
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 09 January 2024
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1307694
This article is part of the Research Topic Early Cervical Cancer: Laparotomic vs Minimally Invasive Surgery and Fertility-Sparing Possible Strategies View all 5 articles
Objective: To explore the factors influencing the successful implementation of same-day discharge in patients undergoing minimally invasive hysterectomy for malignant and non-malignant gynecological diseases.
Method: We searched PubMed, Embase, Cochrane Central Register of Controlled Trials, International Clinical Trials Registry Platform, and Clinical Trials.gov from inception to May 23, 2023. We included case-control and cohort studies published in English reporting same-day discharge factors in patients undergoing minimally invasive hysterectomy for malignant and non-malignant gynecological diseases. STATA 16.0 was used for the meta-analysis. Risk factors were assessed using odds ratios (OR) (relative risk (RR)/hazard ratios (HR)) with 95% confidence intervals (CI), and logistic regression determined the same-day discharge rate (%).
Results: We analyzed 29 studies with 218192 patients scheduled for or meeting same-day discharge criteria. The pooled rates were 50% (95% CI 0.46-0.55), and were similar for malignant and non-malignant gynecological diseases (48% and 47%, respectively). In terms of basic characteristics, an increase in age (OR: 1.03; 95% CI: 1.01–1.05), BMI (OR: 1.02; 95% CI: 1.01–1.03), and comorbidities including diabetes and lung disease were risk factors affecting SDD, while previous abdominal surgery history (OR: 1.54; 95% CI: 0.93–2.55) and hypertension (OR: 1.53; 95% CI: 0.80–2.93) appeared not to affect SDD. In terms of surgical characteristics, radical hysterectomy (OR: 3.46; 95% CI: 1.90–6.29), surgery starting after 14:00 (OR: 4.07; 95% CI: 1.36–12.17), longer surgical time (OR: 1.03; 95% CI: 1.01–1.06), intraoperative complications (OR: 4.68; 95% CI: 1.78–12.27), postoperative complications (OR: 3.97; 95% CI: 1.68–9.39), and surgeon preference (OR: 4.47; 95% CI: 2.08–9.60) were identified as risk factors. However, robotic surgery (OR: 0.44; 95% CI: 0.14–1.42) and intraoperative blood loss (OR: 1.16; 95% CI: 0.98–1.38) did not affect same-day discharge.
Conclusions: An increase in age, body mass index, and distance to home; certain comorbidities (e.g., diabetes, lung disease), radical hysterectomy, surgery starting after 14:00, longer surgical time, operative complications, and surgeon preference were risk factors preventing same-day discharge. Same-day discharge rates were similar between malignant and non-malignant gynecological diseases. The surgery start time and body mass index have a greater impact on same-day discharge for malignant diseases than non-malignant diseases.
Same-day discharge (SDD) for patients who have undergone hysterectomy is becoming more common with the advancement in minimally invasive surgery including its application in treating benign diseases and malignant tumors (1–4). After a minimally invasive hysterectomy, overnight hospitalization is common to monitor perioperative complications such as hemorrhage, blood pressure liability, desaturation, possible intraoperative bladder/ureteral or bowel injuries, or for immediate detection in case of postoperative pain. Experts believe perioperative complications should be identified intraoperatively, requiring immediate admission or a few days after discharge (5). Hence, prolonged hospitalization does not change the readmission rate resulting from complications (6).
There have been reports of lower healthcare costs, better utilization of scarce medical resources, and higher patient satisfaction following the successful application of SDD in minimally invasive hysterectomy without compromising outcomes (7). In the United States, annual hysterectomy costs are over $5 billion (8). A retrospective study by Schiavone et al., reported that the cost of a one-day discharge following a laparoscopic hysterectomy was $207 greater than that of patients discharged on the same day (9). In addition, SDD implementation can allow more patients to obtain high-quality medical resources in areas with limited medical resources.
Minimally invasive hysterectomy is a method to treat many benign or malignant gynecological diseases (10–13). Although the safety, feasibility, and economy of implementing SDD in minimally invasive hysterectomy for benign or malignant gynecological diseases have been confirmed in a series of studies, further research is required on patient and surgical factors that affect its application (14, 15). This study explored the factors affecting the successful implementation of SDD in minimally invasive hysterectomies to enhance the promotion and application of SDD.
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines and was registered with the International Prospective Register of Systematic Reviews (CRD42023425260) (16).
All potentially eligible studies, including case-control and cohort studies, published in English were considered. The inclusion criteria were: (a) evaluation of factors influencing SDD in patients undergoing minimally invasive hysterectomy for malignant and non-malignant gynecological diseases, (b) effect data including odds ratios (OR), relative risk (RR), or hazard ratios (HR) with 95% confidence intervals (CI) provided or calculation of these data enabled, and (c) if data subsets had been published in more than one article, that with the largest sample size was included. The exclusion criteria were as follows: (a) redundant publications, (b) incomplete data, (c) and conference abstracts and reviews.
We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), International Clinical Trials Registry Platform, and Clinical Trials.gov from inception to May 23, 2023. The reference lists of published reviews and retrieved articles were checked for additional trials. The search terms were: “hysterectomy,” “same day discharge,” “outpatient surgery,” “influencing factor,” “risk factor,” “related factor,” and “factor.”
Two researchers (HC and LH) independently screened titles and abstracts to assess the eligibility of the studies and independently read the full texts of all potential articles for further evaluation. Disagreements between the authors were resolved through discussion with a third researcher (XT).
Two independent reviewers (JL and YC) extracted data in duplicate and recorded it in a standardized database. We used a predefined extraction form that included the methods, study quality, participants, and outcomes. The authors were blinded to the trial authors, institutions, sources of funding, and acknowledgments. We attempted to acquire missing data by contacting the authors via email; however, no replies were received.
Two reviewers (JL and YC) independently assessed the quality of the included studies. Differences were resolved by discussion, and if no consensus was reached, a third review author (XT) was involved. Cohort studies included in the prognosis analysis were assessed using the Newcastle–Ottawa Scale (NOS) based on three categories: selected cases, comparability of groups, and assessment of outcomes. Studies awarded six or more stars were classified as high-quality.
We conducted the meta-analysis using STATA 16.0 (StataCorp., College Station, TX). We employed ORs (relative risks (RRs)/hazard ratios (HRs)) with 95% CI to combine data assessing risk factors. Only those risk factors investigated in at least two studies were included in the meta-analysis. Statistical significance was set at P <0.05. The SDD rate (%) was determined using logistic regression analysis. The heterogeneity between studies was assessed using the I2 test: I2 <30% was considered low heterogeneity, I2 30–50% was designated to have moderate heterogeneity, and I2 ≥50% was considered high heterogeneity (17). When there was substantial heterogeneity, a random-effects model was used to combine the data. Otherwise, a fixed effects model was used. Publication bias was evaluated using a funnel plot, and statistical assessment was performed using the Egger test (18).
The study selection process is illustrated in Figure 1. A total of 665 articles were retrieved after removing duplicates. After screening the titles and abstracts, 48 full texts were retrieved for subsequent assessment. After reading the full texts, 19 articles were excluded. Finally, 29 studies were included with 218,192 patients scheduled for SDD or who met the SDD criteria (5, 7, 8, 19–44). No randomized controlled trials were found. All the included studies were retrospective cohort studies and were awarded six or more stars according to the NOS criteria. The general characteristics of the included studies are summarized in Table 1.
The pooled SDD rates were 50% (95% CI 0.46–0.55; I2 = 99.8%; 29 studies with 218,192 participants; Figure 2A) (5, 7, 8, 19–44). The publication bias in these studies was assessed using a funnel plot (Figure 2B), followed by the Egger test (P=0.011), indicating that a high risk of publication bias existed among these studies. Subgroup analysis showed the SDD rates for malignant (OR 48%; 95% CI 0.38-0.59; I2 = 99.8%; 10 studies, 172,770 participants; Figure 2C) and non-malignant gynecological diseases (OR 47%; 95% CI 0.41-0.53; I2 = 99.7%; 10 studies, 33253 participants; Figure 2D) were similar.
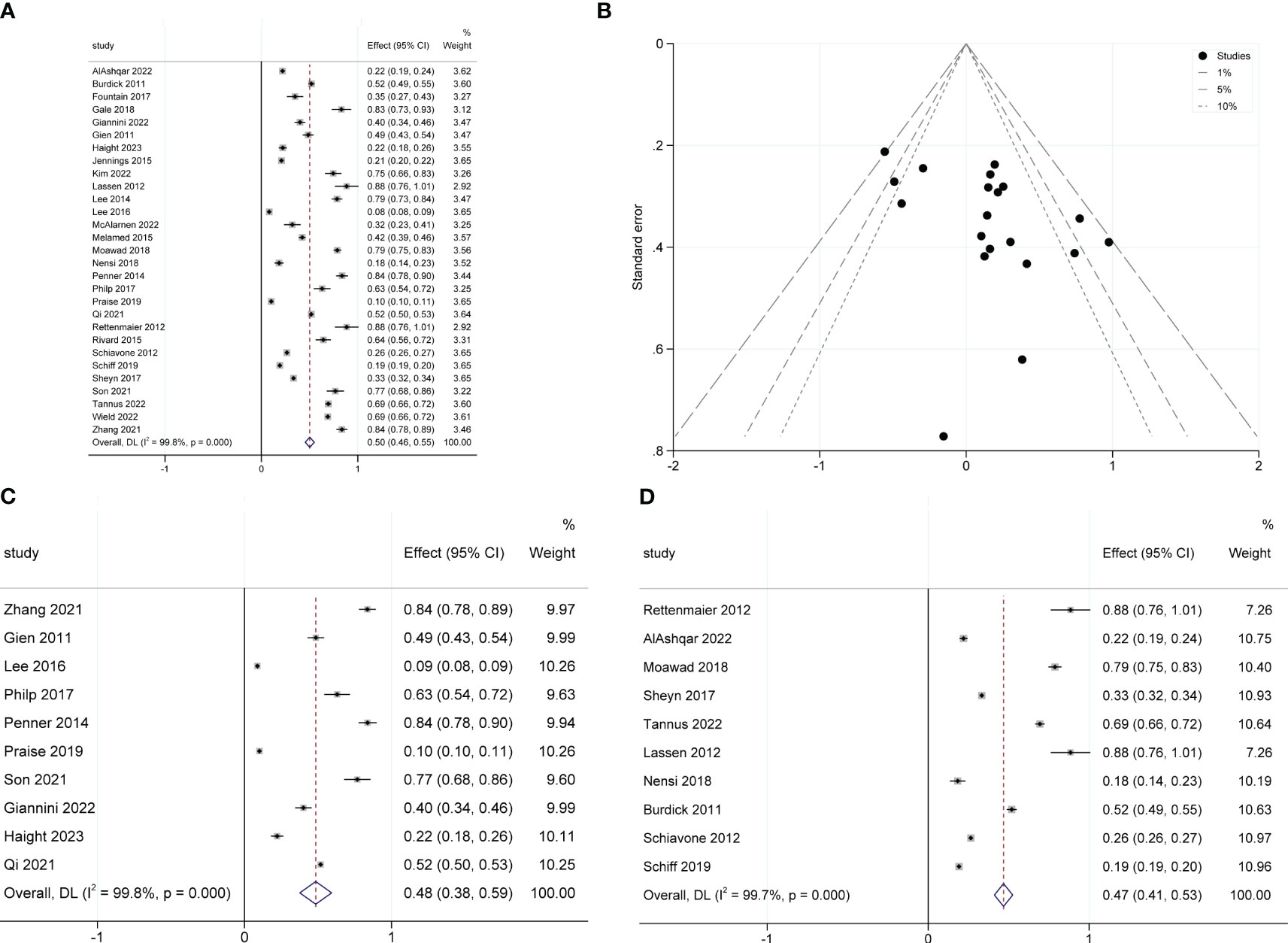
Figure 2 Forest plot of pooled same-day discharge rates (A), funnel plot (B), and the SDD rates for malignant subgroup (C) and non-malignant subgroup (D).
The meta-analysis revealed that an increase in age (OR: 1.03; 95% CI: 1.01–1.05; I2 = 85.7%; Figure 3A), BMI (OR: 1.02; 95% CI: 1.01–1.03; I2 = 0.0%; Figure 3B), and distance from home (OR: 1.01; 95% CI: 1.00–1.01; I2 = 0.0%; Figure 3C) were disadvantageous factors for SDD (5, 7, 19–21, 23, 26, 27, 31).
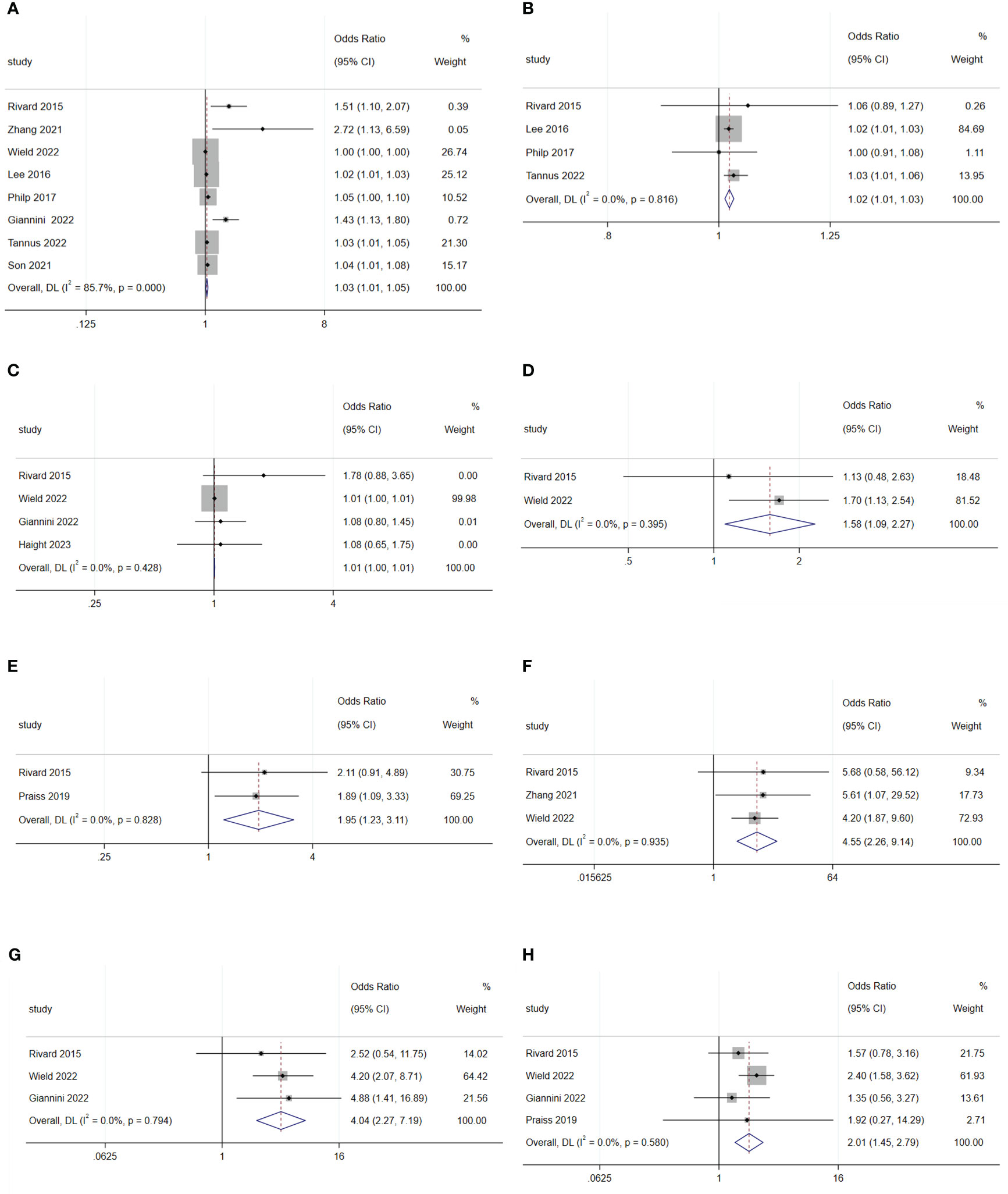
Figure 3 Forest plot of baseline characteristics influencing same-day discharge after minimally invasive hysterectomy in patients: (A) age; (B) BMI; (C) distance to home; (D) diabetes; (E) lung disease; (F) cerebral vascular event; (G) deep-vein thrombosis; (H) heart disease.
There was no difference in rates of SDD between Black and White patients (OR: 1.11; 95% CI: 0.79–1.56; I2 = 54.0%; Figure 4A), between Hispanic and non-Hispanic people (OR: 0.66; 95% CI: 048–0.91; I2 = 59.0%; Figure 4B), and between smoking and non-smoking populations (OR: 1.02; 95% CI: 0.88–1.19; I2 = 0.0%; Figure 4C) (5, 19, 25, 29, 30).
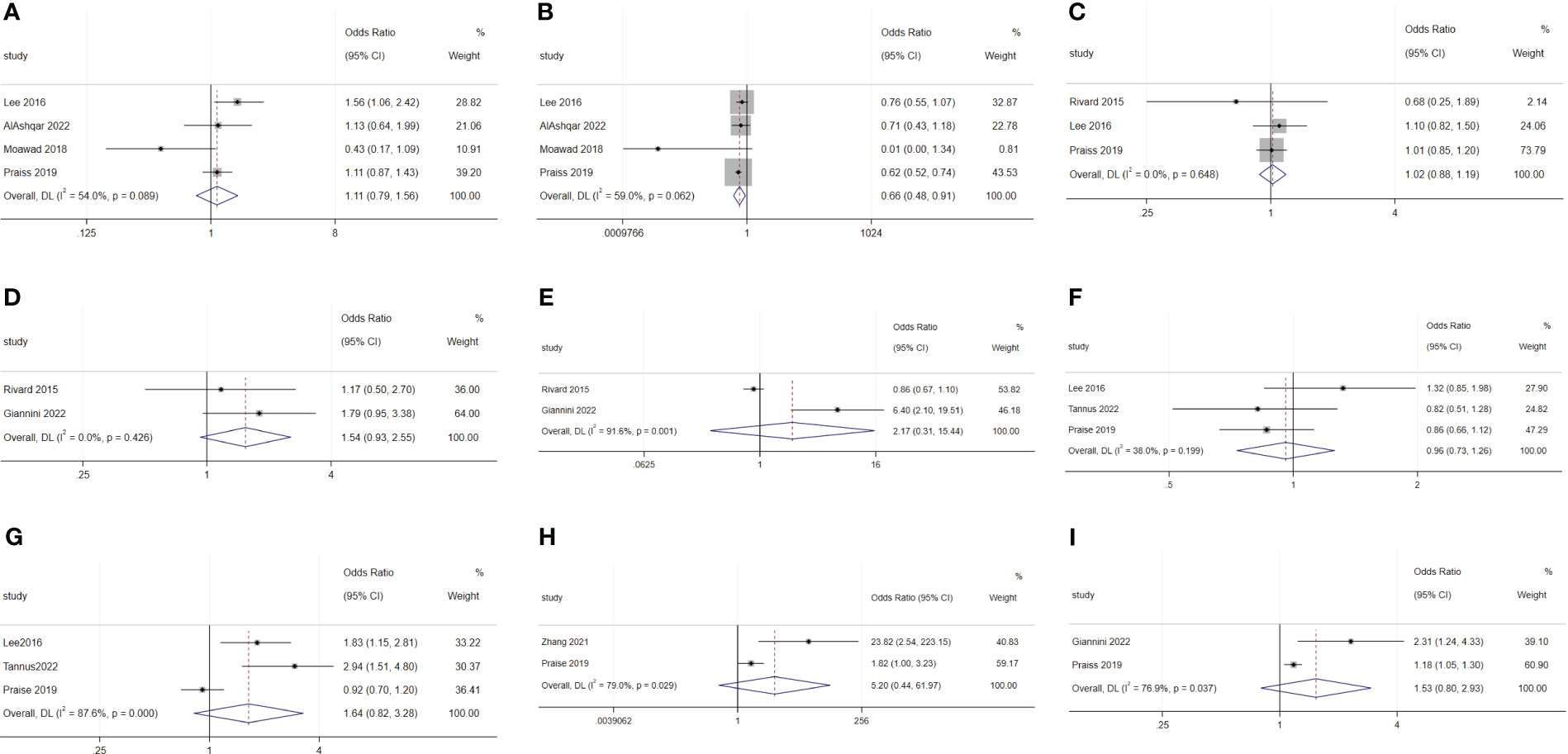
Figure 4 Forest plot of baseline characteristics not influencing same-day discharge after minimally invasive hysterectomy in patients: (A) black or white; (B) Hispanic and non-Hispanic people; (C) smoking; (D) previous abdominal surgery history; (E) preoperative hemoglobin levels; (F) ASA 2; (G) ASA 3; (H) ASA 4; (I) hypertension.
Previous abdominal surgery (OR: 1.54; 95% CI: 0.93–2.55; I2 = 0.0%; Figure 4D) and preoperative hemoglobin levels (OR: 2.17; 95% CI: 0.31–15.44; I2 = 91.6%; Figure 4E) did not affect SDD (19, 27).
Diabetes (OR: 1.58; 95% CI: 1.09–2.27; I2 = 0.0%; Figure 3D), lung disease (OR: 1.95; 95% CI: 1.23–3.11; I2 = 0.0%; Figure 3E), cerebral vascular events (OR: 4.55; 95% CI: 2.26–9.14; I2 = 0.0%; Figure 3F), deep-vein thrombosis (OR: 4.04; 95% CI: 2.27–7.19; I2 = 0.0%; Figure 3G), and heart disease (OR: 2.01; 95% CI: 1.45–2.79; I2 = 0.0%; Figure 3H) were disadvantageous factors for SDD (16, 18, 22, 24). Moreover, different American Society of Anesthesiologists physical status classification system (ASA) status, and hypertension were not predictive factors for SDD (Figure 4F-I) (5, 25, 31).
The meta-analysis showed that radical hysterectomy (OR: 3.46; 95% CI: 1.90–6.29; I2 = 0.0%; Figure 5A) was disadvantageous for SDD, while lymphadenectomy (OR: 1.70; 95% CI: 0.39–7.45; I2 = 90.9%; Figure 6A) and adhesiolysis (OR: 1.48; 95% CI: 0.91–2.41; I2 = 56.0%; Figure 6B) did not affect SDD (21, 22, 25, 27, 29, 30).
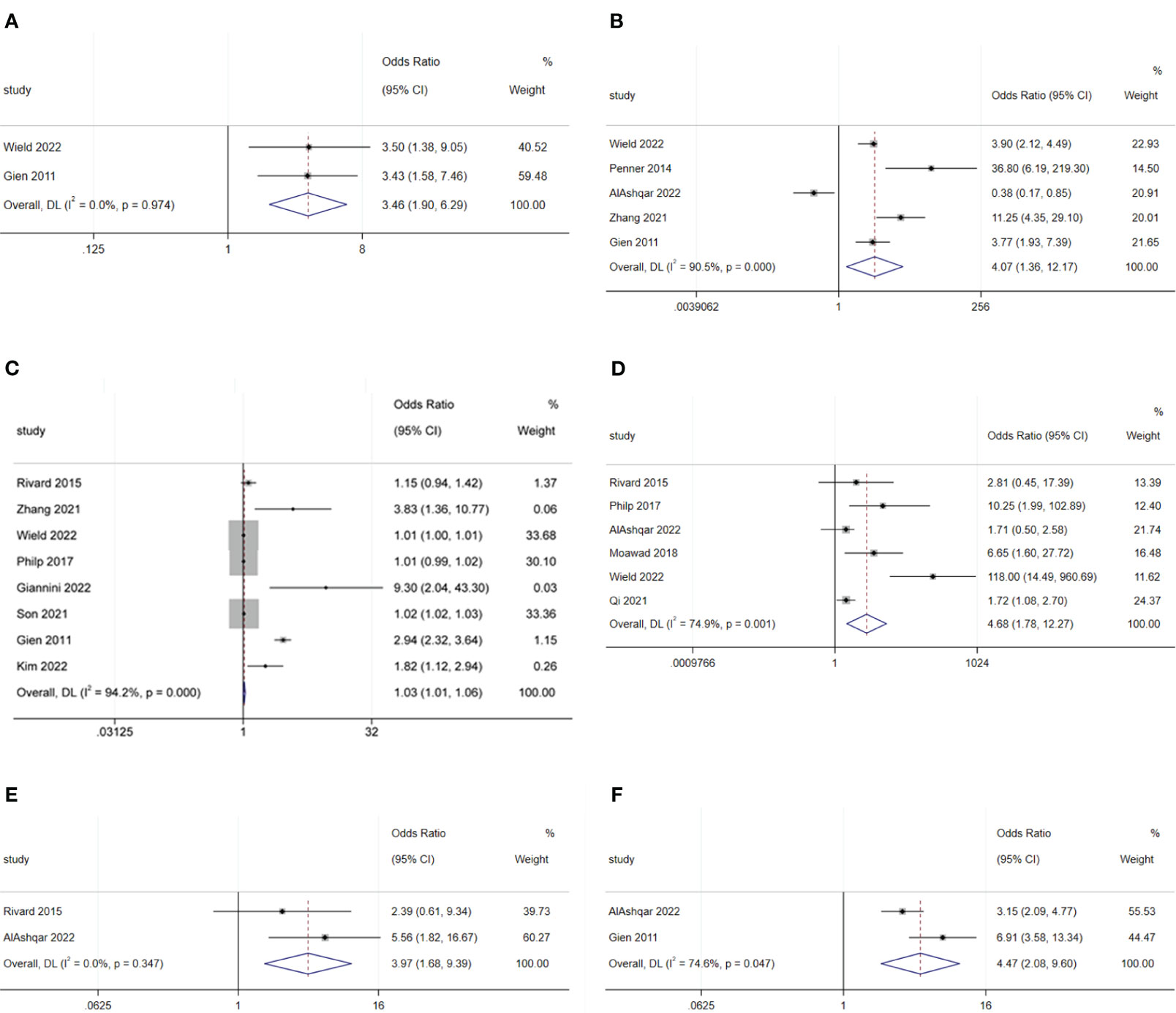
Figure 5 Forest plot of surgical characteristics influencing same-day discharge after minimally invasive hysterectomy in patients: (A) radical hysterectomy; (B) start of surgery after 14:00; (C) increase in surgical time; (D) intraoperative complications; (E) postoperative complications; (F) preferences of surgeons.
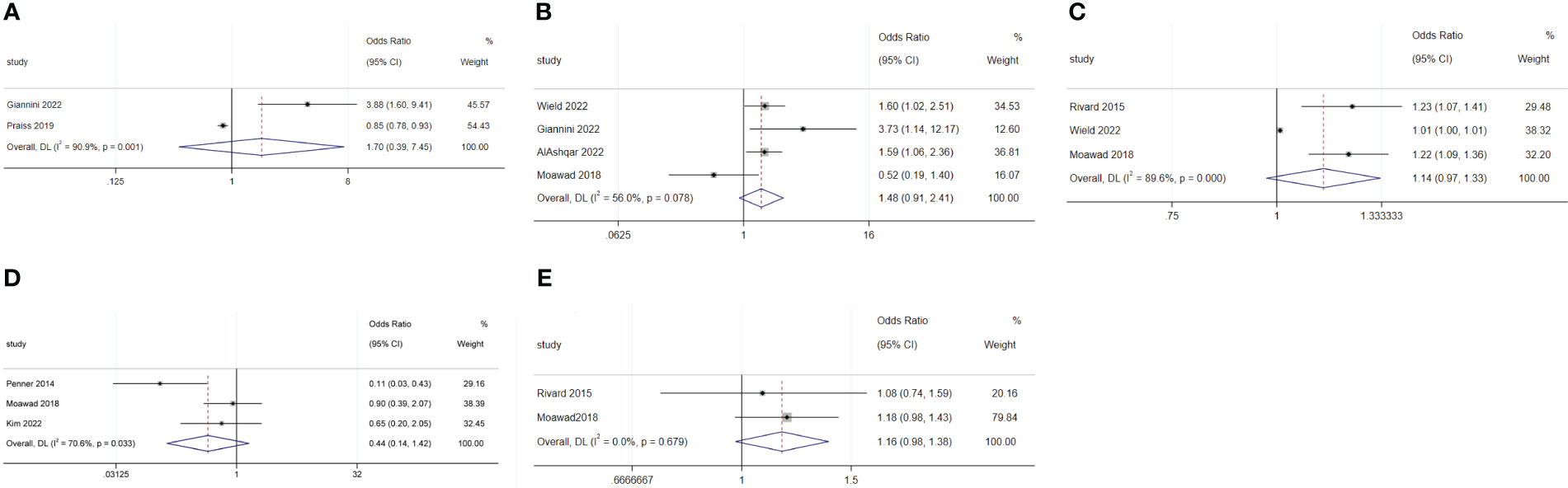
Figure 6 Forest plot of surgical characteristics not influencing same-day discharge after minimally invasive hysterectomy in patients: (A) lymphadenectomy; (B) adhesiolysis; (C) end time of surgery; (D) robotic surgery; (E) intraoperative blood loss.
Surgery starting after 14:00 (OR: 4.07; 95% CI: 1.36–12.17; I2 = 90.5%; Figure 5B) and longer surgical time (OR: 1.03; 95% CI: 1.01–1.06; I2 = 94.2%; Figure 5C) were disadvantageous factors for SDD (19–24, 26, 27, 29, 44). The end time of surgery did not affect SDD (OR: 1.14; 95% CI: 0.97–1.33; I2 = 89.6%; Figure 6C) (19, 21, 30).
Robotic surgery had no impact on SDD (OR: 0.44; 95% CI: 0.14–1.42; I2 = 70.6%; Figure 6D) compared to conventional minimally invasive hysterectomy (24, 30, 44). Intraoperative blood loss (OR: 1.16; 95% CI: 0.98–1.38; I2 = 0.00%; Figure 6E) also did not affect SDD (19, 30).
Intraoperative complications (OR: 4.68; 95% CI: 1.78 to 12.27; I2 = 74.9%; Figure 5D) and postoperative complications (OR: 3.97; 95% CI: 1.68 to 9.39; I2 = 0.0%; Figure 5E) were disadvantageous factors for SDD (19, 21, 23, 28–30). Surgeon preference was an important influencing factor for SDD (OR: 4.47; 95% CI: 2.08–9.60; I2 = 74.6%; Figure 5F) (22, 29).
For malignant diseases, BMI (OR: 1.02; 95% CI: 1.01–1.02; I2 = 0.0%; Figure 7A) and surgery starting after 14:00 (OR: 15.84; 95% CI: 5.53–45.4; I2 = 24.3%; Figure 7B) were disadvantage factors for SDD. Hispanic people were more likely to leave hospital on the same day of surgery (OR: 0.65; 95% CI: 0.55–0.78; I2 = 10.9%; Figure 7C) (5, 20, 23–25). Age (OR: 1.07; 95% CI: 0.99–1.16; I2 = 78.9%; Figure 8A), race (Black or White) (OR: 1.26; 95% CI: 0.91 to 1.74; I2 = 47.8%; Figure 8B), smoking (OR: 1.03; 95% CI: 0.89–1.20; I2 = 0.0%; Figure 8C), heart disease (OR: 1.43; 95% CI: 0.64–3.21; I2 = 0.0%; Figure 8D), hypertension (OR: 1.53; 95% CI: 0.80–2.93; I2 = 76.9%; Figure 8E), ASA 2 (OR: 1.03; 95% CI: 0.68–1.56; I2 = 64.3%; Figure 8F), ASA 3 (OR: 1.27; 95% CI: 0.64–2.49; I2 = 85.1%; Figure 8G), ASA 4 (OR: 5.20; 95% CI: 0.44–61.97; I2 = 79.0%; Figure 8H), lymphadenectomy (OR: 1.70; 95% CI: 0.39–7.45; I2 = 90.9%; Figure 8I), and length of surgery (OR: 2.67; 95% CI: 0.31–23.14; I2 = 87.7%; Figure 8J) did not affect SDD.
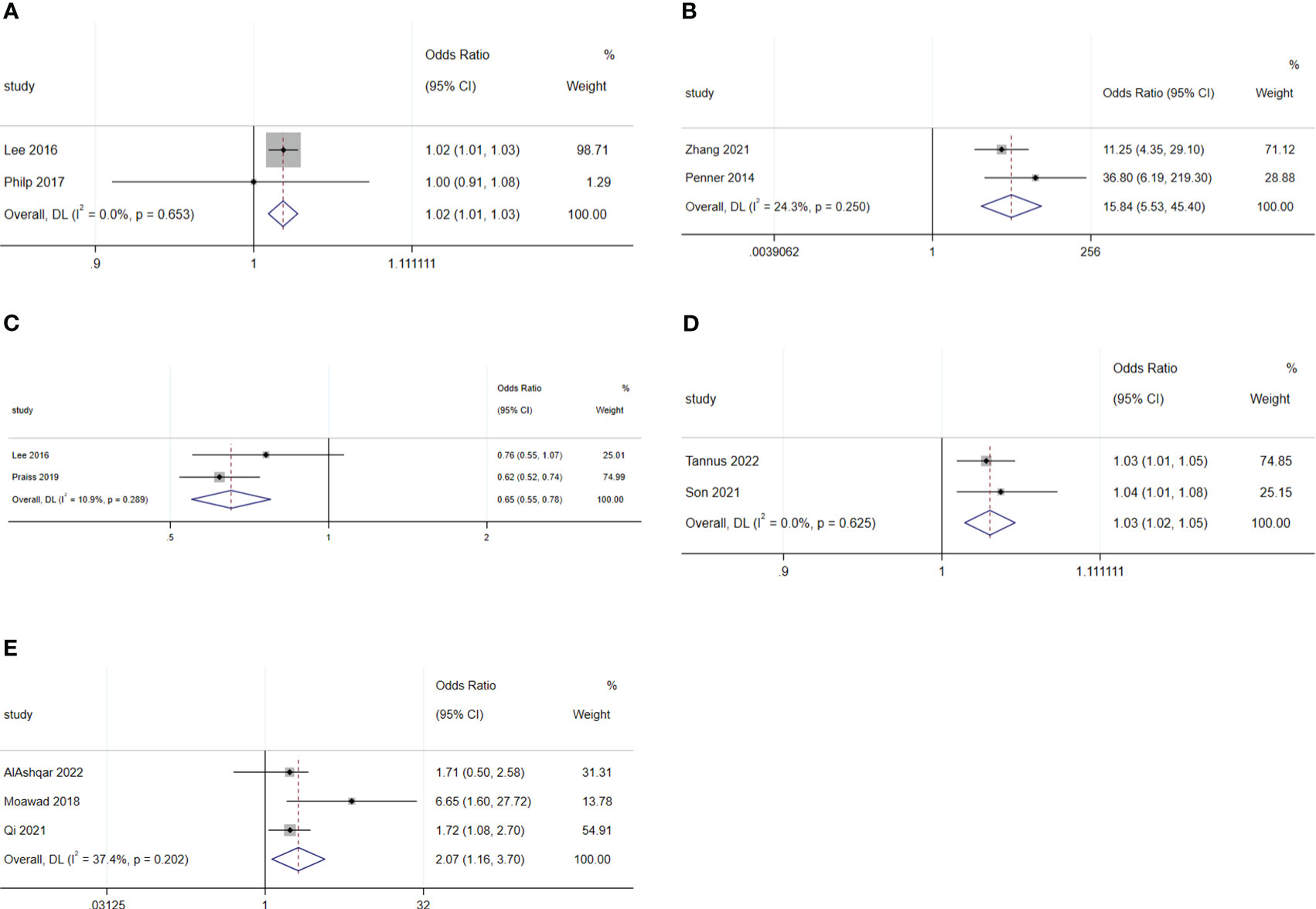
Figure 7 Forest plot of factors influencing same-day discharge after minimally invasive hysterectomy in subgroups: (A) (BMI), (B) (start time of surgery after 14:00) and (C) (Hispanic and non-Hispanic people) for malignant diseases; (D) (age) and (E) (intraoperative complication) for non-malignant diseases.

Figure 8 Forest plot of factors not influencing same-day discharge after minimally invasive hysterectomy in subgroups: (A) (age), (B) (black or white), (C) (smoking), (D) (heart disease), (E) (hypertension), (F) (ASA 2), G (ASA 3), (H) (ASA 4); (I) (lymphadenectomy) and (J) (length of surgery) for malignant diseases; (K) (black or white), (L) (adhesiolysis) and (M) (length of surgery) for non-malignant diseases.
For non-malignant diseases, age (OR: 1.03; 95% CI: 1.02–1.05; I2 = 0.0%; Figure 7D) and intraoperative complications (OR: 2.07; 95% CI: 1.16–3.70; I2 = 37.4%; Figure 7E) were disadvantageous factors for SDD (26, 28–31). Race (Black or White) (OR: 0.75; 95% CI: 0.30–1.90; I2 = 67.0%; Figure 8K), adhesiolysis (OR: 0.10; 95% CI: 0.00–8.83; I2 = 83.9%; Figure 8L), and length of surgery (OR: 1.78; 95% CI: 0.49–6.40; I2 = 84.1%; Figure 8M) did not affect SDD.
We comprehensively reviewed the currently available literature on the risk factors influencing SDD after minimally invasive hysterectomy in patients with malignant and non-malignant gynecological diseases. The pooled SDD rates were 49%, similar to the gynecological malignant and non-malignant diseases. The main risk factors influencing SDD were an increase in age, BMI, and distance to home; certain comorbidities (e.g., diabetes, lung disease, cerebral vascular event, and deep-vein thrombosis); radical hysterectomy; surgery starting after 14:00; longer surgical time; intraoperative complications; postoperative complications; and surgeon preference. However, factors such as previous abdominal surgery history, hypertension, ASA status, robotic surgery, and intraoperative blood loss did not appear to impact SDD. The surgical procedures for gynecological malignancies are usually more complex than the non-malignant diseases; hence, the start time of the surgery and BMI had a greater impact on SDD in these cases.
Since the first report of SDD after a minimally invasive hysterectomy in 1993, despite studies demonstrating the safety and efficacy of SDD in minimally invasive hysterectomy, the SDD rate has not been widely adopted (4, 32). Our study revealed a pooled SDD rate of 50%. A high publication bias existed between the included studies, and the reasons are multifactorial. First, the publication years of the included studies ranged from 2011 to 2022. A growing body of literature has reported increasing rates of SDD over the years (26, 40, 45). Giannini et al. reported that the SDD rate for minimally invasive hysterectomies increased from 13.8% to 88% between 2012 and 2021 (27). Second, there is a paucity of standard patient protocols for SDD in minimally invasive hysterectomies. Patient demographics and preoperative, perioperative, and postoperative characteristics were associated with different SDD rates. Third, the acceptance by doctors and patients varies across regions and medical institutions.
Our review of available evidence indicated that patient demographic variables, such as an increase in age, BMI, and distance to home; comorbidities including diabetes; lung disease; cerebrovascular events; deep-vein thrombosis; and heart disease were also disadvantageous factors for SDD (45). Rivard et al. revealed that an age gap of 10 years increases the admission rate by 50% (19). Praise et al., Matern et al., and Rivard et al. reported that patients aged 80-, 75-, and 70-years respectively, are at an increased risk of admission (19, 25, 46). This may provide a cutoff age when patients with SDD are included. Similarly, the rate of SDD decreases with increasing BMI, with rates of 16.3%, 13.7%, and 11.0% among normal-weight, overweight, and obese women, respectively (16). However, another study reported that BMI >40 kg/m (2) did not increase the admission rate. The study illustrated this by introducing robotic surgery, which reduced the conversion rate in obese patients (47, 48). Thus, in the future, BMI may not be a contraindication for SDD in minimally invasive surgery (49). A cohort of studies revealed that comorbidities are unfavorable factors for SDD in minimally invasive hysterectomy (5, 6, 26, 31). Ji et al. and Lee et al. reported that the admitted group had older age, higher BMI, and more comorbidities associated with more complex surgical procedures that affected the SDD rate (5, 26). Our meta-analysis revealed that distance to family is a negative factor for SDD. Patients may refuse SDD because of concerns regarding postoperative complications and inconvenient readmission. However, the effect of distance to family remains controversial.7.19,21,27 A low 30 day-readmission rate and preoperative consultations for SDD surgery may weaken the impact of family distance. A meta-analysis including 16423 patients who underwent minimally invasive surgery and SDD by a gynecological oncologist showed no statistically significant differences in complications and readmission rates within 30 days after surgery when compared with those in patients who underwent SDD.
The surgical risk factors for SDD in minimally invasive hysterectomy include preoperative, intraoperative, and postoperative variables. In our meta-analysis, radical hysterectomy, surgery starting after 14:00, increased surgical time, and postoperative/intraoperative complications negatively affected SDD. One study found that the risk of hospitalization increased for every 30-minute increase in surgical time and every 1-hour delay in surgical completion time (16). This can be used as a guide for the surgery starting time and serve as a reference for the cutoff point of SDD. For radical hysterectomy with a longer surgical time, surgeons should provide sufficient preoperative consultation to patients (19, 50).
Surgeon preference is an important factor in SDD. Although it has been reported that the rehospitalization rate and incidence of postoperative complications after minimally invasive hysterectomy with SDD are very low, SDD is not implemented in 38.3% of patients because of doctors concerns about patient safety (27). This reminds us that establishing standardized inclusion procedures, ensuring the smoothness of postoperative readmission channels, and sufficient doctor-patient communication may reduce surgeons’ anxiety and improve the rate of SDD implementation.
Our meta-analysis found that the SDD rates for malignant and non-malignant gynecological diseases are similar, which will enhance the confidence of doctors and patients in SDD for malignant gynecological diseases. The surgical procedures for gynecological malignancies are usually more complex than those for benign diseases; hence, the start time of the surgery and BMI of the patient have greater impacts on SDD.
We followed a review protocol for the study selection, data extraction, and analysis. Two review authors independently performed study selection, data extraction, and assessment of the risk of bias. Standardized data extraction forms were used in this study. However, this study has some limitations. First, all the included studies were retrospective which have intrinsic restrictions. Second, a random-effects model was used for most analyses. The limitations of this approach were the down-weighting of large studies when statistical heterogeneity was present and assigning equal weighting to the combined studies.
An increase in age, BMI, distance to home, certain comorbidities (including diabetes, lung disease, cerebral vascular event, deep-vein thrombosis, and heart disease), radical hysterectomy, surgery starting after 14:00, longer surgical time, operative complications, and surgeon preference were risk factors preventing SDD. In contrast, previous abdominal surgery, hypertension, ASA status, robotic surgery, and intraoperative blood loss do not appear to affect SDD. The SDD rates of malignant and non-malignant gynecological diseases are similar, which will enhance the confidence of doctors and patients on the day of discharge after surgery for malignant gynecological diseases. Compared to non-malignant diseases, the start time and BMI have a greater impact on SDD for malignant diseases.
In conclusion, sufficient preoperative consultation, skilled surgeons’ participation, early surgical times, and avoidance of complications are beneficial for the successful implementation of SDD. Notably, gynecological malignancies are not a risk factor affecting successful SDD, but relatively complex surgeries should begin before 14:00. Adequate operative support can reduce patients’ and surgeons’ concerns about the safety of SDD and improve its successful application.
JL: Conceptualization, Data curation, Methodology, Writing – original draft. YC: Data curation, Writing – review & editing. XT: Writing – review & editing. HC: Methodology, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to express our appreciation to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nahas S, Feigenberg T, Park S. Feasibility and safety of same-day discharge after minimally invasive hysterectomy in gynecologic oncology: a systematic review of the literature. Gynecol Oncol (2016) 143:439–42. doi: 10.1016/j.ygyno.2016.07.113
2. Golia D'Augè T, Giannini A, Bogani G, Di Dio C, Laganà AS, Di Donato V, et al. Prevention, screening, treatment and follow-up of gynecological cancers: state of art and future perspectives. Clin Exp Obstet Gynecol (2023) 50(8):160. doi: 10.31083/j.ceog5008160
3. Pecorino B, D'Agate MG, Scibilia G, Scollo P, Giannini A, Di Donna MC, et al. Evaluation of surgical outcomes of abdominal radical hysterectomy and total laparoscopic radical hysterectomy for cervical cancer: A retrospective analysis of data collected before the LACC trial. Int J Environ Res Public Health (2022) 19(20):13176. doi: 10.3390/ijerph192013176
4. Giannini A, D'Oria O, Chiantera V, Margioula-Siarkou C, Di Donna MC, Terzic S, et al. Minimally invasive surgery for cervical cancer: should we look beyond squamous cell carcinoma? J Invest Surg (2022) 35(7):1602–3. doi: 10.1080/08941939.2022.2075495
5. Lee J, Aphinyanaphongs Y, Curtin JP, Chern JY, Frey MK, Boyd LR. The safety of same-day discharge after laparoscopic hysterectomy for endometrial cancer. Gynecol Oncol (2016) 142:508–13. doi: 10.1016/j.ygyno.2016.06.010
6. Jennings AJ, Spencer RJ, Medlin E, Rice LW, Uppal S. Predictors of 30-day readmission and impact of same-day discharge in laparoscopic hysterectomy. Am J Obstet Gynecol (2015) 213:344.e1–7. doi: 10.1016/j.ajog.2015.05.014
7. Haight PJ, Barrington DA, Graves SM, Piver RN, Baek J, Ardizzone M, et al. Safety and feasibility of same-day discharge following minimally invasive hysterectomy in the morbidly obese patient population. Gynecol Oncol (2023) 170:203–9. doi: 10.1016/j.ygyno.2023.01.013
8. Sheyn D, El-Nashar S, Billow M, Mahajan S, Duarte M, Pollard R. Readmission rates after same-day discharge compared with postoperative day 1 discharge after benign laparoscopic hysterectomy. J Minim Invasive Gynecol (2018) 25:484–90. doi: 10.1016/j.jmig.2017.10.013
9. Schiavone MB, Herzog TJ, Ananth CV, Wilde ET, Lewin SN, Burke WM, et al. Feasibility and economic impact of same-day discharge for women who undergo laparoscopic hysterectomy. Am J Obstet Gynecol (2012) 207:382.e1–9. doi: 10.1016/j.ajog.2012.09.014
10. Wright JD, Burke WM, Tergas AI, Hou JY, Huang Y, Hu JC, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clin Oncol (2016) 34(10):1087–96. doi: 10.1200/JCO.2015.65.3212
11. La Verde M, Riemma G, Tropea A, Biondi A, Cianci S. Ultra-minimally invasive surgery in gynecological patients: a review of the literature. Updates Surg (2022) 74(3):843–55. doi: 10.1007/s13304-022-01248-y
12. Drahonovsky J, Haakova L, Otcenasek M, Krofta L, Kucera E, Feyereisl J. A prospective randomized comparison of vaginal hysterectomy, laparoscopically assisted vaginal hysterectomy, and total laparoscopic hysterectomy in women with benign uterine disease. Eur J Obstet Gynecol Reprod Biol (2010) 148(2):172–6. doi: 10.1016/j.ejogrb.2009.10.019
13. Giannini A, D'Oria O, Bogani G, Di Donato V, Vizza E, Chiantera V, et al. Hysterectomy: let's step up the ladder of evidence to look over the horizon. J Clin Med (2022) 11(23):6940. doi: 10.3390/jcm11236940
14. Sanabria D, Rodriguez J, Pecci P, Ardila E, Pareja R. Same-day discharge in minimally invasive surgery performed by gynecologic oncologists: a review of patient selection. J Minim Invasive Gynecol (2020) 27:816–25. doi: 10.1016/j.jmig.2019.10.023
15. Korsholm M, Mogensen O, Jeppesen MM, Lysdal VK, Traen K, Jensen PT. Systematic review of same-day discharge after minimally invasive hysterectomy. Int J Gynaecol Obstet (2017) 136:128–37. doi: 10.1002/ijgo.12023
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. J Clin Epidemiol (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
17. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods (2006) 11:193–206. doi: 10.1037/1082-989X.11.2.193
18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
19. Rivard C, Casserly K, Anderson M, Isaksson Vogel R, Teoh D. Factors influencing same-day hospital discharge and risk factors for readmission after robotic surgery in the gynecologic oncology patient population. J Minim Invasive Gynecol (2015) 22:219–26. doi: 10.1016/j.jmig.2014.10.001
20. Zhang N, Wilson B, Enty MA, Ketch P, Ulm MA, ElNaggar AC, et al. Same-day discharge after robotic surgery for endometrial cancer. J Robot Surg (2022) 16:543–8. doi: 10.1007/s11701-021-01253-0
21. Wield AM, Cohen MG, Toal CT, Kulinsky M, Holder-Murray JM, Esper SA, et al. Same-day hospital discharge after minimally invasive hysterectomy in a gynecologic oncology practice: feasibility, safety, predictors of admission, and adverse outcomes. J Minim Invasive Gynecol (2022) 29:1043–53. doi: 10.1016/j.jmig.2022.05.010
22. Gien LT, Kupets R, Covens A. Feasibility of same-day discharge after laparoscopic surgery in gynecologic oncology. Gynecol Oncol (2011) 121:339–43. doi: 10.1016/j.ygyno.2010.12.344
23. Philp L, Covens A, Vicus D, Kupets R, Pulman K, Gien LT. Feasibility and safety of same-day discharge after laparoscopic radical hysterectomy for cervix cancer. Gynecol Oncol (2017) 147:572–6. doi: 10.1016/j.ygyno.2017.09.026
24. Penner KR, Fleming ND, Barlavi L, Axtell AE, Lentz SE. Same-day discharge is feasible and safe in patients undergoing minimally invasive staging for gynecologic Malignancies. Am J Obstet Gynecol (2015) 212:186. doi: 10.1016/j.ajog.2014.08.010
25. Praiss AM, Chen L, St Clair CM, Tergas AI, Khoury-Collado F, Hou JY, et al. Safety of same-day discharge for minimally invasive hysterectomy for endometrial cancer. Am J Obstet Gynecol (2019) 221:239.e1–11. doi: 10.1016/j.ajog.2019.05.003
26. Son J, Tran T, Yao M, Michener CM. Factors associated with unplanned admission in patients intended for same day discharge after minimally invasive hysterectomy for endometrial cancer. Surg Innov (2022) 29:336–42. doi: 10.1177/15533506211041882
27. Giannini A, Magrina JF, Magtibay PM, Butler KA. Same-day dismissal for endometrial cancer robotic surgery: feasibility factors. Updates Surg (2023) 75:743–55. doi: 10.1007/s13304-022-01424-0
28. Qi M, Lopa S, Adambekov S, Harris JA, Mansuria S, Edwards RP, et al. Same-day discharge after minimal invasive hysterectomy: applications for improved value of care. Eur J Obstet Gynecol Reprod Biol (2021) 259:140–5. doi: 10.1016/j.ejogrb.2021.02.020
29. AlAshqar A, Wildey B, Yazdy G, Goktepe ME, Kilic GS, Borahay MA. Predictors of same-day discharge after minimally invasive hysterectomy for benign indications. Int J Gynaecol Obstet (2022) 158:308–17. doi: 10.1002/ijgo.13992
30. Moawad G, Park D, Maasen M, Frost A, Tyan P. Predictors of overnight admission after laparoscopic myomectomy in a high-volume minimally invasive gynecologic surgery setting. J Minim Invasive Gynecol (2020) 27:195–9. doi: 10.1016/j.jmig.2019.03.022
31. Tannus S, Giannini A, Magrina JF, Crosson J, Kosiorek H, Yi J, et al. Same-day discharge after robotic hysterectomy for benign conditions: feasibility and safety. J Minim Invasive Gynecol (2023) 30:277–83. doi: 10.1016/j.jmig.2022.12.007
32. Taylor RH. Outpatient laparoscopic hysterectomy with discharge in 4 to 6 hours. J Am Assoc Gynecol Laparosc (1994) 1:S35. doi: 10.1016/s1074-3804(05)80983-1
33. Fountain CR, Havrilesky LJ. Promoting same-day discharge for gynecologic oncology patients in minimally invasive hysterectomy. J Minim Invasive Gynecol (2017) 24:932–9. doi: 10.1016/j.jmig.2017.05.005
34. Gale J, Thompson C, Lortie KJ, Bougie O, Singh SS. Early discharge after laparoscopic hysterectomy: a prospective study. J Obstet Gynaecol Can (2018) 40:1154–61. doi: 10.1016/j.jogc.2017.11.039
35. Lee SJ, Calderon B, Gardner GJ, Mays A, Nolan S, Sonoda Y, et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and Malignant indications. Gynecol Oncol (2014) 133:552–5. doi: 10.1016/j.ygyno.2014.04.006
36. McAlarnen LA, Maurer JE, Knaub A, Hopp E, Streitenberger K, Bishop E, et al. Same-day discharge after robotic hysterectomy: a resource utilization and quality improvement project. WMJ (2022) 121:243–6.
37. Lassen PD, Larsen HM, Nully PD. Same-day discharge after laparoscopic hysterectomy. Acta Obstet Gynecol Scand (2012) 91:1339–41. doi: 10.1111/j.1600-0412.2012.01535.x
38. Nensi A, Coll-Black M, Leyland N, Sobel ML. Implementation of a same-day discharge protocol following total laparoscopic hysterectomy. J Obstet Gynaecol Can (2018) 40:29–35. doi: 10.1016/j.jogc.2017.05.035
39. Burdick MP, Yamamoto M, Zaritsky E. Same-day discharge after laparoscopic hysterectomy. Obstet Gynecol (2011) 117:1136–41. doi: 10.1097/AOG.0b013e31.-8215dd4e
40. Melamed A, Katz Eriksen JL, Hinchcliff EM, Worley MJ Jr, Berkowitz RS, Horowitz NS, et al. Same-day discharge after laparoscopic hysterectomy for endometrial cancer. Ann Surg Oncol (2016) 23:178–85. doi: 10.1245/s10434-015-4582-4
41. Rettenmaier MA, Mendivil AA, Brown JV 3rd, Abaid LN, Micha JP, Goldstein BH. Same-day discharge in clinical stage I endometrial cancer patients treated with total laparoscopic hysterectomy, bilateral salpingo-oophorectomy and bilateral pelvic lymphadenectomy. Oncology (2012) 82:321–6. doi: 10.1159/000337573
42. Schiavone MB, Herzog TJ, Ananth CV, Wilde ET, Lewin SN, Burke WM, et al. Feasibility and economic impact of same-day discharge for women who undergo laparoscopic hysterectomy. Am J Obstet Gynecol (2012) 207:382. doi: 10.1016/j.ajog.2012.09.014
43. Schiff LD, Voltzke KJ, Strassle PD, Louie M, Carey ET. Effect of length of hospital stay on infection and readmission after minimally invasive hysterectomy. Int J Gynaecol Obstet (2019) 145:293–9. doi: 10.1002/ijgo.12812
44. Kim SR, Laframboise S, Nelson G, McCluskey SA, Avery L, Kujbid N, et al. Enhanced recovery after minimally invasive gynecologic oncology surgery to improve same day discharge: a quality improvement project. Int J Gynecol Cancer (2022) 32:457–65. doi: 10.1136/ijgc-2021-003065
45. Di Donato V, D'Oria O, Giannini A, Bogani G, Fischetti M, Santangelo G, et al. Age-adjusted charlson comorbidity index predicts survival in endometrial cancer patients. Gynecol Obstet Invest (2022) 87(3-4):191–9. doi: 10.1159/000525405
46. Luchristt D, Kenton KS, Bretschneider CE. Historical and forecasted changes in utilization of same-day discharge after minimally invasive hysterectomy. J Minim Invasive Gynecol (2022) 29:855–61.e1. doi: 10.1016/j.jmig.2022.03.011
47. Sinno AK, Fader AN. Robotic-assisted surgery in gynecologic oncology. Fertil Steril (2014) 102(4):922–32. doi: 10.1016/j.fertnstert.2014.08.020
48. Cianci S, Arcieri M, Vizzielli G, Martinelli C, Granese R, La Verde M, et al. Robotic pelvic exenteration for gynecologic Malignancies, anatomic landmarks, and surgical steps: A systematic review. Front Surg (2021) 8:790152. doi: 10.3389/fsurg.2021.790152
49. Matern T, Kang E, Lim PC. Factors in the feasibility and safety of outpatient robotic-assisted hysterectomy for endometrial or cervical carcinoma. Gynecol Oncol (2020) 157:482–6. doi: 10.1016/j.ygyno.2020.01.028
Keywords: same-day discharge, hysterectomy, minimally invasive surgery, systematic review, meta-analysis
Citation: Liu J, Chen Y, Tan X and Chen H (2024) Factors influencing same-day discharge after minimally invasive hysterectomy for malignant and non-malignant gynecological diseases: a systematic review and meta-analysis. Front. Oncol. 13:1307694. doi: 10.3389/fonc.2023.1307694
Received: 05 October 2023; Accepted: 18 December 2023;
Published: 09 January 2024.
Edited by:
Andrea Giannini, Umberto 1 Hospital, ItalyReviewed by:
Marco La Verde, Università degli Studi della Campania “Luigi Vanvitelli”, ItalyCopyright © 2024 Liu, Chen, Tan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hengxi Chen, d293b2NoeEAxMjYuY29t; Xin Tan, MzQ4OTkxNDE3QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.