- Department of Neurology, Focus Program Translational Neuroscience (FTN) and Immunotherapy (FZI), Rhine-Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
Background: Combination treatment with BRAF/MEK inhibitors favorably impact progression-free survival in malignant melanoma. However, it may cause paradoxical activation of the MAPK/ERK pathway in immune cells without BRAF mutation, which may lead to over activation of the immune system, especially in patients with pre-existing autoimmune conditions. In this case report, treatment of malignant melanoma with BRAF/MEK inhibitors was associated with radiological disease exacerbation of pre-existing multiple sclerosis (MS).
Case presentation: A 47-year-old patient with pre-existing MS was diagnosed with malignant melanoma in June 2020. Anti-tumor treatment was initiated with a combination therapy of BRAF inhibitor dabrafenib and MEK inhibitor trametinib. In February 2022, the patient presented at our neurological clinic after routine MRI revealed exacerbation of radiological MS disease activity with ten new and gadolinium-enhancing lesions, and concomitant high levels of neurofilament light chain (NfL) in serum, a marker for axonal damage. In-depth analysis of immune cells in both peripheral blood and cerebrospinal fluid was performed by multi-color flow cytometry. After treatment with the B cell-depleting antibody ocrelizumab, MS disease stability was obtained and anti-tumor medication could be continued.
Conclusions: Immunomodulatory treatment in cancer patients is highly effective from an oncological point of view, but may be associated with autoimmune side effects. This is of special importance in patients with pre-existing autoimmune diseases, as reflected by our case of MS disease reactivation under treatment with BRAF/MEK inhibitors. In our case, sequential modulation of immune cell subsets by B cell depletion, associated with marked shifts in B and T cell subsets, allowed for stabilization of disease and continuation of anti-tumor treatment.
Introduction
Immune modulation has become a mainstay for treatment of many oncological diseases, including malignant melanoma. While enhancing anti-tumor immune response, these treatments can also cause autoimmune side effects by promoting self-antigen driven over-activity of the immune system. Special caution is required in treating cancer patients with pre-existing autoimmune diseases. Here, we describe a case in which treatment of malignant melanoma with BRAF/MEK inhibitors led to radiological exacerbation of pre-existing multiple sclerosis (MS). A timeline of events is shown in Figure 1A. By in depth-analysis of immune cells both during exacerbation and after successful treatment, we aimed to advance our understanding of balancing immunomodulatory interventions.
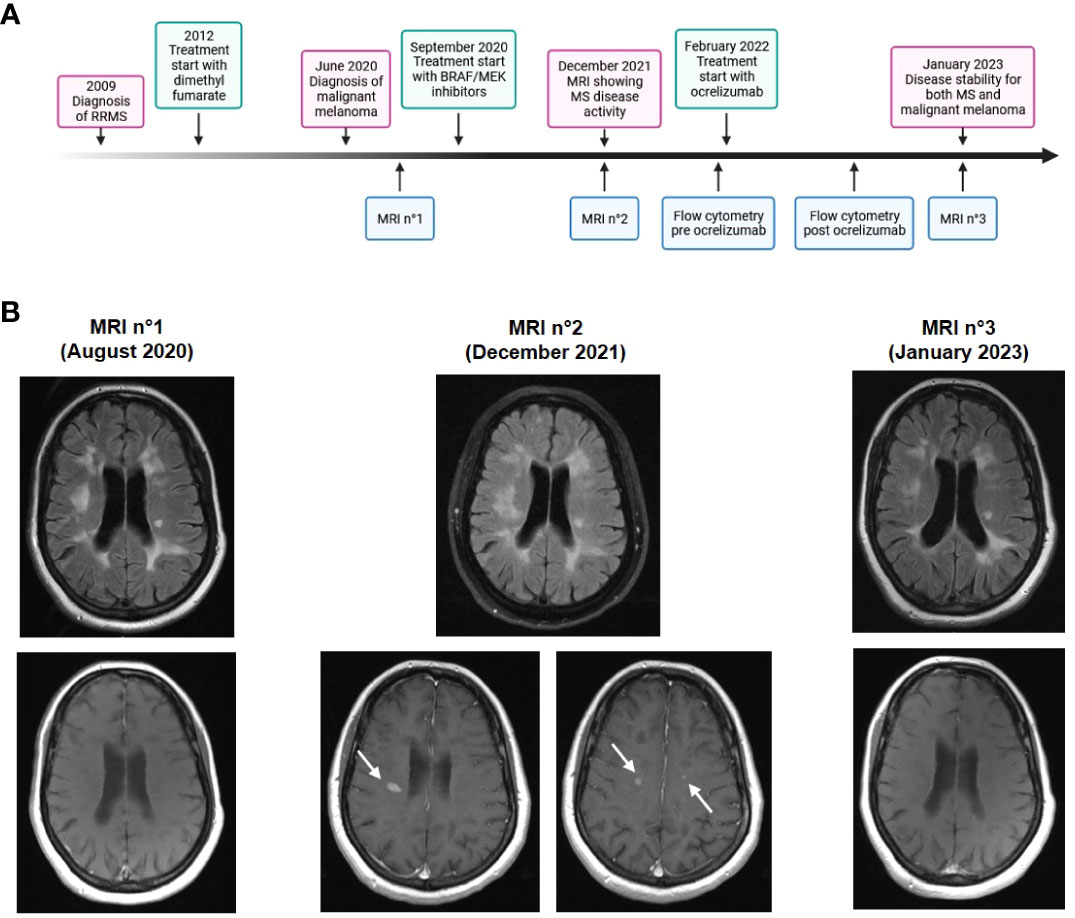
Figure 1 Time course and cerebral imaging. (A) Timeline showing clinical, diagnostic and therapeutic developments. (B) Cerebral MRI scans at different time points. Upper panels: T2 fluid-attenuated inversion recovery (FLAIR)-weighted image, lower panels: T1-weighted image with contrast enhancement. MRI n°2 shows multiple new and contrast-enhancing lesions (arrows).
Case description
In February 2022, a 47-year-old female patient presented at the neurological clinic of the University hospital in Mainz, Germany. The patient had been diagnosed with relapsing-remitting MS in 2009. Disease-modifying treatment was initiated with glatiramer acetate and escalated to dimethyl fumarate in 2012, after which the patient had a stable disease course without relapses or disease progression. In June 2020, malignant melanoma was diagnosed located on the right lower leg (Breslow tumor thickness 1.3 mm, not ulcerated, Clark’s level IV, pT2a pN2a M1, stage IV according to 2017 AJCC classification). After resection of tumor and lymph nodes, anti-tumor medication was started in September 2020 with a combination therapy of BRAF inhibitor dabrafenib and MEK inhibitor trametinib. In December 2021, a routine MRI scan showed an exacerbation of radiological disease activity with ten new and gadolinium-enhancing lesions (Figure 1B). There were no new focal neurological deficits in clinical examination. At this time point, levels of neurofilament light chain (NfL) in serum were 94.4 pg/ml, indicating axonal damage.
Serological analyses, including antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), anti-dsDNA antibodies, rheumatoid factor, soluble interleukin (IL)-2 receptor, anti-cardiolopin IgG and IgM, β2-glycoprotein I IgG and IgM, myelin oligodendrocyte glycoprotein (MOG) antibodies, anti-aquaporin-4 antibodies, screening for HIV, hepatitis B and C, cryptococcus, Lyme borreliosis and syphilis revealed no evidence of other autoimmune or infectious disease. Analysis of cerebrospinal fluid (CSF) showed a mild lymphocytic pleocytosis with positive oligoclonal bands without evidence for atypical or malignant cells.
In order to investigate the patient’s immune compartment in more detail, we performed flow cytometry analysis (FACS) for both peripheral blood mononuclear cells (PBMC) and CSF (Figure 2). CD4+ T cells composed the largest fraction of lymphocytes (PBMC: 58.9%; CSF: 78.9%) followed by CD8+ T cells (PBMC: 14.1%; CSF: 13.0%) and B cells (PBMC: 4.69%; CSF: 1.6%). Among CD4+ T cells, the fraction of CD45RO+CCR6+ Th17 cells was 17.6% in PBMC (CSF: 52.4%) and the fraction of FoxP3+ regulatory T cells was 0.83% (CSF: 9.61%). Activation markers PD-1 and KLRG1 (on both CD4+ and CD8+ T cells) and CD69 (on CD4+ T cells) were elevated in CSF compared to PBMC. In B cells, expression of the activation marker CD86 was also enhanced in CSF (70.0% compared to 6.18% in PBMC).
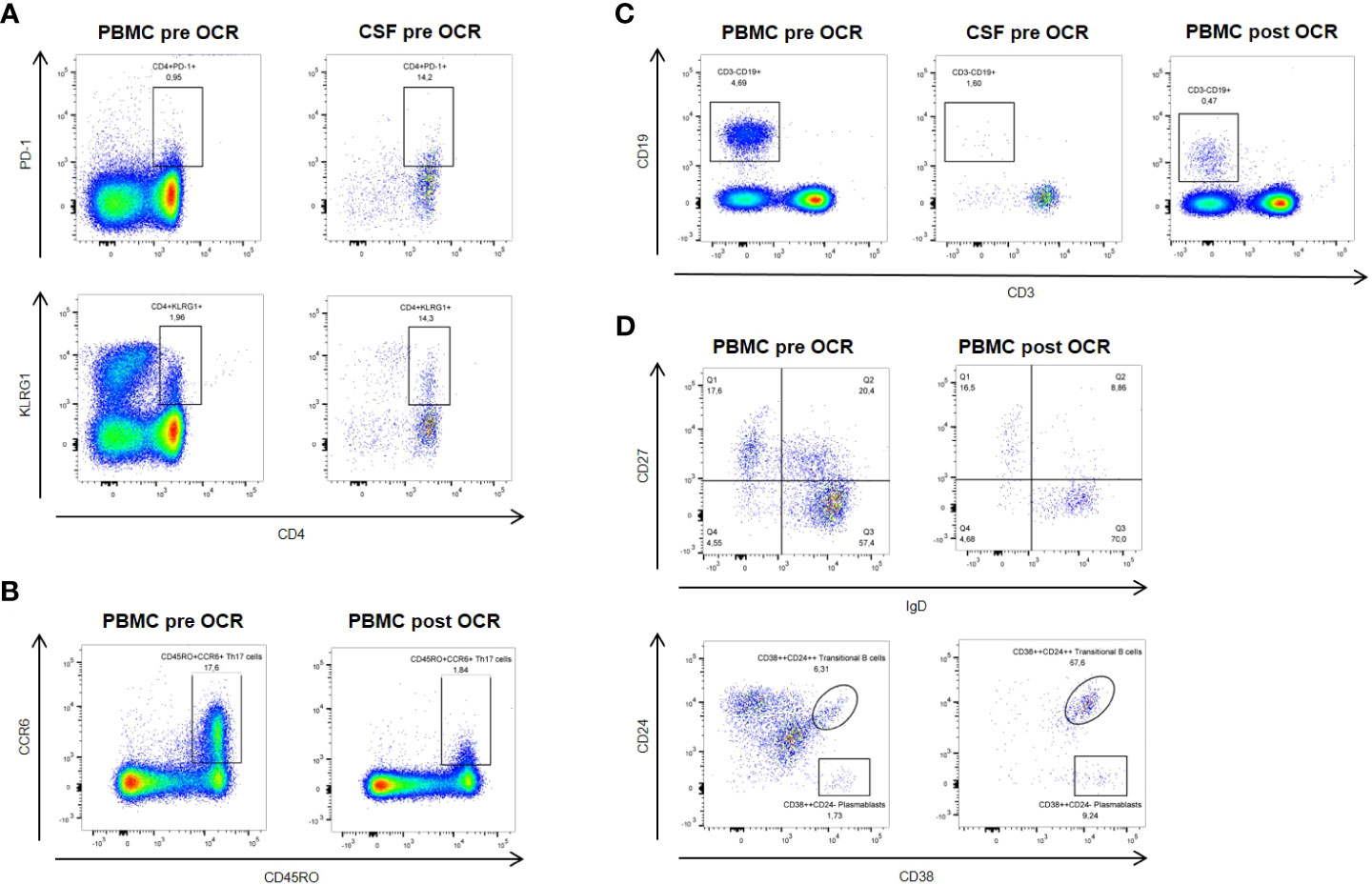
Figure 2 Flow cytometric analysis pre- and post-ocrelizumab (OCR) treatment. (A) Activation markers PD-1 and KLRG1 are elevated on CSF compared to peripheral blood CD4+ T cells at baseline. (B) Six months after ocrelizumab treatment, CD45RO+CCR6+ Th17 cells are reduced compared to baseline. (C) Levels of B cells are reduced under ocrelizumab treatment. (D) Ocrelizumab changes B cell subset composition, with decrease of IgD+CD27+ non-switched memory B cells and proportional increase of CD38++CD24++ transitional B cells and CD38++CD24- plasmablasts.
Due to the large number of new contrast-enhancing lesions, we decided to treat the patient with 500 mg methylprednisolone i.v. for three days, and then escalate disease-modifying therapy for MS to the B cell-depleting antibody ocrelizumab. The patient received the first cycle of ocrelizumab in February 2022. Oncologic treatment with BRAF/MEK inhibitors was continued. Follow-up in September 2022 showed no signs for MS disease activity, and oncologic follow-ups were also stable. In an MRI scan in January 2023 (Figure 1B), no new MS lesions were observed. Levels of NfL in serum were reduced to 11.6 pg/ml.
FACS analysis of PBMC in September 2022 showed a marked reduction of B cells under treatment with ocrelizumab (0.47% of lymphocytes). Subset analysis of B cells revealed that IgD+CD27+ non-switched memory B cells were decreased (8.86% compared to 20.4%) while CD38++CD24++ transitional B cells were strongly increased (67.6% compared to 6.31%), as were CD38++CD24- plasmablasts (9.24% compared to 1.73%). Furthermore, there was a reduction of CD45RO+CCR6+ Th17 cells (1.84% of CD3+CD4+ cells compared to 17.6%) and an increase in FoxP3+ regulatory T cells (2.29% of CD3+CD4+ cells compared to 0.83%). We also observed a slight decrease of activation markers PD-1 and KLRG1 on both CD4+ and CD8+ T cells.
Discussion
About a decade ago, the introduction of targeted treatments greatly improved prognosis for patients with malignant melanoma. Specifically, inhibitors target the protein kinase BRAF, which is often affected by an oncogenic driver mutation leading to constitutive activation of the MAPK/ERK pathway with enhanced cell proliferation and survival (1). Standard treatment regimens combine BRAF inhibitors with inhibitors of the downstream substrate kinase MEK, further improving progression-free survival (2). Inhibition of this signaling cascade favorably impacts the tumor microenvironment by increase of tumor infiltrating lymphocytes, a shift in T cell subsets and cytokines from immunosuppressive towards cytotoxic, and up-regulation of MHC-expression on tumor cells (3, 4).
However, expression of BRAF and MEK is not restricted to tumor cells. In fact, inhibition of BRAF in wild-type cells without mutation causes paradoxical activation of the MAPK/ERK pathway by promoting transactivation of RAF dimers (5). For example, the BRAF inhibitor vemurafenib has been shown to increase cytotoxic activity and cytokine secretion by T cells (6). Similar results have been described for natural killer (NK) cells and macrophages (7, 8). While these mechanisms are beneficial in driving anti-tumor immune response, over-activation of the immune system might also be detrimental in terms of autoimmune side effects. Accordingly, there have been case reports of inflammatory demyelinating sensorimotor polyradiculoneuropathy (9), sarcoidosis (10), granulomatosis with polyangiitis (11) and also one previous case of MS exacerbation (12), in which BRAF/MEK treatment was subsequently discontinued. Similar effects on autoimmune central nervous system (CNS) diseases might be caused by other anti-tumor treatments such as immune checkpoint inhibitors (13).
Chronic autoimmune inflammation in MS is driven by both T and B cells, and ultimately leads to cumulating axonal damage and neurodegeneration already in young adults (14). During exacerbation under BRAF/MEK treatment in our patient, we observed expression of activation markers on peripheral immune cells that was further enhanced in CSF. In order to effectively treat the MS exacerbation without completely abolishing the anti-tumor immune response, we chose therapeutic B cell depletion by the humanized anti-CD20 antibody ocrelizumab. As expected, FACS analysis showed a significant decrease of B cells in peripheral blood even six months after the first treatment cycle. However, further analysis revealed that different B cell subsets were affected differentially by ocrelizumab treatment (15). More specifically, there was a proportional increase in transitional B cells and plasmablasts. While plasmablasts escape anti-CD20 directed depletion, the relative increase in transitional B cells probably reflect an anti-inflammatory remodelling of the repopulating B cell profile. Interestingly, depletion of B cells also markedly reduced the percentage of circulating Th17 cells, a finding in line with previous reports from B cell-depleting treatment with rituximab in rheumatoid arthritis (16), and enhanced the fraction of regulatory CD4+ T cells. Under continuation of BRAF/MEK therapy, expression of activation markers was only slightly affected by B cell depletion.
In sum, targeted anti-tumor treatment can induce autoimmune side effects, an impact of special importance in patients with pre-existing autoimmune conditions. Balancing favorable anti-tumor immune response and potentially devastating exacerbation of autoimmune disease is a major challenge for treating physicians. In our case report, additional targeting of B cells served as a game changer for stabilization of MS, allowing for continuation of anti-tumor treatment (Figure 3). Although a larger number of cases and longer time of follow-up are needed in order to provide general recommendations, this case report highlights the possibilities of sequential modulation of immune cell subsets and their surveillance by flow cytometric analysis paving the way for individualized therapeutic concepts.
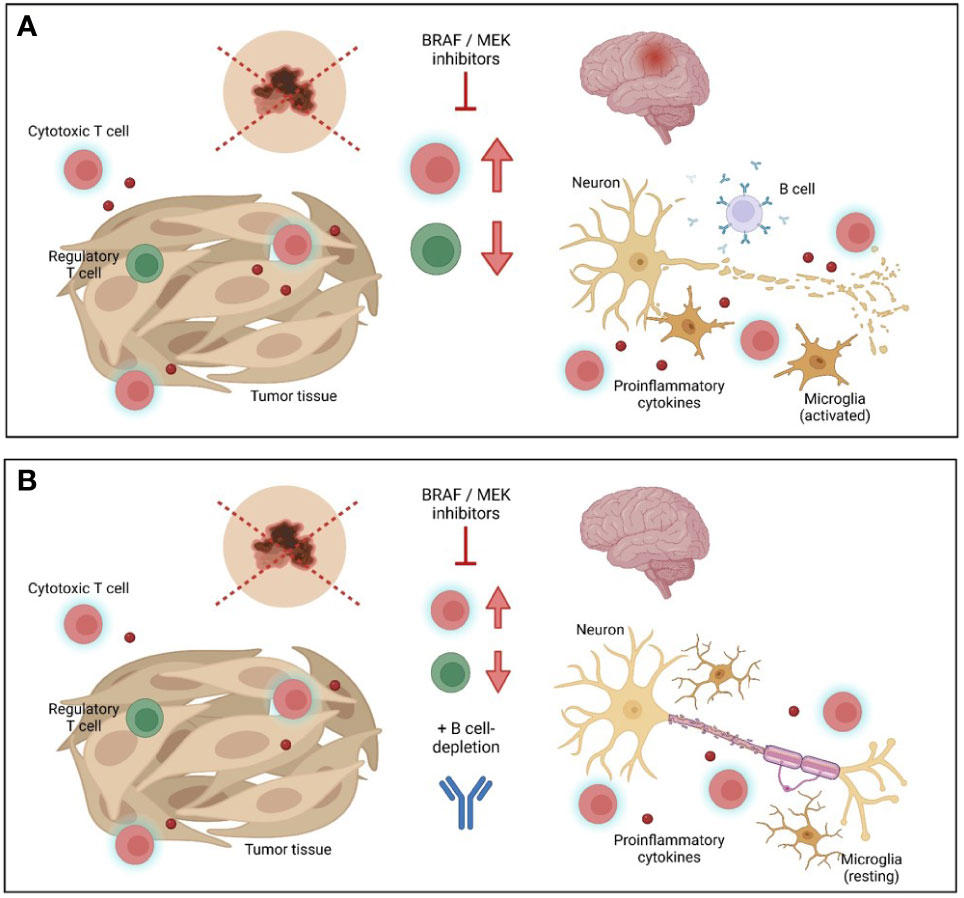
Figure 3 Schematic overview of combined tumor and multiple sclerosis treatment. (A) Inhibition of BRAF/MEK leads to beneficial anti-tumor immune response, but may drive autoimmune inflammation in the central nervous system (CNS) in multiple sclerosis (MS) patients. (B) Additional treatment with the anti-CD20 antibody ocrelizumab might favorably balance detrimental autoimmunity in CNS while preserving anti-tumor immunity. Graphic was created with Biorender.com.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Landesärztekammer Rheinland-Pfalz, ethics vote n° 2020-15206_1, and conducted in accordance with the local legislation and institutional requirements. The participant provided her written informed consent to participate in the study and and consented to publication of the data included in this article.
Author contributions
KP: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft. MP: Conceptualization, Methodology, Writing – review & editing. MS: Conceptualization, Methodology, Writing – review & editing. FS: Formal Analysis, Investigation, Writing – review & editing. FZ: Funding acquisition, Writing – review & editing. SB: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. SB is supported by the Deutsche Forschungsgemeinschaft (DFG, SFB CRC-TR-128 and CRC-TR-355) and the Hermann and Lilly-Schilling Foundation. FZ is supported by the Deutsche Forschungsgemeinschaft (DFG, SFB CRC-TR-128, SFB 1080 and SFB CRC-1292).
Acknowledgments
The authors thank Dr. Cheryl Ernest for proofreading and editing the manuscript. Schematic images were created with Biorender.com.
Conflict of interest
SB has received honoraria from Biogen Idec, Bristol Meyer Squibbs, Hexal, Merck Healthcare, Mylan, Novartis, Roche, Sanofi-Genzyme and TEVA. FZ has recently received research grants and/or consultation funds from Biogen, Ministry of Education and Research (BMBF), Bristol-Meyers-Squibb, Celgene, German Research Foundation (DFG), Janssen, Max-Planck-Society (MPG), Merck Serono, Novartis, Progressive MS Alliance (PMSA), Roche, Sanofi Genzyme, and Sandoz.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANA, antinuclear antibodies; ANCA, anti-neutrophil cytoplasmic antibodies; CNS, central nervous system; CSF, cerebrospinal fluid; FACS, flow cytometry analysis; MS, multiple sclerosis; NfL, neurofilament light chain; NK, natural killer; PBMC, peripheral blood mononuclear cells.
References
1. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature (2002) 417(6892):949–54. doi: 10.1038/nature00766
2. Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med (2012) 367(18):1694–703. doi: 10.1056/NEJMoa1210093
3. Lelliott EJ, McArthur GA, Oliaro J, Sheppard KE. Immunomodulatory effects of BRAF, MEK, and CDK4/6 inhibitors: implications for combining targeted therapy and immune checkpoint blockade for the treatment of melanoma. Front Immunol (2021) 12:661737. doi: 10.3389/fimmu.2021.661737
4. Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res (2012) 18(5):1386–94. doi: 10.1158/1078-0432.CCR-11-2479
5. Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature (2010) 464(7287):427–30. doi: 10.1038/nature08902
6. Koya RC, Mok S, Otte N, Blacketor KJ, Comin-Anduix B, Tumeh PC, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res (2012) 72(16):3928–37. doi: 10.1158/0008-5472.CAN-11-2837
7. Ferrari de Andrade L, Ngiow SF, Stannard K, Rusakiewicz S, Kalimutho M, Khanna KK, et al. Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E-mutant metastatic melanoma. Cancer Res (2014) 74(24):7298–308. doi: 10.1158/0008-5472.CAN-14-1339
8. Wang T, Xiao M, Ge Y, Krepler C, Belser E, Lopez-Coral A, et al. BRAF inhibition stimulates melanoma-associated macrophages to drive tumor growth. Clin Cancer Res (2015) 21(7):1652–64. doi: 10.1158/1078-0432.CCR-14-1554
9. Devic P, Amini-Adle M, Camdessanché JP, Dalle S. Demyelinating polyradiculoneuropathy under combined BRAF/MEK inhibitors. Eur J Cancer (2017) 78:103–4. doi: 10.1016/j.ejca.2017.03.018
10. Rubio-Rivas M, Moreira C, Marcoval J. Sarcoidosis related to checkpoint and BRAF/MEK inhibitors in melanoma. Autoimmun Rev (2020) 19(8):102587. doi: 10.1016/j.autrev.2020.102587
11. Dimou A, Barron G, Merrick DT, Kolfenbach J, Doebele RC. Granulomatosis with polyangiitis in a patient treated with dabrafenib and trametinib for BRAF V600E positive lung adenocarcinoma. BMC Cancer (2020) 20(1):177. doi: 10.1186/s12885-020-6661-6
12. Hemond CC, Bakshi R, Tauhid S, Sarrosa R, Ryan M, Kamath V, et al. Exacerbation of multiple sclerosis by BRAF/MEK treatment for Malignant melanoma: the central vein sign to distinguish demyelinating lesions from metastases. J Investig Med High Impact Case Rep (2021) 9:23247096211033047. doi: 10.1177/23247096211033047
13. Oliveira MCB, de Brito MH, Simabukuro MM. Central nervous system demyelination associated with immune checkpoint inhibitors: review of the literature. Front Neurol (2020) 11:538695. doi: 10.3389/fneur.2020.538695
14. Bar-Or A, Li R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol (2021) 20(6):470–83. doi: 10.1016/S1474-4422(21)00063-6
15. Shinoda K, Li R, Rezk A, Mexhitaj I, Patterson KR, Kakara M, et al. Differential effects of anti-CD20 therapy on CD4 and CD8 T cells and implication of CD20-expressing CD8 T cells in MS disease activity. Proc Natl Acad Sci U S A (2023) 120(3):e2207291120. doi: 10.1073/pnas.2207291120
Keywords: malignant melanoma, BRAF/MEK treatment, multiple sclerosis, B cell depletion, ocrelizumab, multi-color flow cytometry, individualized therapy
Citation: Pape K, Protopapa M, Schraad M, Steffen F, Zipp F and Bittner S (2023) Case Report: Balancing immune responses – multiple sclerosis disease exacerbation under BRAF/MEK treatment for malignant melanoma. Front. Oncol. 13:1303141. doi: 10.3389/fonc.2023.1303141
Received: 27 September 2023; Accepted: 27 October 2023;
Published: 24 November 2023.
Edited by:
Suzie Chen, The State University of New Jersey, United StatesReviewed by:
Jahan S. Khalili, SystImmune Inc, United StatesZeev Elkoshi, Taro Pharmaceutical, Israel
Copyright © 2023 Pape, Protopapa, Schraad, Steffen, Zipp and Bittner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Bittner, Yml0dG5lckB1bmktbWFpbnouZGU=
 Katrin Pape
Katrin Pape Maria Protopapa
Maria Protopapa Muriel Schraad
Muriel Schraad Falk Steffen
Falk Steffen Frauke Zipp
Frauke Zipp Stefan Bittner
Stefan Bittner