- 1Division of Pharmacology, Otto Loewi Research Center, Medical University of Graz, Graz, Austria
- 2BioTechMed, Graz, Austria
Numerous studies in various cancer models have demonstrated that ingredients of cannabis can influence tumor growth through the endocannabinoid system (ECS), a network of molecules (mediators, receptors, transporters, enzymes) that maintains homeostasis and protection in many tissues. The main constituents of the ECS are the classical cannabinoid (CB) receptors, such as CB1 and CB2, their endogenous ligands (endocannabinoids), and the endocannabinoids’ synthesizing and degrading enzymes. The role of the ECS in cancer is still unclear and its effects often depend on the tumor entity and the expression levels of CB receptors. Many studies have highlighted the tumor cell-killing potential of CB1 agonists. However, cannabis is also known as an immunosuppressant and some data suggest that the use of cannabis during immunotherapy worsens treatment outcomes in cancer patients. CB receptors are widely present in immune cells, and together with monoacylglycerol lipase, the 2-arachidonoylglycerol degrading enzyme, they could be critically involved in the regulation of the immune cell profile of the tumor microenvironment (TME), and hence in tumor progression. So far, data on the impact of the ECS in the immune-TME are still vague. In this review, we discuss the current understanding of the ECS on immunoregulation during tumor growth, and how it might affect the outcome of cancer immunotherapy.
Introduction
In ancient medicine, Chinese and Indian cultures widely used cannabis (derived from the plant Cannabis sativa L., mostly known as “marijuana”) for the treatment of various ailments (1). In the 1850s, cannabis was introduced into Western medicine by William B. O’Shaughnessy and Jacques-Joseph Moreau, who described its beneficial effects in treating rheumatism, convulsions, muscular spasms during tetanus and rabies, mental illnesses and cholera (2, 3). At the beginning of the 20th century, the therapeutic use of cannabis considerably declined due to its abuse potential and the variability in the herbal material’s quality. At the same time, its use as a recreational drug increased. Thus, legal restrictions on the use of cannabis were imposed that delayed exploring its medical potential for more than 50 years (4–6). Only after the discovery of cannabinoid (CB) receptors in the early 1990s, attention was payed to this new field of research leading to the introduction of the endocannabinoid system (ECS). Since then, a huge number of studies have been conducted pertaining to the (patho)physiological roles of the ECS (7, 8).
The main constituents of the ECS are the classical CB receptors, CB1 and CB2, their endogenous ligands (endocannabinoids), such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and the endocannabinoids’ synthesizing (diacylglycerol lipase (DAGL), N-acylphosphatidyl-ethanolamine phospholipase D (NAPE-PLD)) and degrading enzymes (fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL, also known as MGL)) (9, 10). CB1 is expressed throughout the body systems, predominantly in the central nervous system (abundant expression), but can be also found in the immune system (low expression) (9, 11). Factors that influence the expression of CB1 in immune cells are the type and activation status of the cells, the immune stimulus, and the presence of endocannabinoids (11, 12). CB2 is highly abundant in cells of the immune system, and its expression level is 10-100-fold higher than that of CB1 (11). B cells show the highest expression of the CB2 mRNA, followed by natural killer (NK) cells, monocytes, neutrophils, CD8+ T and CD4+ T cells (11). Many studies demonstrate that CB1 and CB2 expression may be either increased or decreased under pathological conditions, like in cancer, whereby the level of expression substantially influences tumor progression (13–17). Currently, the main indications for medical cannabis in cancer therapy are for palliative care to manage refractory nausea and pain, and to improve appetite (reviewed in (18)). The synthetic analogue of tetrahydrocannabinol (THC), nabilone (Cesamet), was licensed to suppress nausea and vomiting induced by chemotherapy. Moreover, dronabinol (Marinol), which is a synthetic THC, entered the clinic in 1985 as an anti-emetic, and in 1992 as an appetite stimulant (19).
Importance of the ECS within the tumor microenvironment
The majority of in vitro studies on the ECS and cancer have focused on the direct activation or inhibition of CB receptors expressed in cancer cells, and the subsequent outcome on growth behavior (13–17). However, many questions about the impact of cannabinoids on immune cells in tumors, and hence on tumor progression, still remain unanswered.
Next to malignant cells, tumors consist of a heterogenous group of infiltrated and resident host cells, secreted factors and extracellular matrix, collectively called tumor microenvironment (TME). The composition of the TME changes between tumor entities, but distinctive characteristics of immune and stromal cells, blood vessels, and of the extracellular matrix remain. It is well established that TME cells actively participate in the regulation of cancer progression (20). Adaptive and innate immune cells are important components of the TME that either suppress or promote tumor growth. Within the TME, the adaptive immune response includes actions of T-, B-, and NK cells while the innate immune response works via the actions of myeloid cells like macrophages, neutrophils, eosinophils, dendritic cells (DCs), and other immune cells (20).
Cannabinoids and the ECS: effect on the TME
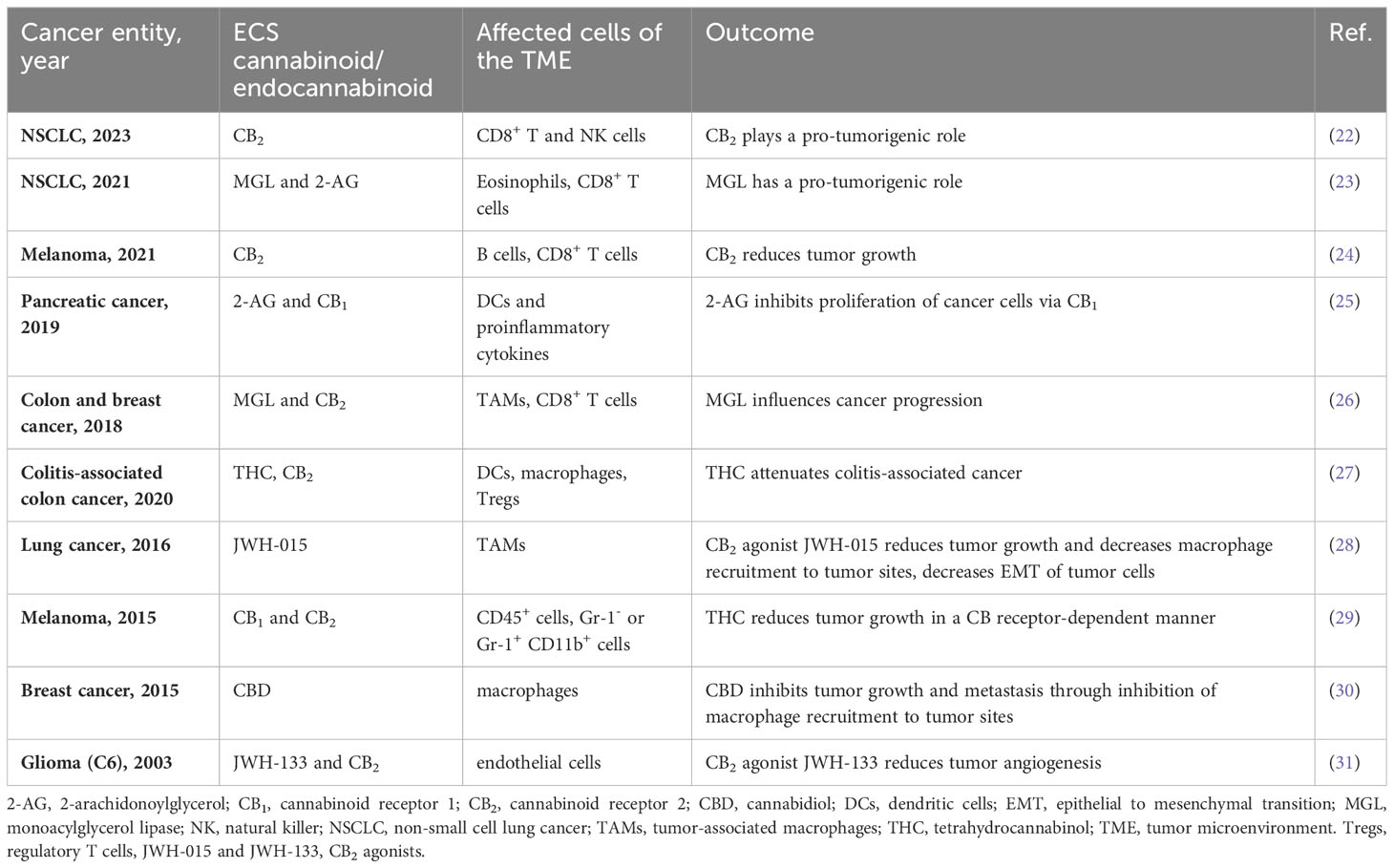
Components of the ECS are virtually expressed in all cells of the tumor mass, including host cells of the TME as well as cancer cells, which makes it challenging to delineate their exact role in tumor development, and to use them as therapeutic targets. To date, only a handful of studies has looked at the influence of endo-/cannabinoids and ECS components on the TME, and their role in tumor development (reviewed in (21)). A summary of these studies and their outcomes are shown in Table 1. Recently, we reported that TME cell-derived CB2 promotes tumor growth in an experimental model of non-small cell lung cancer (NSCLC) by reducing accumulation and cytotoxic activity of CD8+ T and NK cells. We additionally observed, that deficiency of CB2 on host cells enhanced the expression of programmed death-1 (PD-1) and its ligand PD-L1 on lymphoid and myeloid cells, respectively (22). In the same NSCLC model, our group earlier identified that MGL expressed in TME cells was responsible for a pro-tumorigenic environment and modulated the immune cell profile (23). CD8+ T cells were increased in tumors of MGL knockout mice, and showed increased expression of granzyme-B, interferon-γ, and PD-1, signifying enhanced tumoricidal and immune activity (23). Since 2-AG also promotes the migration in these cells (23), MGL could be a major tumor driver in NSCLC. In a pancreatic cancer model, Qiu et al. showed that proliferation of tumors was compromised in the presence of 2-AG via activation of CB1, but not of CB2 (25). In this type of cancer, 2-AG promoted the maturation of DC phenotypes and the production of pro-inflammatory cytokines through up-regulation of the signal transducer and activator of transcription 6 (p-STAT6) (25). In a different study, THC had no direct effect on the growth of melanoma cells, but indirectly affected tumor growth via cells of the TME in a CB receptor-dependent manner (29). In particular, the infiltration of pro-tumorigenic myeloid immune cells was inhibited by THC, demonstrating that the ECS can interact with the TME in modulating tumor growth (29). In contrast to these studies, Xiang et al. demonstrated that MGL in macrophages inhibited CB2 receptor-dependent tumor progression. They described that MGL deficiency enhanced macrophage activation in a CB2/TLR4-dependent manner affecting the exhaustion status of CD8+ T cells in models of colon and breast cancer (26). In murine melanoma, absence of CB2 increased tumor growth and accumulation of immature B cells, reducing infiltration of CD8+ T cells into the TME (24).
Altogether, these reports suggest that components of the ECS like MGL and CB2 are able to affect tumor progression via immune cell activity and immune checkpoints of the TME. However, pro- or anti-tumorigenic effects of the ECS are likely dependent on the type of cancer.
Immunotherapy and the ECS
CD8+ T cells work together with other immune cells of the TME to eradicate tumors. However, at some point during tumor development, immune cells, mainly cytotoxic CD8+ T cells, start losing their cytotoxic ability. Fortunately, targeted therapy has revolutionized the approach to treat cancer through the introduction of immunotherapy, which removes the brake from immune cells to eliminate tumors. Active (vaccines) and passive (monoclonal antibodies and adoptive cell therapy) therapies represent two subcategories of immunotherapy (32). Here, we briefly discuss how the ECS may interfere with the response to treatment with monoclonal antibodies, known as immune checkpoint inhibitors (ICIs).
In various types of tumors, the ECS has been shown to influence the immune cell composition of the TME and to subsequently affect immune cell activity and tumor growth (22–26, 29, 32). The activation and exhaustion status of immune cells, particularly of cytotoxic CD8+ T cells, are determined by the expression of immune checkpoint proteins, such as PD-1, cytotoxic T-lymphocyte antigen-4 (CTLA-4, aka CD152), and others like T cell immunoglobulin and ITIM domain (TIGIT), T-cell immunoglobulin and mucin-domain containing 3 (TIM-3), lymphocyte-activation gene 3 (LAG-3), and B- and T-lymphocyte attenuator (BTLA) (33). The main targets of current ICI therapies are PD-1/PD-L1 and the CTLA-4 receptor (34, 35). In 2011, a CTLA-4 blocking antibody (ipilimumab) was approved to treat melanoma. This was followed by the development of monoclonal antibodies targeting PD-1 (pembrolizumab and nivolumab) and PD-L1 (atezolizumab, durvalumab and avelumab). Furthermore, biomarkers to predict the efficacy of ICIs such as PD-L1 levels in tumor tissue, tumor mutation burden (TMB) and microsatellite instability (MSI) were introduced (36). PD-1/PD-L1 blocking antibodies have become standard treatment of various cancer types, including melanoma, NSCLC, mismatch repair-deficient and/or microsatellite-instable colorectal cancer, triple-negative breast cancer, head and neck squamous cell carcinoma, hepatocellular carcinoma, and several other cancers (37). The rate for effective treatment with anti-PD-1/PD-L1 antibodies varies with the type of tumor and lies around 80% in lymphoma, 60% in high MSI cancers, and approximately 10-30% in other common solid tumors (38). Owing to their reduced toxicity and their lower high-grade immune-related adverse events (irAEs), anti-PD-1/PD-L1 antibodies are used more often than anti-CTLA-4 antibodies (39–41).
Apart of the biomarkers that predict responsiveness to ICI therapy (36), other factors can cause resistance to immunotherapy, which must be taken into consideration before and during therapy. These include genomic factors, tumor heterogeneity, factors related to immune cells and the TME, factors emerging from host cell-cancer cell interactions, and other vital factors, such as advanced age, biological sex, diet, hormones, existing comorbidities, drugs, and the gut microbiome (42).
Influence of cannabinoids and the ECS on immunotherapy
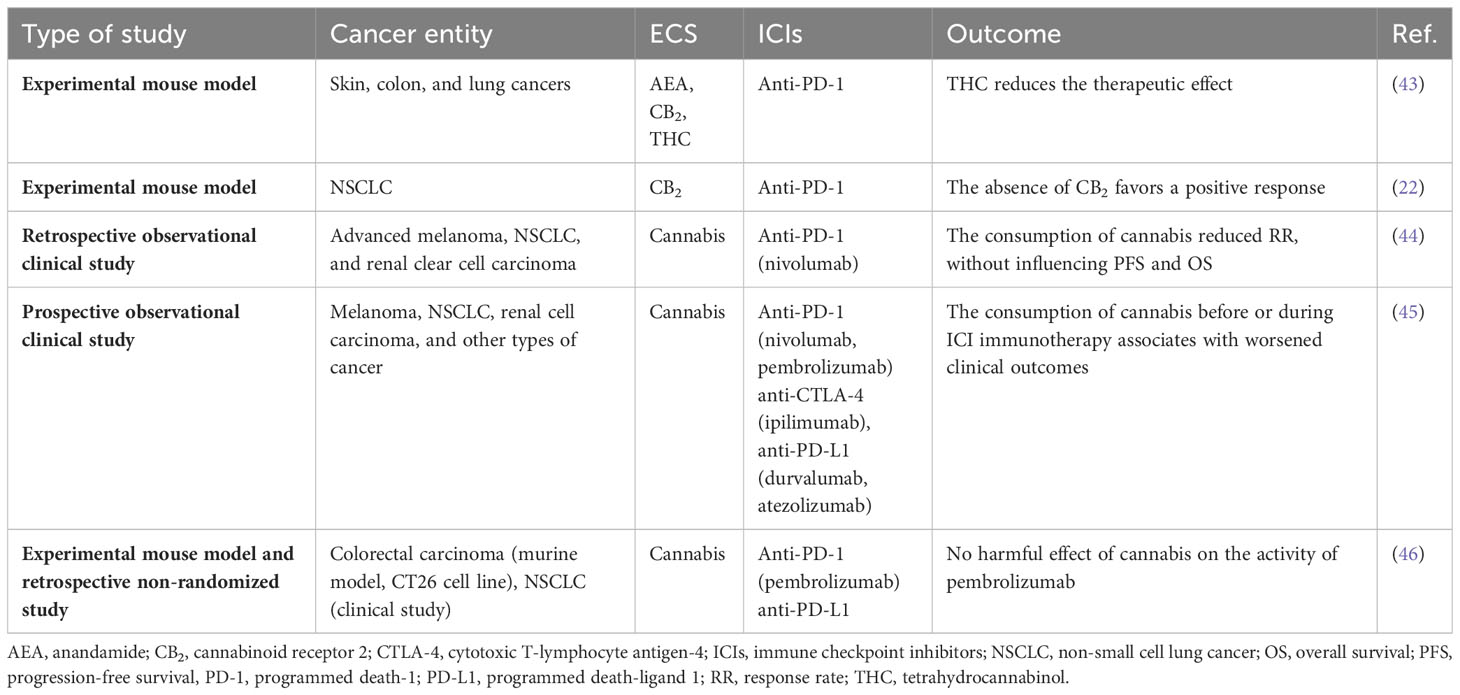
Immuno-oncologic studies aim to find ways to circumvent the development of resistance to immunotherapy and to determine adjuvant targets to increase patient survival. Because of its immunomodulatory effects, the ECS may hold such targets, but up to now, only few experimental or clinical studies have investigated the role of the ECS on the immune-TME and the impact of cannabis or cannabinoids in immunotherapy (shown in Table 2). Xiong and colleagues identified that exogenous (THC) as well as endogenous (AEA) cannabinoids negatively affect antitumor immunity by impairing the function of tumor-specific T cells via CB2. They also observed that THC reduces the therapeutic effect of anti-PD-1 therapy (43). In our NSCLC model, the response to anti-PD-1 treatment was improved in CB2 knockout mice suggesting that CB2 may act as an immunosuppressor in NSCLC. Additionally, anti-PD-1 antibody treatment increased the accumulation of cytotoxic immune cells (CD8+ T and NK cells) in CB2 knockout mice, suggesting that the presence of CB2 in the TME interferes with the response to PD-1/PD-L1 blockade (22). Targeting CB2 during immunotherapy could, therefore, have an additional benefit for NSCLC patients, and increase the response rate (22).
Our experimental data largely fit with a clinical study, in which Taha et al. retrospectively demonstrated the effect of cannabis during immunotherapy (nivolumab) and evaluated the response rates (RR), progression-free survival (PFS), and overall survival (OS) of cancer patients. The group found that cannabis use during nivolumab therapy reduced RR without affecting PFS or OS (44). In a recent prospective observational clinical study, Bar-Sela et al. enrolled 102 advanced cancer patients (68 cannabis non-users and 34 cannabis users) who started ICI treatment. The findings showed that cannabis users had a significant decrease in time to treatment progression (TTP) and OS vs. cannabis non-users. The consumption of cannabis, however, reduced irAEs. The authors suggested to use cannabis with attentiveness before and during ICI immunotherapy of advanced malignances (45, 47). In contrast, a recent study by Waissengrin et al. described that concomitant use of medical cannabis with pembrolizumab had no harmful effect in advanced NSCLC. TTP of cannabis users did not differ from cannabis non-users. However, the median OS was numerically higher for cannabis non-users vs. cannabis users, but did not reach statistical difference (p = 0.08) (46).
Low response to ICI during cannabis use may be, therefore, linked to the fact that cannabinoids exert immunosuppressive effects (48). These effects may involve CB2 activity in immune or other TME cells.
Discussion
Inflammation promotes tumorigenesis, but an active immune system is also a powerful weapon to combat tumor development. The fact that cells of the immune system are present in a shared microenvironment with cancer cells has been known since the 19th century (49). However, not until recently, immune cells began to be used as tools against cancer, as Jennifer Couzin-Frankel wrote in Science magazine: “Immunotherapy marks an entirely different way of treating cancer- by targeting the immune system, not the tumor itself” (50). Today, many effective antibodies against checkpoint proteins are already at hand for antitumor treatment (37).
Downsides of immunotherapy include the development of resistance and a low responder rate (like in NSCLC) (51). Molecules other than checkpoint proteins like CB2 and MGL may, therefore, act as targets to aid the response to immunotherapy (22, 24–26, 29). CB2 is abundantly expressed in immune cells (52) and upon activation, it has the ability to influence immune functions, such as cytokine production, proliferation/differentiation, migration, apoptosis, and antibody production (reviewed in (12, 53). These functions are all amenable to CB2 receptor agonism/antagonism. Also MGL has been implicated as a driver in many types of tumors (23, 54, 55). A MGL inhibitor (ABX-1431) (56) has been already tested in the clinics (clinicaltrials.gov: NCT03625453). However, tumor type, the TME, or both seem to influence the behavior of CB2 and MGL towards tumors. This introduces an obstacle to the universal use of CB2 or MGL blockers for the treatment of cancer without prior knowledge of the ECS component’s role. Genetic modification of CB receptors in immune cells and adoptive cell therapy (e.g., CD8+ T and NK cells, known to express CB receptors (21)) could be one possible way of application. Investigating other members of the ECS, like DAGL, the 2-AG producing enzyme, and proteins involved in CB2 receptor signaling pathways would also be a step towards identifying new targets that could support ICI treatment.
It is known that co-medications such as proton pump inhibitors, glucocorticoids, antibiotics, psychotropic drugs and opioids must be carefully assessed at the time of ICI treatment initiation, as they may significantly change the antitumoral response (57–59) Likewise, the use of medical cannabis or cannabis-derived products during immunotherapy should be reconsidered as they might interfere with the ICIs’ mechanism. As cannabis use is widespread in cancer patients, ICI treatment may be unsuccessful if cannabis consumption is continued.
Further efforts are required to unravel the exact role of the ECS within the TME and to understand the importance of the ECS in tumor development and immunotherapy.
Author contributions
AS: Writing – original draft, Writing – review & editing. RS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Austrian Science Fund (FWF; grants P33325 and KLI887).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Touw M. The religious and medicinal uses of cannabis in China, India and Tibet. J Psychoactive Drugs (1981) 13(1):23–34. doi: 10.1080/02791072.1981.10471447
3. Moreau JJ. Du Hachisch et de l’Alienation Mentale: Etudes Psychologiques. Paris: Librarie de Fortin Mason. English Ed. New York: Raven Press (1972). p. 1845.
4. Zuardi AW. History of cannabis as a medicine: A review. Braz J Psychiatry (2006) 28(2):153–7. doi: 10.1590/S1516-44462006000200015
5. Robinson SM, Adinoff B. The classification of substance use disorders: Historical, contextual, and conceptual considerations. Behav Sci (Basel) (2016) 6(3):18. doi: 10.3390/bs6030018
6. Dariš B, Verboten MT, Knez Ž, Ferk P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn J Basic Med Sci (2019) 19(1):14–23. doi: 10.17305/bjbms.2018.3532
7. Birdsall SM, Birdsall TC, Tims LA. The use of medical marijuana in cancer. Curr Oncol Rep (2016) 18(7):40. doi: 10.1007/s11912-016-0530-0
8. Davis MP. Cannabinoids for symptom management and cancer therapy: the evidence. J Natl Compr Canc Netw (2016) 14(7):915–22. doi: 10.6004/jnccn.2016.0094
9. Lu HC, Mackie K. Review of the endocannabinoid system. Biol Psychiatry Cognit Neurosci Neuroimaging (2021) 6(6):607–15. doi: 10.1016/j.bpsc.2020.07.016
10. Cristino L, Bisogno T, Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol (2020) 16(1):9–29. doi: 10.1038/s41582-019-0284-z
11. Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem (1995) 232(1):54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x
12. Kaplan BLF. The role of CB1 in immune modulation by cannabinoids. Pharmacol Ther (2013) 137(3):365–74. doi: 10.1016/j.pharmthera.2012.12.004
13. Laezza C, Pagano C, Navarra G, Pastorino O, Proto MC, Fiore D, et al. The endocannabinoid system: A target for cancer treatment. Int J Mol Sci (2020) 21(3):747. doi: 10.3390/ijms21030747
14. Ramer R, Wittig F, Hinz B. The endocannabinoid system as a pharmacological target for new cancer therapies. Cancers (Basel) (2021) 13(22):5701. doi: 10.3390/cancers13225701
15. Cherkasova V, Wang B, Gerasymchuk M, Fiselier A, Kovalchuk O, Kovalchuk I. Use of cannabis and cannabinoids for treatment of cancer. Cancers (Basel) (2022) 14(20):5142. doi: 10.3390/cancers14205142
16. Heider CG, Itenberg SA, Rao J, Ma H, Wu X. Mechanisms of cannabidiol (CBD) in cancer treatment: A review. Biol (Basel) (2022) 11(6):817. doi: 10.3390/biology11060817
17. O’Brien K. Cannabidiol (CBD) in cancer management. Cancers (Basel) (2022) 14(4):885. doi: 10.3390/cancers14040885
18. Vinette B, Côté J, El-Akhras A, Mrad H, Chicoine G, Bilodeau K. Routes of administration, reasons for use, and approved indications of medical cannabis in oncology: a scoping review. BMC Cancer (2022) 22(1):319. doi: 10.1186/s12885-022-09378-7
19. Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol (2009) 156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x
20. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol (2020) 30(16):R921–5. doi: 10.1016/j.cub.2020.06.081
21. Sheik A, Farani MR, Kim E, Kim S, Gupta VK, Kumar K, et al. Therapeutic targeting of the tumor microenvironments with cannabinoids and their analogs: Update on clinical trials. Environ Res (2023) 231(Pt 1):115862. doi: 10.1016/j.envres.2023.115862
22. Sarsembayeva A, Kienzl M, Gruden E, Ristic D, Maitz K, Valadez-Cosmes P, et al. Cannabinoid receptor 2 plays a pro-tumorigenic role in non-small cell lung cancer by limiting anti-tumor activity of CD8+ T and NK cells. Front Immunol (2023) 13:997115. doi: 10.3389/fimmu.2022.997115
23. Kienzl M, Hasenoehrl C, Maitz K, Sarsembayeva A, Taschler U, Valadez-Cosmes P, et al. Monoacylglycerol lipase deficiency in the tumor microenvironment slows tumor growth in non-small cell lung cancer. Oncoimmunology (2021) 10(1):e1965319. doi: 10.1080/2162402X.2021.1965319
24. Gruber T, Robatel S, Kremenovic M, Bäriswyl L, Gertsch J, Schenk M. Cannabinoid receptor type-2 in B cells is associated with tumor immunity in melanoma. Cancers (Basel) (2021) 13(8):1934. doi: 10.3390/cancers13081934
25. Qiu C, Yang L, Wang B, Cui L, Li C, Zhuo Y, et al. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. BioMed Pharmacother (2019) 115:108952. doi: 10.1016/j.biopha.2019.108952
26. Xiang W, Shi R, Kang X, Zhang X, Chen P, Zhang L, et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat Commun (2018) 9(1):2574. doi: 10.1038/s41467-018-04999-8
27. Becker W, Alrafas HR, Wilson K, Miranda K, Culpepper C, Chatzistamou I, et al. Activation of cannabinoid receptor 2 prevents colitis-associated colon cancer through myeloid cell de-activation upstream of IL-22 production. iScience (2020) 23(9):101504. doi: 10.1016/j.isci.2020.101504
28. Ravi J, Elbaz M, Wani NA, Nasser MW, Ganju RK. Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol Carcinog (2016) 55(12):2063–76. doi: 10.1002/mc.22451
29. Glodde N, Jakobs M, Bald T, Tüting T, Gaffal E. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci (2015) 138:35–40. doi: 10.1016/j.lfs.2015.04.003
30. Elbaz M, Nasser MW, Ravi J, Wani NA, Ahirwar DK, Zhao H, et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol Oncol (2015) 9(4):906–19. doi: 10.1016/j.molonc.2014.12.010
31. Blázquez C, Casanova ML, Planas A, Gómez Del Pulgar T, Villanueva C, Fernández-Aceñero MJ, et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J (2003) 17(3):529–31. doi: 10.1096/fj.02-0795fje
32. Zaiachuk M, Pryimak N, Kovalchuk O, Kovalchuk I. Cannabinoids, medical cannabis, and colorectal cancer immunotherapy. Front Med (2021) 8:713153. doi: 10.3389/fmed.2021.713153
33. Anderson AC, Joller N, Kuchroo VK. Lag-3, tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity (2016) 44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001
34. Zhao Y, Yang W, Huang Y, Cui R, Li X, Li B. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell Physiol Biochem (2018) 47(2):721–34. doi: 10.1159/000490025
35. Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev (2008) 224(1):166–82. doi: 10.1111/j.1600-065X.2008.00662.x
36. Buder-Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-A review from the melanoma perspective and beyond. Front Immunol (2018) 9:1474. doi: 10.3389/fimmu.2018.01474
37. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun (2020) 11(1):3801. doi: 10.1038/s41467-020-17670-y
38. Yu Y. Molecular classification and precision therapy of cancer: immune checkpoint inhibitors. Front Med (2018) 12(2):229–35. doi: 10.1007/s11684-017-0581-0
39. Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol (2016) 27(4):559–74. doi: 10.1093/annonc/mdv623
40. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol (2022) 33(12):1217–38. doi: 10.1016/j.annonc.2022.10.001
41. Oiseth SJ, Aziz MS. Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J Cancer Metastasis Treat (2017) 3(10):250. doi: 10.20517/2394-4722.2017.41
42. Dobosz P, Stępień M, Golke A, Dzieciątkowski T. Challenges of the immunotherapy: perspectives and limitations of the immune checkpoint inhibitor treatment. Int J Mol Sci (2022) 23(5):2847. doi: 10.3390/ijms23052847
43. Xiong X, Chen S, Shen J, You H, Yang H, Yan C, et al. Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal Transduct Target Ther (2022) 7(1):99. doi: 10.1038/s41392-022-00918-y
44. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist (2019) 24(4):549–54. doi: 10.1634/theoncologist.2018-0383
45. Bar-Sela G, Cohen I, Campisi-Pinto S, Lewitus GM, Oz-Ari L, Jehassi A, et al. Cannabis consumption used by cancer patients during immunotherapy correlates with poor clinical outcome. Cancers (Basel) (2020) 12(9):2447. doi: 10.3390/cancers12092447
46. Waissengrin B, Leshem Y, Taya M, Meiri D, Merimsky O, Shamai S, et al. The use of medical cannabis concomitantly with immune checkpoint inhibitors in non-small cell lung cancer: A sigh of relief? Eur J Cancer (2023) 180:52–61. doi: 10.1016/j.ejca.2022.11.022
47. Abu-Amna M, Salti T, Khoury M, Cohen I, Bar-Sela G. Medical cannabis in oncology: a valuable unappreciated remedy or an undesirable risk? Curr Treat Options Oncol (2021) 22(2):16. doi: 10.1007/s11864-020-00811-2
48. Eisenstein TK, Meissler JJ. Effects of cannabinoids on T-cell function and resistance to infection. J Neuroimmune Pharmacol Springer New York LLC; (2015) 10:204–16. doi: 10.1007/s11481-015-9603-3
49. Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol (2020) 20(8):483–97. doi: 10.1038/s41577-019-0271-z
50. Couzin-Frankel J. Breakthrough of the year 2013. Cancer Immunother Sci (2013) 342(6165):1432–3. doi: 10.1126/science.342.6165.1432
51. Onoi K, Chihara Y, Uchino J, Shimamoto T, Morimoto Y, Iwasaku M, et al. Immune checkpoint inhibitors for lung cancer treatment: A review. J Clin Med (2020) 9(5):1362. doi: 10.3390/jcm9051362
52. Grill M, Hasenoehrl C, Kienzl M, Kargl J, Schicho R. Cellular localization and regulation of receptors and enzymes of the endocannabinoid system in intestinal and systemic inflammation. Histochem Cell Biol (2019) 151(1):5–20. doi: 10.1007/s00418-018-1719-0
53. Basu S, Dittel BN. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol Res (2011) 51(1):26–38. doi: 10.1007/s12026-011-8210-5
54. Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell (2010) 140(1):49–61. doi: 10.1016/j.cell.2009.11.027
55. Grabner GF, Zimmermann R, Schicho R, Taschler U. Monoglyceride lipase as a drug target: At the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther (2017) 175:35–46. doi: 10.1016/j.pharmthera.2017.02.033
56. Cisar JS, Weber OD, Clapper JR, Blankman JL, Henry CL, Simon GM, et al. Identification of ABX-1431, a selective inhibitor of monoacylglycerol lipase and clinical candidate for treatment of neurological disorders. J Med Chem (2018) 61(20):9062–84. doi: 10.1021/acs.jmedchem.8b00951
57. Kostine M, Mauric E, Tison A, Barnetche T, Barre A, Nikolski M, et al. Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur J Cancer (2021) 157:474–84. doi: 10.1016/j.ejca.2021.08.036
58. Cani M, Bironzo P, Garetto F, Buffoni L, Cotogni P. Immune checkpoint inhibitors and opioids in patients with solid tumours: is their association safe? A systematic literature review. Healthcare (2022) 11(1):116. doi: 10.3390/healthcare11010116
Keywords: cannabis, cannabinoid receptors, tumor microenvironment, endocannabinoid system, immune checkpoint inhibitor
Citation: Sarsembayeva A and Schicho R (2023) Cannabinoids and the endocannabinoid system in immunotherapy: helpful or harmful? Front. Oncol. 13:1296906. doi: 10.3389/fonc.2023.1296906
Received: 19 September 2023; Accepted: 06 November 2023;
Published: 22 November 2023.
Edited by:
Simona Kranjc Brezar, Senior Research Associate, Ljubljana, SloveniaReviewed by:
Pankaj Gaur, Georgetown University, United StatesCopyright © 2023 Sarsembayeva and Schicho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rudolf Schicho, cnVkb2xmLnNjaGljaG9AbWVkdW5pZ3Jhei5hdA==
†ORCID: Arailym Sarsembayeva, orcid.org/0000-0001-9793-0789
Rudolf Schicho, orcid.org/0000-0002-5726-4731
 Arailym Sarsembayeva
Arailym Sarsembayeva Rudolf Schicho
Rudolf Schicho