
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 22 November 2023
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1284493
This article is part of the Research Topic Advances in Imaging of Cervical Cancer View all 6 articles
 Canyang Lin1†
Canyang Lin1† Fengling Yang1†
Fengling Yang1† Baoling Guo1
Baoling Guo1 Nan Xiao1
Nan Xiao1 Dongxia Liao1
Dongxia Liao1 Pengfei Liu1
Pengfei Liu1 Yunshan Jiang1
Yunshan Jiang1 Jiancheng Li2
Jiancheng Li2 Xiaolei Ni1*
Xiaolei Ni1*Objective: This study aimed to develop non-invasive predictive tools based on clinical characteristics and magnetic resonance imaging (MRI) features to predict survival in patients with locally advanced cervical cancer (LACC), thereby facilitating clinical decision-making.
Methods: We conducted a retrospective analysis of clinical and MRI data from LACC patients who underwent radical radiotherapy at our center between September 2012 and May 2020. Prognostic predictors were identified using single-factor and multifactor Cox analyses. Clinical and MRI models were established based on relevant features, and combined models were created by incorporating MRI factors into the clinical model. The predictive performance of the models was evaluated using the area under the curve (AUC), consistency index (C-index), and decision curve analysis (DCA).
Results: The study included 175 LACC patients. Multivariate Cox analysis revealed that patients with FIGO IIA-IIB stage, ECOG score 0-1, CYFRA 21-1<7.7 ng/ml, ADC ≥ 0.79 mm^2/s, and Kep ≥ 4.23 minutes had a more favorable survival prognosis. The clinical models, incorporating ECOG, FIGO staging, and CYFRA21-1, outperformed individual prognostic factors in predicting 5-year overall survival (AUC: 0.803) and 5-year progression-free survival (AUC: 0.807). The addition of MRI factors to the clinical model (AUC: 0.803 for 5-year overall survival) increased the AUC of the combined model to 0.858 (P=0.011). Similarly, the combined model demonstrated a superior predictive ability for 5-year progression-free survival, with an AUC of 0.849, compared to the clinical model (AUC: 0.807) and the MRI model (AUC: 0.673). Furthermore, the C-index of the clinical models for overall survival and progression-free survival were 0.763 and 0.800, respectively. Upon incorporating MRI factors, the C-index of the combined model increased to 0.826 for overall survival and 0.843 for progression-free survival. The DCA further supported the superior prognostic performance of the combined model.
Conclusion: Our findings indicate that ECOG, FIGO staging, and CYFRA21-1 in clinical characteristics, as well as ADC and Kep values in MRI features, are independent prognostic factors for LACC patients undergoing radical radiotherapy. The combined models provide enhanced predictive ability in assessing the risk of patient mortality and disease progression.
Cervical cancer is the fourth most common malignant tumor among women (1). In recent years, the incidence rate of cervical cancer has declined, but it still remains high. Due to the lack of effective screening in some areas, the mortality rate of young women with cervical cancer has increased (2). Cervical cancer staging is primarily based on the International Federation of Gynecology and Obstetrics (FIGO) staging or the International Union for Cancer Control (UICC) TNM staging (3, 4). Research suggests that different clinical stages are associated with varying prognostic outcomes, with 5-year survival rates of approximately 92%, 65%, and 17% for early-stage, locally advanced, and metastatic cervical cancer, respectively (5, 6). Despite receiving platinum-based concurrent chemoradiotherapy (CCRT) followed by intracavitary brachytherapy (7), nearly 40% of patients with locally advanced cervical cancer (LACC) still experience poor outcomes such as recurrence and metastasis (8, 9). Currently, there is a lack of ideal treatment methods for recurrence or metastasis (10). Additionally, radiotherapy or surgery often leads to side effects and sequelae that significantly impact the patients’ quality of life. These unsatisfactory results highlight the need for more refined diagnostic and treatment management approaches for cervical cancer.
General clinical characteristics, such as FIGO stage, lymph node metastasis, histopathology, tumor size, and age, are common prognostic factors for survival in LACC patients (11, 12). However, there are variations in clinical outcomes among patients with the same characteristics after treatment. This suggests the importance of identifying additional independent prognostic factors based on clinical features to improve the ability to predict patient recurrence and metastasis. Magnetic resonance imaging (MRI) is an important diagnostic tool for evaluating cervical cancer, offering the advantage of assessing overall tumor imaging characteristics (13). Traditional MRI techniques such as T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) provide detailed anatomical images (14, 15). With advancements in technology, emerging MRI techniques like diffusion-weighted MRI (DWI-MRI) and dynamic contrast-enhanced MRI (DCE-MRI) can provide physiological, metabolic, and functional information (16). Previous studies have demonstrated the potential value of MRI in predicting treatment efficacy and survival (17). Therefore, it is crucial to integrate clinical features with MRI imaging features to construct more accurate predictive models for guiding the treatment of LACC and improving prognosis.
The objective of this study is to analyze the clinical characteristics and MRI features of LACC patients receiving radical radiotherapy and construct an effective prognostic model for predicting long-term survival and disease progression.
Between January 2012 and December 2020, a total of 175 patients diagnosed with histopathologically confirmed cervical cancer (stage IIA-IVA CC at diagnosis) were included in this retrospective study. The patients were restaged in accordance with the 8th edition of the FIGO system. To be eligible for inclusion, patients had to meet the following criteria: 1) histologically diagnosed with cervical squamous cell carcinoma or adenocarcinoma, and 2) undergone imaging examinations, such as pelvic MRI, blood routine examination, biochemical examination, and tumor marker examination, two weeks before treatment initiation. Patients were excluded if they: 1) had received any antitumor treatment prior to their initial evaluation, 2) did not complete the planned radical radiotherapy, or 3) had obvious MRI artifacts or were unable to undergo MRI examination. The retrospective study was approved by the Institutional Review Board of our hospital, and all patients provided informed consent to participate in the study.
All patients underwent a combination of External Beam Radiation Therapy (EBRT) and high-dose brachytherapy. The clinical target volume (CTV) for EBRT included the cervical mass, the entire cervix, uterus, part of the vagina, parametrium, and draining lymph nodes (internal iliac, external iliac, common iliac, and presacral). The prescribed dose to the CTV was 4860-5040 cGy in 27-28 fractions, and lymph nodes involved were considered for Simultaneous Integrated Boost (SIB) to a dose of 5670-6160 cGy in 27-28 fractions. After 20 fractions of EBRT, all patients received brachytherapy, with a dose of 2600-2800 cGy in 4 fractions (once weekly) delivered to the point A of the pelvic dose reference point. Concurrent chemotherapy regimens for these patients included weekly cisplatin (CDDP 40 mg/m2) for 6 cycles or cisplatin and taxane (CDDP 75 mg/m2 + paclitaxel 175 mg/m2) administered every 3 weeks for 2-3 cycles. Out of the 175 patients, 110 (62.9%) received cisplatin-based concurrent chemoradiotherapy (CCRT), while the remaining 65 patients (30.8%) received neoadjuvant chemotherapy.
Basic information and clinical characteristics of cervical cancer patients treated at our hospital were obtained from our hospital’s information management system and laboratory management system. Clinical features included age, family history of cancer, ECOG score, FIGO stage, general tumor classification, maximum tumor diameter, pathological type, pathological differentiation, carbohydrate antigen 125 (CA125), carcinoembryonic antigen (CEA), cytokeratin fragment antigen 21-1 (CYFRA21-1), hemoglobin, leukocyte count, serum albumin, CCRT, and others. All the mentioned data represent the baseline characteristics of patients before treatment.
MRI scans were conducted within 2 weeks before the initiation of treatment. GE Signa HDI Echospeed 1.5T or Philips Achieva 3.0T superconducting MR scanners with an 8-channel bulk phase array coil were used, along with GE AW4.6 or Philips ISP V7 workstations. The conventional MRI parameters primarily included the apparent diffusion coefficient (ADC), volume transfer constant (Ktrans), rate constant (Kep), extracellular volume fraction (Ve), and plasma volume fraction (Vp). Tumor volume delineation in T2-enhanced images was performed by tracing the tumor area on the workstation for each slice using a trackball.
In this study, statistical analysis was conducted using SPSS (version 26.0) and R software (version 4.4.1). Univariate and multivariate Cox analyses were performed to identify effective prognostic variables for the model. The “Forest Map” software package was used to generate a forest plot. AUC curves were plotted using Medcalc software, and differences in AUC prediction performance were compared using the DeLong method. Model comparison was performed using the consistency index (C-index) and decision curve analysis (DCA) methods. A bilateral p-value of less than 0.05 was considered statistically significant.
Table 1 displays the baseline clinical and MRI features of the 175 LACC patients enrolled in this study. The median follow-up time was 59.5 months (range: 13.17-106.70 months). Among the 175 LACC patients, 35 (20.0%) succumbed to the disease, 14 (8.0%) experienced local recurrence, and 16 (9.1%) developed distant metastasis. Univariate analysis followed by multivariate COX analysis incorporating clinically significant variables revealed that ECOG score, FIGO staging, and CYFRA 21-1 were independent prognostic factors for both overall survival (OS) and progression-free survival (PFS) in LACC patients (as shown in Table 2). Moreover, MRI features such as ADC and Kep were also identified as independent prognostic factors for OS and PFS (as shown in Table 3).
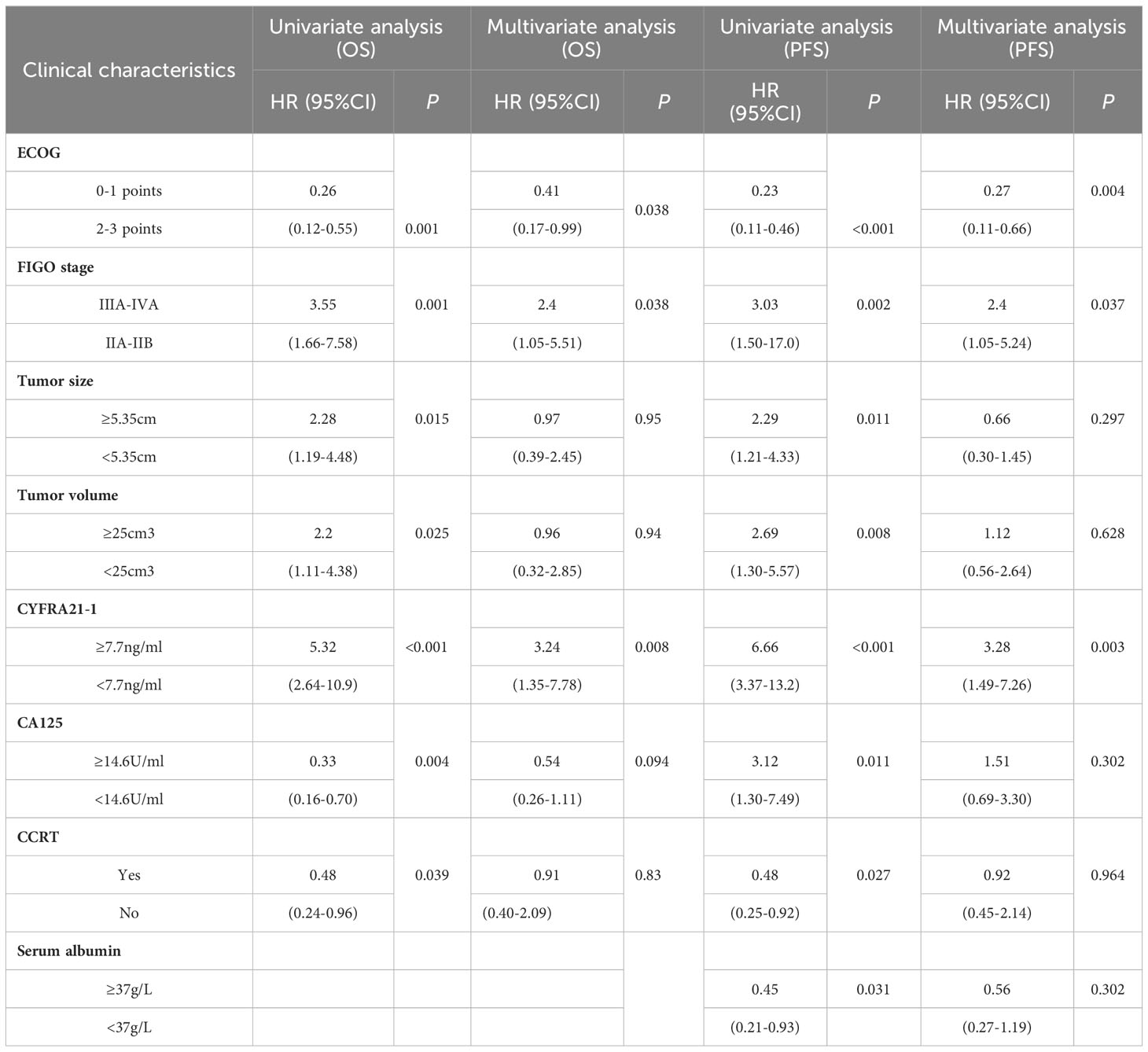
Table 2 Univariate and multivariate COX analysis of clinical characteristics in cervical cancer patients.
The multifactor forest plot resulting from the multivariate analysis included three clinical features (ECOG score, FIGO staging, CYFRA 21-1) and two MRI features (ADC, Kep). Regardless of OS or PFS, patients with LACC who had FIGO stage IIA-IIB, ECOG score 0-1, CYFRA 21-1 < 7.7 ng/ml, ADC ≥ 0.79 mm^2/s, and Kep ≥ 4.23 min exhibited more favorable survival outcomes, as indicated by the multivariate COX analysis (Figure 1).
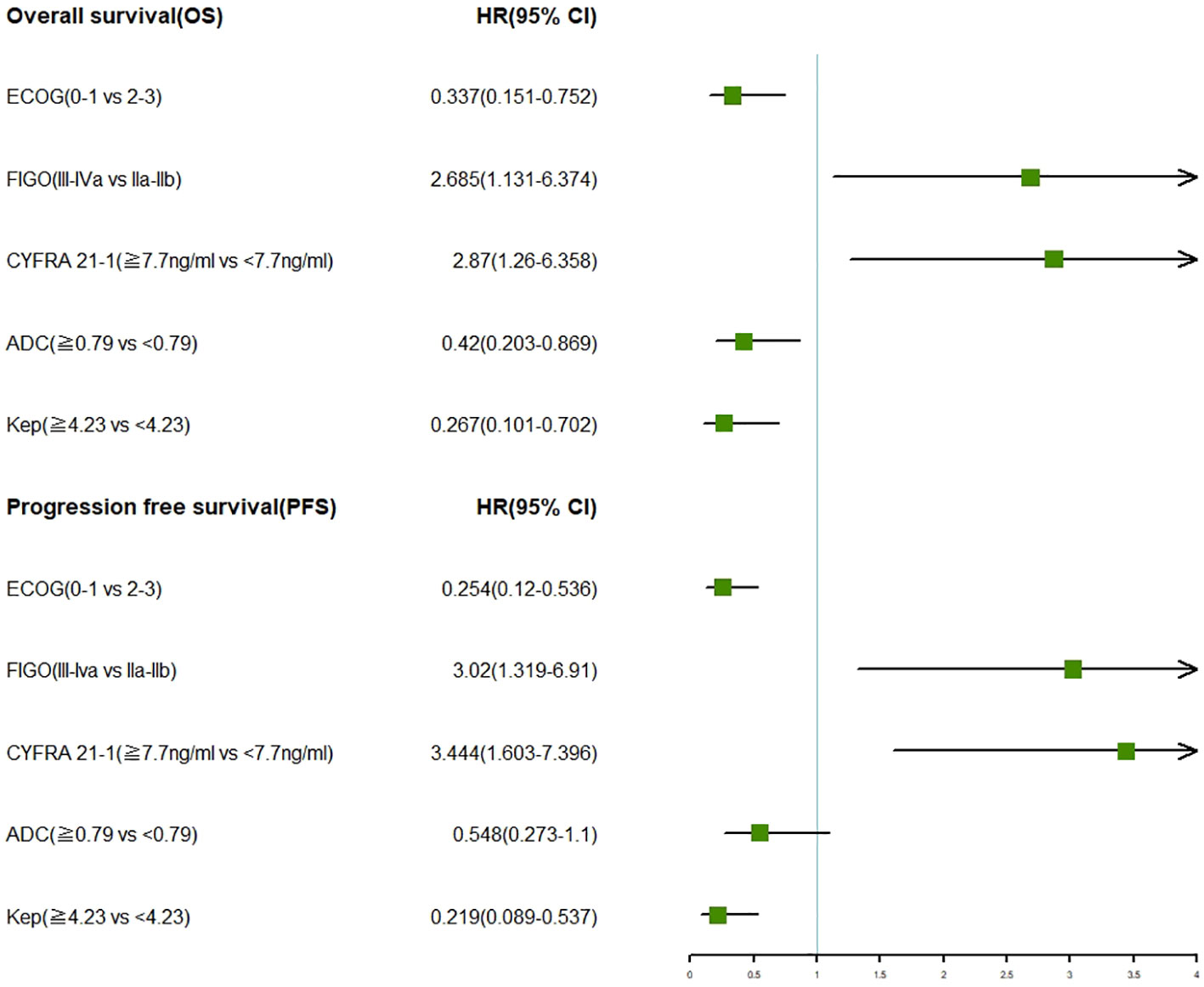
Figure 1 Forest plots showcasing multivariate analysis of clinical characteristics and MRI features in relation to OS and PFS in LACC patients.
The clinical model, incorporating ECOG score, FIGO staging, and CYFRA 21-1 as independent prognostic factors, was developed to predict 5-year OS and PFS. The clinical model exhibited an AUC value of 0.803 for predicting 5-year OS, which outperformed the individual prognostic factors, namely CYFRA 21-1 (AUC: 0.662), FIGO staging (AUC: 0.642), and ECOG score (AUC: 0.73) (P < 0.05). Similarly, the clinical model achieved an AUC value of 0.807 for predicting 5-year PFS, surpassing the individual prognostic factors (CYFRA 21-1: AUC 0.671, FIGO staging: AUC 0.663, ECOG score: AUC 0.715) (P < 0.05). The corresponding curves are illustrated in Figures 2A, B.
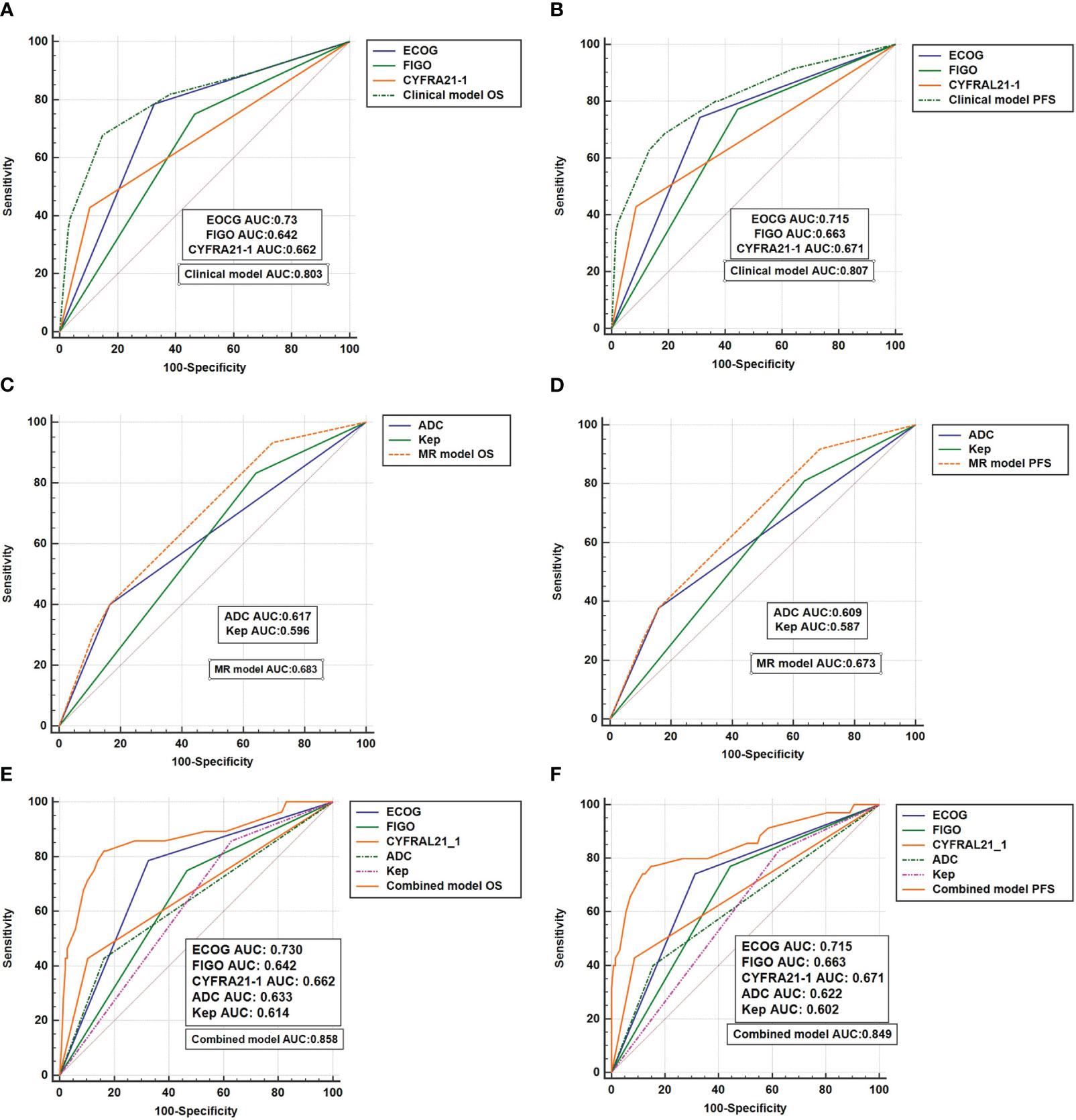
Figure 2 The ROC curves (A, B) reflect the efficacy of clinical models in predicting OS and PFS; (C, D) reflects the effectiveness of the MR model in predicting OS and PFS; (E, F) reflects the effectiveness of the combinated model in predicting OS and PFS.
The MRI model, based on independent prognostic factors ADC and Kep, was developed to predict survival outcomes. The MRI model demonstrated an AUC of 0.683 for 5-year OS, outperforming the individual prognostic factor ADC (AUC: 0.617) (P = 0.0065) and exhibiting a trend compared to Kep (AUC: 0.596) (P = 0.09). Additionally, the MRI model predicted a 5-year PFS AUC of 0.673, which surpassed the individual prognostic factor ADC (ADC: 0.609) and showed a trend compared to Kep (AUC: 0.587) (P = 0.0078 for ADC and P = 0.068 for Kep). Please refer to Figures 2C, D for the corresponding curves.
By integrating the clinical model and the MRI model, a combined prediction prognosis model (the combination model) was developed. The combined model yielded a 5-year OS AUC of 0.858 for LACC patients, which was higher than the AUC of the clinical model (0.803) (P = 0.0099) and the MRI model (0.683) (P = 0.0109). Similarly, the combined model predicted a 5-year PFS AUC of 0.849, surpassing the clinical model (AUC: 0.807) (P = 0.0032) and the MRI model (AUC: 0.673) (P = 0.0056). Figures 2E, F display the corresponding curves. .
The prognostic prediction models were further evaluated using the C-index. The combined model achieved a C-index of 0.826 (0.761-0.890) for predicting OS survival in LACC patients, which was superior to the clinical model (0.763, 0.685-0.841) and the MRI model (0.665, 0.585-0.745). For PFS, the combined model demonstrated a C-index of 0.843 (0.78-0.90), outperforming the clinical model (0.8, 0.735-0.865) and the MRI model (0.646, 0.568-0.724). Please refer to Table 4 for detailed results. Additionally, the decision curve analysis (DCA) results, as depicted in Figures 3A, B, illustrate the superior predictive performance of the combined model compared to the clinical and MRI models for both OS and PFS.
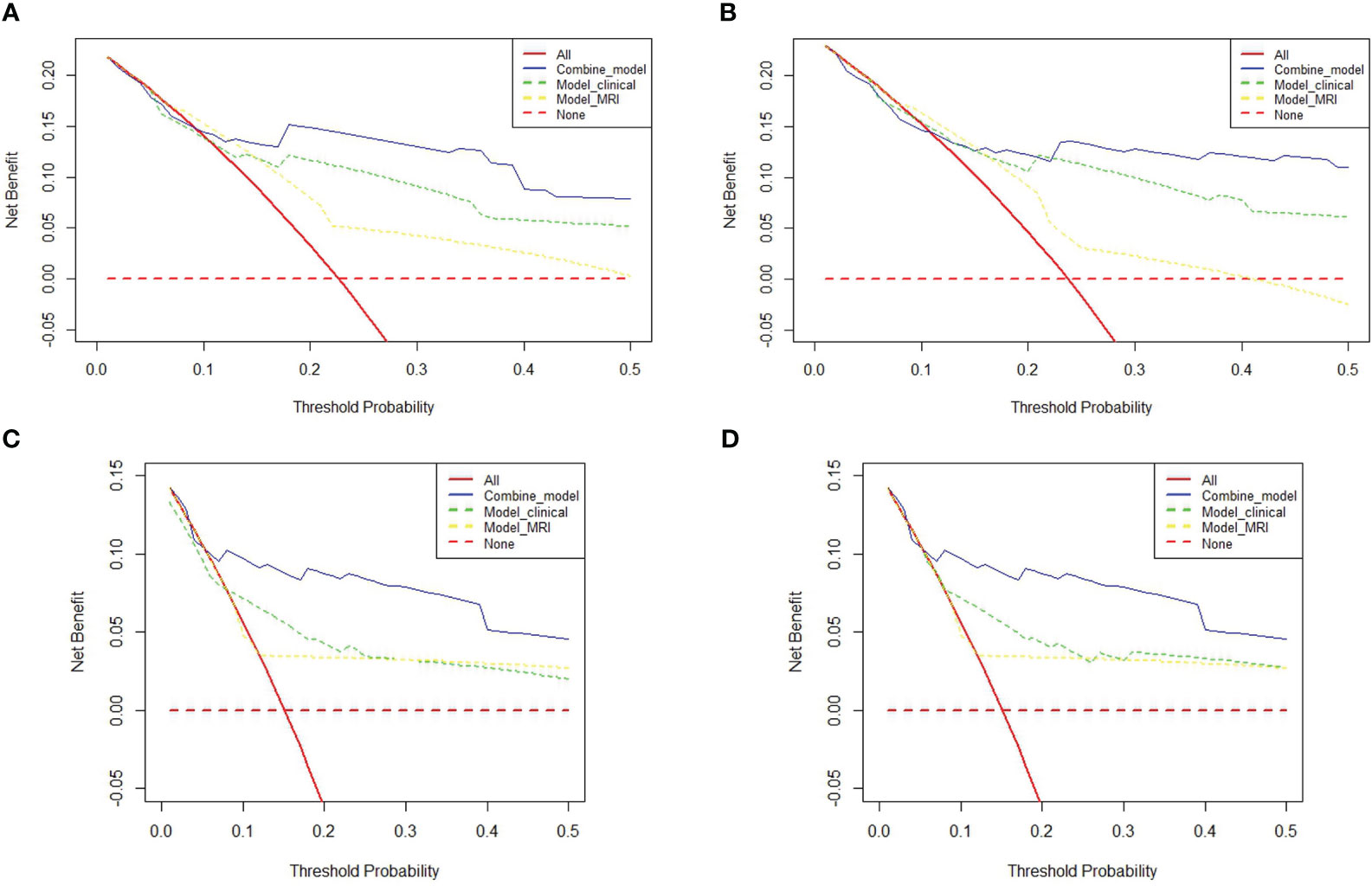
Figure 3 Decision Curve Analysis (DCA) plots: (A, B) contrast the predictive efficacy for OS and PFS among different models for 175 LACC patients. (C, D) delineate the comparative efficacy of different models in predicting OS and PFS in a subset of 110 LACC patients undergoing concurrent radiotherapy and chemotherapy (CCRT).
This study delved deeper into a subgroup analysis of 110 LACC patients who underwent concurrent chemoradiation (CCRT). Figures 4A, B reveal that the 5-year OS AUC for the combined model stood at 0.929, surpassing both the clinical model (AUC: 0.788) and the MRI model (AUC: 0.7). Similarly, the combined model’s 5-year PFS AUC was 0.873, outdoing the clinical (AUC: 0.762) and MRI (AUC: 0.659) models. Among the 110 LACC patients treated with CCRT, the C-index for OS was 0.886 (0.723-1.00), which was better than both the clinical model’s 0.886 (0.723-1.00) and the MRI model’s 0.75 (0.481-1.00). Regarding PFS, the combined model’s C-index was 0.969 (0.91-1.00), outperforming the clinical model (0.906, 0.809-1.00) and the MRI model (0.781, 0.659-0.902). Comprehensive results can be found in Table 4. Additionally, the DCA results (Figures 4C, D) suggest that the combined model surpassed the clinical and MRI models in predicting OS and PFS for LACC patients undergoing CCRT.
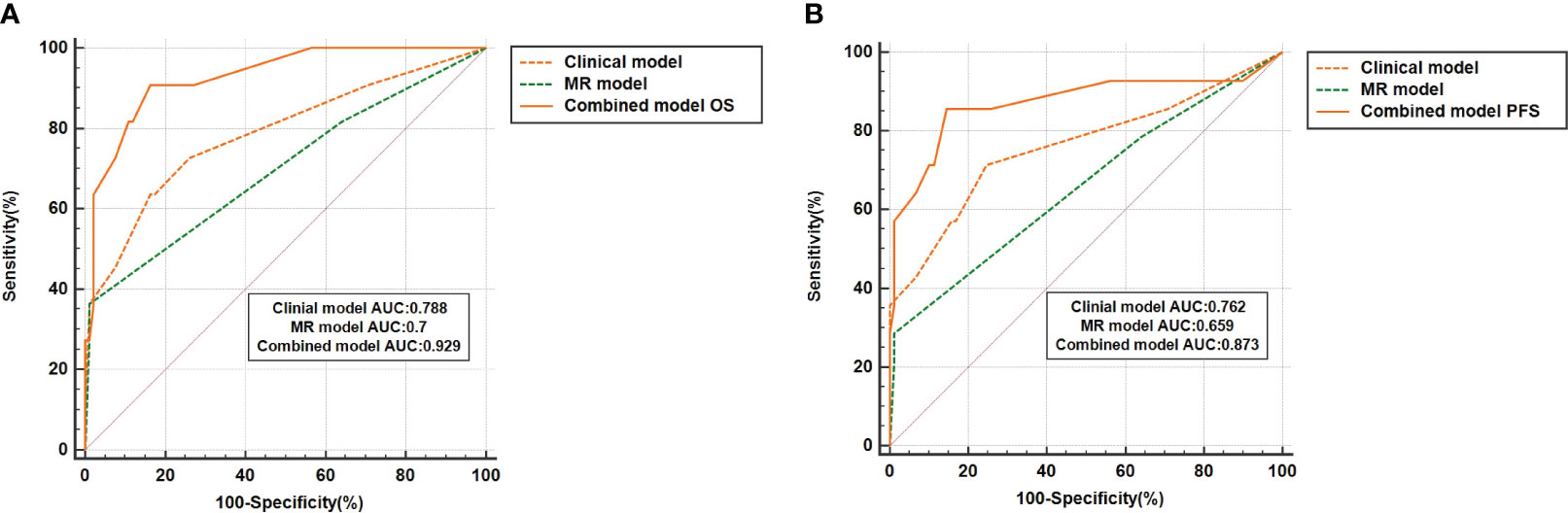
Figure 4 The ROC curve represents the performance of different prognostic models for 110 LACC patients who received concurrent radiotherapy and chemotherapy (CCRT). (A, B) represent the results of the model's prediction of OS and PFS, respectively.
Despite concurrent chemoradiotherapy (CCRT) treatment, approximately 40% of patients with locally advanced cervical cancer (LACC) still face the risk of recurrence or metastasis (18). This underscores the need for more accurate prognostic models in clinical practice. Our research demonstrates that ECOG score, FIGO staging, and CYFRA21-1 are independent prognostic factors for cervical cancer patients. The clinical model constructed using these factors exhibits superior predictive performance compared to any single factor, facilitating the identification of LACC patients with a poor prognosis. Furthermore, we have incorporated magnetic resonance imaging (MRI) factors such as ADC and Kep into the clinical model to develop a combined model. The results indicate that the combined model surpasses both the clinical and MRI models in predicting 5-year overall survival (OS) or progression-free survival (PFS) and C-index in LACC patients. Additionally, Decision Curve Analysis (DCA) demonstrates that the inclusion of MRI factors in clinical models significantly enhances predictive performance.
Accurate staging plays a crucial role in determining treatment strategies, estimating prognosis, and guiding follow-up for malignant tumors. The revised FIGO cervical cancer staging standard in 2018 introduced imaging and pathology options (19). It divided stage IB into three groups based on tumor diameter and added a new stage IIIC to reflect the survival heterogeneity associated with lymph node involvement (4). Tang et al. (20) conducted a retrospective analysis of 3,238 cervical cancer patients, revealing FIGO staging as an independent prognostic factor for 5-year survival, with a C-index value of 0.721 indicating good predictive ability. Consistently, our study demonstrates that FIGO staging is an independent prognostic factor for 5-year OS and PFS in LACC. Patients in stages IIIA-IVA exhibit a 2.4 times higher risk of death and disease progression compared to those in stages IIA-IIB. Moreover, unlike previous studies, our research incorporates factors such as ECOG score, tumor markers, and MRI imaging.
The ECOG score serves as a scale to assess whether cancer patients’ physical condition can tolerate anti-tumor therapy (21). Research suggests that ECOG scores are associated with chemotherapy response, tolerance, survival rates, and quality of life (22). However, limited studies have explored the correlation between ECOG scores and long-term survival in cervical cancer. Our study reveals the ECOG score as an independent prognostic factor for long-term survival in LACC patients undergoing radical radiotherapy. Patients with an ECOG score of 0-1 demonstrate better 5-year OS and PFS compared to those with a score of 2-3 (P<0.05). Prior studies have indicated a correlation between pre-treatment CYFRA21-1 levels and tumor size, staging, and worse pathological classification (23, 24). In our study, we determined the optimal cutoff value for CYFRA21-1 to be 7.7 umol/l, with patients having ≥7.7 umol/l exhibiting worse 5-year OS and PFS (P<0.001). The clinical model constructed based on the three clinical factors—ECOG score, FIGO staging, and CYFRA21-1—outperforms any single prognostic factor, achieving an AUC of 0.803 for 5-year OS and 0.799 for 5-year PFS.
Magnetic Resonance Dynamic Contrast-Enhanced Imaging (DCE) employs swift, recurring enhancements to evaluate tumor vascular permeability and perfusion, offering a prognostic assessment for cancer patients (25). Mayr et al. (26) identified tumor heterogeneous regions with diminished DCE values, correlating with a risk of treatment failure, using DCE functional MRI. They also quantified the functional risk volume (FRV). The findings point out that FRV is an innovative functional imaging heterogeneity metric, superior to the anatomical tumor volume (ATV). It holds the potential for clinical transition into personalized early outcome forecasts before treatment initiation or as early as 2-5 weeks post-treatment. This might clarify why the ATV didn’t hold significance in our multivariate analysis, leading to its exclusion from the model analysis. Beyond FRV, contemporary research highlights DWI-based ADC, K(el), and Ktrans as stand-alone prognostic indicators for LACC patients (14) (27). In line with these insights, our investigation underscores that both DWI ADC and DCE Kep values are pivotal prognostic elements for the extended survival of LACC patients. Nevertheless, our research boasts a considerably larger cohort and an extended overall monitoring duration compared to preceding investigations. Furthermore, unlike certain prior studies, we’ve integrated clinical characteristics, especially those linked with radiation therapy, for collective scrutiny and juxtaposition.
Yu W et al. (28) gathered data on 13,802 LACC patients from the SEER database and developed a prediction model using a machine learning (ML) algorithm to estimate the 5-year survival of LACC patients. Their findings indicated that the XGBoost model displayed the most superior predictive capability, achieving an AUC of 0.8365 — a performance that surpassed both the LR and SVM models. In our research, when we incorporated MRI indicators into the clinical model, we noted an enhancement in the AUC for the 5-year OS predictive capacity of the combined model to 0.858 (P=0.011), marginally outperforming the XGBoost model. Similarly, the combined model registered a 5-year PFS AUC value of 0.849, overtaking the clinical model’s 0.807 and the MRI model’s 0.673. As further validated by the DCA curve analysis, the combined model consistently exhibited the most commendable predictive accuracy. Additionally, a noteworthy limitation of the XGBoost model is its omission of a clear delineation regarding the treatment approach for LACC. Variances in treatment strategies can considerably influence cervical cancer outcomes. Consequently, we executed a subgroup analysis focusing on 110 LACC patients who underwent CCRT. This deep dive revealed AUC values for the 5-year OS and 5-year PFS from the combined model as 0.886 and 0.969, respectively. This methodology stands out as it proficiently discerns patients facing elevated risks of recurrence and mortality.
There are several limitations to our study. Firstly, it is a retrospective study, and rigorous prospective cohort studies are needed for further validation. Secondly, external validation from other institutions is necessary to confirm our findings. Lastly, our study includes a limited number of MRI parameter items. Therefore, our future research will focus on incorporating additional multi-parameter imaging features, such as imaging omics features, to establish a more effective prognostic prediction model for LACC.
Clinical factors, including ECOG score, FIGO staging, and CYFRA21-1, are independent prognostic factors for LACC patients, with the clinical model exhibiting superior predictive power compared to individual factors. Furthermore, the addition of MRI factors to the clinical model to construct a combined model yields the best predictive performance, providing valuable guidance for clinical decision-making.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Longyan First Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CL: Writing – original draft, Conceptualization, Formal analysis, Investigation, Software, Supervision, Writing – review & editing. FY: Data curation, Writing – original draft. BG: Writing – original draft, Formal analysis, Methodology. NX: Methodology, Writing – original draft. DL: Investigation, Writing – original draft. FY: Writing – original draft. PL: Writing – original draft. YJ: Writing – original draft. JL: Conceptualization, Writing – review & editing. XN: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is funded by the Fujian Province Clinical Key Professional Construction Project. This study was also supported by the Natural Science Foundation of Fujian Province (2020J011325) and the Science and Technology Plan of Longyan City (2020LYF17041).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N. Cervical cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann oncology: Off J Eur Soc Med Oncol (2018) 29:iv262. doi: 10.1093/annonc/mdy160
3. Bhatla N, Singhal S, Dhamija E, Mathur S, Natarajan J, Maheshwari A. Implications of the revised cervical cancer figo staging system. Indian J Med Res (2021) 154(2):273–83. doi: 10.4103/ijmr.IJMR_4225_20
4. van Kol K, Ebisch R, van der Aa M, Wenzel H, Piek J, Bekkers R. The prognostic value of the presence of pelvic and/or para-aortic lymph node metastases in cervical cancer patients; the influence of the new figo classification (Stage iiic). Gynecologic Oncol (2023) 171:9–14. doi: 10.1016/j.ygyno.2023.01.023
5. Gennigens C, De Cuypere M, Hermesse J, Kridelka F, Jerusalem G. Optimal treatment in locally advanced cervical cancer. Expert Rev Anticancer Ther (2021) 21(6):657–71. doi: 10.1080/14737140.2021.1879646
6. Mohamud A, Høgdall C, Schnack T. Prognostic value of the 2018 figo staging system for cervical cancer. Gynecologic Oncol (2022) 165(3):506–13. doi: 10.1016/j.ygyno.2022.02.017
7. Kou L, Zhang T, Yang X, Peng S, Wang Y, Yuan M, et al. Role of adjuvant chemotherapy after concurrent chemoradiotherapy in patients with locally advanced cervical cancer. Future Oncol (2022) 18(16):1917–5. doi: 10.2217/fon-2021-0818
8. Verma J, Monk B, Wolfson A. New strategies for multimodality therapy in treating locally advanced cervix cancer. Semin Radiat Oncol (2016) 26(4):344–8. doi: 10.1016/j.semradonc.2016.05.003
9. Meng J, Liu S, Zhu L, Zhu L, Wang H, Xie L, et al. Texture analysis as imaging biomarker for recurrence in advanced cervical cancer treated with ccrt. Sci Rep (2018) 8(1):11399. doi: 10.1038/s41598-018-29838-0
10. Cohen A, Roane B, Leath C. Novel therapeutics for recurrent cervical cancer: moving towards personalized therapy. Drugs (2020) 80(3):217–27. doi: 10.1007/s40265-019-01249-z
11. Quinn B, Deng X, Colton A, Bandyopadhyay D, Carter J, Fields E. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy (2019) 18(1):29–37. doi: 10.1016/j.brachy.2018.08.016
12. Pinto P, Chen M, Santos Neto E, Faloppa C, De Brot L, Guimaraes A, et al. Prognostic factors in locally advanced cervical cancer with pelvic lymph node metastasis. Int J gynecological cancer: Off J Int Gynecological Cancer Soc (2022) 32(3):239–45. doi: 10.1136/ijgc-2021-003140
13. Lu H, Wu Y, Liu X, Huang H, Jiang H, Zhu C, et al. The role of dynamic contrast-enhanced magnetic resonance imaging in predicting treatment response for cervical cancer treated with concurrent chemoradiotherapy. Cancer Manage Res (2021) 13:6065–78. doi: 10.2147/cmar.S314289
14. Onal C, Erbay G, Guler O. Treatment response evaluation using the mean apparent diffusion coefficient in cervical cancer patients treated with definitive chemoradiotherapy. J magnetic resonance imaging: JMRI (2016) 44(4):1010–9. doi: 10.1002/jmri.25215
15. Gu K, Kim C, Choi C, Yoon Y, Park W. Prognostic value of adc quantification for clinical outcome in uterine cervical cancer treated with concurrent chemoradiotherapy. Eur Radiol (2019) 29(11):6236–44. doi: 10.1007/s00330-019-06204-w
16. Ho J, Allen P, Bhosale P, Rauch G, Fuller C, Mohamed A, et al. Diffusion-weighted magnetic resonance imaging as a predictor of outcome in cervical cancer after chemoradiation. Int J Radiat oncology biology Phys (2017) 97(3):546–53. doi: 10.1016/j.ijrobp.2016.11.015
17. Lim K, Chan P, Dinniwell R, Fyles A, Haider M, Cho Y, et al. Cervical cancer regression measured using weekly magnetic resonance imaging during fractionated radiotherapy: radiobiologic modeling and correlation with tumor hypoxia. Int J Radiat oncology biology Phys (2008) 70(1):126–33. doi: 10.1016/j.ijrobp.2007.06.033
18. Liu J, Tang G, Zhou Q, Kuang W. Outcomes and prognostic factors in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. Radiat Oncol (2022) 17(1):142. doi: 10.1186/s13014-022-02115-1
19. Okugawa K, Yahata H, Ohgami T, Yasunaga M, Asanoma K, Kobayashi H, et al. An update of oncologic and obstetric outcomes after abdominal trachelectomy using the figo 2018 staging system for cervical cancer: A single-institution retrospective analysis. J Gynecologic Oncol (2023) 34(3):e41. doi: 10.3802/jgo.2023.34.e41
20. Tang X, Guo C, Liu S, Guo J, Hua K, Qiu J. A novel prognostic nomogram utilizing the 2018 figo staging system for cervical cancer: A large multicenter study. . Int J gynaecology obstetrics: Off Organ Int Fed Gynaecology Obstetrics (2021) 155(1):86–94. doi: 10.1002/ijgo.13644
21. Kelly C, Shahrokni A. Moving beyond karnofsky and ecog performance status assessments with new technologies. J Oncol (2016) 2016:6186543. doi: 10.1155/2016/6186543
22. Quinn S, Crandell C, Blake M, Bontrager A, Dempsey A, Lewis D, et al. The correlative strength of objective physical assessment against the ecog performance status assessment in individuals diagnosed with cancer. Phys Ther (2020) 100(3):416–28. doi: 10.1093/ptj/pzz192
23. Gadducci A, Tana R, Cosio S, Genazzani A. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: A review of the literature. Crit Rev oncology/hematology (2008) 66(1):10–20. doi: 10.1016/j.critrevonc.2007.09.002
24. Molina R, Filella X, Augé J, Bosch E, Torne A, Pahisa J, et al. Cyfra 21.1 in patients with cervical cancer: comparison with scc and cea. Anticancer Res (2005) 25:1765–71.
25. Zhang Q, Guo J, Ouyang H, Chen S, Zhao X, Yu X. Added-value of dynamic contrast-enhanced MRI on prediction of tumor recurrence in locally advanced cervical cancer treated with chemoradiotherapy. Eur Radiol (2022) 32(4):2529–39. doi: 10.1007/s00330-021-08279-w
26. Mayr N, Huang Z, Wang J, Lo S, Fan J, Grecula J, et al. Characterizing tumor heterogeneity with functional imaging and quantifying high-risk tumor volume for early prediction of treatment outcome: cervical cancer as a model. Int J Radiat oncology biology Phys (2012) 83(3):972–9. doi: 10.1016/j.ijrobp.2011.08.011
27. Andersen E, Hole K, Lund K, Sundfør K, Kristensen G, Lyng H, et al. Pharmacokinetic parameters derived from dynamic contrast enhanced MRI of cervical cancers predict chemoradiotherapy outcome. Radiotherapy oncology: J Eur Soc Ther Radiol Oncol (2013) 107(1):117–22. doi: 10.1016/j.radonc.2012.11.007
Keywords: cervical cancer, clinical characteristics, magnetic resonance imaging, prognosis, radiotherapy
Citation: Lin C, Yang F, Guo B, Xiao N, Liao D, Liu P, Jiang Y, Li J and Ni X (2023) Development of a combined model incorporating clinical characteristics and magnetic resonance imaging features to enhance the predictive value of a prognostic model for locally advanced cervical cancer. Front. Oncol. 13:1284493. doi: 10.3389/fonc.2023.1284493
Received: 28 August 2023; Accepted: 06 November 2023;
Published: 22 November 2023.
Edited by:
Alessio G. Morganti, University of Bologna, ItalyReviewed by:
Wenkun Bai, Tongji Hospital Affiliated to Tongji University, ChinaCopyright © 2023 Lin, Yang, Guo, Xiao, Liao, Liu, Jiang, Li and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolei Ni, bml4aWFvbGVpMjAwNUAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.