde CJ. Value of palliative resection in gastric cancer. Br J Surg (2002) 89(11):1438–43. doi: 10.1046/j.1365-2168.2002.02220.x
29. Koga S, Kawaguchi H, Kishimoto H, Tanaka K, Miyano Y, Kimura O, et al. Therapeutic significance of noncurative gastrectomy for gastric cancer with liver metastasis. Am J Surg (1980) 140(3):356–9. doi: 10.1016/0002-9610(80)90167-1
30. Chen S, Li YF, Feng XY, Zhou ZW, Yuan XH, Chen YB. Significance of palliative gastrectomy for late-stage gastric cancer patients. J Surg Oncol (2012) 106(7):862–71. doi: 10.1002/jso.23158
31. Omori H, Tanizawa Y, Makuuchi R, Irino T, Bando E, Kawamura T, et al. Role of palliative resection in patients with incurable advanced gastric cancer who are unfit for chemotherapy. World J Surg (2019) 43(2):571–9. doi: 10.1007/s00268-018-4816-2
32. Picado O, Dygert L, Macedo FI, Franceschi D, Sleeman D, Livingstone AS, et al. The role of surgical resection for stage IV gastric cancer with synchronous hepatic metastasis. J Surg Res (2018) 232:422–9. doi: 10.1016/j.jss.2018.06.067
33. Chang YR, Han DS, Kong SH, Lee HJ, Kim SH, Kim WH, et al. The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol (2012) 19(4):1231–9. doi: 10.1245/s10434-011-2056-x
34. Saidi RF, ReMine SG, Dudrick PS, Hanna NN. Is there a role for palliative gastrectomy in patients with stage IV gastric cancer? World J Surg (2006) 30(1):21–7. doi: 10.1007/s00268-005-0129-3
35. Einama T, Abe H, Shichi S, Matsui H, Kanazawa R, Shibuya K, et al. Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol Clin Oncol (2017) 6(2):163–6. doi: 10.3892/mco.2017.1128
36. Ishigami S, Natsugoe S, Nakajo A, Matsumoto M, Uenosono Y, Arigami T, et al. Salvage gastrectomy following a combination of biweekly paclitaxel and S-1 for stage IV gastric cancer. J Gastrointest Surg (2008) 12(8):1370–5. doi: 10.1007/s11605-008-0539-2
37. Namikawa T, Tsuda S, Fujisawa K, Iwabu J, Uemura S, Tsujii S, et al. Conversion surgery after S-1 plus oxaliplatin combination chemotherapy for advanced gastric cancer with multiple liver metastases. Clin J Gastroenterol (2018) 11(4):297–301. doi: 10.1007/s12328-018-0842-8
38. Tsunematsu M, Takahashi N, Murakami K, Misawa T, Akiba T, Yanaga K. Successful conversion surgery for gastric cancer with multiple liver metastases treated after S-1 plus cisplatin combination chemotherapy: a case report. Surg Case Rep (2017) 3(1):95. doi: 10.1186/s40792-017-0372-5
39. Petrioli R, Francini E, Cherri S, Marrelli D, Rovello F, Fiaschi AI, et al. Feasibility of modified docetaxel, oxaliplatin, capecitabine followed by capecitabine as maintenance chemotherapy as first-line therapy for patients with metastatic gastric or gastroesophageal cancer. Anticancer Drugs (2020) 31(3):292–7. doi: 10.1097/CAD.0000000000000877
40. Seo JY, Jin EH, Jo HJ, Yoon H, Shin CM, Park YS, et al. Clinicopathologic and molecular features associated with patient age in gastric cancer. World J Gastroenterol (2015) 21(22):6905–13. doi: 10.3748/wjg.v21.i22.6905
41. Izuishi K, Mori H. Recent strategies for treating stage IV gastric cancer: roles of palliative gastrectomy, chemotherapy, and radiotherapy. J Gastrointestin Liver Dis (2016) 25(1):87–94. doi: 10.15403/jgld.2014.1121.251.rv2
42. Zhu J, Chen S, Yang B, Mao W, Yang X, Cai J. Molecular mechanisms of lncRNAs in regulating cancer cell radiosensitivity. Biosci Rep (2019) 39(8). doi: 10.1042/BSR20190590
Keywords: gastric cancer, liver metastasis, palliative gastrectomy, survival benefit, propensity score-matched
Citation: Ren B, Yang Y, Lv Y and Liu K (2023) Survival benefits of palliative gastrectomy for gastric cancer patients with liver metastasis: a population-based propensity score–matched cohort analysis. Front. Oncol. 13:1309699. doi: 10.3389/fonc.2023.1309699
Received: 08 October 2023; Accepted: 08 November 2023;
Published: 01 December 2023.
Edited by:
Luigi Marano, Academy of Applied Medical and Social Sciences, PolandReviewed by:
Natale Calomino, University of Siena, ItalyCopyright © 2023 Ren, Yang, Lv and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kang Liu, a2FuZ2xpdUB4anR1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.