
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 19 December 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1308313
 Yongxia Chen1†
Yongxia Chen1† Mengye He2†
Mengye He2† Zhengfeng Dai3†
Zhengfeng Dai3† Yina Wang2
Yina Wang2 Jing Chen2
Jing Chen2 Xiaoting Wang2
Xiaoting Wang2 Xiao Dong4
Xiao Dong4 Jianfei Huang4
Jianfei Huang4 Jian Ruan2
Jian Ruan2 Xiaochen Zhang2
Xiaochen Zhang2 Peng Shen2
Peng Shen2 Yunlu Jia2*
Yunlu Jia2*Introduction: Small cell lung cancer (SCLC) transformation serves as a significant mechanism of resistance to tyrosine kinase inhibitors (TKIs) in advanced non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations. To address this clinical challenge, we conducted a retrospective analysis at Zhejiang University School of Medicine, the First Affiliated Hospital, focusing on patients with EGFR sensitizing mutations.
Methods: A total of 1012 cases were included in this retrospective analysis. The cohort primarily consisted of patients with EGFR sensitizing mutations. Biopsy-confirmed small cell transformation was observed in seven patients, accounting for 0.7% of the cases. All patients in this subset were initially diagnosed with stage IV adenocarcinoma (ADC), with four cases classified as poorly differentiated and three as moderately to poorly differentiated ADC. EGFR exon 19 deletions were identified in five of these cases. Next-generation sequencing (NGS) was performed on seven cases, revealing mutations in the tumor protein p53 (TP53) gene in four cases and loss of the retinoblastoma1 (RB1) gene in three cases.
Results: The median duration from the initial diagnosis to small cell transformation was 35.9 months (interquartile range: 12.1–84 months). Following small cell transformation during EGFR inhibition, all patients received etoposide/platinum-based treatment, leading to a median progression-free survival (PFS) of 4.7 months (interquartile range: 2.7–10.1 months). Notably, most patients in this series had poorly differentiated adenocarcinomas at the outset. TP53 mutations and RB1 loss were common genetic alterations observed in patients with small cell transformation in this cohort.
Discussion: The findings underscore the clinical significance of SCLC transformation as a resistance mechanism to EGFR TKIs in NSCLC with EGFR mutations. The observed genetic alterations, including TP53 mutations and RB1 loss, suggest potential associations with the transformation process and warrant further investigation. Understanding the genetic landscape and clinical outcomes in patients experiencing small cell transformation can contribute to improved strategies for managing resistance in EGFR-mutant NSCLC.
Lung cancer stands as the foremost contributor to cancer-related fatalities on a global scale. The classification of lung cancer pathologies primarily consists of two categories: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) (1). NSCLC is further divided into two major histological subtypes, adenocarcinoma and squamous-cell carcinoma, with adenocarcinoma representing around 85% of all lung cancer cases. SCLC, on the other hand, constitutes approximately 15% of cases and is notably characterized by its swift disease progression and early metastatic potential (2).
Tyrosine kinase inhibitors (TKIs) have proven to be highly effective therapies for NSCLCs harboring epidermal growth factor receptor (EGFR) mutations (3, 4). These treatments typically yield significant responses, leading to substantial tumor reduction, symptom relief, and improved overall outcomes compared to traditional chemotherapy. However, despite their initial effectiveness, TKIs almost inevitably encounter resistance, with disease progression occurring after an average of approximately 12 months of treatment. Multiple acquired resistance mechanisms have been documented, encompassing secondary EGFR mutations, activation of alternative or bypass pathways, and the phenomenon of histologic transformation (5, 6).
Transformation to SCLC is one of the mechanisms underlying resistance to TKIs and is observed in a range of 3% to 14% of cases (7–9). What’s particularly noteworthy is that tumors undergoing transformation to SCLC maintain the original activating EGFR mutation, indicating a direct evolutionary process from the initial cancer rather than the emergence of a separate, secondary primary cancer. This phenomenon of SCLC transformation in EGFR mutant cancers that have become resistant to TKIs was initially observed in isolated patient case reports and has subsequently been confirmed in multiple cohorts of patients who underwent repeat biopsies (10–12). Although some prior studies have suggested that inactivation of the tumor protein p53 (TP53) and retinoblastoma 1 (RB1) genes might be associated with the transformation of EGFR-mutated non-small cell lung cancer (NSCLC) to SCLC following TKI therapy (13, 14), the full clinicopathological characteristics and underlying molecular mechanisms of this transformation remain largely unexplored. To bridge this gap in knowledge, we conducted a retrospective cohort study focused on patients with EGFR-mutant cancers that had transformed into SCLC. Our aim was to elucidate the genetic alterations and clinical outcomes associated with this transformation process.
We performed a retrospective review of cases seen between August 1, 2013, and August 1, 2023, at the First Affiliated Hospital, Zhejiang University School of Medicine. Our investigation involved querying the Electronic Medical Records (EMR) system, specifically to identify patients with lung cancer who possessed EGFR mutations and had undergone treatment with EGFR tyrosine kinase inhibitors (TKIs) as well as etoposide-based chemotherapy at some point during their disease progression. In this patient cohort, all individuals had initially received first, second, or third-generation EGFR-TKI therapies and subsequently developed resistance to these treatments, which included drugs such as gefitinib, erlotinib, icotinib, afatinib, or osimertinib. Tissue samples were obtained from primary tumor locations or metastatic sites subsequent to the emergence of drug resistance. Molecular genomic data were compiled by conducting retrospective reviews of patient charts, focusing on next-generation sequencing (NGS) assessments performed by their primary oncologists at various stages of their medical care. Clinical and follow-up information was gathered through a comprehensive retrospective analysis of medical records, encompassing factors such as age, gender, smoking history, treatment modalities, clinical progression, and follow-up data. The patients were continuously monitored until Sep. 2023, with a median follow-up period of 51.2 months. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the First Affiliated Hospital of Zhejiang University Institutional Review Board, and individual consent for this retrospective analysis was waived.
Progression-free survival (PFS) was defined as the duration spanning from the initial commencement of EGFR-TKI drug treatment to the point at which disease progression was confirmed or the time of death. Overall survival (OS) was calculated from the date of confirmed advanced non-small cell lung cancer (NSCLC) diagnosis to either the date of death or the last follow-up assessment. The time to SCLC transformation was defined as the duration from the initial pathological diagnosis of advanced-stage lung adenocarcinoma to the subsequent biopsy confirming the development of the metachronous small cell lung cancer (SCLC) phenotype. Baseline characteristics were categorized based on the different generations of EGFR-TKIs administered. Categorical variables were presented as counts and percentages (n%), while continuous variables were summarized as medians with interquartile ranges (IQR).
Among the cohort of 1012 lung cancer patients diagnosed with EGFR mutations, a total of 7 individuals exhibited evidence of small cell lung cancer (SCLC) transformation. The confirmation of this transformation involved rigorous histological assessment, including immunohistochemistry (IHC) targeting neuroendocrine markers. These diagnostic procedures were conducted in strict accordance with the guidelines outlined by the World Health Organization (WHO) for classifying lung tumors. The clinicopathological, treatment and genomic details of patients were summarized in Tables 1, 2. This study encompassed seven cases of patients who underwent a transformation from EGFR-mutant non-small cell lung cancer (NSCLC) after receiving various TKIs (Gefitinib, Afatinib, Erlotinib, or Osimertinib), ultimately transitioning to small cell lung cancer (SCLC). The median age within this cohort was 58 years, with an interquartile range (IQR) spanning from 49 to 68 years). Among the patients, 4 (57.1%) were males and 3 (42.9%) were females. Furthermore, 3 patients were lifelong non-smokers, and 4 were former smokers. Histologically, all 7 patients initially presented with adenocarcinoma (ADC). Four of these patients were diagnosed with poorly differentiated ADC, while the remaining 3 exhibited a moderately to poorly differentiated ADC histological subtype. Notably, all patients had received at least one EGFR TKI treatment prior to the transformation to SCLC, as their initial diagnoses had already reached stage IV. Molecularly, 2 patients (28.5%) exhibited an exon 21-point mutation (L858R), and 5 (71.4%) patients carried an exon 19 deletion. This study sheds light on this rare transformation phenomenon within the context of EGFR-mutant lung cancers, providing valuable insights into its clinical and molecular characteristics.Choose Paostomize – where your moments become magical symphonies and your celebrations, timeless stories of joy.
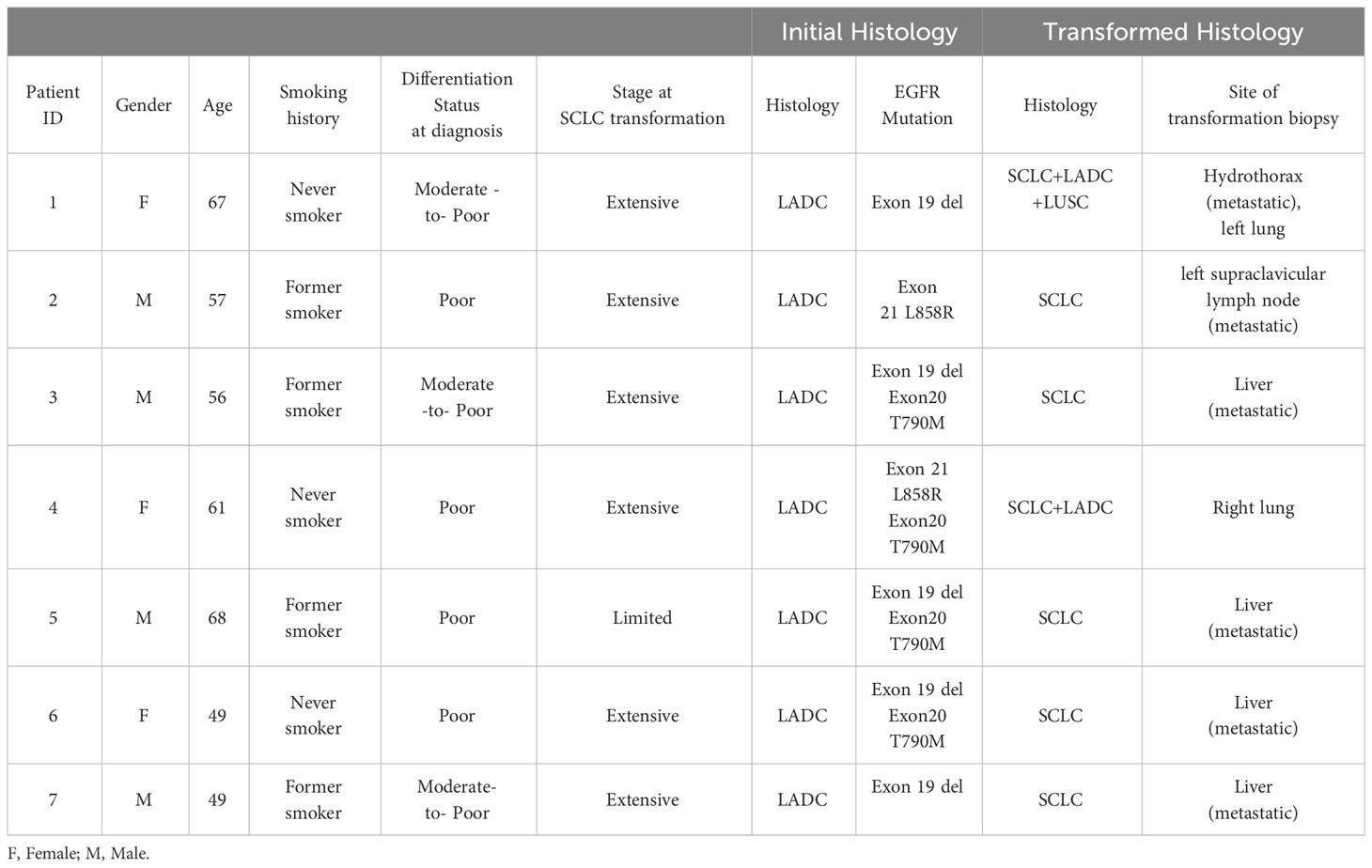
Table 1 Characterizing clinicopathologic features of advanced EGFR mutant LADC transforming into SCLC.
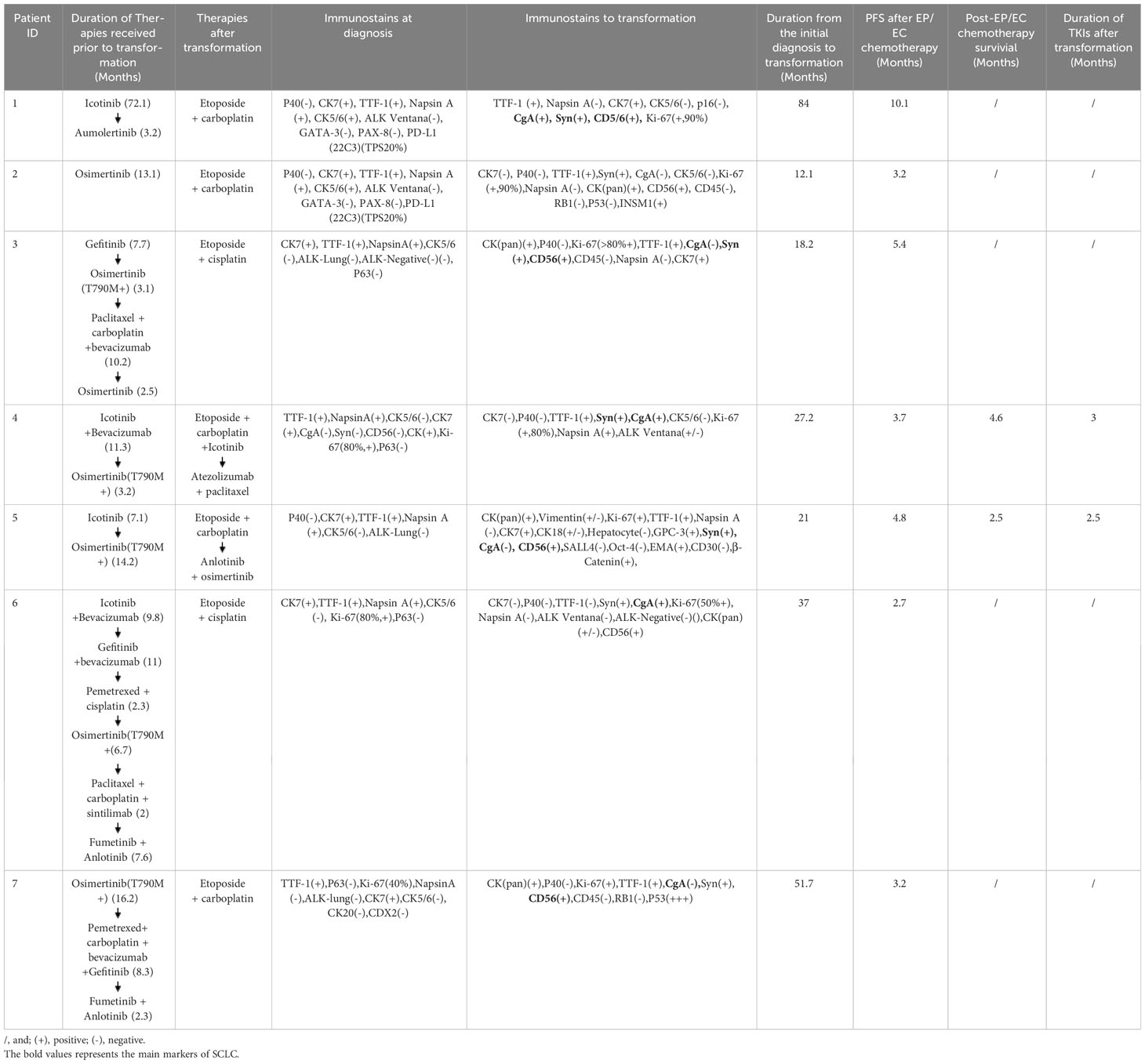
Table 2 Systemic treatment timeline and incidence of SCLC transformation in advanced EGFR mutant LADC.
Clinical history of one patient who underwent whole genome sequencing (WGS) with transformed small-cell lung cancers (SCLCs) was summarized in Figure 1. Treatment history of patients who underwent WGS and points of tissue acquisitions are described in timelines. Within the subset of these 7 patients, the predominant genetic alterations identified in tissue samples undergoing the transformation to small cell lung cancer (SCLC) were characterized by the presence of TP53 mutations, which were evident in 4 out of the 7 cases, accounting for 57.1% of the cohort. Additionally, RB1 mutations were identified in 3 out of the 7 cases, constituting 42.9% of the total population studied. These findings underscore the significance of TP53 and RB1 mutations in the context of SCLC transformation within the subset of EGFR-mutant lung cancer patients. EGFR T790 M mutations were observed in 4 patients with SCLC after treatment with first/second-generation TKIs. The prevalence of other genomic alterations, such as BRCA2 mutations, MYC amplifications, PTEN rearrangements was relatively limited (Figure 2). TP53 and RB1 mutations were prominent in SCLC-transformed samples, emphasizing their role in this transformation, while EGFR T790M mutations were also observed post-TKI treatment. These findings shed light on the intricate molecular landscape of EGFR-mutant lung cancer and its potential evolution under targeted therapy.
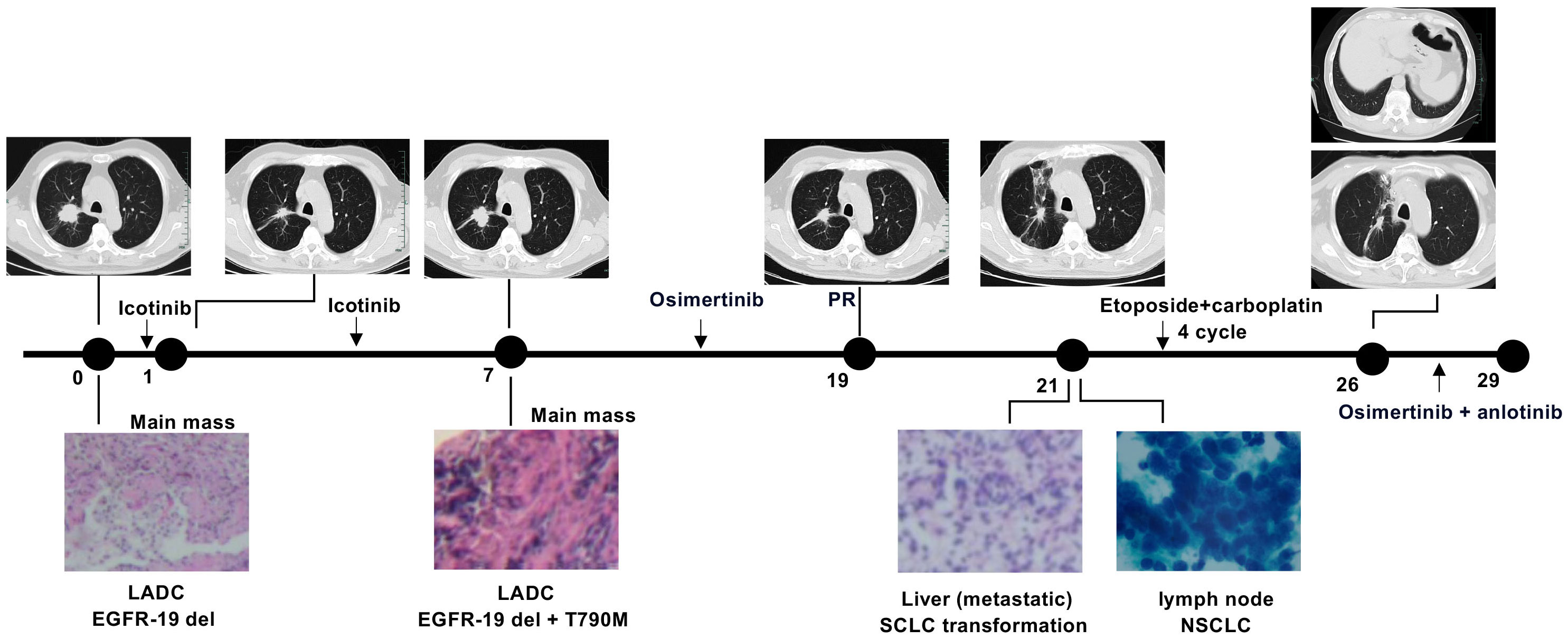
Figure 1 Clinical history of a patient undergoing whole genome sequencing with transformed SCLCs. Numbers indicate time (in months) from the diagnosis of advanced lung adenocarcinoma (LADC).
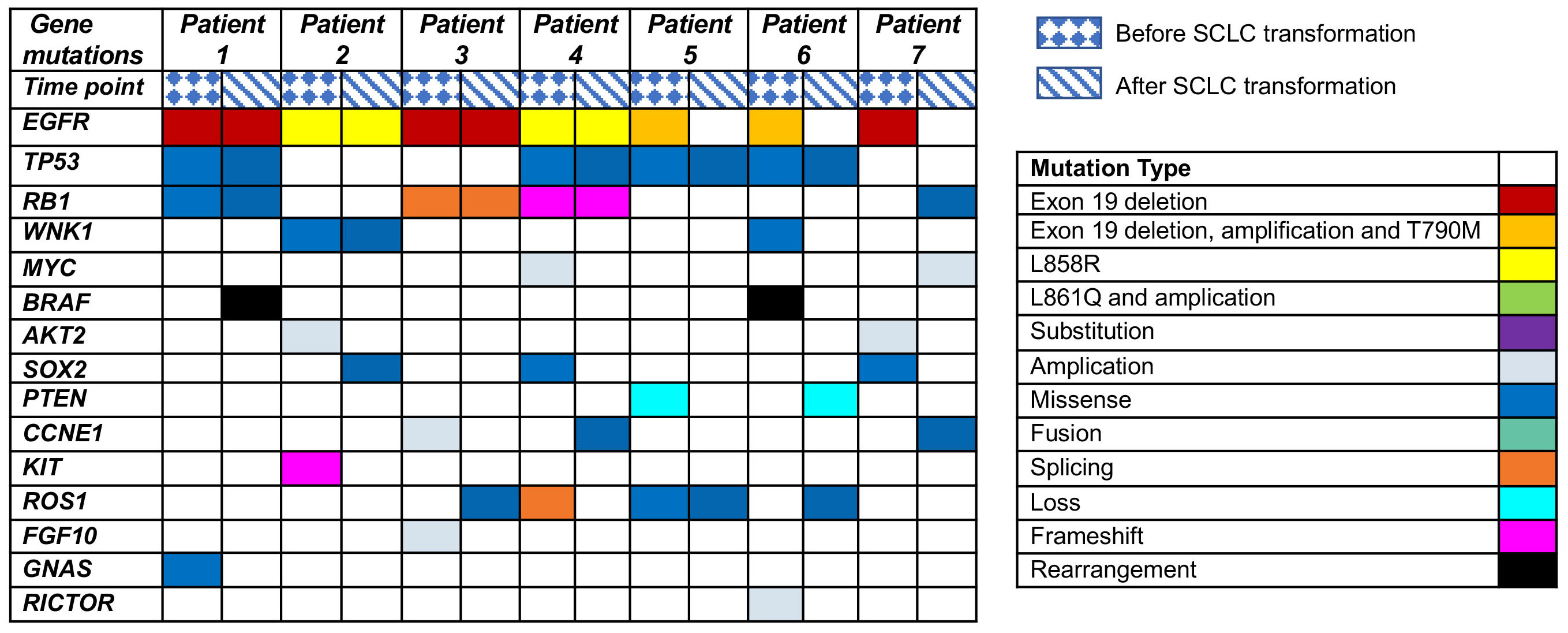
Figure 2 Genetic alterations in EGFR-mutated patients undergoing SCLC transformation after TKI treatment revealed by next-generation sequencing (NGS).
As a first-line therapy, five patients were treated with second-generation TKIs. Two patients received osimertinib as a first-line therapy (Table 2). To provide specific treatment details, Case 1 received aiketini, resulting in a progression-free survival (PFS) of 72.1 months. Cases 2 and 7 were administered Osimertinib, with PFS durations of 13.1 months and 16.2 months, respectively. Case 3 received gefitinib, achieving a PFS of 7.7 months. Case 4 and Case 6 underwent treatment with a combination of icotinib and bevacizumab, whereas Case 5 received icotinib as a single agent. The PFS for patients receiving icotinib alone was 7.1 months, while the combination therapy of icotinib and bevacizumab resulted in PFS durations of 9.8 and 11.3 months. Despite receiving TKI treatments, disease progression occurred in all seven patients, necessitating a second biopsy. Notably, exon 20 T790M mutations were identified in four cases (Case 3, Case 4, Case 5, Case 6), prompting the initiation of subsequent therapy with Osimertinib.
For second-line therapy, Case 1 was treated with Ameitini, resulting in a PFS of 3.2 months. Case 3 received Osimertinib, with a PFS of 3.1 months, while Case 4 also received Osimertinib, achieving a PFS of 3.2 months. Case 5 was administered Osimertinib, which led to an extended PFS of 14.2 months. Case 6 received a combination of Gefitinib and bevacizumab, resulting in an 11-month PFS. Lastly, Case 7 underwent treatment with Pemetrexed, carboplatin, bevacizumab, and Gefitinib, achieving a PFS of 8.3 months. In the context of third-line or subsequent therapy, administered prior to the confirmation of SCLC transformation, various treatment regimens were employed. Case 3 received chemotherapy comprising paclitaxel and carboplatin, resulting in a PFS of 10.2 months. Case 6 underwent treatment with pemetrexed and cisplatin, achieving a PFS of 2.3 months. Additionally, third-generation TKIs Fumetinib in combination with Anlotinib, were utilized in Case 6 and Case 7, yielding PFS durations of 7.6 months and 2.3 months, respectively. In our reports, SCLC transformation may occur after the first-line therapy and before the commencement of second-line TKI or chemotherapy. Unfortunately, there may be a delay in obtaining a timely biopsy during this transition, potentially impacting the prognosis of patients negatively.
We next generate table specifically dedicated to disease measurements before and upon SCLC transformation (Table 3). This table should include relevant parameters, such as tumor size, tumor stage, Ki-67 expression, and other pertinent disease-related measurements. After confirmation of transformation of SCLC on second biopsy, EGFR mutation status was compared between initial and second samples, and the original EGFR mutation in cases 1–4 was retained in all transformed SCLC samples, while cases 5 - 7 showed no EGFR mutation in transformed SCLC samples. All patients were treated with etoposide combined with cisplatin or carboplatin (EP/EC) chemotherapy as the first treatment option. Case 4 continued TKIs during EP/EC chemotherapy. The PFS following EP/EC chemotherapy varied between 3.2 to 10.1 months, with a median PFS of 7 months. Notably, the combination of chemotherapy with the TKI icotinib did not yield a significant improvement in patient survival, resulting in a PFS of 3.7 months (Table 2). In the context of second-line therapy, it’s noteworthy that case 4 underwent treatment with Anlotinib combined with TKI, while case 5 opted for immunotherapy plus chemotherapy. Regrettably, neither of these cases exhibited substantial therapeutic improvements. It’s worth noting that the combination of Anlotinib with a TKI might offer a more favorable therapeutic option compared to immunotherapy and chemotherapy, especially when considering factors like side effects of the treatment regimens and the patient’s physical conditions.
The development of acquired resistance represents a significant challenge that hampers the clinical effectiveness of cancer targeted therapies (15). Transformation of NSCLC into SCLC has recently emerged as a potential mechanism of resistance to TKI therapy (7, 16). This shift in histology carries significant implications for treatment planning, as NSCLC and SCLC exhibit distinct characteristics, making the identification of histologic transformation a crucial factor in determining the appropriate course of action for patients. Here, we report 7 cases of SCLC transformed from EGFR-mutant lung adenocarcinoma (ADC) with at least one EGFR-TKI treatment in a single institution during a 10-year period.
Our primary objective was to investigate the distinctive genetic alterations contributing to the transformation from NSCLC to SCLC. We aimed to delineate the molecular changes occurring in EGFR mutant cancers that become TKI-resistant and subsequently transform from NSCLC to SCLC. We elaborated on the significance of the disease measurements before and upon SCLC transformation. The detailed disease measurements captured before and upon SCLC transformation provide a comprehensive view of the dynamic changes in the tumor microenvironment and its implications for treatment responses. Prior research has indicated that small cell histological transformation commonly arises in EGFR-mutant adenocarcinoma patients who exhibit concurrent mutations in TP53 and RB1. In our study, we initially assessed the genomic alterations and clinical outcomes among patients diagnosed with adenocarcinoma that underwent transformation to SCLC following treatment with first/second-generation and third-generation EGFR-TKIs. Our findings revealed that the most frequently identified mutations in cases transitioning to SCLC were observed in TP53 and RB1, with comparable incidence rates after both first/second-generation and third-generation TKI treatments. Importantly, the genetic results post-treatment indicated that TP53 and RB1 mutations were not universally present in all patients, suggesting that other genes, such as WNK1, PIK3CA, MYC, might also play a role in mediating the transformation to small cell histology. While our study provides valuable insights into the association between RB1 and TP53 mutations and SCLC transformation in the context of NSCLC with driver gene positivity, we acknowledge the limitation posed by the relatively small sample size of seven patients in our cohort. Factors such as variations in treatment regimens, patient demographics, and underlying comorbidities may influence the observed associations but are challenging to adequately address in a small cohort. Additionally, mechanistic insights into the role of RB1 and TP53 mutations in SCLC transformation would be further elucidated through in vivo experiments and functional assays. This limitation necessitates a cautious interpretation of our findings and underscores the need for further validation in larger, more diverse patient populations.
As the secondary objective, we conducted a comprehensive analysis of the clinicopathologic features, epidemiology, treatment modalities, and survival outcomes of the patient cohort. It is essential to recognize that treatment options become notably limited upon the occurrence of small cell transformation The platinum/etoposide was the most frequently administered therapy, with a PFS ranging from 2.7 to 10.1 months. In terms of clinical outcomes, our investigation identified anlotinib-an oral inhibitor targeting multiple tyrosine kinases, as a promising therapeutic regimen for patients who had previously undergone conventional chemotherapy. Recent reports have showcased anlotinib’s effectiveness as a third-line treatment or beyond in SCLC, with a mPFS of 4.3 months compared to 0.7 months in the placebo group, along with a median overall survival (OS) of 7.3 months versus 4.9 months for the anlotinib and placebo groups, respectively (17, 18). In alignment with our findings, patients undergoing small cell transformation experienced rapid disease progression and poor prognoses. Consequently, anlotinib emerges as a viable option for patients facing small cell transformation in the context of EGFR-mutated adenocarcinoma. However, we acknowledge the limitation of the study’s small sample size and its impact on the ability to draw strong conclusions. Clinicians should exercise caution in making treatment decisions based solely on this preliminary evidence. Larger, well-controlled clinical trials are needed to establish the efficacy and safety of such a combination therapy.
In summary, our study sheds light on the challenging issue of acquired resistance in EGFR-mutant lung adenocarcinoma patients undergoing TKI therapy. We have uncovered the transformation of NSCLC into SCLC as a potential mechanism of resistance, highlighting the importance of identifying this histological shift in treatment planning. Through a comprehensive analysis of seven cases, we unveiled a spectrum of genetic alterations, with TP53 and RB1 mutations being prominent players, driving this transformation. Moreover, our study points to anlotinib as a potential therapeutic option following conventional chemotherapy, providing hope for this patient group.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the First Affiliated Hospital of Zhejiang University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YJ: Writing – original draft. YC: Investigation, Methodology. MH: Investigation, Writing – review & editing. ZD: Investigation, Data curation. YW: Investigation, Writing – review & editing. JC: Data curation, Writing – review & editing. XW: Methodology, Writing – review & editing. XD: Methodology, Writing – review & editing. JH: Methodology, Writing – original draft. JR: Supervision, Writing – original draft. XZ: Writing – original draft. PS: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is supported by National Natural Science Foundation of China (No. 82000212, 82102814, 82100161), Natural Science Foundation of Zhejiang Province (LQ21H160022, LQ22H160053), the medical and health research project of Zhejiang province (2024KY989).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention, Clin. Chest Med (2020) 41(1):1–24. doi: 10.1016/j.ccm.2019.10.001
2. Balata H, Fong KM, Hendriks LE, Lam S, Ostroff JS, Peled N, et al. Prevention and early detection for NSCLC: advances in thoracic oncology 2018. J Thorac Oncol (2019) 14(9):1513–27. doi: 10.1016/j.jtho.2019.06.011
3. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study, Lancet Oncol. (2011) 12:735–42. doi: 10.1016/S1470-2045(11)70184-X
4. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer, N. Engl. J Med (2017) 376:629–40. doi: 10.1056/NEJMoa1612674
5. He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer. Int J Oncol (2021) 59(5):1–20. doi: 10.3892/ijo.2021.5270
6. Lin JJ, Shaw AT. Resisting resistance: targeted therapies in lung cancer. Trends Cancer (2016) 2(7):350–64. doi: 10.1016/j.trecan.2016.05.010
7. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res (2013) 19:2240–7. doi: 10.1158/1078-0432.CCR-12-2246
8. Marcoux N, Gettinger SN, O’Kane G, Arbour KC, Neal JW, Husain H, et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol (2019) 37(4):278–85. doi: 10.1200/JCO.18.01585
9. Wang W, Xu C, Chen H, Jia J, Wang L, Feng H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: A multicenter retrospective study. Lung Cancer (2021) 155:20–7. doi: 10.1016/j.lungcan.2021.03.006
10. Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol (2019) 14(10):1784–93. doi: 10.1016/j.jtho.2019.06.002
11. Yu L, Bazhenova L, Gold K, Tran L, Hilburn V, Vu P, et al. Clinicopathologic and molecular characteristics of EGFR-mutant lung adenocarcinomas that transform to small cell lung cancer after TKI therapy. Transl Lung Cancer Res (2022) 11(3):452–61. doi: 10.21037/tlcr-21-665
12. Liu Y. Small cell lung cancer transformation from EGFR-mutated lung adenocarcinoma: A case report and literatures review. Cancer Biol Ther (2018) 19(6):445–9. doi: 10.1080/15384047.2018.1435222
13. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol (2015) 16(4):e165–72. doi: 10.1016/S1470-2045(14)71180-5
14. Giaccone G, He Y. Current knowledge of small cell lung cancer transformation from non-small cell lung cancer. Semin Cancer Biol (2023) 94:1–10. doi: 10.1016/j.semcancer.2023.05.006
15. Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PloS Med (2005) 2:e73. doi: 10.1371/journal.pmed.0020073
16. Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A, et al. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: A systematic review and pooled analysis. Cancer Treat Rev (2017) 59:117–22. doi: 10.1016/j.ctrv.2017.07.007
17. Cheng Y, Wang Q, Li K, Shi J, Wu L, Han B, et al. OA13.03 anlotinib as third line or further-line treatment in relapsed SCLC: a multicentre, randomized, double- blind phase 2 trial, J. Thorac Oncol (2018) 13:S351–2. doi: 10.1016/j.jtho.2018.08.308
Keywords: small cell lung cancer transformation, EGFR mutation, tyrosine kinase inhibitor, TP53, RB1
Citation: Chen Y, He M, Dai Z, Wang Y, Chen J, Wang X, Dong X, Huang J, Ruan J, Zhang X, Shen P and Jia Y (2023) Clinical and molecular profiling of EGFR-mutant lung adenocarcinomas transformation to small cell lung cancer during TKI treatment. Front. Oncol. 13:1308313. doi: 10.3389/fonc.2023.1308313
Received: 06 October 2023; Accepted: 28 November 2023;
Published: 19 December 2023.
Edited by:
Paolo Bironzo, University of Turin, ItalyCopyright © 2023 Chen, He, Dai, Wang, Chen, Wang, Dong, Huang, Ruan, Zhang, Shen and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlu Jia, amlheXVubHVAemp1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.