- 1Department of Oncology, First Affiliated Hospital of Dalian Medical University, Dalian Medical University, Dalian, China
- 2Department of Breast Surgery, First Affiliated Hospital of Dalian Medical University, Dalian Medical University, Dalian, China
- 3Department of Pathology, First Affiliated Hospital of Dalian Medical University, Dalian Medical University, Dalian, China
- 4Imaging and Nuclear Medicine Department, First Affiliated Hospital of Dalian Medical University, Dalian Medical University, Dalian, China
- 5Nuclear Medicine Department, First Affiliated Hospital of Dalian Medical University, Dalian Medical University, Dalian, China
- 6Department of Radiotherapy, First Affiliated Hospital of Dalian Medical University, Dalian Medical University, Dalian, China
Background/aim: Human epidermal growth factor receptor 2 (HER2)-positive breast cancer is associated with a higher risk of metastasis and poorer overall survival (OS) due to HER2 gene overexpression/amplification. Although anti-HER2 targeted therapy has shown survival benefits in HER2-positive advanced breast cancer (ABC) patients, long-term treatment often leads to drug resistance, complicating further treatment options. RC48, an antibody-drug conjugate (ADC), combines the benefits of antibody targeting with the cytotoxic effects of a small molecule drug.
Case report: We present a case involving a female patient with HER2-positive ABC who developed drug resistance and disease progression following multi-line anti-HER2 targeted therapy. In this instance, RC48 exhibited anti-tumor activity in an ABC patient resistant to HER2-targeted therapy. After eight treatment cycles with 120 mg of RC48, the tumor size decreased and stabilized.
Conclusion: This case report underscores the potential clinical value of RC48 as a promising treatment alternative for patients resistant to HER2 targeted therapies.
1 Introduction
Breast cancer is the most common malignant tumor worldwide, with the highest incidence and the second highest mortality in women (1). Human epidermal growth factor receptor 2 (HER2)-positive breast cancer represents approximately 15-20% of all breast cancer cases. This tumor subtype, HER2-positive breast cancer, is more invasive, prone to metastasis, and associated with poorer prognosis (2). Anti-HER2 therapy can greatly improve the survival rate of patients; however, after multi-line treatment, the majority of advanced breast cancer (ABC) patients eventually develop drug resistance, leading to disease progression, and the limited subsequent treatment options (3). Currently, the mechanisms underlying resistance to anti-HER2 targeted therapy are under study. Research has indicated that resistance may be associated with spatial effects (structural mutations of the HER2 protein), overexpression of tyrosine kinase receptors [such as the insulin-like growth factor receptor (IGFR)], and mutations in the HER2 downstream signaling pathway (4). Overcoming resistance to anti-HER2 targeted therapy is crucial, and identifying new effective treatment strategies can provide valuable insights for clinical practice.
Currently, the primary treatment for HER2-positive ABC involves anti-HER2 targeted therapy drugs, often utilizing a combination of trastuzumab and pertuzumab as first-line treatment (3). The combination of trastuzumab and pertuzumab synergistically enhances the inhibition of the HER2 pathway (5).
The study of von Minckwitz et al. (6) showed that the HER2 pathway could be further inhibited when the first-line treatment disease progresses, and subsequent targeted therapy still has application value. Trastuzumab emtansine (T-DM1) is a novel antibody-drug conjugate (ADC). Verma et al. (7) assessed the utility of T-DM1 in patients who experienced disease progression following trastuzumab treatment. The T-DM1 treatment group had an objective response rate (ORR) of 43.6%, a median progression-free survival (PFS) of 9.6 months, and overall survival (OS) of 30.9 months, with P < 0.0017. These results indicate that T-DM1 could serve as a preferred drug for targeted therapy after drug resistance develops. DS-8201 (T-DXd) is another novel ADC drug. In a phase III clinical trial (NCT03529110), T-DXd treatment demonstrated significant OS and PFS benefits (28.8 months vs. 6.8 months) compared to T-DM1, and T-DXd substantially reduced the risk of death by 36% (HR, 0.64) (8).
Disitamab Vedotin (RC48) is an ADC with a higher affinity for HER2 than the targeted drug trastuzumab (KD, 5.0E-10M vs KD, 1.9E-09M) (9). By utilizing antibodies, RC48 can block the downstream signaling pathway activated by HER2, work in conjunction with cytotoxic drugs to kill tumor cells and interfere with the transcription, division, proliferation, and growth of cancer cells to exert anti-tumor effects. In a clinical trial (NCT02881190), RC48 demonstrated stronger antitumor activity in HER2-positive breast cancer, gastric cancer, and trastuzumab and lapatinib-resistant xenograft tumor models than the FDA-approved T-DM1 (10, 11).
However, to the best of our knowledge, there have been limited studies reporting the efficacy of RC48 monotherapy in ABC patients resistant to HER2-targeted therapy. Therefore, we present a case of a female patient with HER2-positive ABC who developed resistance to multiple lines of anti-HER2 therapy. This patient experienced significant benefits and ultimately became eligible for surgery following treatment with RC48. This case report offers new insights into addressing resistance to HER2 targeted therapy in clinical practice.
2 Case report
A 54-year-old female patient was admitted to an external hospital on December 15, 2019 due to the discovery of a right breast tumor for 2 weeks. A physical examination revealed a tumor in the upper quadrant of the right breast. Ultrasound-guided right breast tumor biopsy pathology indicated non-specific invasive breast cancer in the right breast; immunohistochemical staining results showed that estrogen receptor (ER) (70% weak-moderate intensity +), progesterone receptor (PR) (40% weak-moderate intensity +), HER2 (3 +), proliferation index (Ki67) (80%); right axillary lymph node biopsy cytology showed cancer metastasis. The patient was diagnosed with right breast invasive breast cancer (cT4N1M0 stage III). The patient began anti-tumor treatment in July 2020 after half a year, and had been treated with multiple lines. The treatment is shown in Table 1.
On July 14, 2020, the patient began neoadjuvant chemotherapy, receiving two cycles of albumin-bound paclitaxel combined with fluorouracil (specific dose: albumin-bound paclitaxel 125mg/m2 d1 d8 ivgtt, 5FU 500mg/m2 d1 ivgtt, with 21 days per cycle), during which the tumor continued to grow. On August 27, 2020, the patient underwent a PET-CT examination, which revealed right breast cancer with right axillary lymph node metastasis and a response of progressive disease (PD).
On September 1, 2020, the neoadjuvant treatment regimen was changed to albumin-bound paclitaxel, carboplatin, and trastuzumab for two cycles (actual dose: albumin-bound paclitaxel 200mg d1, d8 ivgtt, carboplatin 700mg d1 ivgtt, trastuzumab 580mg for the first dose, followed by 430mg ivgtt d1 q21d). Since the tumor did not shrink after two chemotherapy cycles, pertuzumab (initially 840mg, followed by 420mg ivgtt d1) was added for two cycles. After completing four treatment cycles, the PET-CT findings from November 20, 2020, were juxtaposed with the data from August 27, 2020, for comparative analysis. The right breast tumor had reduced in size, and the right axillary lymph node had also decreased in size (Figure 1), achieving the best response of partial response (PR). For economic reasons, the patient discontinued treatment on their own accord.

Figure 1 PET-CT results of patients at different time. On August 27, 2020, (A, B) multiple lymph nodes in the right axilla were enlarged, with a large diameter of about 1.5 cm, SUVmax: 9.1; (C) 3.7 cm x 3.8 cm tumor was seen in the right breast nipple, SUVmax: 22.4; (D-F) after four cycles of treatment (November 20, 2020), the right breast tumor and axillary lymph nodes were smaller than before.
In September 2021, the right breast tumor locally enlarged and ulcerated. A chest CT scan revealed multiple enlarged lymph nodes in the bilateral axilla, which were larger and more numerous than before. On September 27, 2021, the patient began first-line treatment with two cycles of albumin-bound paclitaxel, capecitabine, trastuzumab, and pertuzumab (actual dose: albumin-bound paclitaxel 200mg d1, d8 ivgtt, capecitabine 1000mg/m2 d1-d14 bid po, trastuzumab 430mg ivgtt d1 q21d, pertuzumab 420mg ivgtt d1). Due to economic factors, the chemotherapy drug was switched to docetaxel, and the actual medication regimen was docetaxel, trastuzumab, and pertuzumab for two cycles. After four cycles of treatment, the tumor was visibly larger, and the bleeding became severe. A chest CT scan showed that the bilateral axillary lymph nodes had increased in size compared to before, with a response of PD.
Due to disease progression following first-line treatment, the patient underwent second-line treatment on December 27, 2021, with four cycles of capecitabine, pyrotinib, trastuzumab, and pertuzumab (actual dose: capecitabine 1000mg/m2 d1-d14 bid po, pyrotinib 400mg qd po, trastuzumab 430mg ivgtt d1 q21d, pertuzumab 420mg ivgtt d1). During treatment, the tumor ruptured, and bleeding was stopped using an external application of Yunnan Baiyao powder. On March 17, 2022, a chest CT scan was reviewed, showing that the bilateral axillary lymph nodes had increased in size compared to before, with a response of PD. Considering the possibility of drug resistance, the patient was advised to undergo genetic testing. The results revealed a PIK3CA exon 10 c.1624G>A p. E542K missense mutation, but related targeted drugs were not yet available to us.
On March 18, 2022, the patient underwent third-line chemotherapy with two cycles of Inetetamab, vinorelbine, and cisplatin (specific drugs: Inetetamab 550mg ivgtt d1 q21d, vinorelbine 40mg ivgtt d1, d5 q21d, cisplatin 60mg ivgtt d1 q21d). On June 9, 2022, PET-CT results showed that the original right breast tumor had significantly increased in size compared to before, and the original right axillary lymph nodes were larger and more numerous than before. New lymph nodes with increased FDG metabolism appeared in the bilateral chest wall, right internal mammary region, and left axilla, indicating disease progression once again, with a response of PD.
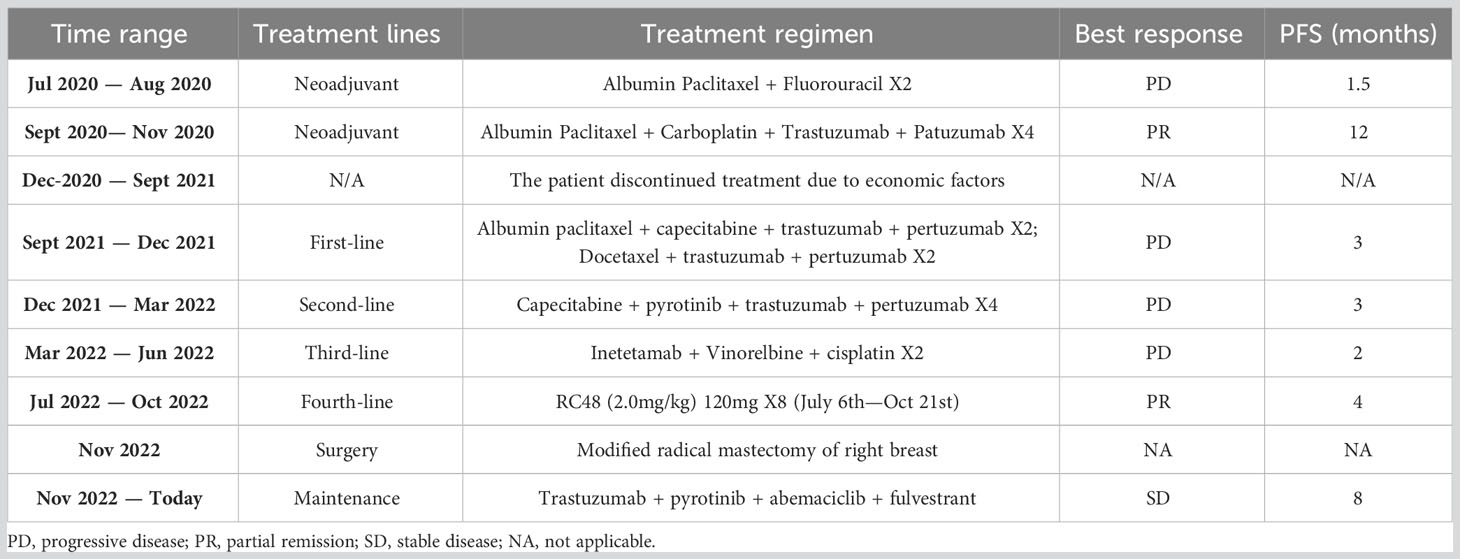
On June 16, 2022, a right axillary lymph node biopsy revealed poorly differentiated carcinoma. Immunohistochemistry results were as follows: ER (3 + 80%), PR (–), HER2 (3+), and Ki-67 (+ 2%). The immunohistochemical results confirmed that the patient still had HER2 overexpression. Considering the chemotherapy and targeted resistance after previous multi-line chemotherapy, the patient was given RC48 as a fourth-line treatment, which is administered in a cycle every 14 days with the treatment being given on the first day of each cycle. On July 6, 2022, eight cycles of RC48 treatment were initiated, with the planned medication regimen as follows: RC48 (2.0 mg/kg) 120mg ivgtt d1 q14d. Before treatment, the patient experienced significant pain and required analgesic drugs for pain control. After RC48 treatment, the pain considerably improved, and the dosage of analgesics was significantly reduced. During the treatment period, the ulcerated tumor gradually healed (Figure 2A), the patient tolerated the treatment well, and no obvious adverse reactions occurred. After six cycles, a chest CT scan showed that the breast tumor and bilateral axillary lymph nodes had significantly reduced in size, with the best response of PR (Figure 2B).
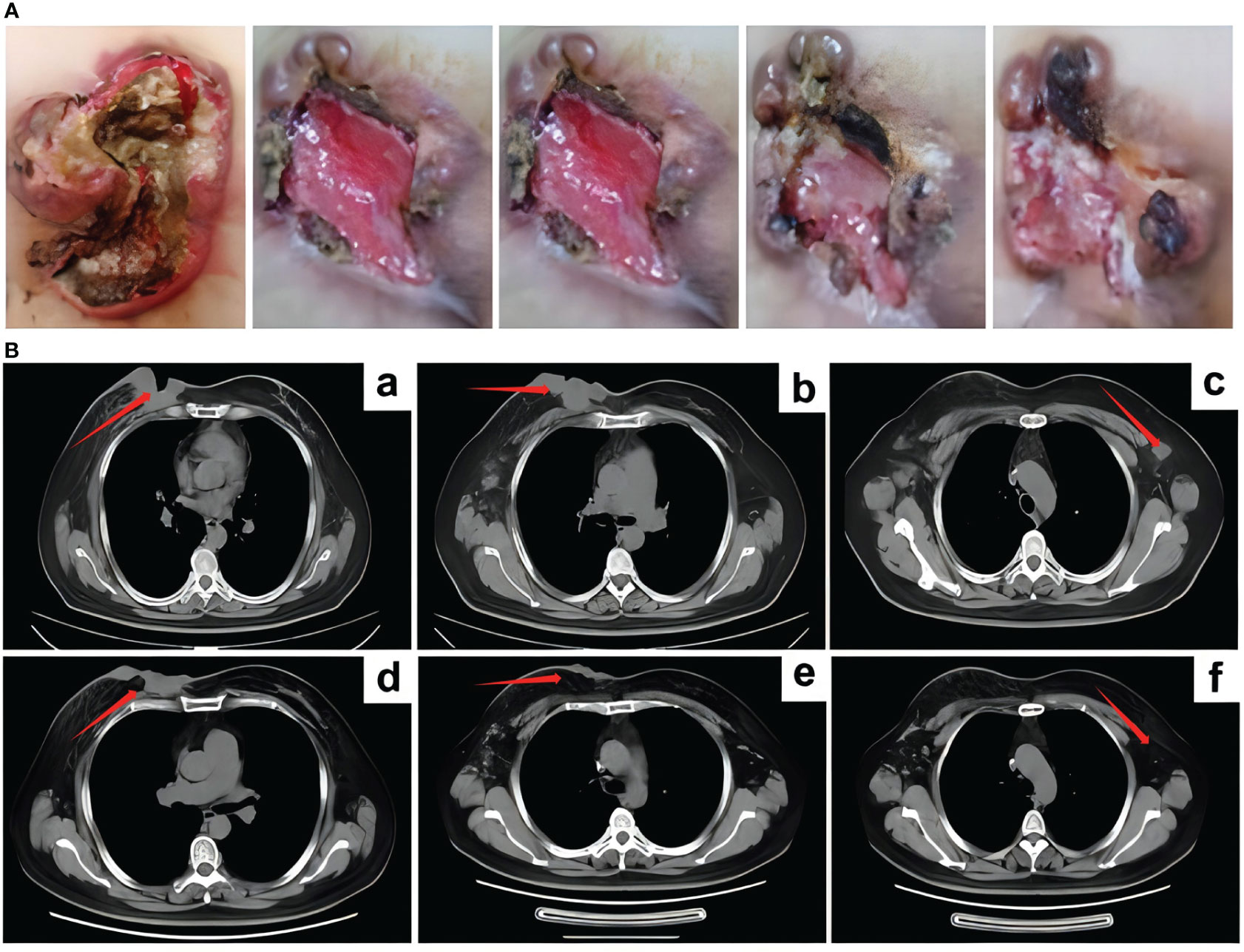
Figure 2 The curative effect of RC48 treatment. (A) After RC48 treatment, the tumor rupture was smaller than before. (B) CT efficacy evaluation after 6 cycles of RC48 treatment. (a-c) Patient status before RC48 treatment; (d-f) after RC48 treatment, the breast tumor and bilateral axillary lymph nodes were significantly reduced.
In order to explore the improvement in quality of life brought about by RC48, we used the FACT-B scale to evaluate the patient-reported outcomes (PROs). After using RC48, the patient experienced significant improvements in the five functional areas and overall health compared to their previous quality of life (Figure 3A). In the process of multi-line anti-HER-2 treatment, the patient often has adverse reactions such as bone marrow suppression and limb end numbness, among which nausea and vomiting most affect the quality of life. During the treatment with RC48, the patient feels good about herself, no obvious systemic adverse reactions occurred, and the overall quality of life was significantly improved.
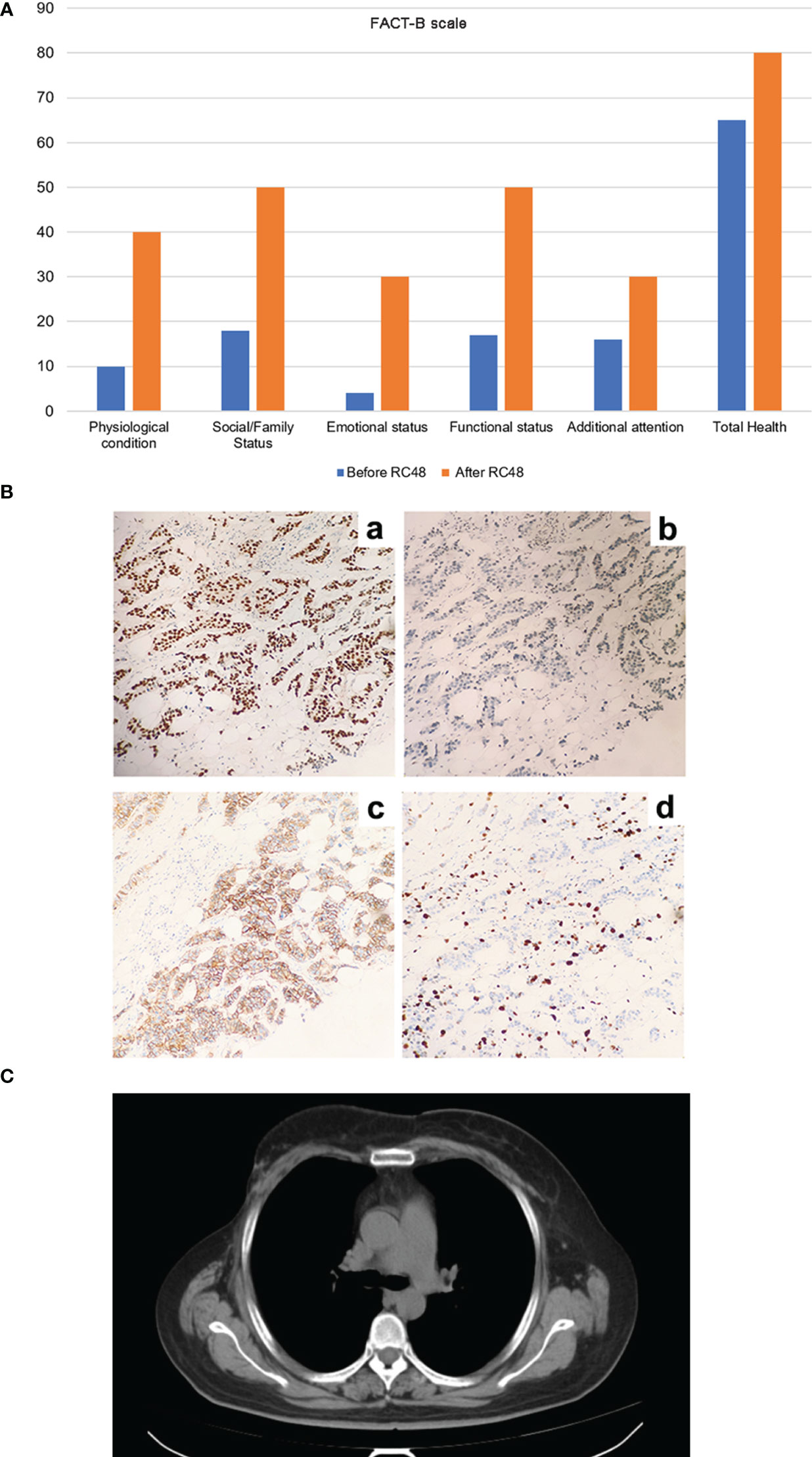
Figure 3 The improvement of patient’s life quality and postoperative immunohistochemical results of right breast cancer after RC48 treatment, as well as the CT results after half a year (July 12, 2023). (A) The five functional areas and overall health level of patients were significantly improved, and the quality of life was significantly improved. (B) The postoperative immunohistochemical results of right breast cancer. (a) ER (2 + 60%), (b) PR (5%), (c) HER2 (2 +), (d) Ki67 proliferation index (60%). (C) The patient ‘s CT results showed no enlarged lymph nodes, and the condition was stable.
After six cycles of RC48 treatment, the tumor shrank and stabilized, with the 7th and 8th cycles continuing to show stability. In order to further improve the quality of life, a multidisciplinary team of breast cancer specialists (MDT) recommended a modified radical mastectomy of the right breast cancer, which was performed on November 21, 2022. Postoperative pathology revealed non-specific invasive carcinoma in the right breast and metastatic carcinoma in the right axillary and subclavian lymph nodes. Immunohistochemical results for the right breast lesions showed: ER (2 + 60%), PR (+ 5%), HER2 (2+), and Ki67 (60%) (Figure 3B); HER2 FISH was positive.
Considering that the patient’s contralateral axillary enlarged lymph node was a metastatic lymph node, it was recommended that the patient continue maintenance treatment after surgery. The patient’s postoperative pathology was positive for hormone receptor (HR+) and HER2 overexpression. The recommended treatment was trastuzumab + pyrotinib targeted therapy, combined with abemaciclib + fulvestrant targeted dual pathway endocrine therapy (specific medications: trastuzumab 430mg ivgtt d1 q21d, pyrotinib 400mg qd po, abemaciclib 50mg bid po, fulvestrant 500mg intramuscular injection). The patient’s condition was stable half a year after operation. The CT results showed that the right breast was changed after operation (Figure 3C).
3 Discussion
HER2 is a transmembrane protein with tyrosine kinase activity. It mediates signal transduction activation and downstream signaling pathways through heterodimer and tyrosine kinase autophosphorylation (12). HER2-positive tumors are prone to metastasis, and most have a poor prognosis. Anti-HER2 targeted therapy can greatly improve the survival rate of breast cancer (13). Trastuzumab combined with chemotherapy in the treatment of HER2-positive ABC patients can significantly prolong the disease-free survival (DFS) and OS. However, most patients may develop drug resistance after treatment. More than 70% of HER2-positive breast cancer patients have tumor recurrence, lymph node metastasis, etc (4).
The issue of drug resistance is crucial for patients’ prognosis. Currently, the mechanisms of targeted therapy resistance mainly involve changes in the HER2 receptor molecular structure, alterations in the PI3K/AKT/mTOR signaling pathway, and influences of the HER family on downstream signaling pathways. Other mechanisms include incomplete blocking of the HER2 signaling pathway, involvement of immune mechanisms, and changes in oncogenes/tumor suppressor genes (14–19). The patient’s genetic test suggested a missense mutation in PIK3CA, which may be the cause of drug resistance. However, due to the unavailability of related signaling pathway inhibitors, targeted treatment was not possible.
In addition, other factors affecting drug resistance and efficacy, which are clinically important, should not be overlooked. Our case report highlights a treatment gap in one patient, which may have also contributed to the observed drug resistance. Due to financial difficulties and adherence issues, our patient discontinued treatment, which may lead to a decrease in drug efficacy and the development of resistance, especially to targeted therapies such as anti-tumor necrosis factor-2 (anti-HER-2). Suspension of treatment after initial efficacy of neoadjuvant therapy may lead to the development of drug resistance in tumor cells, which may affect the efficacy of subsequent anti-HER-2 therapy. In addition, the coronavirus pandemic poses a healthcare challenge. Our patient’s treatment interruption coincided with the coronavirus epidemic. Although the treatment interruption was not directly caused by the epidemic, the economic impact may have exacerbated the patient’s financial stress, which may have influenced her decision to continue treatment.
RC48 is an antibody-coupled drug with both antibody targeting and small molecule drug killing properties. Relevant clinical studies have confirmed its efficacy in the treatment of ABC. Two phase-I clinical studies, NCT02881138 and NCT03052634 (20, 21), evaluated the efficacy of RC48 in patients with HER2-positive (IHC 3+ or IHC 2+ and FISH amplification) locally advanced or metastatic breast cancer. We performed a combined analysis of the two studies (20, 21) to assess the efficacy. Among the 70 patients treated with RC48, the ORR was 31.4% (22/70), the clinical benefit rate (CBR) was 38.6%, and the median PFS was 5.8 months. Among the 64 patients receiving ≥ 1.5 mg/kg dose, the ORR was 34.4% (22/64), and the median PFS was 6.2 months. The ORR of patients receiving 1.5 mg/kg, 2.0 mg/kg, and 2.5 mg/kg doses was 22.2%, 42.9%, and 36.0%, respectively, and the median PFS was 6.2 months, 6.0 months, and 6.3 months, respectively. It can be observed that RC48 demonstrates a favorable effect in HER2 positive metastatic breast cancer, and 2.0 mg/kg Q2W is the optimal choice. The author’s intention here is to convey an integrated analysis of the two aforementioned studies.
In another phase I clinical study (NCT02881190) (11), 57 patients with solid tumors were enrolled and treated with RC48 at a dose of 2.0 mg/kg Q2W. The ORR and disease control rate (DCR) were 21.0% (12/57) and 49.1% (28/57), respectively. Notably, the efficacy of RC48 in HER2+/FISH- patients was similar to that of IHC2+/FISH+ and IHC3+ patients, with ORRs of 35.7% (5/14), 20.0% (2/10), and 13.6% (3/22), respectively. RC48 also demonstrated promising efficacy in patients who had not previously received HER2-targeted therapy, with an ORR of 15.0% (3/20) and a DCR of 45.0% (9/20). These results suggest that RC48 may have a beneficial effect on HER2-positive or low-expressing solid tumors, further highlighting its potential as a therapeutic option for these patients.
The study by Parise C et al. (22) demonstrated that approximately 50% of HER2-positive breast cancer patients also express hormone receptors (HR), that is, HER2+/HR+ or HER2+/ER+/PR+, accounting for 10%-15% of all breast cancers. HER2 and ER-mediated signaling pathways in breast cancer patients intersect at multiple nodes. ER can affect apoptosis by activating downstream pathways such as HER2, thereby influencing the efficacy of anti-HER2 targeted therapy (23). The Monarch HER study included ABC patients who received at least two anti-HER2 regimens and those who did not receive CDK4/6 inhibitors and fulvestrant. The results showed that, compared to standard chemotherapy plus trastuzumab, abemaciclib plus trastuzumab plus fulvestrant significantly improved PFS in HER2+/ER+ ABC patients resistant to anti-HER2 therapy (8.3 months vs. 5.7 months, P=0.051) (24). According to the final OS results presented at the European Society for Medical Oncology (ESMO) in 2022 (25), abemaciclib plus trastuzumab combined with or without fulvestrant significantly improved the OS of HR+/HER2+ ABC patients compared to standard chemotherapy plus trastuzumab (31.1 months vs. 20.7 months). These findings provide guidance for our postoperative maintenance therapy.
For HER2-positive breast cancer patients, chemotherapy combined with targeted therapy is predominantly used in first-line treatment, significantly prolonging survival and improving the quality of life (26). In our case, the patient was initially diagnosed with invasive breast cancer and axillary lymph node metastasis. Immunohistochemical results showed HER2 (3+), indicating that dual-target combined chemotherapy could provide the greatest benefits (27). The patient experienced disease progression after neoadjuvant therapy and third-line anti-HER2 combined chemotherapy, and the local tumor ulceration severely impacted her quality of life. Faced with multiple drug resistance, we opted for 8 cycles of RC48 treatment. The tumor shrank significantly, the ulceration improved considerably, the patient became eligible for surgical treatment, and her quality of life improved substantially. For postoperative treatment, we employed trastuzumab + pyrotinib targeted therapy in conjunction with abemaciclib + fulvestrant maintenance therapy. This approach resulted in acceptable tumor control and a stable condition for the patient. However, if the patient experiences a relapse, determining the subsequent treatment plan will be a significant challenge.
We report the successful treatment of a HER2-positive ABC patient using RC48 monotherapy. After the failure of multiple lines of anti-HER2 treatment, the use of ADC drugs was proved effective, providing the opportunity for surgical treatment and improving the patient’s quality of life. Our case offers new therapeutic options for other ABC patients resistant to HER2 treatment, holding clinical reference value and presenting new treatment ideas for patients with refractory HER2-targeted therapy resistance.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of First Affiliated Hospital of Dalian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Conceptualization, Data curation, Methodology, Writing – original draft. JZ: Conceptualization, Software, Writing – original draft. ZC: Validation, Writing – review & editing. XG: Validation, Writing – review & editing. LZ: Validation, Writing – review & editing. ZL: Formal Analysis, Writing – review & editing. XW: Formal Analysis, Writing – review & editing. PY: Investigation, Writing – review & editing. JL: Conceptualization, Writing – review & editing. FF: Conceptualization, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful to patient and their family.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
3. Loibl S, Gianni L. HER2-positive breast cancer. Lancet (2017) 389(10087):2415–29. doi: 10.1016/S0140-6736(16)32417-5
4. Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol (2012) 2:62. doi: 10.3389/fonc.2012.00062
5. Leung HWC, Chan ALF, Muo CH, Leung JH. Cost-effectiveness of pertuzumab combined with trastuzumab and docetaxel as a first-line treatment for HER-2 positive metastatic breast cancer. Expert Rev Pharmacoecon Outcomes Res (2018) 18(2):207–13. doi: 10.1080/14737167.2018.1386559
6. von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol (2009) 27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618
7. Wang LC, Kuo CN, Ko Y. Cost-effectiveness analysis of trastuzumab emtansine (T-DM1) in treating HER-2 positive advanced breast cancer in Taiwan. Breast J (2020) 26(10):2099–102. doi: 10.1111/tbj.14053
8. Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet (2023) 401(10371):105–17. doi: 10.1016/S0140-6736(22)02420-5
9. Li L, Xu MZ, Wang L, Jiang J, Dong LH, Chen F, et al. Conjugating MMAE to a novel anti-HER2 antibody for selective targeted delivery. Eur Rev Med Pharmacol Sci (2020) 24(24):12929–37. doi: 10.26355/eurrev_202012_24196
10. Yao X, Jiang J, Wang X, Huang C, Li D, Xie K, et al. A novel humanized anti-HER2 antibody conjugated with MMAE exerts potent anti-tumor activity. Breast Cancer Res Treat (2015) 153(1):123–33. doi: 10.1007/s10549-015-3503-3
11. Xu Y, Wang Y, Gong J, Zhang X, Peng Z, Sheng X, et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer (2021) 24(4):913–25. doi: 10.1007/s10120-021-01168-7
12. De Santis MC, Gulluni F, Campa CC, Martini M, Hirsch E. Targeting PI3K signaling in cancer: Challenges and advances. Biochim Biophys Acta Rev Cancer (2019) 1871(2):361–6. doi: 10.1016/j.bbcan.2019.03.003
13. Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Delivery (2022) 29(1):1335–44. doi: 10.1080/10717544.2022.2069883
14. Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol (2020) 235(4):3142–56. doi: 10.1002/jcp.29216
15. Schettini F, Prat A. Dissecting the biological heterogeneity of HER2-positive breast cancer. Breast (Edinburgh Scotland) (2021) 59:339–50. doi: 10.1016/j.breast.2021.07.019
16. Yin W, Xu T, Altai M, Oroujeni M, Zhang J, Vorobyeva A, et al. The influence of domain permutations of an albumin-binding domain-fused HER2-targeting affibody-based drug conjugate on tumor cell proliferation and therapy efficacy. Pharmaceutics (2021) 13(11):1974. doi: 10.3390/pharmaceutics13111974
17. Bose R, Ma CX. Breast cancer, HER2 mutations, and overcoming drug resistance. New Engl J Med (2021) 385(13):1241–3. doi: 10.1056/NEJMcibr2110552
18. Collins DM, Madden SF, Gaynor N, AlSultan D, Le Gal M, Eustace AJ, et al. Effects of HER family-targeting tyrosine kinase inhibitors on antibody-dependent cell-mediated cytotoxicity in HER2-expressing breast cancer. Clin Cancer Res (2021) 27(3):807–18. doi: 10.1158/1078-0432.CCR-20-2007
19. Ahmed S, Mohamed HT, El-Husseiny N, El Mahdy MM, Safwat G, Diab AA, et al. IL-8 secreted by tumor associated macrophages contribute to lapatinib resistance in HER2-positive locally advanced breast cancer via activation of Src/STAT3/ERK1/2-mediated EGFR signaling. Biochim Biophys Acta Mol Cell Res (2021) 1868(6):118995. doi: 10.1016/j.bbamcr.2021.118995
20. Xu B, Wang J, Zhang Q, Liu Y, Feng J, Wang W, et al. An open-label, multicenter, phase Ib study to evaluate RC48-ADC in patients with HER2-positive metastatic breast cancer. J Clin Oncol (2018) 36:1028–. doi: 10.1200/JCO.2018.36.15_suppl.1028
21. Wang J, Liu Y, Zhang Q, Feng J, Fang J, Chen X, et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. J Clin Oncol (2021) 39:1022–. doi: 10.1200/JCO.2021.39.15_suppl.1022
22. Parise C, Caggiano V. Breast Cancer Mortality among Asian-American Women in California: Variation according to Ethnicity and Tumor Subtype. J Breast Cancer (2016) 19(2):112–21. doi: 10.4048/jbc.2016.19.2.112
23. Zhao S, Liu XY, Jin X, Ma D, Xiao Y, Shao ZM, et al. Molecular portraits and trastuzumab responsiveness of estrogen receptor-positive, progesterone receptor-positive, and HER2-positive breast cancer. Theranostics (2019) 9(17):4935–45. doi: 10.7150/thno.35730
24. Tolaney SM, Wardley AM, Zambelli S, Hilton JF, Troso-Sandoval TA, Ricci F, et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol (2020) 21(6):763–75. doi: 10.1016/S1470-2045(20)30112-1
25. André F, Nadal J, Denys HG, Goel S, Litchfield LM, Appiah AK, et al. LBA18 Final overall survival (OS) for abemaciclib plus trastuzumab +/- fulvestrant versus trastuzumab plus chemotherapy in patients with HR+, HER2+ advanced breast cancer (monarcHER): A randomized, open-label, phase II trial. Ann Oncol (2022) 33:S1386–2387. doi: 10.1016/j.annonc.2022.08.013
26. Mustacchi G, Biganzoli L, Pronzato P, Montemurro F, Dambrosio M, Minelli M, et al. HER2-positive metastatic breast cancer: a changing scenario. Crit Rev Oncology/hematol (2015) 95(1):78–87. doi: 10.1016/j.critrevonc.2015.02.002
27. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr., et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol (2014) 32(33):3744–52. doi: 10.1200/JCO.2014.55.5730
Keywords: Disitamab Vedotin (RC48), advanced breast cancer, human epidermal growth factor receptor 2, targeted therapy, tumor resistance, case report
Citation: Li Y, Zhang J, Cai Z, Gao X, Zhang L, Lu Z, Wang X, Yu P, Li J and Fang F (2023) Disitamab Vedotin (RC48) for HER2-positive advanced breast cancer: a case report and literature review. Front. Oncol. 13:1286392. doi: 10.3389/fonc.2023.1286392
Received: 31 August 2023; Accepted: 06 November 2023;
Published: 24 November 2023.
Edited by:
Shengxi Chen, Arizona State University, United StatesReviewed by:
Rong Mu, Pfizer Australia, AustraliaXinpei Deng, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2023 Li, Zhang, Cai, Gao, Zhang, Lu, Wang, Yu, Li and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengqi Fang, MTgwOTg4NzY3MjNAMTYzLmNvbQ==; Jia Li, MzMyMzYxNDc3QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yang Li1†
Yang Li1† Lina Zhang
Lina Zhang Xiaojie Wang
Xiaojie Wang Jia Li
Jia Li Fengqi Fang
Fengqi Fang