- 1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Radiation Oncology, Peking University Cancer Hospital and Institute, Beijing, China
- 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Endoscopy Center, Peking University Cancer Hospital and Institute, Beijing, China
- 3State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers, Beijing Key Laboratory of Carcinogenesis and Translational Research, Department of Radiation Oncology, Peking University Cancer Hospital and Institute, Beijing, China
Background: The aim of this article was to establish the clinical prognostic models and identify the predictive radiation dosimetric parameters for thrombocytopenia during concurrent chemoradiotherapy for rectal cancer.
Methods: In this retrospective cohort study, patients with rectal adenocarcinoma undergoing concurrent long-term chemoradiotherapy were included. The primary outcome of interest was grade 2 or higher (2+) thrombocytopenia (platelet(PLT) count <75,000/μL). Secondary outcomes included: grade 1 or higher thrombocytopenia (PLT count<100,000/μL) and the PLT count during chemoradiotherapy and its nadir. The risk prediction model was developed by logistic regression to identify clinical predictors of 2+ thrombocytopenia. Univariate linear regression models were used to test correlations between radiation dosimetric parameters and the absolute PLT count at nadirs.
Results: This retrospective cohort comprised 238 patients. Fifty-four (22.6%) patients developed thrombocytopenia during concurrent chemoradiotherapy, while 15 (6.3%) patients developed 2+ thrombocytopenia. Four independently associated risk factors, including age, Alb level, PLT count, and chemotherapy regimen, were included in the final model and used to form a 2+ thrombocytopenia probability estimation nomogram. The C‐index was 0.87 (95% CI: 0.78–0.96). The calibration plot showed a moderate agreement, and the Brier score was 0.047 (95% CI: 0.025–0.070). The total absolute volume of bone marrow irradiated by 5 Gy, 10 Gy and 15 Gy of radiation (BM-V5ab, BM-V10ab, BM-V15ab), calculated by the volume of bone marrow multiplied by the corresponding Vx, were identified as new predictors. The nadir of PLT was found to be negatively correlated with BM-V5ab (β = -0.062, P =0.030), BM-V10ab (β = -0.065, P =0.030) and BM-V15ab (β = -0.064, P =0.042).
Conclusion: The occurrence of 2+ thrombocytopenia during concurrent chemoradiotherapy for rectal cancer can be predicted by the patient’s baseline status and chemoradiotherapy regimen, and low dose irradiation of bone marrow can affect the level of platelets during the treatment.
Introduction
Concurrent chemoradiotherapy (CRT) is the standard of care for locally advanced and resectable metastatic rectal cancer. Both radiotherapy and chemotherapy are myelosuppressive. It is known that the pelvic bones, sacrum and lumbar vertebrae, which are located in the irradiated region in rectal cancer, play an important role in hematopoiesis (1). Previous studies have demonstrated that the dose-volume parameters of the pelvic bone marrow (BM) may predict the hematologic toxicity of rectal cancer (2–5). When chemotherapy is prescribed concurrently, the risk of acute hematologic toxicity (HT) increases due to additional BM injury.
Different types of HT pose different risks to the patients, and have different effects on treatment. Among them, cancer therapy-induced thrombocytopenia (CTIT) represents a troublesome toxicity. Platelets are a scarce resource, and recovery of platelets by TPO or IL-11 takes a long time (6, 7). Therefore, once thrombocytopenia occurs, it may cause chemotherapy and radiotherapy dose reduction or treatment delays that are more severe than those related to other types of HT. Treatment breaks may ultimately result in inferior oncological outcomes. In addition, CRT is not the end of rectal cancer treatment. Neoadjuvant chemoradiotherapy has lasting effects on the bone marrow, which are demonstrable during adjuvant chemotherapy, and patients experience more severe thrombocytopenia in adjuvant chemotherapy (8, 9). In addition, under total neoadjuvant therapy (TNT) treatment strategy, thrombocytopenia occurring during concurrent chemoradiotherapy may result in delayed or missed chemotherapy cycles and treatment breaks in consolidation chemotherapy, which follows closely. The incidence rate of thrombocytopenia is also greater in consolidation chemotherapy than in CRT (10).
We assumed that a better understanding of thrombocytopenia would be conducive to its prevention and management. The aim of this article was to analyze the incidence, pattern and risk factors (including clinical factors, radiation dose and target volumes) for thrombocytopenia during CRT in patients with rectal cancer, based on retrospective data from our institution.
Methods
Patient population
For this retrospective cohort study, consecutive patients undergoing concurrent long-term chemoradiotherapy with a capecitabine and oxaliplatin (XELOX) regimen for histologically confirmed rectal adenocarcinoma at Beijing Cancer Hospital between Apr 1, 2015, and Nov 30, 2021, were included. Other inclusion criteria were as follows: tumor located <12 cm from the anal verge; T2-4 N0-2, M0 or resectable M1 according to the 8th edition of the American Joint Committee on Cancer TNM Classification; and age ≥ 18 and ≤ 75 years. The exclusion criteria were as follows: diagnosis of double primary cancer, history of any other tumor, previous radiation, or low baseline platelet(PLT) count (<100,000/μL).
Radiotherapy planning and chemotherapy regimen
Patients received radiotherapy planning computed tomography (CT) and pelvic magnetic resonance (MR) scanning with 5 mm slice thickness. The MR images were subsequently fused with the CT images. Patients were treated in the prone position with a full bladder and an empty rectum Simultaneous integrated boost intensity-modulated radiation therapy (SIB-IMRT) was performed. The gross tumor volume (GTV) was defined as the primary tumor and metastatic lymph nodes. The clinical target volume (CTV) included: mesorectal and presacral regions, internal iliac and obturator lymph node drainage areas, ≥2 cm margins from the cephalic and caudal extents of the primary lesion in the rectum and 1-2 cm margins around all identified areas of invasion. The IMRT regimens included: a total dose of 50.6 Gy (GTV)/41.8 Gy (CTV) in 22 fractions, or a total dose of 50 Gy (GTV)/45 Gy (CTV) in 25 fractions. This schedule was described in our previous work (11–14).
The concurrent chemotherapy regimen was daily capecitabine (1650 mg/m2/day, orally twice daily during the RT course) and oxaliplatin. The dosage of oxaliplatin (50 mg/m2/qw, 85 mg/m2/q2w, or 100-130 mg/m2/q3w) was prescribed.
Bone marrow delineation and dose calculation
To quantify the volume of pelvic bone marrow irradiated in the IMRT plans, the medullary space within the pelvic bones and lumbar spines was contoured. BM delineation was performed at the eclipse planning station. The window was adjusted to the bone range to contour the low-density region inside the bone. The pelvic BM was delineated as the inner cavity of bone from the top of the L4 vertebra to the bottom of the ischial tuberosity, using previously described methods (Figure 1). The total volume of PTV (VPTV), total volume of BM (VBM), mean dose of BM (BM-Dmean), and volume of BM receiving different radiation dose from 5 Gy to 40 Gy (BM-V5 to BM-V40) were estimated (recorded as percentages). Additionally, the total absolute volume of bone marrow irradiated by different doses was defined as BM-V5ab to BM-V40ab (recorded as absolute volume), calculated by: VBM multiplied by BM-V5 to BM-V40.
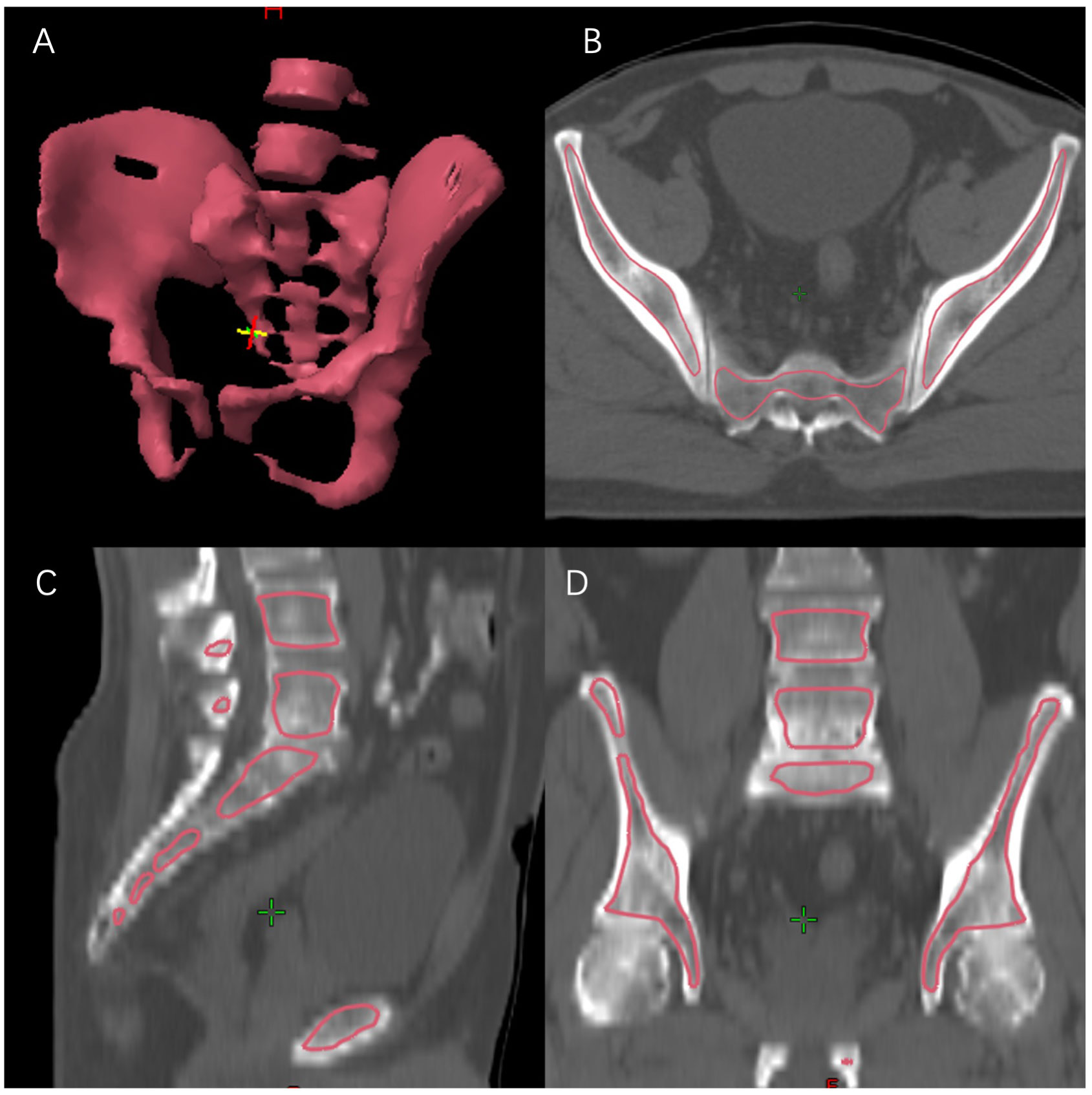
Figure 1 Bone marrow delineation. (A) 3D reconstruction; (B) transverse section; (C) sagittal section; (D) coronal section.
Study endpoints and safety assessment
The primary outcome of interest was the grade 2 or higher (2+) thrombocytopenia (PLT<75,000/μL) during chemoradiotherapy and within 14 days of the end of the last treatment, according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Secondary outcomes included: grade 1 of higher thrombocytopenia (PLT<100,000/μL), PLT count during radiotherapy and its nadir, effects of thrombocytopenia on chemotherapy dose and dose delays, and radiotherapy delays and reduction. Patients underwent complete blood counts at baseline and weekly during chemoradiotherapy. Liver and renal functions were assessed at least every 2 weeks. Hematologic toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events.
Statistical analyses
Multivariate logistic regression analysis was performed to identify predictors of 2+ thrombocytopenia. All eligible variables with univariable P values <0.1 were incorporated into a multivariate regression model and sequentially removed using backward elimination techniques.
The risk prediction model of thrombocytopenia was developed by logistic regression. A final model selection was performed by a backward stepwise selection process with the Akaike information criterion (AIC). The model was presented as a nomogram. We assessed nomogram model performance by examining overall accuracy (Brier score), calibration (calibration plots and Hosmer–Lemeshow calibration test), and discrimination (Harrell C index and its 95% CI).
Correlations between radiation dosimetric parameters and absolute PLT counts at the nadirs was tested by univariate linear regression models. P<0.05 was defined as statistically significant. Statistical analyses were performed with SPSS Statistics software (version 24, IBM Corp., Armonk, NY) and R software (version 4.2.2).
Results
Clinical characteristics
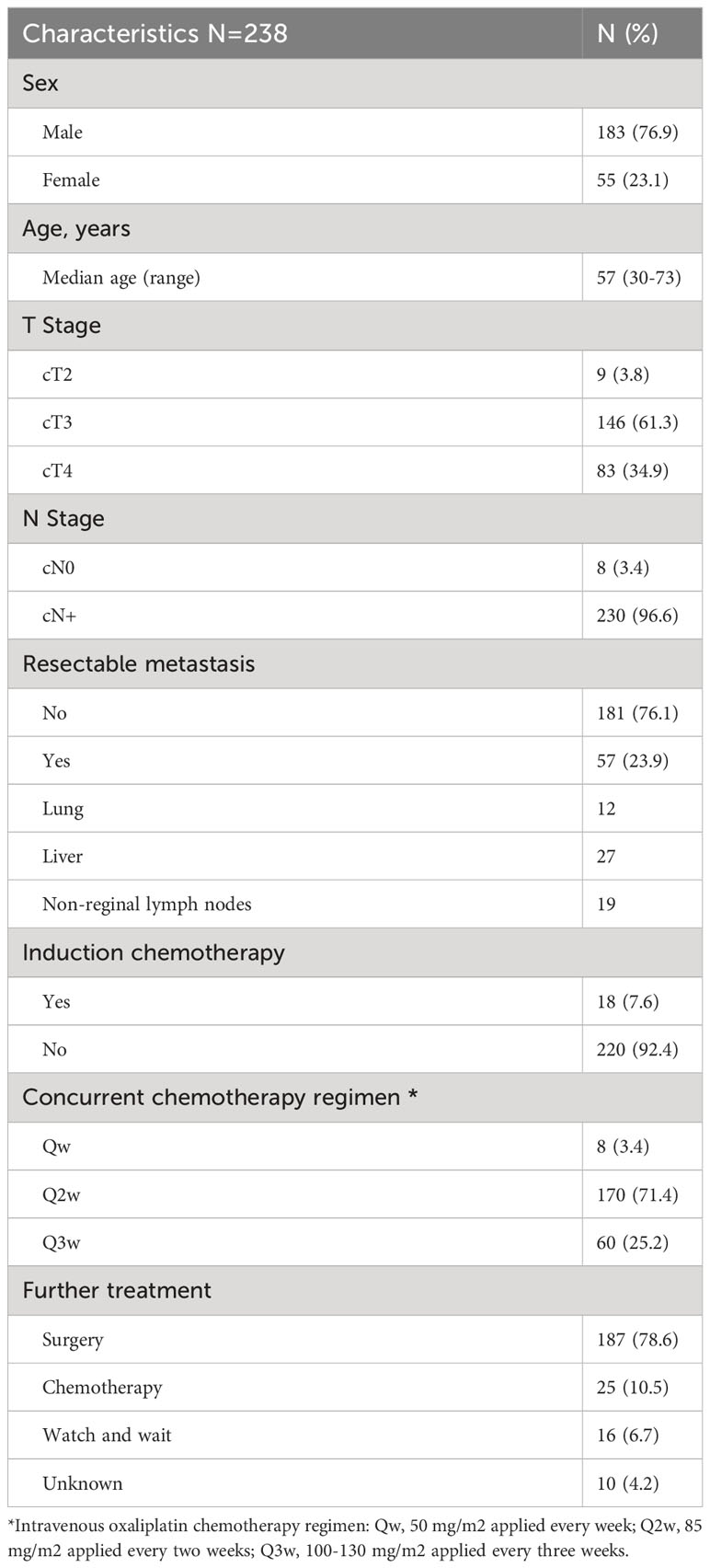
This retrospective cohort comprised 238 patients. Table 1 shows the baseline patient and clinical characteristics. The median age of the cohort was 57 (range, 30-73) years. Two hundred and twenty-nine (96.6%) patients had stage T3-4 disease, and 230 (96.6%) patients had node-positive disease. Eighteen (7.6%) patients received induction chemotherapy. All patients received concurrent chemotherapy with different regimens, and the dosing frequency of oxaliplatin was decided by the physician. Most patients (71.4%) received intravenous oxaliplatin every two weeks.
Incidence of thrombocytopenia and dynamic changes in PLT count during CRT
Fifty-four (22.7%) patients developed thrombocytopenia during CRT, while 15 (6.3%) patients developed grade 2+ thrombocytopenia. Among the patients who developed thrombocytopenia (N=54), treatment was affected by the disease in 17 (31.5%) patients, including radiotherapy (N=5) or chemotherapy interruption (N=10), chemotherapy delay (N=6), and radiotherapy dose reduction (N=1).
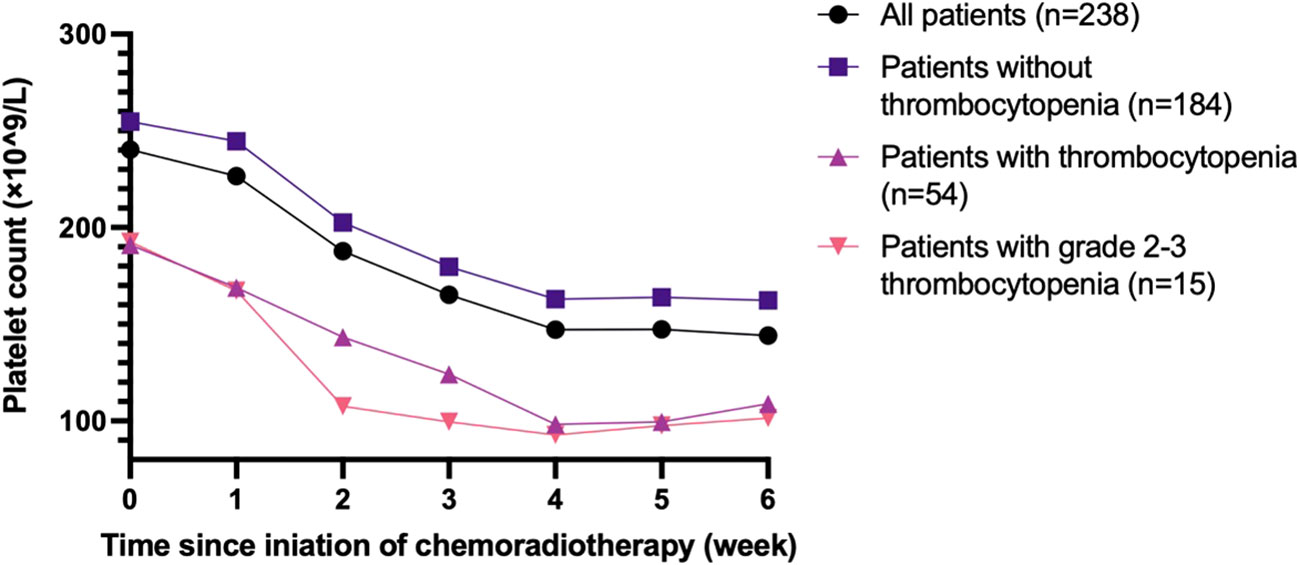
Figure 2 demonstrates the changes in the PLT count during chemoradiotherapy in every week. Generally, the PLT count decreased gradually as the treatment progressed, declining rapidly between week 1 and week 4. Patients who developed thrombocytopenia during treatment typically had a lower PLT count at baseline.
Risk factors associated with grade 2+thrombocytopenia
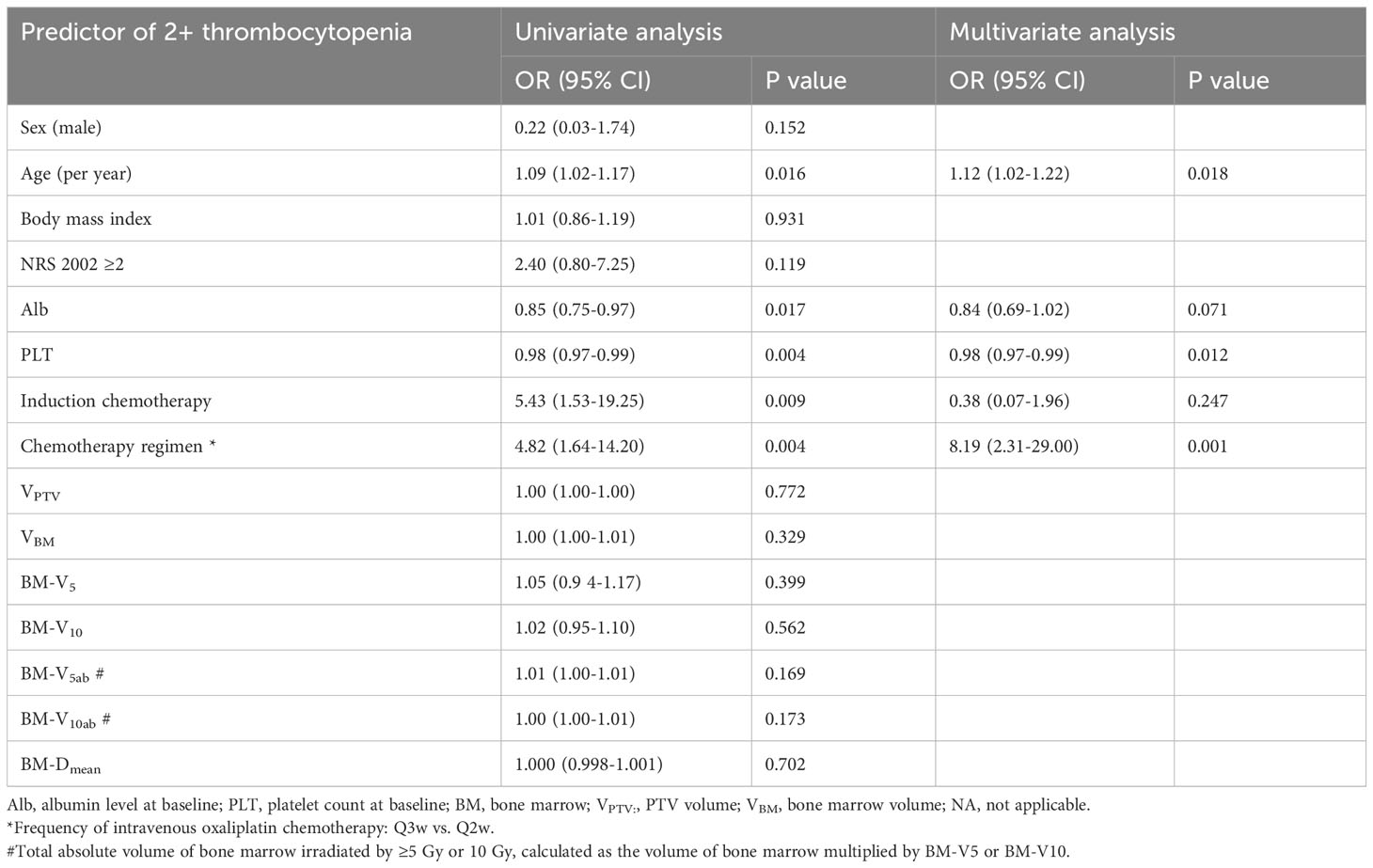
On univariate analysis, older age, low albumin (Alb) level at baseline, low PLT count at baseline, induction chemotherapy, and intravenous oxaliplatin chemotherapy every three weeks were statistically significantly associated with increased risks of grade 2+ thrombocytopenia (Table 2). On multivariate analysis, older age, low PLT count at baseline, and intravenous oxaliplatin chemotherapy every three weeks remained as significant, independent predictors of the risk of disease (Table 2). In the risk receiver-operating characteristic (ROC) curve analysis of the PLT count for grade 2+ thrombocytopenia, the area under the ROC curve (AUC) indicated the prognostic value of the PLT count, with an AUC of 0.718 (P = 0.005), and a cutoff value of 194.5×109/L.
Prediction model development and validation
Based on the results of the backward stepwise selection process with the AIC, four risk factors, including age, Alb level, PLT count, and chemotherapy regimen, were included in the final model and used to form a 2+ thrombocytopenia probability estimation nomogram (Figure 3A).
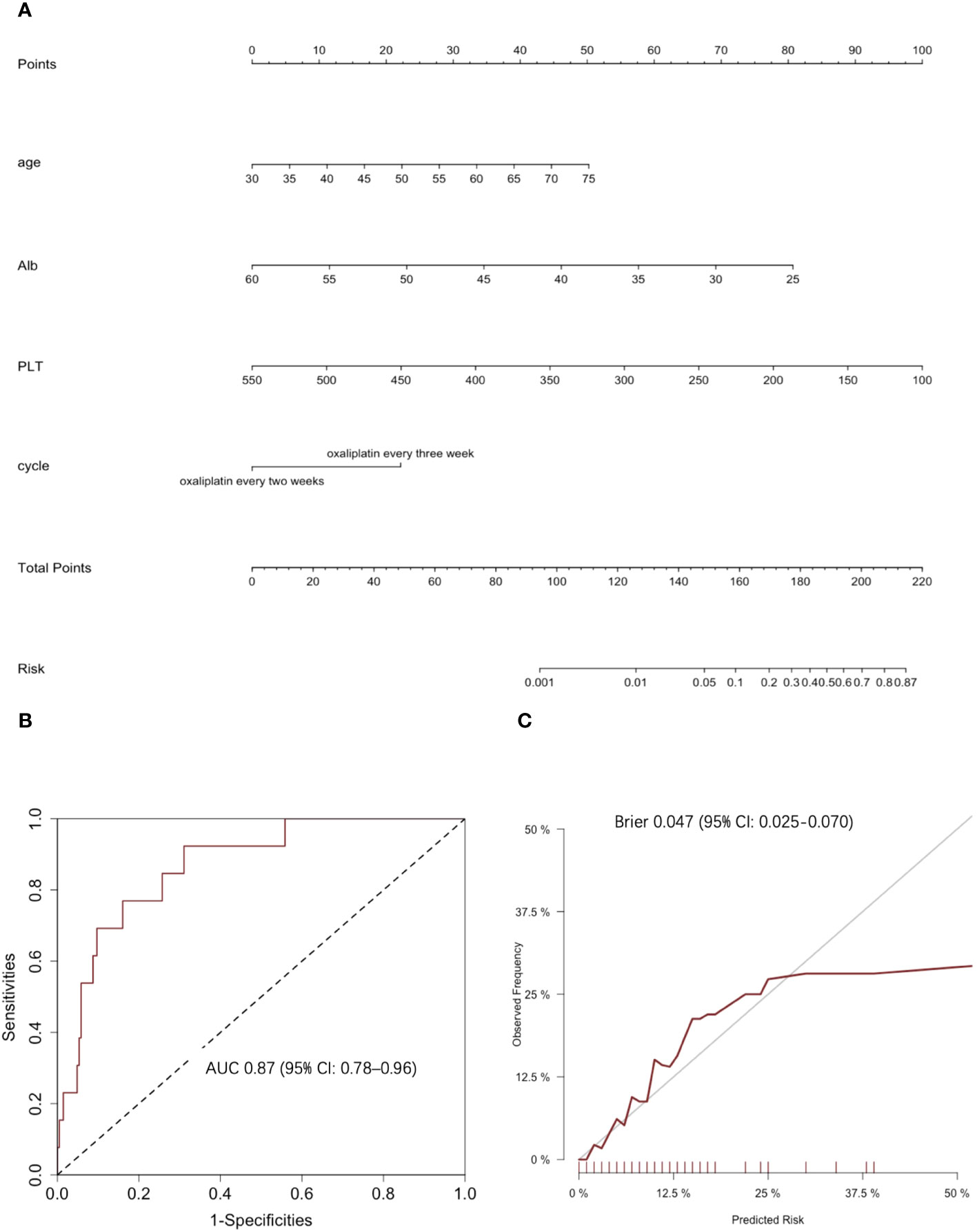
Figure 3 Clinical prediction model of 2+ thrombocytopenia. (A) The nomogram for predicting 2+ thrombocytopenia: This nomogram provides a method for calculating the risk of developing 2+ thrombocytopenia. To use, locate the patient’s age, draw a line straight up to the points axis to establish the score associated with that age. Repeat for the other three covariates (Alb level at baseline, PLT count at baseline and chemotherapy cycle). Add the score of each covariate together and locate the total score on the total points axis. Draw a line straight down to the risk axis to obtain the probability. (B) ROC curves and corresponding AUC statistics. (C) Calibration plots: nomogram-predicted 2+ thrombocytopenia is plotted on the x-axis, with observed 2+ thrombocytopenia on the y-axis. Dashed lines along the diagonal line through the origin point represent the perfect calibration models in which the predicted probabilities are identical to the observed probabilities.
The predictive accuracy for 2+ thrombocytopenia as measured by the C‐index was 0.87 (95% CI: 0.78–0.96) (Figure 3B). The Hosmer‐Lemeshow calibration test was significant (χ2 = 2.27, p = 0.97) and the calibration plot for the probability of 2+ thrombocytopenia showed a moderate agreement between the actual observed outcome and the prediction by the nomogram (Figure 3C). The overall prediction performance was good, with a mean Brier score of 0.047 (95% CI: 0.025–0.070).
PLT count nadir and its associations with radiation dosimetric parameters
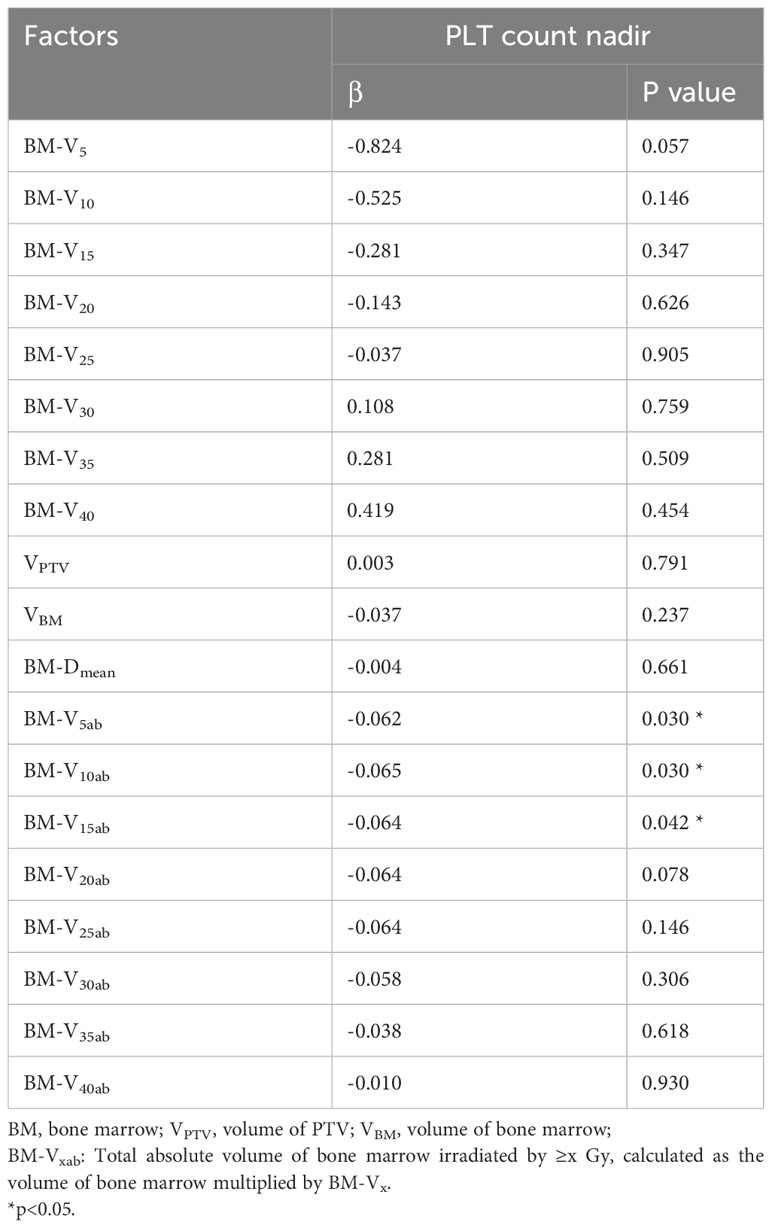
Table 3 further illustrates the role of radiation dosimetric parameters in predicting the PLT count nadirs during CRT. Traditional radiation dosimetric parameters, including V5-V40, BM-DMean, Volume of PTV and BM volume, were not associated with a lower PLT count nadir. However, the total absolute volume of bone marrow irradiated by 5 Gy, 10 Gy and 15 Gy (BM-V5ab, BM-V10ab, BM-V15ab), calculated as the volume of bone marrow multiplied by the corresponding Vx, were predictors of the PLT count nadir. The nadir of PLT was found to be negatively correlated with BM-V5ab (β = -0.062, P =0.030), BM-V10ab (β = -0.065, P =0.030) and BM-V15ab (β = -0.064, P =0.042).
Discussion
In the present study, we focused on thrombocytopenia induced by CRT of rectal cancer, investigating its clinical characteristics of occurrence, impact on treatment and relevant risk factors.
Chemotherapy induced thrombocytopenia (CIT) is a common hematologic toxicity in long-term chemotherapy that has been noted by medical oncologists. The incidence of CIT observed in colorectal cancer patients treated with the adjuvant XELOX regimen was 72.3% (15), and the incidence of grade 3-4 thrombocytopenia was 5.3%-18.5% (16–18) in previous studies. However, the narrow definition of CIT can no longer cover the current diversified tumor treatment methods. In the guidelines of the Chinese Society of Clinical Oncology (CSCO) 2022, the concept of CTIT was proposed. CTIT, cancer therapy-induced thrombocytopenia, is an extensional concept of CIT, which was extended from chemotherapy to the all kinds of antitumor treatment, including radiotherapy, targeted therapy and immunotherapy.
CTIT during radiotherapy for rectal cancer has not received enough attention. This is because traditional neoadjuvant long-term CRT has a short treatment cycle and long interval before surgery for patient recovery. However, under the trend of the total neoadjuvant treatment (TNT) strategy (19–21), preoperative chemoradiation is actually more intensive, which would theoretically lead to more severe thrombocytopenia. The associated dual-therapy modality results in a greater number of hematologic toxicities than monotherapy in the neoadjuvant treatment of rectal cancer (22). Therefore, this enhanced combination treatment strategy has increased radiotherapists’ interest in thrombocytopenia. In our study, 54 (22.6%) patients developed grade 1-3 thrombocytopenia during CRT. This is lower than the data reported for the TNT strategy (10). In our cohort, 49 (20.5%) patients received TNT-like CRT plus induction or consolidation chemotherapy. Among these patients, 17 (34.7%) developed CTIT during TNT-like treatments, and most of these patients (12 patients, 24.5%) developed 2+ thrombocytopenia. These results suggest that greater attention needs to be paid to the clinical impact of thrombocytopenia after adding higher-intensity neoadjuvant chemotherapy.
According to current guidelines, only grade 2+ thrombocytopenia requires clinical intervention. The low incidence of grade 2+ thrombocytopenia may lead to underestimation of impact of thrombocytopenia by radiotherapists. In real-world clinical practice, however, the treatment of patients affected by thrombocytopenia is more than expected. In a large sample size study of a secondary analysis of data from prospective clinical trials, 62% of CIT adverse events (AEs) led to chemotherapy dose delay or change and/or discontinuation in metastatic colorectal cancer patients (23). In our cohort, the treatments of 19 (35.2%) patients were affected by CTIT, and for 5 patients, both chemotherapy and radiotherapy were impacted. Based on our analysis, PLT declined gradually within four weeks after the beginning of CRT and stabilized in the later periods of treatment. Among the 13 patients who experienced chemotherapy interruption, most (84.6%) dropped the last cycle. Thus, patients who received intravenous oxaliplatin chemotherapy every three weeks experienced a greater dose reduction. Sequential chemotherapy or adjuvant chemotherapy could compensate for the dose reduction in CRT, but the efficacy is unknown. Relatively, the radiotherapy regimens were only slightly impacted. One patient had the last 2 fractions dropped, and 2 patients had radiotherapy delays of more than 3 days due to CTIT. However, in many patients in our cohort, the date of radiotherapy delay was not consistent with the date of thrombocytopenia in our cohort. Radiotherapy, unlike chemotherapy, is a continuous process. Patients may not see the doctor in time to suspend radiotherapy when thrombocytopenia occurs, or they may make their own decision to stop radiotherapy because of their own worries. This should convince doctors of the need for more detailed patient education.
Recognizing the risk factors for thrombocytopenia helps in the conduction of reasonable management and prevention. Therefore, we further investigated the predictive factors for grade 2+ thrombocytopenia. Risk factors for CIT found by previous studies included tumor type, stage, chemotherapy regimen, chemotherapy cycles, and high lactate dehydrogenase levels (24–26). In our study, patient-specific factors were also significantly associated with the incidence of 2+ thrombocytopenia, including age and baseline Alb level, which revealed the nutrition level of the patient. Baseline PLT count remained a strong predictor in multivariate analysis, as shown in Figure 1. Chemotherapy, as we expected, made patients prone to thrombocytopenia. Specifically, different frequencies of intravenous oxaliplatin chemotherapy led to different susceptibilities to thrombocytopenia for the patients. Patients receiving intravenous oxaliplatin every two weeks had a lower incidence rate of 2+ thrombocytopenia than those receiving oxaliplatin every three weeks. In addition, patients often had their last cycle of chemotherapy dropped once they began experiencing thrombocytopenia, as described earlier. From this point of view, oxaliplatin 85 mg/m2 applied every two weeks may be a safer choice. Induction chemotherapy was associated with 2+ thrombocytopenia in univariate analysis, as we expected, but did not reach statistical significance in multivariate analysis. This may be due to its small sample size (n=18) and its effect on PLT count at baseline (the beginning of CRT).
Our prediction model performs well in predicting a low risk of 2+ thrombocytopenia, which means that these patients may receive CRT or even total neoadjuvant therapy without excessive worries of severe thrombocytopenia. However, this model failed to filter out patients with high risk, so that it is hard to determine who is suitable for prophylactic use of TPO. This may be due to the relatively low risk of thrombocytopenia in CRT of rectal cancer because most patients only received CRT instead of total neoadjuvant therapy during the study period. However, this model may show a greater significance in the era of TNT. Other regression modeling techniques will also be explored to determine whether predictive accuracy can be further improved.
It is generally believed that pelvic bone marrow irradiation will affect hematopoietic function, thus theoretically leading to thrombocytopenia. In many studies involving pelvic radiotherapy, thrombocytopenia was mixed with leukopenia and/or anemia for analysis or was neglected directly in risk factor analysis (4, 5, 27, 28). However, treatments are different between thrombocytopenia and leukopenia, and thrombocytopenia usually requires a longer recovery process than leukopenia and thus needs to be independently predicted. The conclusions among previous studies have been inconsistent. In a study of cervical cancer patients treated with CRT, no radiation dosimetric parameters were recognized as risk factors for thrombocytopenia or PLT count nadirs (29). However, in another rectal cancer cohort, the V5 and V10 of the BM predicted the PLT count nadir% (specified as a percentage of the baseline value) (3). V5 was also identified as a factor associated with PLT nadir (2). Patients with V40>23% (lower pelvic bone marrow) had a higher rate of grade 2+ thrombocytopenia in another study (30). The reasons for these conflicting findings may include the different contouring strategies for bone marrow and the various endpoints defined in different studies. Active BM delineation on MR images seems to be more accurate than delineation on CT (2). The dose-volume parameters under this strategy may serve as better predictors (3), but more evidence is still needed. In this study, we contoured the medullary space for radiation dosimetric analysis (31). Interestingly, the traditional radiation dosimetric parameters did not serve as significant predictors. However, when we multiplied the V5~15 and BM volume, the new parameters BM-V5ab, BM-V10ab and BM-V15ab were significantly associated with the PLT count nadir and had a lower p value in the univariate analysis in predicting grade 2+ thrombocytopenia. This new parameter is the total absolute volume of bone marrow irradiated by ≥5-15 Gy. This result suggested that the decrease in platelet counts is associated with low-dose irradiation of the bone marrow, but the traditional radiation dosimetric parameters failed to reveal this relationship.
Our study has its limitations. Given that this was a single-center retrospective study and the incidence of 2+ thrombocytopenia was relatively low, the results need to be validated in a larger prospective cohort. In addition, during the study period, most patients received only CRT instead of total neoadjuvant therapy. Thrombocytopenia may be a more important issue in the future, but its importance was not fully reflected in this study. Extra attention should be given to the treatment of relatively high-risk patients identified in our study, especially in total neoadjuvant therapy. Due to the small number of positive cases, this model was not externally validated. Future efforts will seek to test our model performance in external validation using other patient databases.
Nevertheless, this is a comprehensive and detailed analysis of thrombocytopenia induced by both chemotherapy and radiotherapy in rectal cancer patients. We analyzed the incidence rate, pattern and risk factors for thrombocytopenia during CRT to ensure the safe and timely treatment of patients in the future. The occurrence of 2+ thrombocytopenia during concurrent chemoradiotherapy for rectal cancer can be predicted by the patient’s baseline status and chemoradiotherapy regimen, and low dose irradiation of bone marrow can affect the level of platelets during the treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethics approval was not required due to the retrospective nature of the study.
Author contributions
YT: Data curation, Formal Analysis, Methodology, Writing – original draft. DM: Data curation, Writing – original draft. YY: Formal Analysis, Writing – original draft. JG: Writing – review & editing. ZL: Resources, Writing – review & editing. XZ: Resources, Writing – review & editing. SL: Methodology, Writing – review & editing. YZ: Resources, Software, Writing – review & editing. HW: Writing – review & editing. YC: Writing – review & editing. HY: Formal analysis, Writing – review & editing. YL: Conceptualization, Funding acquisition, Investigation, Writing – review & editing. WW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by the programs below in collecting data and writing the manuscript: Thrombocytopenia Funding from Yeehong Business School of Shenyang Pharmaceutical University (TCP funding); Capital’s Funds for Health Improvement and Research No. 2020-2-1027.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AEs, Adverse events; AIC, Akaike information criterion; Alb, Albumin; AUC, Area under the ROC curve; BM, Bone marrow; BM-Dmean, Mean dose of bone marrow; BM-Vx, Volume of bone marrow receiving radiation dose ≥x Gy, recorded as percentages; BM-Vxab, Total absolute volume of bone marrow irradiated by ≥x Gy, calculated as the volume of bone marrow multiplied by BM-Vx; CIT, Chemotherapy induced thrombocytopenia; CRT, Concurrent chemoradiotherapy; CSCO, Chinese Society of Clinical Oncology; CT, Computed tomography; CTCAE, Common Terminology Criteria for Adverse Events; CTIT, Cancer therapy-induced thrombocytopenia; CTV, Clinical target volume; GTV, Gross tumor volume; HT, Hematologic toxicity; MR, Magnetic resonance;
PLT, Platelet; ROC, Receiver-operating characteristics; SIB-IMRT, Boost intensity-modulated radiation therapy; TNT, Total neoadjuvant therapy; VBM, Volume of bone marrow; VPTV, Volume of PTV; XELOX, Capecitabine and oxaliplatin; 2+, Grade 2 or higher.
References
1. Hayman JA, Callahan JW, Herschtal A, Everitt S, Binns DS, Hicks RJ, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys (2011) 79(3):847–52. doi: 10.1016/j.ijrobp.2009.11.040
2. Jianyang W, Yuan T, Yuan T, Xin W, Ning L, Hua R, et al. A prospective phase II study of magnetic resonance imaging guided hematopoietical bone marrow-sparing intensity-modulated radiotherapy with concurrent chemotherapy for rectal cancer. Radiologia Med (2016) 121(4):308–14. doi: 10.1007/s11547-015-0605-2
3. Kuncman L, Stawiski K, Masłowski M, Kucharz J, Fijuth J. Dose–volume parameters of MRI-based active bone marrow predict hematologic toxicity of chemoradiotherapy for rectal cancer. Strahlentherapie und Onkologie (2020) 196(11):998–1005. doi: 10.1007/s00066-020-01659-z
4. Yang TJ, Oh JH, Apte A, Son CH, Deasy JO, Goodman KA. Clinical and dosimetric predictors of acute hematologic toxicity in rectal cancer patients undergoing chemoradiotherapy. Radiother Oncol (2014) 113(1):29–34. doi: 10.1016/j.radonc.2014.09.002
5. Wan J, Liu K, Li K, Li G, Zhang Z. Can dosimetric parameters predict acute hematologic toxicity in rectal cancer patients treated with intensity-modulated pelvic radiotherapy? Radiat Oncol (2015) 10(1):162. doi: 10.1186/s13014-015-0454-0
6. Terrault N, Chen YC, Izumi N, Kayali Z, Mitrut P, Tak WY, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology (2018) 155(3):705–18. doi: 10.1053/j.gastro.2018.05.025
7. Li VJ, Miao Y, Wilkins C, Soff GA. Efficacy and thrombotic adverse events of romiplostim use in patients with thrombocytopenia related to underlying Malignancies. Blood blood (2017) 130(Suppl_1):2324. doi: 10.1182/blood.V130.Suppl_1.2324.2324
8. Newman NB, Sidhu MK, Baby R, Moss RA, Nissenblatt MJ, Chen T, et al. Long-Term Bone Marrow Suppression during Postoperative Chemotherapy in Rectal Cancer Patients after Preoperative Chemoradiation Therapy. Int J Radiat Oncol Biol Phys (2016) 94(5):1052–60. doi: 10.1016/j.ijrobp.2015.12.374
9. Rödel C, Liersch T, Hermann RM, Arnold D, Reese T, Hipp M, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol (2007) 25(1):110–7. doi: 10.1200/JCO.2006.08.3675
10. Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ArO/AIO-12. J Clin Oncol (2019) 37(34):3212–22. doi: 10.1200/JCO.19.00308
11. Zhang Y-Z, Song M, Geng J-H, Zhu X-G, Li S, Li Y-H, et al. Patterns of failure and implications for clinical target volume definition of locally advanced T4b rectal cancer identified with magnetic resonance imaging and treated using neoadjuvant chemoradiotherapy and surgery. Radiother Oncol (2021) 161:132–9. doi: 10.1016/j.radonc.2021.06.017
12. Li JL, Ji JF, Cai Y, Li XF, Li YH, Wu H, et al. Preoperative concomitant boost intensity-modulated radiotherapy with oral capecitabine in locally advanced mid-low rectal cancer: A phase II trial. Radiother Oncol (2012) 102(1):4–9. doi: 10.1016/j.radonc.2011.07.030
13. Wu AW, Cai Y, Li YH, Wang L, Li ZW, Sun YS, et al. Pattern and management of recurrence of mid-low rectal cancer after neoadjuvant intensity-modulated radiotherapy: single-center results of 687 cases. Clin Colorectal Cancer (2018) 17(2):e307–13. doi: 10.1016/j.clcc.2018.01.006
14. Wang L, Li ZY, Li ZW, Li YH, Sun YS, Ji JF, et al. Efficacy and safety of neoadjuvant intensity-modulated radiotherapy with concurrent capecitabine for locally advanced rectal cancer. Dis Colon Rectum (2015) 58(2):186–92. doi: 10.1097/DCR.0000000000000294
15. Suenaga M, Akiyoshi T, Shinozaki E, Fujimoto Y, Matsusaka S, Konishi T, et al. A feasibility study of capecitabine and oxaliplatin for patients with stage II/III colon cancer –actor study–. Anticancer Res (2018) 38(3):1741–7. doi: 10.21873/anticanres.12410
16. Zhao G, Gao P, Yang KH, Tian JH, Ma B. Capecitabine/oxaliplatin as first-line treatment for metastatic colorectal cancer: A meta-analysis. Colorectal Dis (2010) 12(7):615–23. doi: 10.1111/j.1463-1318.2009.01879.x
17. Arkenau HT, Arnold D, Cassidy J, Diaz-Rubio E, Douillard JY, Hochster H, et al. Efficacy of oxaliplatin plus capecitabine or infusional fluorouracil/leucovorin in patients with metastatic colorectal cancer: A pooled analysis of randomized trials. J Clin Oncol (2008) 26(36):5910–7. doi: 10.1200/JCO.2008.16.7759
18. Kibudde S, Begg W. Capecitabine plus oxaliplatin in the treatment of metastatic colorectal cancer at Tygerberg Hospital: a retrospective study. Pan Afr Med J (2022) 42:141. doi: 10.11604/pamj.2022.42.141.31234
19. Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol (2022) 40(23):2546–56. doi: 10.1200/JCO.22.00032
20. Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol (2022) 8(1):e215445. doi: 10.1001/jamaoncol.2021.5445
21. Wang L, Zhang X-Y, Zhao Y-M, Li S-J, Li Z-W, Sun Y-S, et al. Intentional watch & wait or organ preservation surgery following neoadjuvant chemoradiotherapy plus consolidation CAPEOX for MRI-defined low-risk rectal cancer: findings from a prospective phase 2 trial (PKUCH-R01 trial, NCT02860234). Ann Surg (2022) 277(4):647–54. doi: 10.1097/SLA.0000000000005507
22. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: Initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol (2016) 34(27):3300–7. doi: 10.1200/JCO.2016.66.6198
23. Kilpatrick K, Shaw JL, Jaramillo R, Toler A, Eisen M, Sangaré L, et al. Occurrence and management of thrombocytopenia in metastatic colorectal cancer patients receiving chemotherapy: secondary analysis of data from prospective clinical trials. Clin Colorectal Cancer (2021) 20(2):170–6. doi: 10.1016/j.clcc.2020.10.004
24. Wang Z, Cai XJ, Chen L, Cheng B, Shi L, Lei L, et al. Factors potentially associated with gemcitabine-based chemotherapy-induced thrombocytopenia in Chinese patients with nonsmall cell lung cancer. J Cancer Res Ther (2018) 14(10):S656–60. doi: 10.4103/0973-1482.187338
25. Hitron A, Steinke D, Sutphin S, Lawson A, Talbert J, Adams V. Incidence and risk factors of clinically significant chemotherapy-induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract (2011) 17(4):312–9. doi: 10.1177/1078155210380293
26. Lu R, Lin Q, Chen S, Ye X. Chemotherapy-induced thrombocytopenia and platelet transfusion in patients with diffuse large B-cell lymphoma. Transl Cancer Res (2020) 9(3):1640–51. doi: 10.21037/tcr.2020.01.64
27. Mell LK, Schomas DA, Salama JK, Devisetty K, Aydogan B, Miller RC, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys (2008) 70(5):1431–7. doi: 10.1016/j.ijrobp.2007.08.074
28. Franco P, Ragona R, Arcadipane F, Mistrangelo M, Cassoni P, Rondi N, et al. Dosimetric predictors of acute hematologic toxicity during concurrent intensity-modulated radiotherapy and chemotherapy for anal cancer. Clin Trans Oncol (2017) 19(1):67–75. doi: 10.1007/s12094-016-1504-2
29. Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys (2006) 66(5):1356–65. doi: 10.1016/j.ijrobp.2006.03.018
30. Lee AY, Golden DW, Bazan JG, Kopec M, Pelizzari CA, Aggarwal S, et al. Hematologic nadirs during chemoradiation for anal cancer: temporal characterization and dosimetric predictors. Int J Radiat Oncol Biol Phys (2017) 97(2):306–12. doi: 10.1016/j.ijrobp.2016.10.010
Keywords: rectal cancer, thrombocytopenia, concurrent chemoradiotherapy, risk factors, clinical predictors, radiation dosimetric parameters, bone marrow
Citation: Teng Y, Ma D, Yan Y, Geng J, Liu Z, Zhu X, Li S, Zhang Y, Wang H, Cai Y, Yue H, Li Y and Wang W (2024) Retrospective cohort study for thrombocytopenia during concurrent chemoradiotherapy for rectal cancer. Front. Oncol. 13:1289824. doi: 10.3389/fonc.2023.1289824
Received: 06 September 2023; Accepted: 14 November 2023;
Published: 02 January 2024.
Edited by:
Rakesh Kapoor, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaCopyright © 2024 Teng, Ma, Yan, Geng, Liu, Zhu, Li, Zhang, Wang, Cai, Yue, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongheng Li, eW9uZ2hlbmdsZWVAMTYzLmNvbQ==; Weihu Wang, d2FuZ3dlaWh1ODhAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Yue Teng1†
Yue Teng1† Yan Yan
Yan Yan Zhiyan Liu
Zhiyan Liu Xianggao Zhu
Xianggao Zhu Haizhen Yue
Haizhen Yue Yongheng Li
Yongheng Li Weihu Wang
Weihu Wang