- 1Department of Gynecologic Oncology and Surgery, Cangzhou Central Hospital, Cangzhou, Hebei, China
- 2Department of Pharmacy, Cangzhou Central Hospital, Cangzhou, Hebei, China
- 3Department of Medical Laboratory, Beijing Origin-Poly Bio-Tec Co., Ltd., Beijing, China
Objective: Developing a non-invasive and reliable triage test for endometrial malignant lesions is an important goal, as it could help to reduce the number of invasive diagnostic procedures required and improve patient survival. We aimed to estimate the diagnostic value of DNA methylation levels in cervical cytological samples of endometrial cancer (EC) and endometrial atypical hyperplasia (AH).
Methods: A total of 607 women who had indications for endometrial biopsy in the Department of Obstetrics and Gynecology of Cangzhou Central Hospital from October 2022 to April 2023 were enrolled in this study. The cervical exfoliated cells were collected for gene methylation before endometrial biopsy. Clinical information, tumor biomarkers, and endometrial thickness (ET) of transvaginal ultrasonography (TVS) were also collected. With endometrial histopathology as the gold standard, multivariate unconditional logistic regression was applied to analyze the risk factors of endometrial malignant lesions. The role of cysteine dioxygenase type 1 (CDO1) and CUGBP Elav-like family member 4 (CELF4) gene methylation as a triage strategy biomarker in endometrial malignant lesions was specifically explored.
Results: Multivariate logistic regression analysis showed that premenopausal ET ≥ 11 mm or postmenopausal ET ≥ 5 mm, CDO1 ΔCt ≤ 8.4, or CELF4 ΔCt ≤ 8.8 were the risk factors for AH and EC, with odds ratios (ORs) (95%CI) of 5.03 (1.83–13.82) and 6.92 (1.10–43.44), respectively (p-values < 0.05). The sensitivity and specificity of CDO1/CELF4 dual-gene methylation assay for AH and EC reached 84.9% (95%CI: 75.3%–94.5%) and 86.6% (95%CI: 83.8%–89.5%), respectively. ET combined with DNA methylation detection further improved the specificity to (94.9%, 95%CI: 93.1%–96.8%).
Conclusion: The accuracy of cervical cytology DNA methylation is superior to that of other clinical indicators in the non-invasive examination of endometrial malignant lesions. DNA methylation combined with TVS can further improve the specificity and is a promising biomarker triage strategy in women with suspected endometrial lesions.
Introduction
Endometrial cancer (EC) is the most prevalent gynecological cancer in countries with high income, and its occurrence is increasing worldwide (1–3). In 2017, the worldwide incidence and mortality rates of EC were 35.7 and 5.3 per 100,000, respectively. As reported by the National Cancer Center in 2019, the incidence and mortality rates of EC were respectively 10.28 and 1.9 per 100,000 in China, and both rates are increasing (4). Approximately 70% of EC patients are diagnosed in the early clinical stage and have a good prognosis because they are confined to the uterus. The prognosis of EC is influenced by factors such as age, stage, tumor differentiation, and pathological type. Patients with advanced age, late stage, and low differentiation tend to have a worse prognosis (5, 6). There is a significant prognostic difference between the histological types of endometrial cancers. Most ECs are well to moderately differentiated and develop in the presence of endometrial hyperplasia. These tumors are also known as type I (low-grade) endometrial carcinomas, as the main pathological type (7). They are associated with long-duration unopposed estrogenic stimulation. Early detection and treatment can result in a 5-year survival rate greater than 80% (8). Type II tumors are non-hormone-dependent, mainly including serous carcinoma, clear cell carcinoma, and carcinosarcoma, which are highly graded and have a tendency to recur even at an early stage, accounting for 70% of EC deaths (9). The chance of developing endometrial hyperplasia without atypia into EC is approximately 3%, but the chance increases up to 23% in developing into endometrial atypical hyperplasia (AH) (10). It is crucial to screen for AH and early EC in women at high risk (11).
Risk factors for endometrial cancer include high body mass index (BMI) (12, 13), metabolic syndrome (14, 15), diabetes mellitus (16), nulliparity and infertility, and polycystic ovarian syndrome (PCOS) (17, 18). Additional factors that increase the risk of EC include unopposed estrogen therapy, estrogen-producing tumors, and early menarche or late menopause (19). Abnormal uterine bleeding (AUB) (20) and postmenopausal bleeding (PMB) (21) are common clinical symptoms of EC. As part of routine health surveillance, clinicians should ask patients about postmenopausal and abnormal bleeding (22). Annual transvaginal ultrasound (TVS) is recommended for monitoring endometrial thickness in those with the above-mentioned factors of increased risk of endometrial cancer. Screening for endometrial cancer is recommended if ultrasonography reveals endometrial thickness (ET) >11 mm in the premenopausal (>5 mm in postmenopausal) (23) or increased blood vessels, uneven endometrium, or poor sound transmission of uterine cavity effusion (13).
There is currently a lack of well-established and universally accepted standardized screening methods for EC that exhibit both high sensitivity and standardization (24). TVS is a non-invasive diagnostic test, but the cutoff value for endometrial thickness remains uncertain, although TVS has a high sensitivity but a low specificity of 24.3%–74.0%. As a result, it has a low positive predictive value and a high rate of false-positive results, making it unreliable for identifying malignant lesions (25, 26). Traditional dilation and curettage are relatively effective methods, and hysteroscopic biopsy can be conducted to obtain a definite diagnosis, which is considered the most reliable for diagnosing EC (27). Biopsy is a highly accurate method (with a high sensitivity of 90%–100% and a specificity of 98%–100%) for detecting EC. Hysteroscopic localization biopsy or diagnostic curettage may be considered for patients at high risk of EC or with ultrasound abnormalities, but these invasive procedures to obtain endometrial tissue are also usually painful and frightening for the patients (28, 29).
Early detection is crucial for improving outcomes and reducing mortality rates, and therefore, researchers are actively investigating biomarkers that can be used for early screening and detection. Recent studies on epigenetics have shown a strong connection between DNA methylation and the progression of cancer (30). Advances in understanding the mechanisms of tumorigenesis have also led to the identification of biomarkers that can be used for early detection, diagnosis, and prognosis of cancer patients (31). Some of these methylation biomarkers have been successfully used to differentiate cancerous tissue from normal tissue with a high accuracy rate of over 95% in common cancers (32). Furthermore, there are noticeable differences in methylation levels between tissue specimens of EC and precancerous lesions compared to benign endometrium (BE) (33, 34). DNA methylation markers can be utilized to assess women with abnormal vaginal bleeding in order to differentiate between those women with endometrial carcinoma from the majority of women without malignancy (35). Recent studies have indicated that endometrial lesions can be detected through cervical cytology samples. Reijene C et al. showed that cervical cytology is a less invasive method that yields comparable results to histology in terms of diagnosis performance (with a sensitivity of 78% and a specificity of 97%). Even with cervicovaginal self-sampling, a significant number of EC cases can be diagnosed (with a sensitivity of 67% and a specificity of 97%) (36). In summary, the use of DNA methylation testing in cervical cytology samples shows promise for the diagnosis of EC and AH. Two hypermethylated candidate genes, namely, cysteine dioxygenase type 1 (CDO1) and CUGBP Elav-like family member 4 (CELF4), have been identified as potential biomarkers for endometrial cancer (33, 37–39). As a key enzyme in cysteine catabolism, CDO1 belongs to the non-heme Fe(II) dioxygenase family. CDO1 is involved in a spectrum of physiological processes, including lipid metabolism and adipogenesis (40), osteoblast differentiation (41), regulation of redox homeostasis, fertility (42), bile acid metabolism, sulfide metabolism, and growth. Many of these processes are regulated directly or indirectly by the CDO1-mediated metabolism of cysteine (43). The degree of methylation of the CDO1 promoter is closely related to tumor progression and malignancy, and overexpression of CDO1 promotes ferroptosis in cancer cells (43). CELF4 is involved in both co-transcriptional and post-transcriptional RNA processing. Although CELF4 proteins all appear to affect pre-mRNA splicing, they play different roles in regulating mRNA stability and translation (44). CELF4 was a prognostic factor for EC patients; when combined with other clinical factors, the expression level of CELF4 could effectively predict the prognosis of EC patients (38).
In the present study, we aim to propose a promising method for detecting early endometrial malignant lesions. The sensitivity and specificity of the two gene assays of CDO1 and CELF4 were examined on endometrial pathological results. The area under the curve (AUC) of methylation assay receiver operating characteristic (ROC) was also detected to estimate the diagnostic value for EC and AH. Finally, its methylation performance was combined with TVS to explore the possibility of multiple scenarios of methylation detection.
Methods
Study design and sample collection
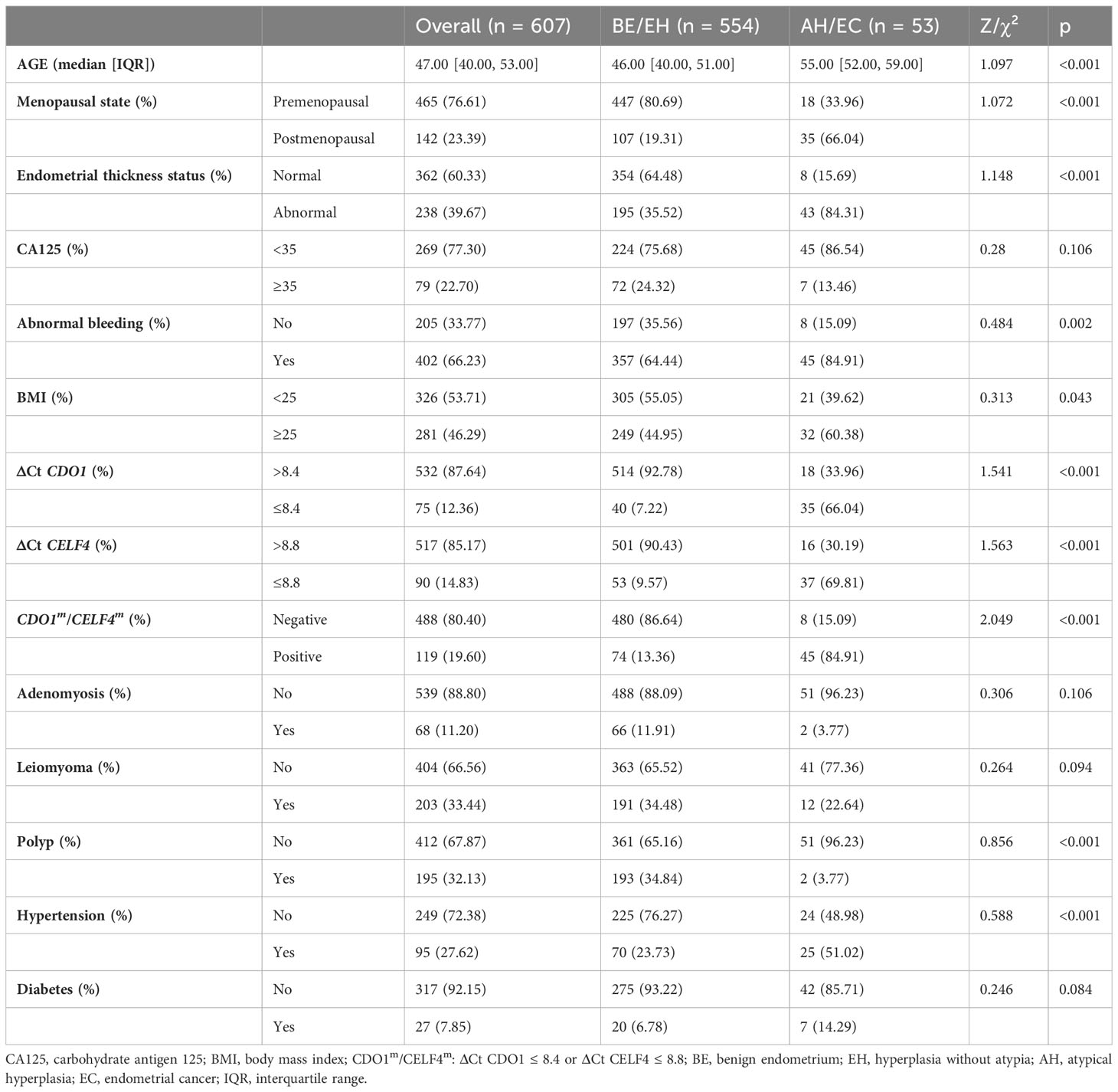
This prospective cross-sectional study uses a non-invasive clinical method to verify the clinical efficacy of CDO1 and CELF4 gene methylation detection. A total of 607 women with indications for endometrial biopsy in the Department of Obstetrics and Gynecology of Cangzhou Central Hospital from October 2022 to April 2023 were enrolled in this study. Indications for endometrial biopsy include irregular vaginal hemorrhage or blood secretions after menopause or before menopause for non-cervical lesions, patients with no ovulation infertility for many years, persistent vaginal discharge, and endometrial abnormal thickening or uterine cavity mass (45–47). Collected patient clinical information and cervical exfoliated cells were collected before surgery for DNA methylation detection. The study was approved by the Institutional Review Board (IRB) of the Ethics Committee, Cangzhou Central Hospital, Hebei, China [No. 2023-144-02(z)]. Based on the Chinese Guidelines for Menopause Management and Menopausal Stimulant Therapy (48), the menopausal status of patients was determined according to clinical manifestations and examinations. Patients with indications for endometrial biopsy were enrolled, and they provided signed informed consent according to the Standards for Reporting of Diagnostic Accuracy Studies (STARD) in clinics (49). Exclusion criteria were as follows: women with a previous diagnosis of cancer in any organ, who had previously undergone hysterectomy, who did not complete all the examinations, who were receiving hormonal therapy for menopausal symptoms in 1 year, or who were receiving immunosuppressive therapy 1 year before enrollment were excluded.
Demographic characteristics and clinical assessment
The general information and clinical manifestations of patients related to the onset of endometrial malignant lesions were collected by a special case collection table. It includes age, menopausal status, past medical history, BMI, endometrial thickness assessed by TVS within 1 month before hysteroscopy, and carbohydrate antigen 125 (CA125) value within 1 month before hysteroscopy. It also includes the history of gynecological diseases, with special attention to uterine, ovarian, and vaginal diseases. For EC patients, the EC tissue type, International Federation of Gynecology and Obstetrics (FIGO) grade and stage, and tumor history were also recorded. BMI was calculated as weight in kilograms divided by height in meters squared. The level of CA125 in serum was detected using a CA125 ELISA kit (Coibo Bio Co., Shanghai, China) according to the manufacturer’s instructions. ET was measured by TVS. In this study, the positive findings of TVS evaluation were defined as endometrial thickness ≥ 11 mm (premenopausal) and endometrial thickness ≥ 5 mm (postmenopausal). BMI ≥ 25 kg/m2, CA125 ≥ 35 U/ml is defined as abnormal.
CDO1 and CELF4 hypermethylation detection
Methylation detection was performed in a certified DNA laboratory, and the operators and staff members were blinded to the patient’s clinical information, TVS, hysteroscopy, and histopathology results. CDO1 and CELF4 methylation detection (CISENDO®) used the specimens collected in the PreservCyt® solution. Genomic DNA (gDNA) was extracted from the exfoliated cervical cell sample using the JH-DNA Isolation and Purifying kit (OriginPoly Bio-Tec Co., Ltd., Beijing, China) per the manufacturer’s instructions. The DNA concentration was quantified using the NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). Briefly, 500 ng of gDNA was subjected to bisulfite conversion using JH-DNA Methylation-Lightning MagPrep (OriginPoly Bio-Tec Co., Ltd., Beijing, China). Subsequently, the levels of CDO1m and CELF4m were determined using the CISENDO DNA Methylation Detection Kit for Endometrial Cancer (real-time PCR) with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control (OriginPoly Bio-Tec Co., Ltd., Beijing, China) using the ABI 7500 real-time PCR System platform (Life Technologies, Foster City, CA, USA) per the manufacturer’s instructions. The hypermethylation level of the COD1 gene was determined by the difference between the two Ct values (ΔCt CDO1 = Ct CDO1 − Ct GAPDH and ΔCt CELF4 = Ct CELF4 − Ct GAPDH). A positive result of the CISENDO methylation (CISENDO®) test is defined as either CDO1m(+): ΔCt CDO1 ≤ 8.4 or CELF4m(+): ΔCt CELF4 ≤ 8.8.
Statistical analysis
SPSS 27.0 (IBM Corp., Armonk, NY, USA) and R (version 4.1.2, Vienna, Austria) were used for all statistical analyses. The participants were characterized using descriptive statistics, and patients and tumor characteristics were tabulated. Use case (%) of counting data was used for comparison between groups χ2 test. The normality test was used to determine whether the variance of the population was equal to the Kolmogorov–Smirnov test, the measurement data of non-normal distribution was expressed by M (Q1, Q3), and the comparison between the two groups adopts the non-parametric Mann–Whitney U test. A multivariate logistic regression model was used to analyze the related factors of AH and EC. ROC curves were used to evaluate the AUCs of ET, CDO1m, or CELF4m. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for detecting AH and EC and their 95% confidence intervals were calculated. The methylation cutoff value for the final clinical statistical analysis was based on the CISENDO methylation (CISENDO®) test defined as either ΔCt CDO1 ≤ 8.4 or ΔCt CELF4 ≤ 8.8. All differences were considered two-sided and statistically significant at p < 0.05.
Results
Participant characteristics
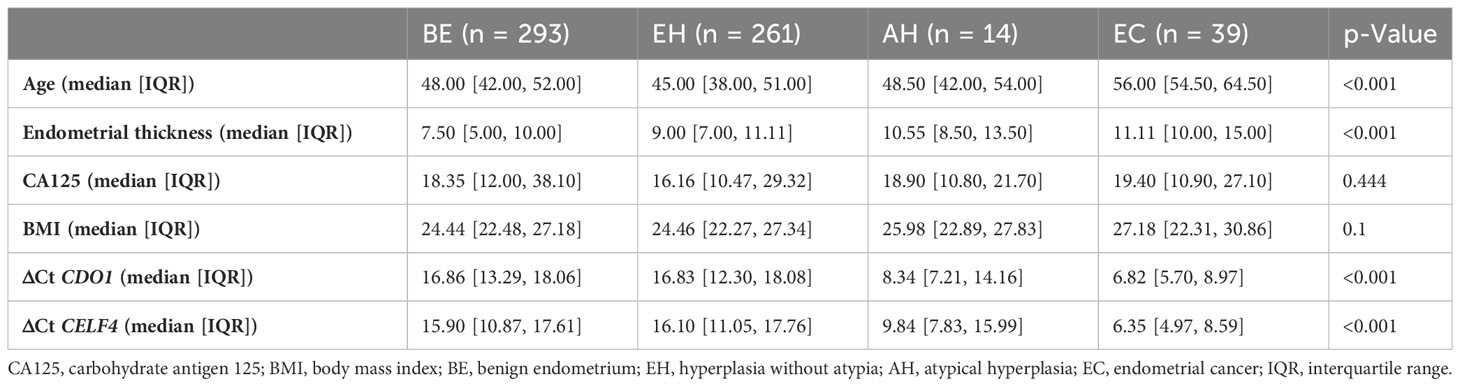
The enrollment flowcharts and the baseline characteristics are shown in Figure 1; Supplementary S1. A total of 607 women (median age [range], 47.0 [40.0–53.0] years) were enrolled and analyzed in the study: 293 (48.3%) with benign endometrial (BE) (48.0 [42.0–52.0] years), 261 (49.2%) with hyperplasia without atypia (EH) (45.0 [38.0–51.0] years), 14 (2.3%) with AH (48.5 [42.0–54.0] years), and 39 (6.0%) with EC (56.0 [54.5–64.5] years). There is a significant difference in age between each group (p < 0.05, Table 1). In Figure 2A, there was a significant difference in ΔCt CDO1 value between EH with AH (p < 0.05), but there were no significant differences in the remaining adjacent subgroups. There was a significant difference at ΔCt CELF4 value between EH with AH, and AH with EC (p < 0.05, Figure 2B). The positivity rate of CDO1m/CELF4m in AH (71.43%) and EC (89.74%) was significantly higher than that in the BE (14.33%) and EH (12.26%) groups (p < 0.05, Figure 2C).
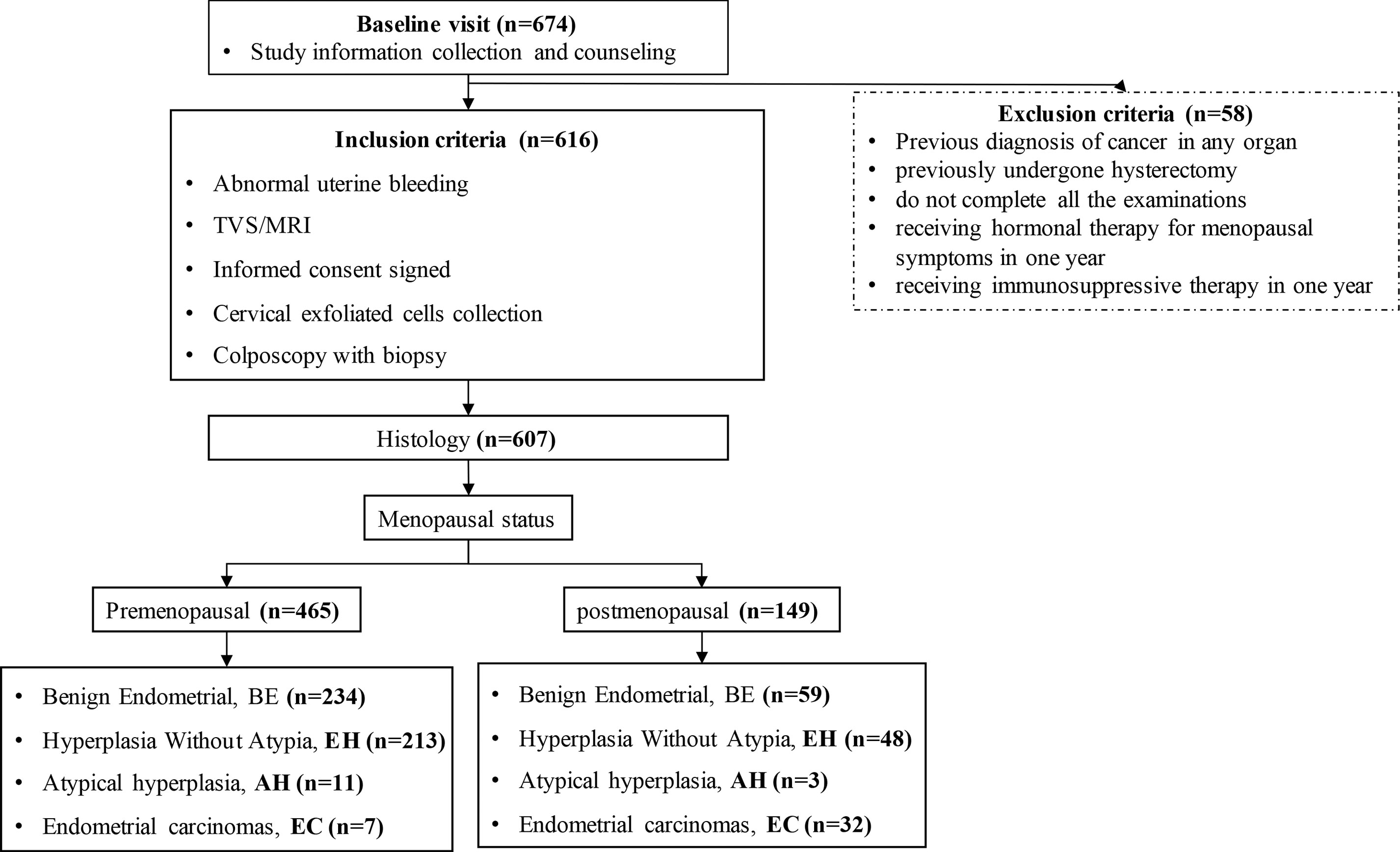
Figure 1 STARD diagram showing the flow of participants in the study. STARD, Standards for Reporting of Diagnostic Accuracy Studies.
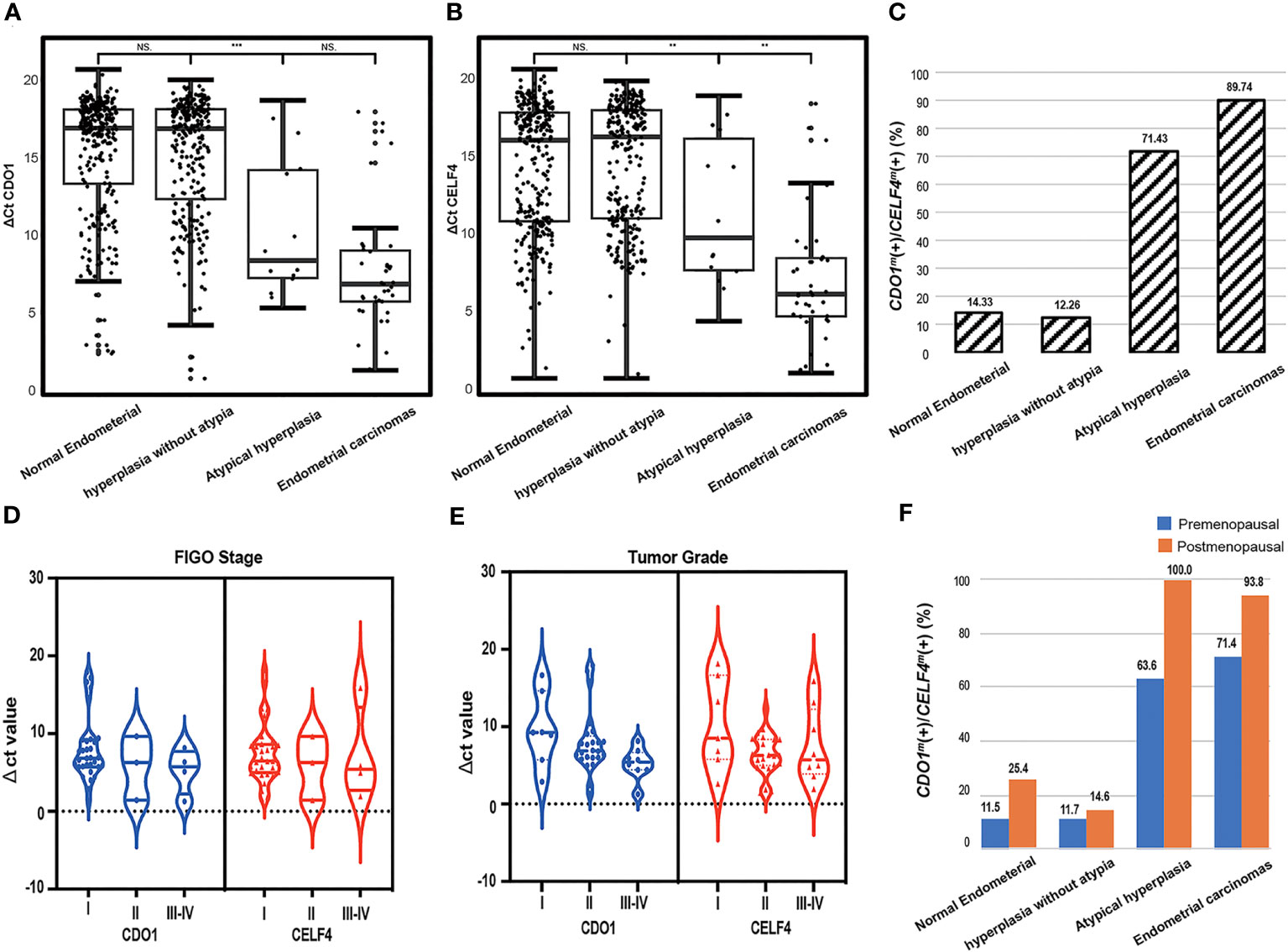
Figure 2 The dot plots show the distribution of DNA methylation in different degrees of pathological groups; the median and interquartile ranges are depicted by boxes. (A) Distribution of the value of methylated CDO1 in different degrees of pathological results. (B) Distribution of the value of methylated CELF4 in different degrees of pathological results. (C) The positive rate of CDO1m(+)/CELF4m(+) in different groups of pathological results, CDO1m(+)/CELF4m(+): ΔCt CDO1 ≤ 8.4 or ΔCt CELF4 ≤ 8.8. (D) Distribution of the value of methylated CDO1 and CELF4 in different FIGO stages. (E) Distribution of the value of methylated CDO1 and CELF4 in different tumor grades. (F) The positive rate of CDO1m(+)/CELF4m(+) in different degrees of pathological results with different menopausal states. FIGO, International Federation of Gynecology and Obstetrics. NS, no statistical significance; **p < 0.01, ***p < 0.001.
According to the FIGO classification, of the 39 women with EC, 32 (82.1%) were in stage I, 3 (7.7%) in stage II, 3 (7.7%) in stage III, and 1 (2.5%) in stage IV. Regarding tumor grading, 7 (17.9%) were G1, 24 (61.5%) were G2, and 8 (20.5%) were G3 (Supplementary S1). There were 37 (97.4%) type I and 2 (2.6%) type II EC patients. No significant difference in DNA methylation (ΔCt CDO1 value or ΔCt CELF4 value) was found between different FIGO classifications (Figure 2D) and tumor grade (p > 0.05, Figure 2E). The methylation positivity rate was higher in postmenopausal women than in premenopausal women in all different pathological subgroups (Figure 2F).
Analysis of clinical factors related to endometrial malignant lesions
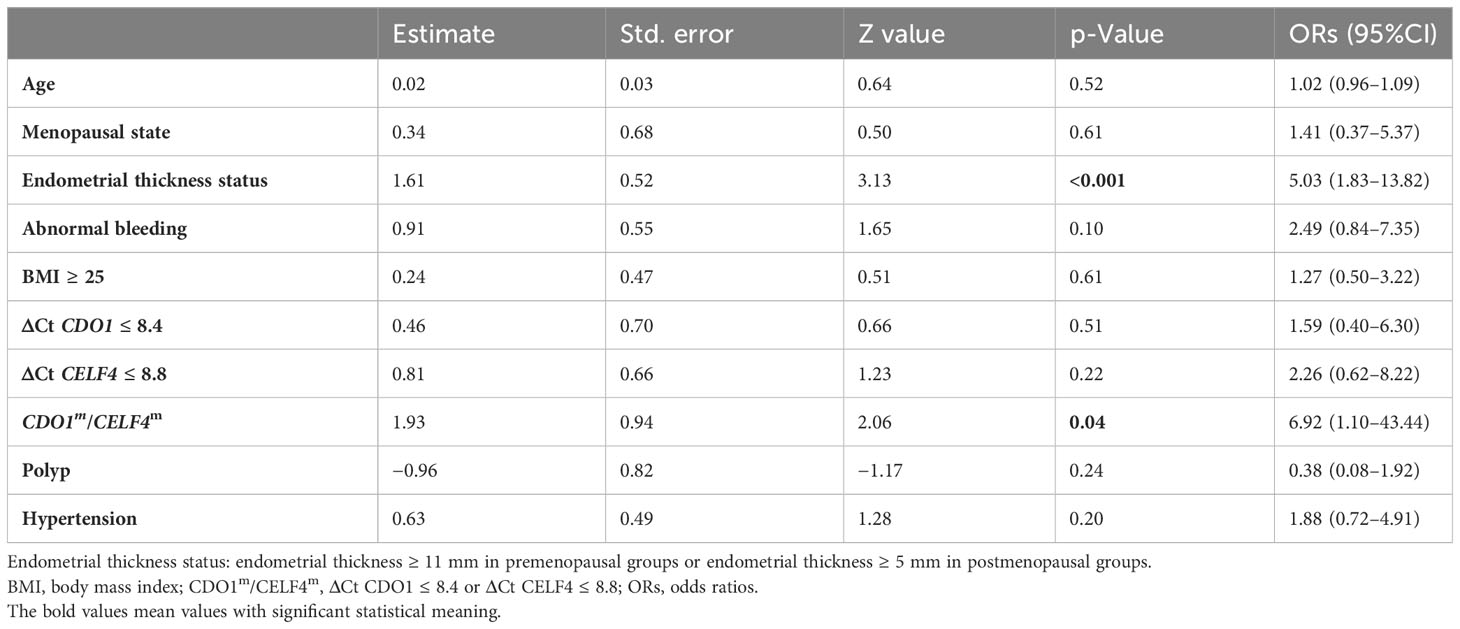
A total of 607 patients were divided into the endometrium malignant group (AH and EC, 53 patients) and the endometrium benign group (BE and EH, 554 patients). As shown in Table 2, women in the malignant group were aged 55.0 [52.0–59.0] years, and those in the benign group were aged 46.0 [40.0–51.0] years. Age was significantly different between the two groups (p < 0.05). In addition to age, menopausal state, endometrial thickness ≥ 11 mm (premenopausal), endometrial thickness ≥ 5 mm (postmenopausal), BMI ≥ 25 kg/m2, ΔCt CDO1 ≤ 8.4, ΔCt CELF4 ≤ 8.8, CDO1m/CELF4m, polyp, and hypertension were significantly different in the malignant group and the benign group (all p < 0.05). Multifactorial analysis revealed that endometrial thickness ≥ 11 mm (premenopausal) and endometrial thickness ≥ 5 mm (postmenopausal), ΔCt CDO1 ≤ 8.4, and ΔCt CELF4 ≤ 8.8 were risk factors for the development of endometrial malignant lesions, with odds ratios (ORs) of 5.03 (95%CI: 1.83–13.82) (p < 0.05) and 6.92 (95%CI: 1.10–43.44) (p = 0.04) in Table 3.
Clinical performance of different tests for endometrial malignant lesion detection
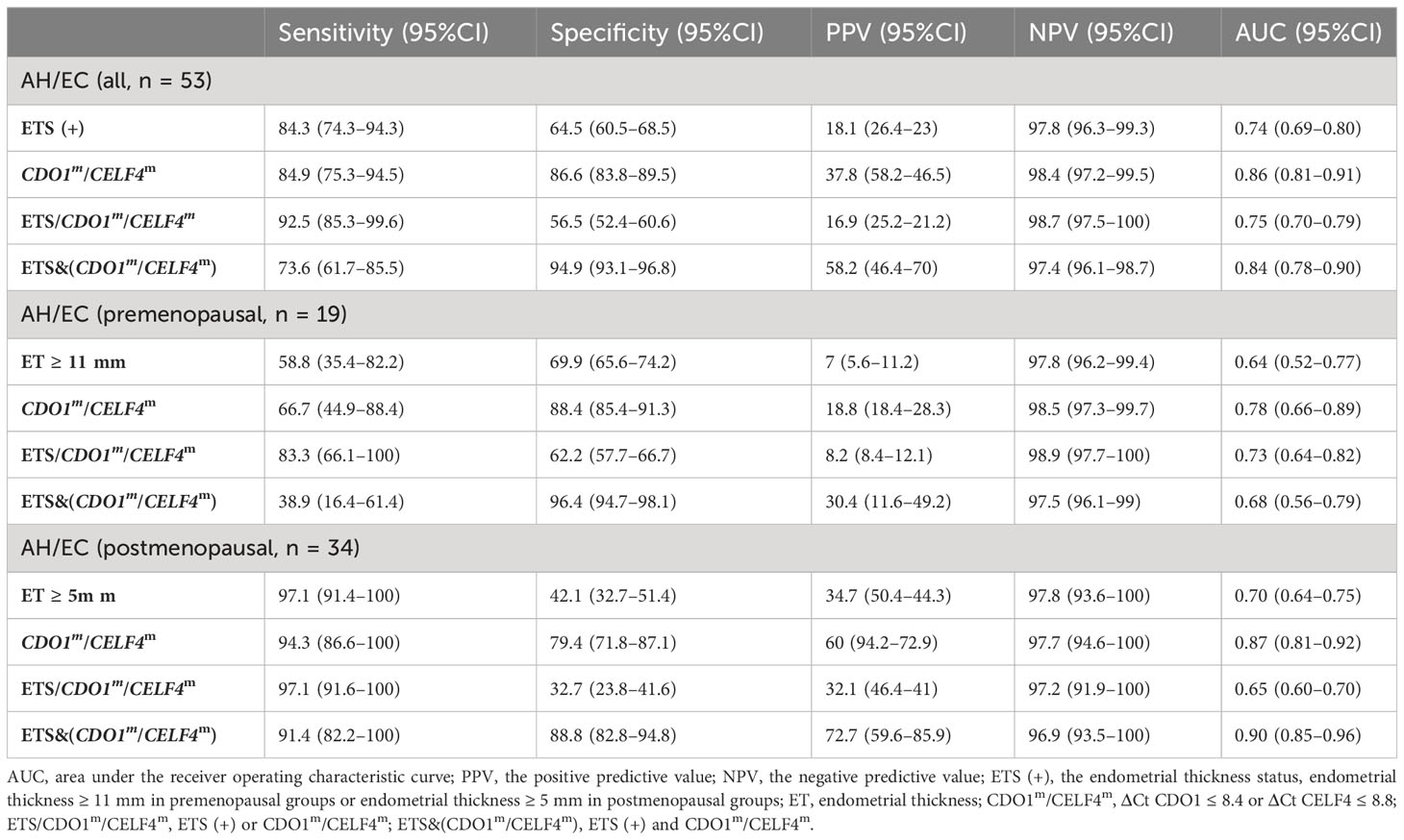
Table 4 reports the risk factors for the development of endometrial malignant lesions as the diagnostic performance test when applied to the study. In the study, the best AUC was 0.86 (0.81–0.91), with a sensitivity of 84.9% (75.3%–94.5%) and a specificity of 86.6% (83.8%–89.5%) by CDO1m/CELF4m for test endometrial malignant lesion patients. ETS/CDO1m/CELF4m test had the best sensitivity of 92.5% (85.3%–99.6%) and the best specificity of 94.9% (93.1%–96.8%) by ETS&(CDO1m/CELF4m) in all patients no matter the menopausal status. Compared with the premenopausal and postmenopausal groups, the AUC was 0.78 (0.66–0.89) by CDO1m/CELF4m, ET ≥ 11 mm/CDO1m/CELF4m test had the best sensitivity 83.8% (66.1%–100.0%), and ET ≥ 11 mm&(CDO1m/CELF4m) had the best specificity 96.4% (94.7%–98.1%) in the premenopausal group. ET ≥ 5 mm/CDO1m/CELF4m test had the best sensitivity of 97.1% (91.6%–100.0%), and ET ≥ 5 mm&(CDO1m/CELF4m) had the best AUC of 0.90 (0.85–0.96) and the best specificity of 88.8% (82.8%–94.8%) in the postmenopausal group.
Comparison of the accuracy of DNA methylation and transvaginal ultrasound
Validation of the diagnostic accuracy of the CDO1m/CELF4m test in 607 exfoliated cervical cell samples in the detection of EC and AH (Figure 3A), with endometrial malignant lesions as the outcome, resulted in an AUC of 0.86 (0.81–0.91), comparing endometrial benign groups. Stratification by menopausal status premenopausal and postmenopausal led to AUCs of 0.78 (0.66–0.89) and 0.87 (0.81–0.92), respectively (Figure 3B). DNA methylation combined with TVS could not further improve diagnostic accuracy in the premenopausal state [CDO1m/CELF4m: 0.78 (0.66–0.89) vs. ETS/CDO1m/CELF4m: 0.73 (0.64–0.82)] but can further improve diagnostic accuracy in the postmenopausal state (CDO1m/CELF4m: 0.87 (0.81–0.92) vs. ETS&(CDO1m/CELF4m): 0.90 (0.85–0.96)). The diagnostic accuracy of the CDO1m/CELF4m in the detection of endometrial malignant lesions has no significant difference in premenopausal and postmenopausal states (p = 0.1453).

Figure 3 Receiver operating characteristic (ROC) curve analysis of difference testing. (A) ROC for endometrial malignant lesions of difference testing. (B) ROC for endometrial malignant lesions of difference testing in different menopausal states. ETS, the endometrial thickness status, endometrial thickness ≥ 11 mm in premenopausal groups or endometrial thickness ≥ 5 mm in postmenopausal groups; CDO1m/CELF4m: ΔCt CDO1 ≤ 8.4 or ΔCt CELF4 ≤ 8.8; ETS/CDO1m/CELF4m: ETS (+) or CDO1m/CELF4m; ETS&(CDO1m/CELF4m): ETS (+) and CDO1m/CELF4m.
Discussion
In this study, a non-invasive liquid biopsy protocol with cervical exfoliative cytology was used to detect endometrium malignant lesions by targeted genetic testing in women with indications for hysteroscopy. We proved that CDO1 or CELF4 methylation tests have relatively high sensitivity, specificity, and AUC in cervical cytological detection for EC and AH. When these two candidate genes are tested in combination, they perform better in clinical applications due to higher negative predictive value and AUC.
The expert consensus on endometrial cancer screening and early diagnosis identified anovulatory abnormal uterine bleeding as a high-risk factor for endometrial cancer [16]. For screening with symptoms of vaginal bleeding or endometrial thickening on ultrasound, progestin therapy can be tried first, and hysteroscopic detection is recommended for those who fail to be treated. Pathological examination after diagnostic curettage is important for the diagnosis and guidance of treatment of abnormal uterine bleeding [17]. Although diagnostic curettage is short, it can cause pain in patients, thus making the procedure more difficult for the surgeon and increasing the risk of surgery for the patient. In this study, women with abnormal uterine bleeding, vaginal drainage, and imaging suggestive of abnormalities during routine gynecological examination with indications for hysteroscopic detection were selected for enrollment, and the advantages of TVS versus DNA methylation testing in the screening and early diagnosis of endometrial malignant lesions were compared and analyzed.
The selection of cervical exfoliated cells for DNA methylation testing for the diagnosis of endometrial cancer is an important innovation in this study. Studies have been conducted on the use of exfoliated cells for cytopathological analysis, target gene detection and genetic syndrome confirmation, exosome analysis, microsatellite instability (MSI) testing, and multi-omics testing (50–55). Recent foreign studies suggest that cervical exfoliative cell DNA methylation testing may be a patient-friendly tool for screening and triaging women with endometrial atypical hyperplasia or endometrial cancer-like conditions or at risk for endometrial malignancy, and because it is suitable for self-collected samples, it may be an appropriate tool for managing women with abnormal uterine bleeding, and future implementation of such testing protocols in preventive screening and early detection settings is being considered (56). However, no studies related to epigenetic analysis of exfoliated cytology have been conducted in China, and there is no reliable screening protocol for clinical application. The use of cervical exfoliated cells for endometrial cancer screening and diagnosis has the outstanding advantages of being completely non-invasive, easy to obtain, and highly accurate (57), having adequate cell volume, and being highly consistent with histological findings [15]. The association of epigenetic alterations in CDO1 and CELF4, the target genes selected in this study, with endometrial malignant lesions has been supported in the literature (33, 58). In histological specimens from EC and AH patients, CDO1 and ZNF454 are hypermethylated compared with benign and normal endometrium (p < 0.001). Also, in a total of 120 cytological specimens, the AUC of the diagnostic test and the methylation biomarker panel was 0.931, with a sensitivity of 9.91% and a specificity of 86.84% (39). In addition, another panel of three genes, BHLHE22, CDO1, and CELF4, including any two of the three hypermethylated genes, reached a sensitivity of 91.8% and a specificity of 95.5%. Different from previous studies, this study confirms the effectiveness of CDO1m/CELF4m in screening and triage of women with endometrial biopsy indications, and they can effectively diagnose and shunt women with symptoms or risks of AH and EC. In our research, the AUC was 0.86 (0.81–0.91), with a sensitivity of 84.9% (75.3%–94.5%) and a specificity of 86.6% (83.8%–89.5%) in CDO1m/CELF4m testing for endometrial malignant lesion patients.
Traditionally, ET is a very important indicator in endometrial cancer screening. TVS has become the preferred screening method for clinical diagnosis of endometrial disease with the advantages of being non-invasive, economical, and easy to perform (59). TVS is highly sensitive for screening endometrial cancer but has low specificity and often results in unnecessary invasive operations. The 2018 Cancer Report published by the International Federation of Obstetrics and Gynecology in October 2018 on endometrial cancer screening states that TVS combined with endometrial diagnostic scraping biopsy has a negative predictive value of 96%, but some patients do not want to undergo the invasive operation of segmental scraping (60, 61). In this study, DNA methylation assay (CDO1m/CELF4m) had a high sensitivity of 84.9% (75.3%–94.5%) and a specificity of 86.6% (83.8%–89.5%), and the combination of ETS/CDO1m/CELF4m could further improve the sensitivity 92.5% (85.3%–99.6%) of endometrial malignant lesion detection. Furthermore, the combined ETS&(CDO1m/CELF4m) can achieve a higher level of specificity of 94.9% (93.1%–96.8%). By combining with TVS in different ways, the specificity can be further enhanced, and the possibility of invasive manipulation is reduced. Whether this situation can be repeated and improved remains to be confirmed by large cohort studies. In this study, DNA methylation was an independent predictor of endometrial malignant lesions. DNA methylation had high sensitivity of 97.1% (91.6%–100%) in the postmenopausal subgroup, detecting more patients with endometrial malignant lesions, while in the non-menopausal group, it had higher specificity of 88.4% (85.4%–91.3%), providing better triage for patients with abnormal bleeding and endometrial thickening, avoiding overly invasive consultations in women of reproductive age, and serving to protect female fertility.
Previous studies have shown a correlation between serum glycoantigen CA125 and the pathological stage of endometrial cancer, and according to the trend of CA125, patients with endometrial cancer can be effectively screened to some extent. However, the rate of misdiagnosis and leakage is also relatively high, and it needs to be combined with other detection methods to improve diagnostic efficacy (62). In this study, there was no statistically significant difference in CA125 levels in the benign endometrial group, hyperplasia without atypia group, atypical hyperplasia group, and endometrial cancer group (p = 0.444). It may be related to the elevated CA125 levels in patients with uterine smooth muscle tumors, adenomyoma (or combined adenomyosis) in the benign endometrial group, and hyperplasia without the atypia group. BMI was not significantly different in the benign endometrial group, hyperplasia without atypia group, atypical hyperplasia group, and endometrial cancer group (p = 0.1). When patients were divided into the endometrium malignant group (AH and EC) and the endometrium benign group (BE and EH), the proportion of BMI ≥ 25 kg/m2 in the endometrium malignant group was 32 (60.38%), which was significantly higher than in the endometrium benign group at 249 (44.95%), χ2 = 0.313 and p = 0.043. A recent meta-analysis of 30 prospective studies reported that each 5 kg/m2 increase in BMI was associated with a 54% (95%CI: 47%–61%) higher risk of endometrial cancer (63, 64). The occurrence of endometrial polyps in the endometrium malignant group at 2 (3.77%) was significantly lower than in the endometrium benign group at 193 (34.84%), χ2 = 0.856 and p < 0.05. This is consistent with previous reports in the literature that most endometrial polyps are benign overgrowths of endometrial mucosa and that spontaneous regression can occur (65, 66).
In this study, cytological methylation testing was used for the detection of malignant endometrial lesions, which can reduce invasive testing of the uterus in women of reproductive age and provide a positive impetus to protect female fertility. The enrollment criteria for this study were the women with indications for endometrial biopsy, the vast majority of which were benign lesions, but malignant lesions were also easily overlooked. Methylation testing is objective, non-invasive, and accurate and can be accurately diagnosed for graded treatment in hospitals where pathologists have limited diagnostic skills. This study also has one limitation: this study selected patients screened in a single hospital gynecology department within 1 year, and the sample size of cancer (n = 39) and atypical hyperplasia patients (n = 14) included in the cohort study is small, with certain geographical limitations. There is an urgent need for future promotion in a large cohort and other problems.
Conclusions
We demonstrated the effectiveness of CDO1m/CELF4m in screening and triaging women with endometrial biopsy indications, and they can be effective in diagnosing and triaging women with symptoms or at risk for AH and EC. The CDO1 and CELF4 dual-gene methylation test provides a simple and highly accurate non-invasive management method for women with abnormal uterine bleeding, which reduces most invasive operations, reduces the psychological burden on patients, and improves patient compliance during the testing process.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Ethics statement
The study was approved by the Ethics Committee of the Cangzhou Central Hospital (No. 2023-144-02(z)), and it was conducted in accordance with the Declaration of Helsinki. Patients with indications for endometrial biopsy were enrolled and provided signed informed consent according to the Standards for Reporting of Diagnostic Accuracy Studies (STARD) in clinics.
Author contributions
BQ: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. YS: Formal analysis, Methodology, Writing – review & editing. YHL: Data curation, Methodology, Writing – review & editing. PH: Data curation, Formal analysis, Writing – review & editing. YM: Investigation, Methodology, Writing – review & editing. WG: Investigation, Methodology, Writing – review & editing. SML: Data curation, Software, Writing – review & editing. XZ: Conceptualization, Data curation, Writing – review & editing. XJ: Conceptualization, Data curation, Writing – original draft. YLL: Conceptualization, Writing – review & editing. PL: Funding acquisition, Writing – review & editing. SKL: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Authors XJ, YL, PL were employed by the company Beijing Origin-Poly Bio-Tec Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1289366/full#supplementary-material
References
1. Makker V, MacKay H, Ray-Coquard I, Levine DA, Westin SN, Aoki D, et al. Endometrial cancer. Nat Rev Dis Primers (2021) 7(1):88. doi: 10.1038/s41572-021-00324-8
2. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet (2022) 399(10333):1412–28. doi: 10.1016/s0140-6736(22)00323-3
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin (2017) 67(1):7–30. doi: 10.3322/caac.21387
4. Sobel M, Simpson AN, Ferguson SE. Endometrial cancer. Cmaj (2021) 193(36):E1423. doi: 10.1503/cmaj.202731
5. Doll KM, Tseng J, Denslow SA, Fader AN, Gehrig PA. High-grade endometrial cancer: revisiting the impact of tumor size and location on outcomes. Gynecol Oncol (2014) 132(1):44–9. doi: 10.1016/j.ygyno.2013.10.023
6. Benedetti Panici P, Basile S, Salerno MG, Di Donato V, Marchetti C, Perniola G, et al. Secondary analyses from a randomized clinical trial: age as the key prognostic factor in endometrial carcinoma. Am J Obstet Gynecol (2014) 210(4):363.e1–363.e10. doi: 10.1016/j.ajog.2013.12.025
7. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol (1983) 15(1):10–7. doi: 10.1016/0090-8258(83)90111-7
8. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet (2005) 366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8
9. Lu KH, Broaddus RR. Endometrial cancer. New Engl J Med (2020) 383(21):2053–64. doi: 10.1056/NEJMra1514010
10. Daud S, Jalil SS, Griffin M, Ewies AA. Endometrial hyperplasia - the dilemma of management remains: a retrospective observational study of 280 women. Eur J Obstet Gynecol Reprod Biol (2011) 159(1):172–5. doi: 10.1016/j.ejogrb.2011.06.023
11. Gentry-Maharaj A, Karpinskyj C. Current and future approaches to screening for endometrial cancer. Best Pract Res Clin Obstet Gynaecol (2020) 65:79–97. doi: 10.1016/j.bpobgyn.2019.12.006
12. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ (2007) 335(7630):1134. doi: 10.1136/bmj.39367.495995.AE
13. Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Ann Oncol (2016) 27(1):16–41. doi: 10.1093/annonc/mdv484
14. Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Giugliano D. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine (2014) 45(1):28–36. doi: 10.1007/s12020-013-9973-3
15. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care (2012) 35(11):2402–11. doi: 10.2337/dc12-0336
16. Luo J, Beresford S, Chen C, Chlebowski R, Garcia L, Kuller L, et al. Association between diabetes, diabetes treatment and risk of developing endometrial cancer. Br J Cancer (2014) 111(7):1432–9. doi: 10.1038/bjc.2014.407
17. Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecol Oncol (2009) 114(1):121–7. doi: 10.1016/j.ygyno.2009.03.039
18. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update (2014) 20(5):748–58. doi: 10.1093/humupd/dmu012
19. Zucchetto A, Serraino D, Polesel J, Negri E, De Paoli A, Dal Maso L, et al. Hormone-related factors and gynecological conditions in relation to endometrial cancer risk. Eur J Cancer Prev (2009) 18(4):316–21. doi: 10.1097/cej.0b013e328329d830
20. Achanna KS, Nanda J. Evaluation and management of abnormal uterine bleeding. Med J Malaysia (2022) 77(3):374–83.
21. Breijer MC, Mol BW. Transvaginal ultrasound measurement of the endometrium remains the first line test for investigating postmenopausal bleeding but integration of patient characteristics into testing may further improve diagnostic algorithms. BJOG (2016) 123(3):447. doi: 10.1111/1471-0528.13438
22. Singh S, Best C, Dunn S, Leyland N, Wolfman WL. No. 292-abnormal uterine bleeding in pre-menopausal women. J Obstet Gynaecol Can (2018) 40(5):e391–415. doi: 10.1016/j.jogc.2018.03.007
23. Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician (2016) 93(6):468–74.
24. Costas L, Frias-Gomez J, Guardiola M, Benavente Y, Pineda M, Pavon MA, et al. New perspectives on screening and early detection of endometrial cancer. Int J Cancer (2019) 145(12):3194–206. doi: 10.1002/ijc.32514
25. Park YR, Lee SW, Kim Y, Bae IY, Kim HK, Choe J, et al. Endometrial thickness cut-off value by transvaginal ultrasonography for screening of endometrial pathology in premenopausal and postmenopausal women. Obstet Gynecol Sci (2019) 62(6):445–53. doi: 10.5468/ogs.2019.62.6.445
26. Wong AS, Lao TT, Cheung CW, Yeung SW, Fan HL, Ng PS, et al. Reappraisal of endometrial thickness for the detection of endometrial cancer in postmenopausal bleeding: a retrospective cohort study. BJOG (2016) 123(3):439–46. doi: 10.1111/1471-0528.13342
27. Tangjitgamol S, Anderson BO, See HT, Lertbutsayanukul C, Sirisabya N, Manchana T, et al. Management of endometrial cancer in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol (2009) 10(11):1119–27. doi: 10.1016/s1470-2045(09)70290-6
28. van Hanegem N, Prins MM, Bongers MY, Opmeer BC, Sahota DS, Mol BW, et al. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol (2016) 197:147–55. doi: 10.1016/j.ejogrb.2015.12.008
29. Miyamoto T, Abiko K, Murakami R, Furutake Y, Baba T, Horie A, et al. Hysteroscopic morphological pattern reflects histological grade of endometrial cancer. J Obstet Gynaecol Res (2019) 45(8):1479–87. doi: 10.1111/jog.13998
30. Pan Y, Liu G, Zhou F, Su B, Li Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin Exp Med (2018) 18(1):1–14. doi: 10.1007/s10238-017-0467-0
31. Wang Y, Li L, Douville C, Cohen JD, Yen TT, Kinde I, et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci Transl Med (2018) 10(433):eaap8793. doi: 10.1126/scitranslmed.aap8793
32. Hao X, Luo H, Krawczyk M, Wei W, Wang W, Wang J, et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc Natl Acad Sci U.S.A. (2017) 114(28):7414–9. doi: 10.1073/pnas.1703577114
33. Huang RL, Su PH, Liao YP, Wu TI, Hsu YT, Lin WY, et al. Integrated epigenomics analysis reveals a DNA methylation panel for endometrial cancer detection using cervical scrapings. Clin Cancer Res (2017) 23(1):263–72. doi: 10.1158/1078-0432.Ccr-16-0863
34. Multinu F, Chen J, Madison JD, Torres M, Casarin J, Visscher D, et al. Analysis of DNA methylation in endometrial biopsies to predict risk of endometrial cancer. Gynecol Oncol (2020) 156(3):682–8. doi: 10.1016/j.ygyno.2019.12.023
35. Wentzensen N, Bakkum-Gamez JN, Killian JK, Sampson J, Guido R, Glass A, et al. Discovery and validation of methylation markers for endometrial cancer. Int J Cancer (2014) 135(8):1860–8. doi: 10.1002/ijc.28843
36. Reijnen C, van der Putten LJM, Bulten J, Snijders M, Kusters-Vandevelde HVN, Sweegers S, et al. Mutational analysis of cervical cytology improves diagnosis of endometrial cancer: A prospective multicentre cohort study. Int J Cancer (2020) 146(9):2628–35. doi: 10.1002/ijc.32686
37. Liu J, Ji C, Wang Y, Zhang C, Zhu H. Identification of methylation-driven genes prognosis signature and immune microenvironment in uterus corpus endometrial cancer. Cancer Cell Int (2021) 21(1):365. doi: 10.1186/s12935-021-02038-z
38. Yuan S, Sun X, Wang L. Prognostic values from integrated analysis of the nomogram based on RNA-binding proteins and clinical factors in endometrial cancer. Clin Med Insights Oncol (2022) 16:11795549221123620. doi: 10.1177/11795549221123620
39. Wang L, Dong L, Xu J, Guo L, Wang Y, Wan K, et al. Hypermethylated CDO1 and ZNF454 in cytological specimens as screening biomarkers for endometrial cancer. Front Oncol (2022) 12:714663. doi: 10.3389/fonc.2022.714663
40. Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, et al. Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology (2006) 147(7):3276–84. doi: 10.1210/en.2005-1007
41. Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res (2011) 343(2):289–302. doi: 10.1007/s00441-010-1086-1
42. Stipanuk MH, Ueki I, Roman HB, Valli A, Fieselmann K, Lam J, et al. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab (2011) 301(4):E668-84. doi: 10.1152/ajpendo.00151.2011
43. Chen M, Zhu JY, Mu WJ, Guo L. Cysteine dioxygenase type 1 (CDO1): Its functional role in physiological and pathophysiological processes. Genes Dis (2023) 10(3):877–90. doi: 10.1016/j.gendis.2021.12.023
44. Wagnon JL, Briese M, Sun W, Mahaffey CL, Curk T, Rot G, et al. CELF4 regulates translation and local abundance of a vast set of mRNAs, including genes associated with regulation of synaptic function. PloS Genet (2012) 8(11):e1003067. doi: 10.1371/journal.pgen.1003067
45. Yu M, Xiang Y, Ma XX, Xue FX, Feng LM, Wang DB, et al. Advices on standards of endometrial cancer screening. Chin J Obstetrics Gynecology (2020) 55(05):307–7. doi: 10.3760/cma.j.cn112141-20200201-00070
46. Association GEGotOaGBotCM. Guideline on diagnosis and treatment of abnormal uterine bleeding: 2022 revisions. Chin J Obstetrics Gynecology (2022) 57(07):481–90. doi: 10.3760/cma.j.cn112141-20220421-00258
47. Li L CX, Cui MH. Chinese guideline on the management of endometrial hyperplasia. Chin J Obstetrics Gynecology (2022) 57(08):566–74. doi: 10.3760/cma.j.cn112141-20220628-00418
48. Menopause Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Chinese guideline on menopause management and menopause hormone therapy. Chin J Obstet Gynecol (2018) 53(11):729–39. doi: 10.3760/cma.j.issn.0529-567x.2018.11.001
49. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Bmj (2015) 351:h5527. doi: 10.1136/bmj.h5527
50. Odashima H, Yoshioka H, Ota K, Goto Y, Noro M, Horie K, et al. Morphological differences between liquid-based cytology and conventional preparation in endometrial endometrioid carcinoma grade 1 and grade 3, and the differentiation of grades in each method. Acta Cytol (2021) 65(3):227–34. doi: 10.1159/000512867
51. Akahane T, Kitazono I, Yanazume S, Kamio M, Togami S, Sakamoto I, et al. Next-generation sequencing analysis of endometrial screening liquid-based cytology specimens: a comparative study to tissue specimens. BMC Med Genomics (2020) 13(1):101. doi: 10.1186/s12920-020-00753-6
52. Buglyó G, Styk J, Pös O, Csók Á, Repiska V, Soltész B, et al. Liquid biopsy as a source of nucleic acid biomarkers in the diagnosis and management of lynch syndrome. Int J Mol Sci (2022) 23(8):4284. doi: 10.3390/ijms23084284
53. Silveira AB, Bidard FC, Kasperek A, Melaabi S, Tanguy ML, Rodrigues M, et al. High-accuracy determination of microsatellite instability compatible with liquid biopsies. Clin Chem (2020) 66(4):606–13. doi: 10.1093/clinchem/hvaa013
54. Srivastava A, Moxley K, Ruskin R, Dhanasekaran DN, Zhao YD, Ramesh R. A non-invasive liquid biopsy screening of urine-derived exosomes for miRNAs as biomarkers in endometrial cancer patients. AAPS J (2018) 20(5):82. doi: 10.1208/s12248-018-0220-y
55. Martinez-Garcia E, Lesur A, Devis L, Cabrera S, Matias-Guiu X, Hirschfeld M, et al. Targeted proteomics identifies proteomic signatures in liquid biopsies of the endometrium to diagnose endometrial cancer and assist in the prediction of the optimal surgical treatment. Clin Cancer Res (2017) 23(21):6458–67. doi: 10.1158/1078-0432.Ccr-17-0474
56. Herzog C, Marín F, Jones A, Evans I, Reisel D, Redl E, et al. A simple cervicovaginal epigenetic test for screening and rapid triage of women with suspected endometrial cancer: validation in several cohort and case/control sets. J Clin Oncol (2022) 40(33):3828–38. doi: 10.1200/jco.22.00266
57. Verhoef VM, Bosgraaf RP, van Kemenade FJ, Rozendaal L, Heideman DA, Hesselink AT, et al. Triage by methylation-marker testing versus cytology in women who test HPV-positive on self-collected cervicovaginal specimens (PROHTECT-3): a randomised controlled non-inferiority trial. Lancet Oncol (2014) 15(3):315–22. doi: 10.1016/s1470-2045(14)70019-1
58. Liew PL, Huang RL, Wu TI, Liao CC, Chen CW, Su PH, et al. Combined genetic mutations and DNA-methylated genes as biomarkers for endometrial cancer detection from cervical scrapings. Clin Epigenet (2019) 11(1):170. doi: 10.1186/s13148-019-0765-3
59. ACOG Committee Opinion No. 734: The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol (2018) 131(5):e124–9. doi: 10.1097/AOG.0000000000002631
60. Bhatla N, Denny L. FIGO cancer report 2018. Int J Gynaecol Obstet (2018) 143 Suppl 2:2–3. doi: 10.1002/ijgo.12608
61. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet (2021) 155(Suppl 1):28–44. doi: 10.1002/ijgo.13865
62. Kakimoto S, Miyamoto M, Einama T, Takihata Y, Matsuura H, Iwahashi H, et al. Significance of mesothelin and CA125 expression in endometrial carcinoma: a retrospective analysis. Diagn Pathol (2021) 16(1):28. doi: 10.1186/s13000-021-01093-4
63. Aune D, Navarro Rosenblatt DA, Chan DSM, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose–response meta-analysis of prospective studies. Ann Oncol (2015) 26(8):1635–48. doi: 10.1093/annonc/mdv142
64. Shams-White MM, Brockton NT, Mitrou P, Romaguera D, Brown S, Bender A, et al. Operationalizing the 2018 world cancer research fund/american institute for cancer research (WCRF/AICR) cancer prevention recommendations: a standardized scoring system. Nutrients (2019) 11(7):1572. doi: 10.3390/nu11071572
65. Lieng M, Istre O, Sandvik L, Qvigstad E. Prevalence, 1-year regression rate, and clinical significance of asymptomatic endometrial polyps: cross-sectional study. J Minim Invasive Gynecol (2009) 16(4):465–71. doi: 10.1016/j.jmig.2009.04.005
Keywords: abnormal uterine bleeding, postmenopausal bleeding, atypical hyperplasia, endometrial cancer, diagnosis, DNA methylation biomarker, cysteine dioxygenase type 1 (CDO1), UGBP Elav-like family member 4 (CELF4)
Citation: Qi B, Sun Y, Lv Y, Hu P, Ma Y, Gao W, Li S, Zhang X, Jin X, Liou Y, Liu P and Liu S (2023) Hypermethylated CDO1 and CELF4 in cytological specimens as triage strategy biomarkers in endometrial malignant lesions. Front. Oncol. 13:1289366. doi: 10.3389/fonc.2023.1289366
Received: 05 September 2023; Accepted: 16 November 2023;
Published: 01 December 2023.
Edited by:
Mihaela Carmen Cristea, Regeneron Pharmaceuticals, Inc., United StatesReviewed by:
Nupur Mukherjee, National Institute for Research in Reproductive Health (ICMR), IndiaYara Lucia Furtado, Federal University of Rio de Janeiro, Brazil
Copyright © 2023 Qi, Sun, Lv, Hu, Ma, Gao, Li, Zhang, Jin, Liou, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shikai Liu, Y29tbWFzbGxAMTYzLmNvbQ==
 Bingli Qi
Bingli Qi Ye Sun1
Ye Sun1