- Department of Urology, Minda Hospital of Hubei Minzu University, En Shi, China
Background: The primary aim of this present study is to undertake a comprehensive comparative analysis of the perioperative, functional, and oncologic outcomes associated with laparoscopic partial nephrectomy (LPN) and open partial nephrectomy (OPN) as interventions for the treatment of complex renal tumors, defined as PADUA or RENAL score ≥ 7.
Methods: We systematically carried out an extensive search across four electronic databases, namely PubMed, the Cochrane Library, Embase, and Web of Science. Our objective was to identify pertinent studies published in the English language up to December 2023, and encompassed controlled trials comparing LPN and OPN as interventions for complex renal tumors.
Results: This study encompassed a total of seven comparative trials, involving 934 patients. LPN exhibited a noteworthy reduction in the length of hospital stay (weighted mean difference [WMD] -2.06 days, 95% confidence interval [CI] -2.62, -1.50; p < 0.00001), blood loss (WMD -34.05mL, 95% CI -55.61, -12.48; p = 0.002), and overall complications (OR 0.38, 95% CI 0.19, 0.79; p = 0.009). However, noteworthy distinctions did not arise between LPN and OPN concerning parameters such as warm ischemia time, renal function, and oncological outcomes.
Conclusions: This study reveals that LPN presents several advantages over OPN. These benefits encompass a shortened hospital stay, diminished blood loss, and a reduced incidence of complications. Importantly, LPN achieves these benefits while concurrently upholding comparable renal function and oncological outcomes.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=457716, identifier CRD42023453816.
1 Introduction
Renal tumors constitute a prevalent form of urinary system neoplasm, ranking second in incidence only to bladder cancer. Owing to the inherently low biological aggressiveness of renal tumors, their malignancy is relatively subdued (1). In recent years, the continuous advancements in medical diagnostic technology have contributed to heightened rates of early kidney tumor detection and favorable clinical prognoses. Clinical investigations affirm the inherent resistance of renal tumors to multiple pharmaceutical agents and their reduced sensitivity to radiation, thereby constraining options for biological targeting and immunotherapy (2). Consequently, surgical intervention has emerged as the foremost efficacious modality for managing renal tumors. Nephron-sparing surgery represents a surgical approach endorsed in the clinical treatment paradigm for renal tumors. This technique adeptly conserves the patient’s nephron while concurrently extending their survival duration to a significant degree (3, 4).
RENAL and PADUA score rank among the most frequently employed scoring systems in the assessment of renal tumors. Complex renal tumors are defined by a PADUA or RENAL score of ≥ 7 (5, 6). Owing to the deep encapsulation of the renal parenchyma and the intricate proximity to the anatomically elaborate renal collection system, the challenge of excising or resecting complex renal tumors while preserving nephrons is evident. Laparoscopic partial nephrectomy (LPN) has gained broad acceptance across numerous medical institutions due to its straightforward requirements for surgical equipment. Its effectiveness has been validated in managing non-complex renal tumors (7). Nonetheless, the utilization of LPN for the management of complex renal masses continues to be a subject of ongoing debate and intricacy (8). Despite numerous recent endeavors to scrutinize and compare the perioperative and functional outcomes of LPN and open partial nephrectomy (OPN) in addressing complex renal tumors, a substantial portion of these investigations remains constrained within the boundaries of single medical institutions or the realm of proficient surgical practitioners (9).
Hence, the primary objective of this investigation is to systematically aggregate data from comparative studies and conduct a comprehensive assessment of the effectiveness and safety profiles pertaining to LPN and OPN in the management of exceedingly intricate renal tumors. The outcomes derived from this inquiry are intended to serve as all-encompassing guide for clinical deliberations, thereby aiding medical practitioners in the judicious determination of the optimal surgical modality for their respective patient cohorts.
2 Methods
This study was conducted in strict adherence to the protocols outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (10, 11). Moreover, it was registered in the PROSPERO registry (ID: CRD42023457716) in accordance with established practices.
2.1 Literature search strategy, study selection and data collection
A rigorous and exhaustive exploration was conducted across a multitude of databases, encompassing PubMed, Embase, Web of Science, and the Cochrane Library. Our objective was to identify pertinent studies published in the English language up to December 2023. We formulated the subsequent search query through the amalgamation of intervention and patient-centric search phrases: ((Laparoscopic PN OR Endoscopic PN) AND (Renal carcinoma OR Renal tumor OR Renal cancer OR Kidney cancer) AND (Complex)). In order to ascertain comprehensiveness, we also carried out a manual examination of pertinent references, proceedings, and summaries.
We used the PICOS approach to define inclusion criteria. P (patients): adult patients diagnosed with complex renal tumor, defined specifically by a PADUA or RENAL score of ≥ 7; I (intervention): the intervention involves patients undergoing LPN; C (comparator): OPN was performed for comparison; O (outcome): one or more of the ensuing outcomes: perioperative, complications, renal functional, and oncologic outcomes; S (study type): This investigation encompasses prospective comparative studies, retrospective analyses, or randomized controlled trials (RCTs). Exclusion criteria were as follows: (1) studies lacking comparative designs were systematically excluded. (2) editorial commentaries, correspondences with the editor, abstracts from meetings, and isolated case reports were not incorporated into the analytical framework. (3) studies that did not assess the designated outcome metrics were deliberately excluded from consideration.
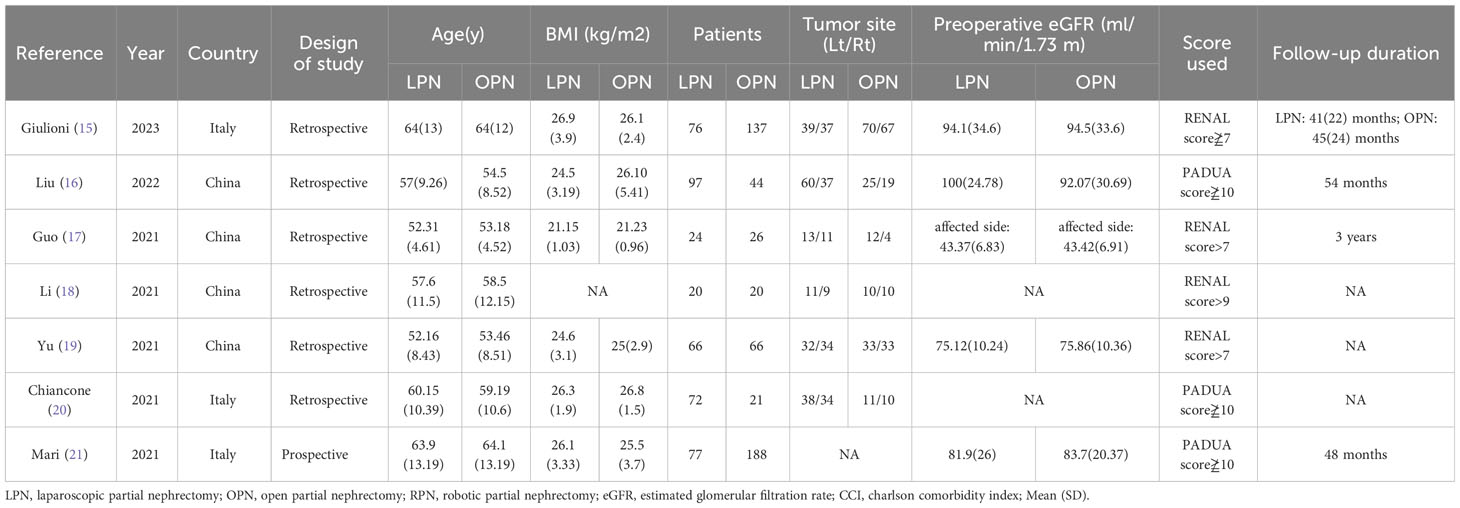
Subsequently, two independent reviewers meticulously extracted the following dataset from the included studies: (1) General manuscript details, including the year of publication, author, and country of origin. (2) Attributes of the study cohort, including sample size, age distribution, and body mass index (BMI). (3) Tumor site, preoperative estimated glomerular filtration rate (eGFR), RENAL score, and follow-up duration. (4) Perioperative consequences: Duration of the operation, volume of blood loss, instances of transfusion, length of hospital stay, warm ischemia, conversion to radical nephrectomy, positive surgical margins (PSM), overall complications (defined by Clavien grade ≥ 1), and major complications (defined by Clavien grade ≥ 3) (12). The process of data extraction was autonomously conducted by the two reviewers to ensure meticulousness and consistency.
In order to appraise the quality of the literature, a comprehensive evaluation was undertaken on the studies incorporated in the analysis, employing the “risk of bias in non-randomized studies of interventions” (ROBINS-I) framework (13). This assessment was independently conducted by two evaluators (F.Z. and J.H.), who scrupulously scrutinized the studies for potential biases, encompassing confounding variables or other plausible origins of systematic divergence. Any incongruities or disparities that emerged during the assessment procedure were resolved through thorough deliberations.
2.2 Statistical analysis
For the purpose of data analysis in this study, we used the Cochrane Collaborative RevMan 5.4 software. Odds ratios (OR) were employed to assess dichotomous outcomes, while weighted mean differences (WMD) were used to quantify continuous outcomes, accompanied by 95% confidence intervals (CI) for all measured parameters. The evaluation of inter-study heterogeneity was conducted using the I2 test (14). In anticipation of potential inter-trial heterogeneity, we adopted the random-effects model for all analytical procedures, establishing a level of statistical significance at p < 0.05. In scenarios where substantial heterogeneity was identified among outcomes (I2 > 75%), sensitivity analyses were executed to pinpoint the origins of inter-study variance and to authenticate the steadfastness of our conclusions. It’s noteworthy, however, that sensitivity analyses were precluded for outcomes derived from three or fewer studies.
2.3 Publication bias
To appraise the likelihood of publication bias, we employed Begg’s method funnel plot.
3 Results
3.1 Baseline characteristics
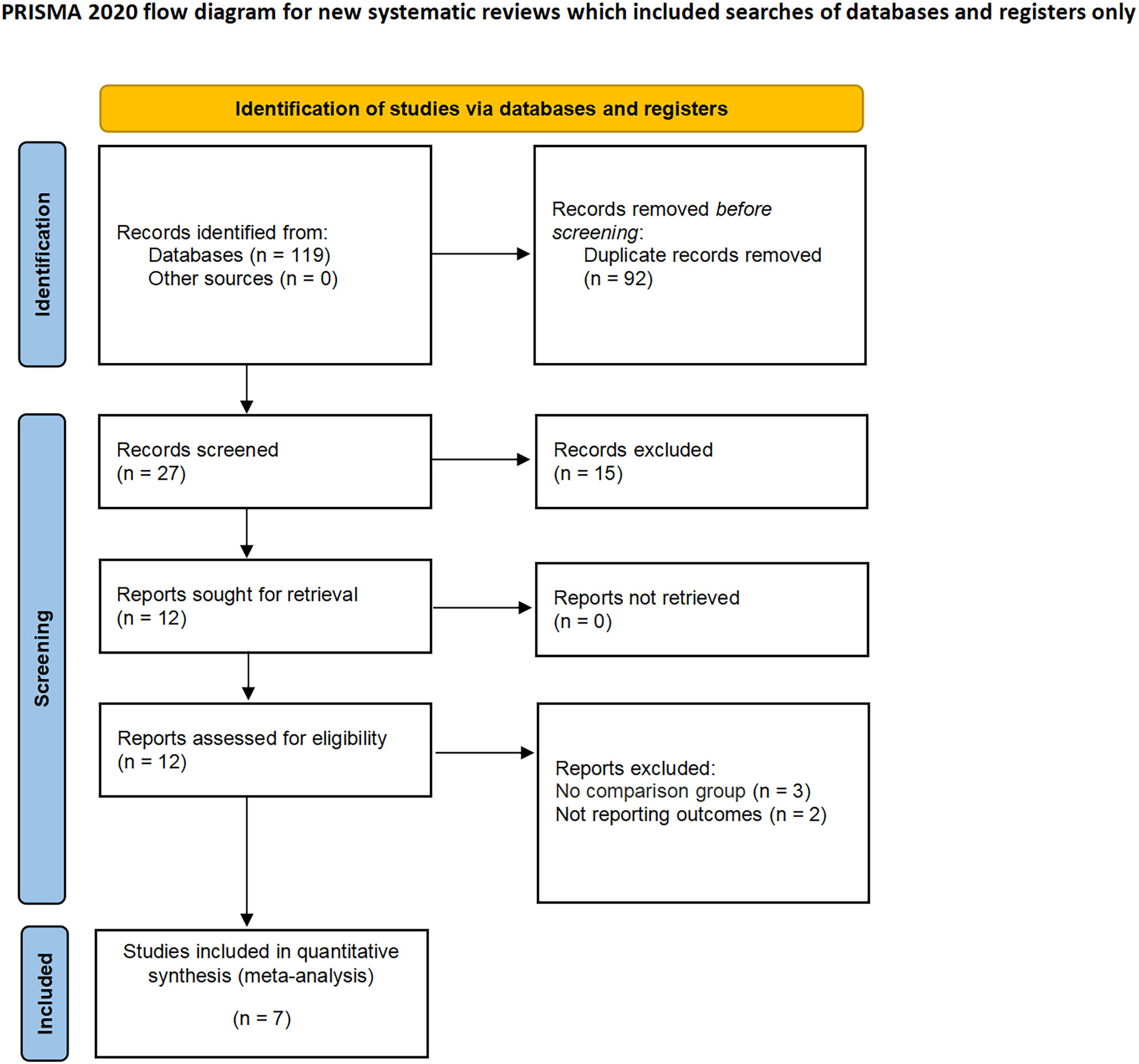
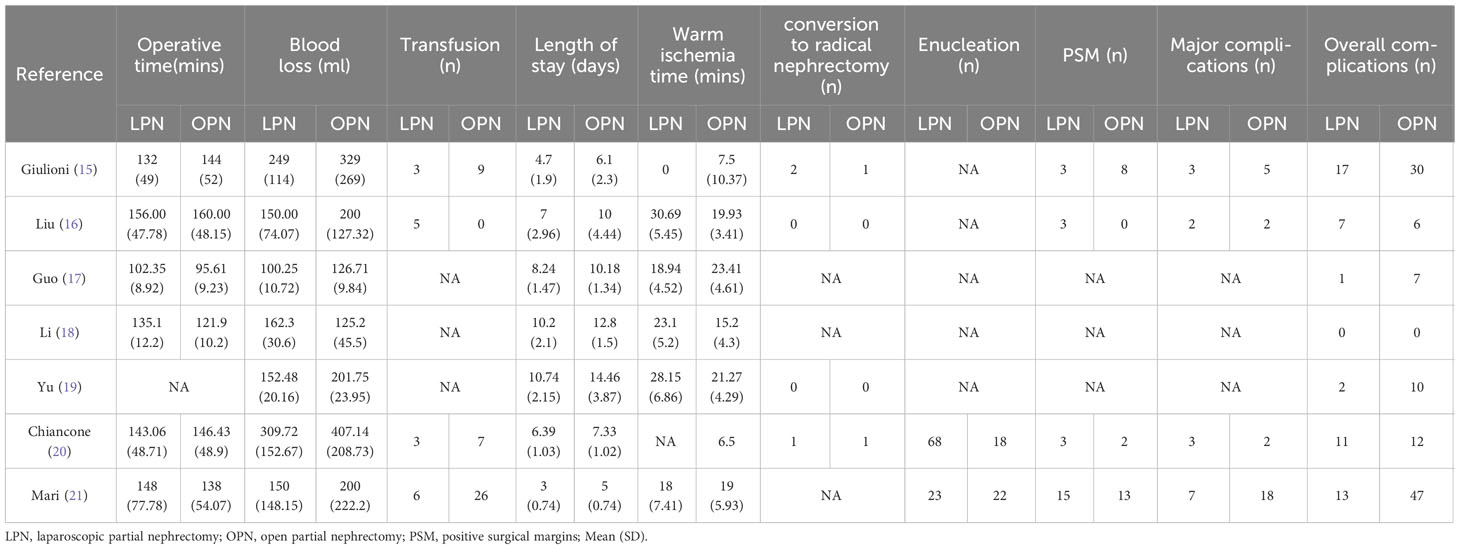
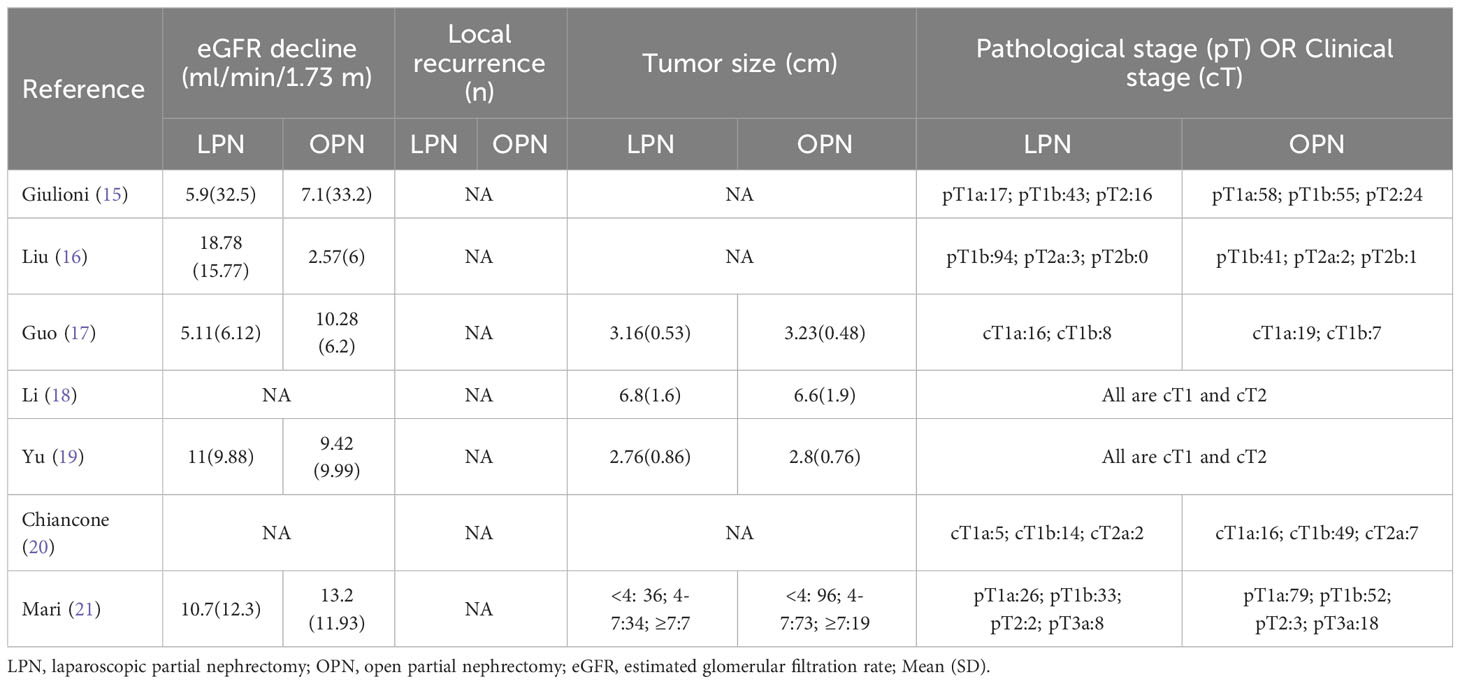
The applied search algorithm initially identified a total of 116 studies within the databases. Following an extensive review of full-text materials and a meticulous screening process, seven studies, comprising 934 patients in total (432 LPN vs. 502 OPN), were deemed suitable for inclusion in the comprehensive meta-analysis (Figure 1) (15–21). The succinct overview in Table 1 offers a synopsis of fundamental patient characteristics, accompanied by the corresponding interventions and associated preoperative variables (including sample size, age, BMI, tumor diameter, preoperative eGFR, RENAL score, and follow-up duration). These studies were published between 2021 and 2023. Table 2 delineates perioperative and surgical outcomes, encompassing pivotal parameters such as operative temporal metrics, blood loss, instances of transfusion, duration of hospitalization, warm ischemic intervals, cases necessitating conversion to the more extensive radical nephrectomy, enucleation, PSM, and complications. The renal functional and oncologic outcomes is presented in Table 3.
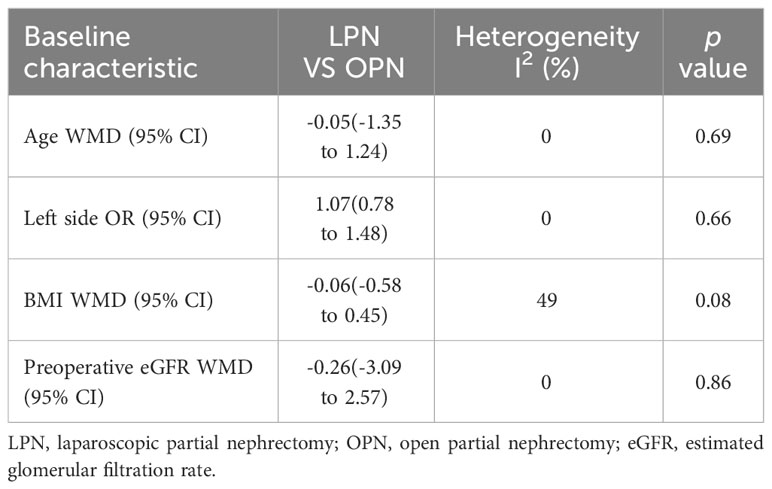
No significant differences were observed in terms of age (p = 0.69), laterality (p = 0.66), BMI (p = 0.08), and preoperative eGFR (p = 0.86) between the LPN and OPN groups (Table 4).
3.2 Assessment of quality
All of the studies conducted a comparative analysis and were published between 2021 and 2023. Out of these, three studies were found to exhibit a moderate level of bias risk, while four studies demonstrated a significantly high risk of bias (Supplementary Table 1).
3.3 Outcome analysis
3.3.1 Perioperative effectiveness
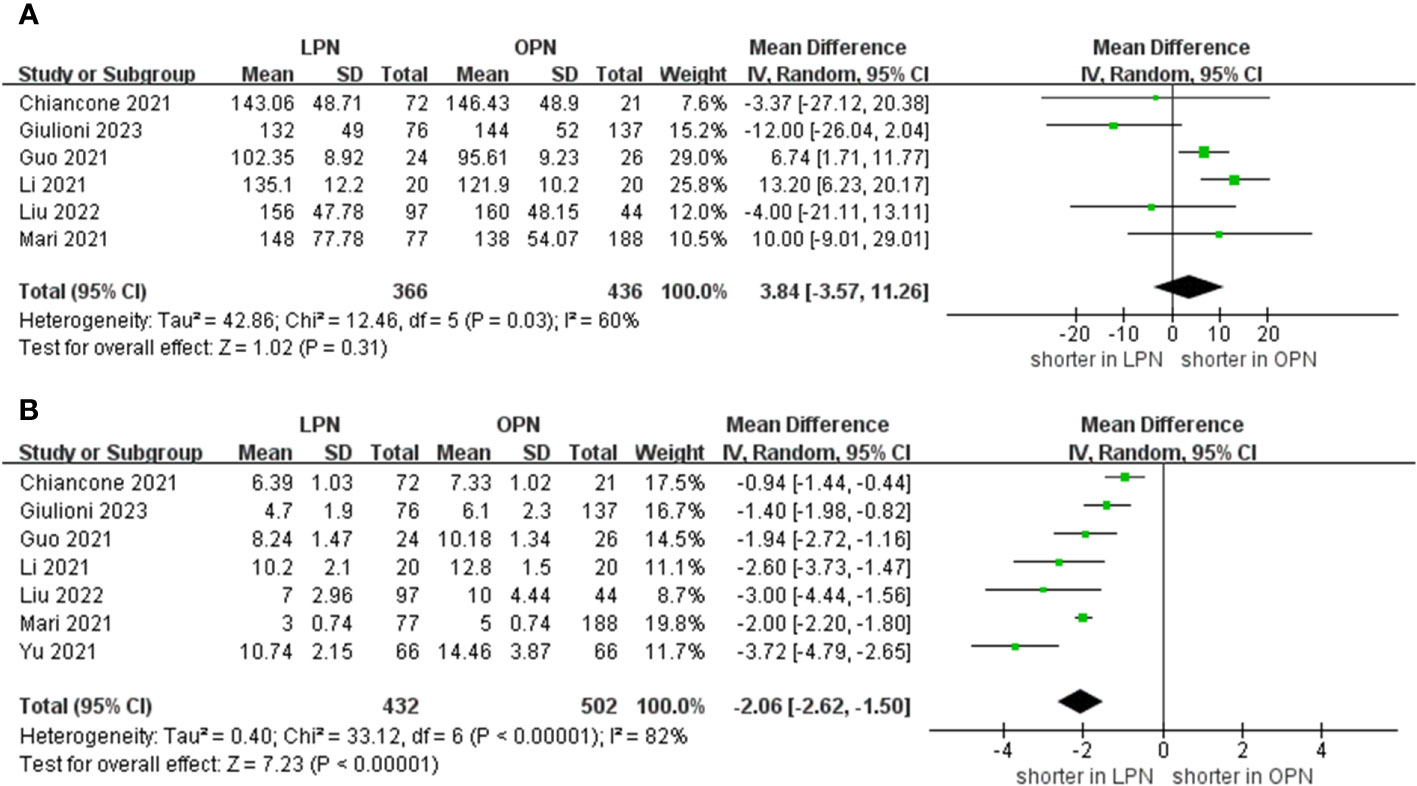
The meta-analysis encompassed six studies that provided data on operative time. Additionally, the results demonstrated that there was no statistically significant distinction in operative time between the groups that underwent LPN and OPN (WMD 3.84 mins, 95% CI -3.57 to 11.26; p = 0.31) (15–18, 20, 21). After pooling data from seven separate studies, the LPN cohort demonstrated a shorter duration of hospitalization in comparison to their OPN counterparts (WMD -2.06 days, 95% CI -2.62 to -1.50; p < 0.00001) (Figure 2) (15–21).
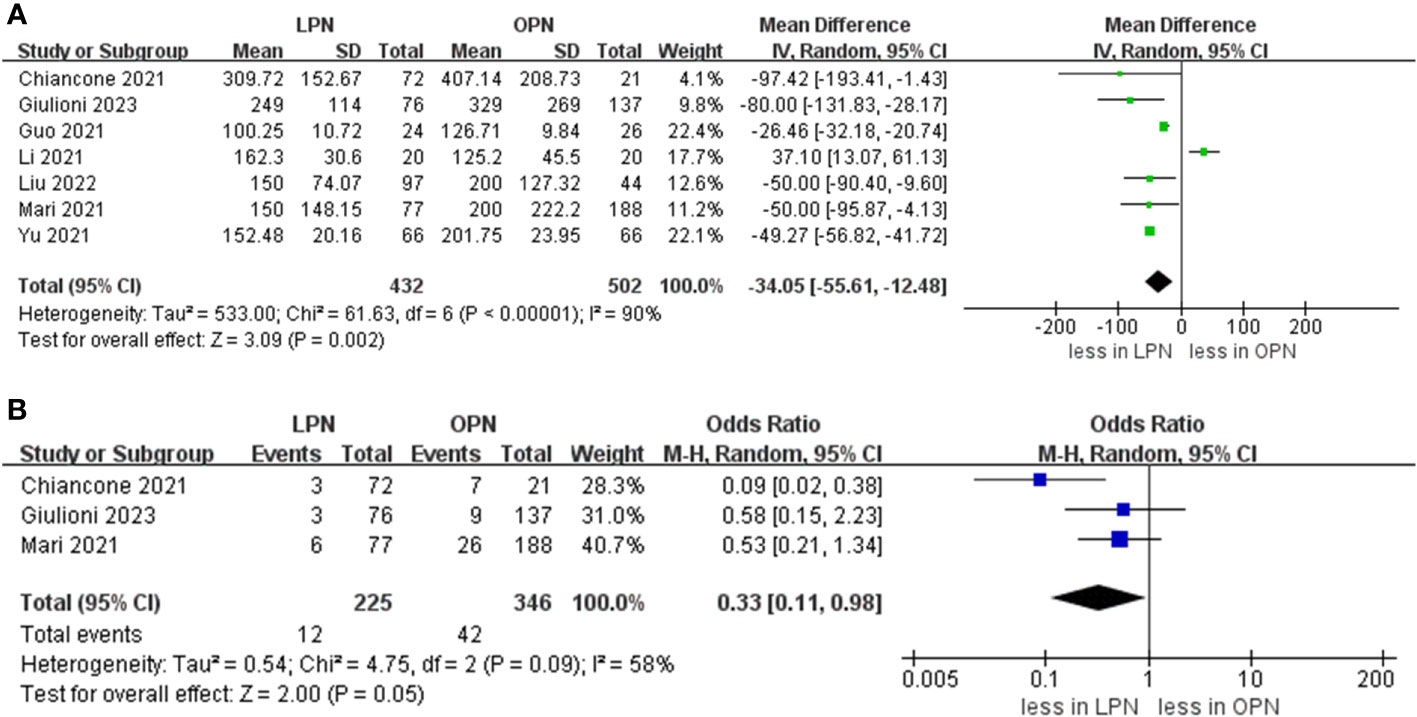
Data concerning blood loss were extracted from seven studies (15–21). The collective findings indicated that LPN was linked to lower blood loss compared to OPN (WMD: -34.05 mL, 95% CI: -55.61 to -12.48; p = 0.002). Nevertheless, no significant distinction was observed in the transfusion rate prevalence between LPN and OPN (OR 0.33, 95% CI 0.11, 0.98; p = 0.05) (three studies; Figure 3) (15, 20, 21). The analysis revealed that a discernible disparity in the prevalence of warm ischemia time between LPN and OPN was not evident (WMD 4.03 mins, 95% CI -1.72 to 9.78; p = 0.17) (based on findings from six studies; Figure 4) (15–19, 21).
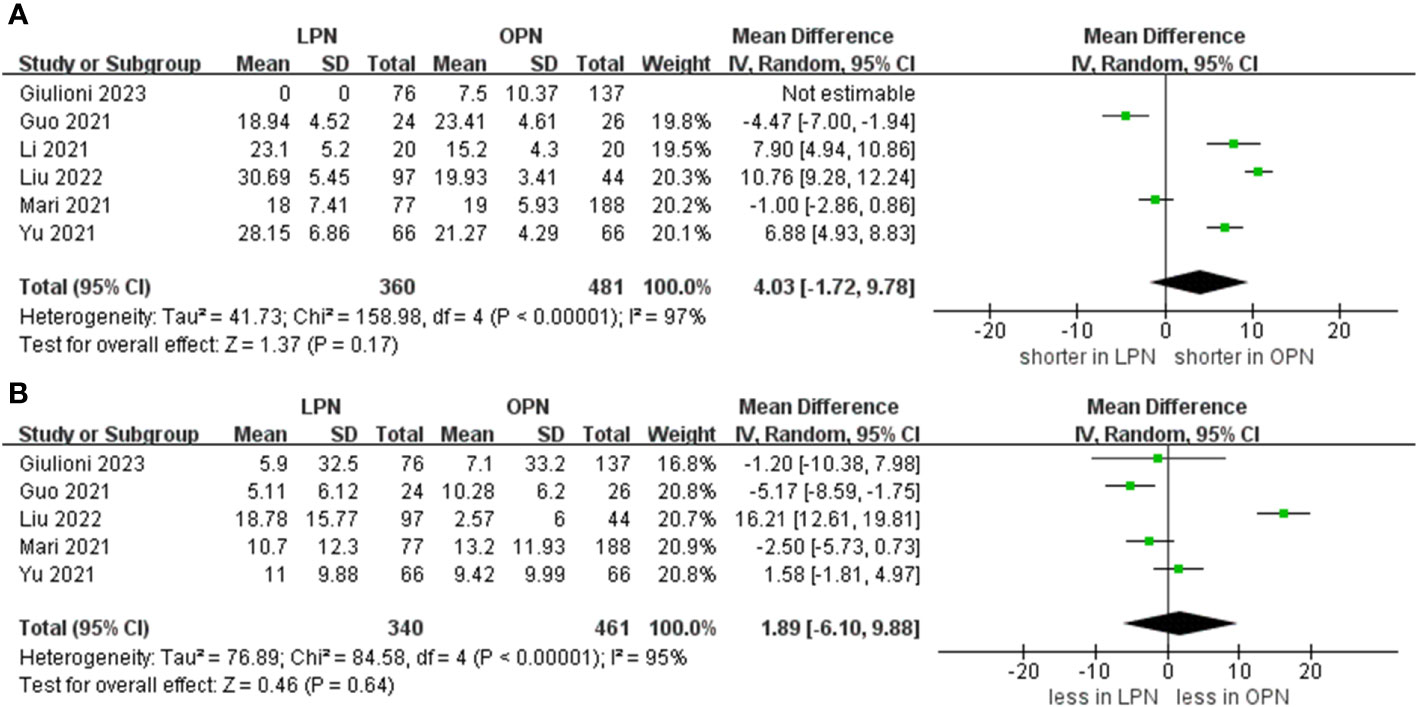
Figure 4 Forest plots of perioperative and renal functional outcomes (A) warm ischemia time, (B) eGFR decline.
3.3.2 Renal functional
The meta-analysis encompassed four studies that reported the rate of decline in eGFR. The results demonstrated that there was no statistically significant distinction in the eGFR decline between the groups that underwent LPN and OPN (WMD 1.89 ml/min/1.73 m, 95% CI -6.10 to 9.88; p = 0.64) (Figure 4) (15–17, 19, 21).
3.3.3 Complications
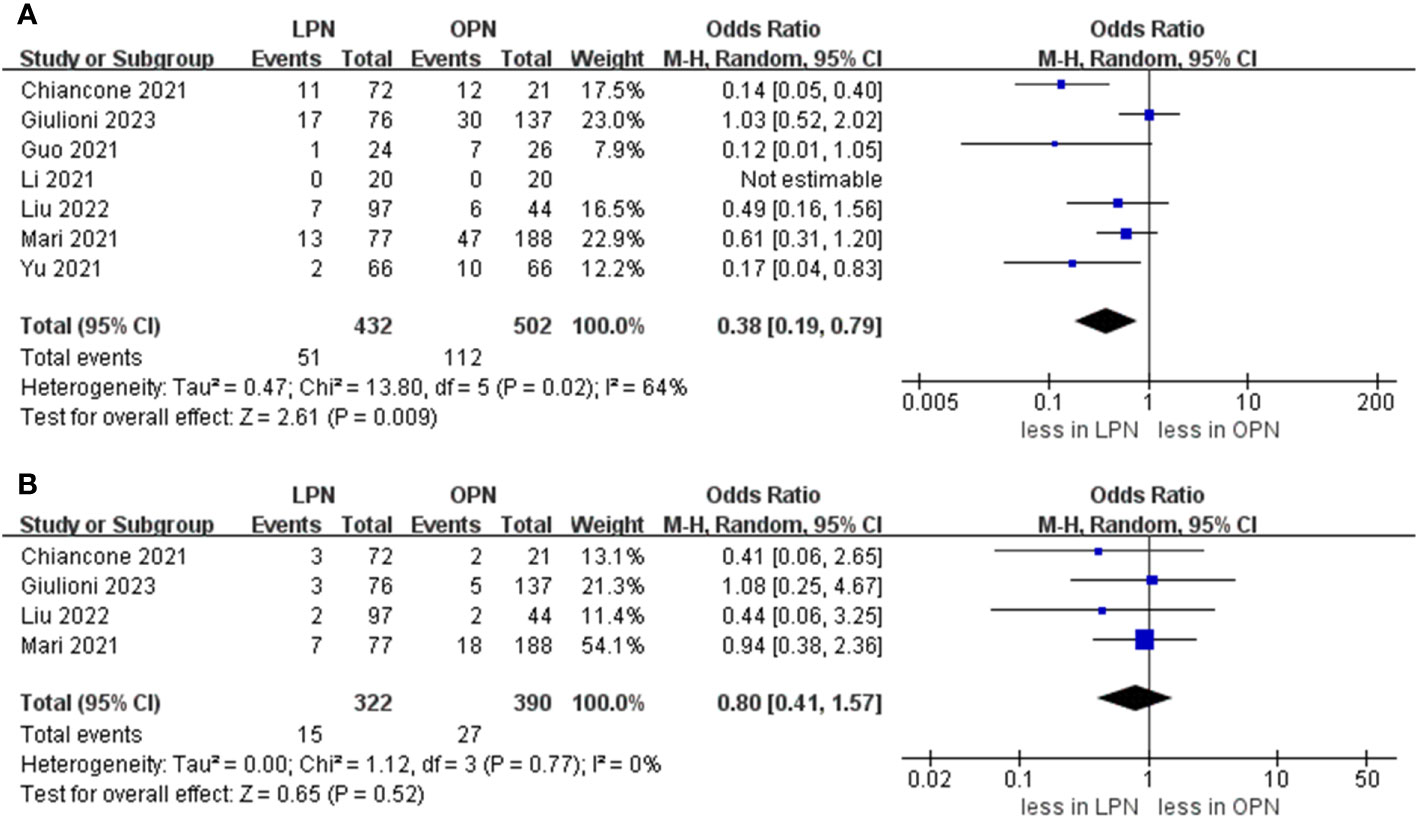
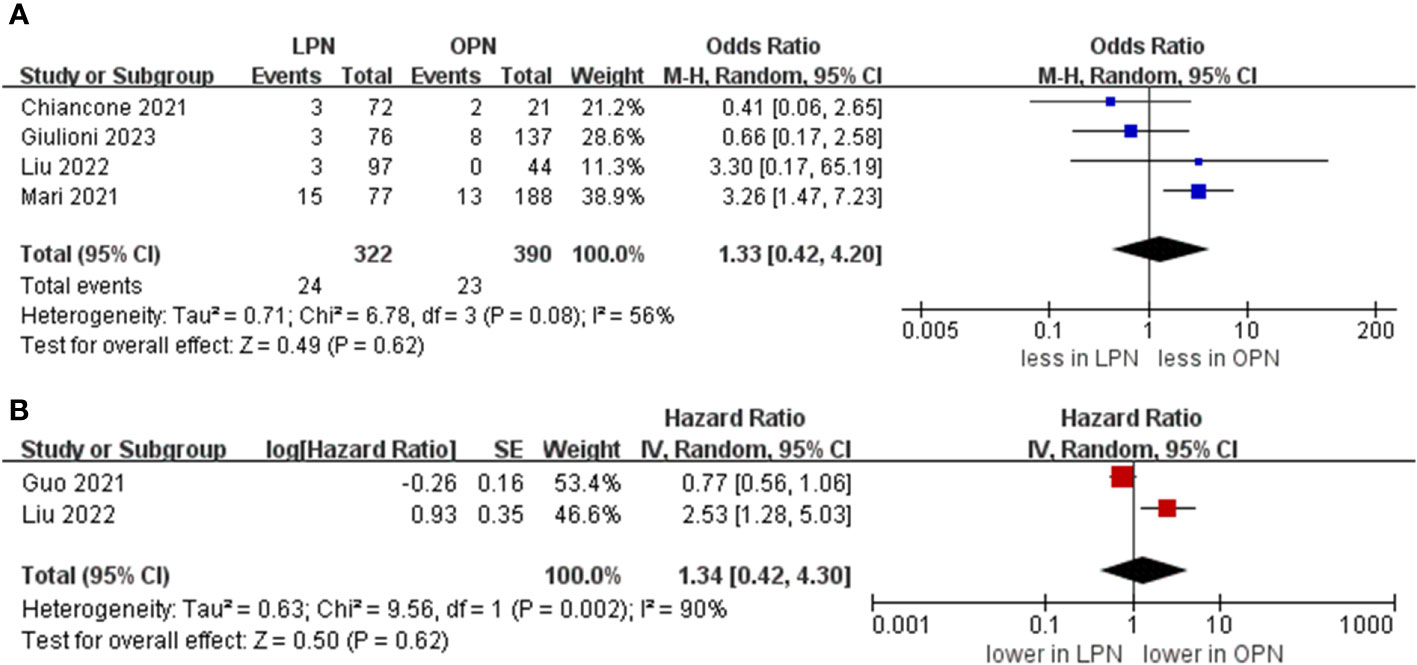
The cumulative incidence of overall complications was 11.8% (51 out of 432 cases) for LPN and 22.3% (112 out of 502 cases) for OPN. Across seven studies (16–21), LPN exhibited a lower incidence of overall complications in comparison to OPN (OR 0.38, 95% CI 0.19, 0.79; p = 0.009) (15–21). Furthermore, the rates of major complications were 4.5% (15 out of 332 cases) for LPN and 6.9% (27 out of 390 cases) for OPN. However, no statistically noteworthy disparities in the occurrence of significant complications between LPN and OPN were detected (OR 0.80, 95% CI 0.41, 1.57; p = 0.52) (Figure 5) (15, 16, 20, 21).
3.3.4 Oncologic outcomes
A PSM-based meta-analysis demonstrated that there existed no statistically meaningful distinction between LPN and OPN (four studies; OR 0.80, 95% CI 0.41, 1.57; p = 0.52) (15, 16, 20, 21). Moreover, no statistically notable differentiation was witnessed regarding overall survival (OS) (5 years) between LPN and OPN (two studies; HR 1.34, 95% CI 0.42, 4.30; p = 0.62) (Figure 6) (16, 17).
3.4 Heterogeneity
A prevailing inclination toward mild to moderate heterogeneity was noticeable across the majority of the outcomes. Although studies of intermediate and high quality were incorporated, substantial heterogeneity emerged in four of the outcomes: duration of hospitalization (I2 = 82%), blood loss (I2 = 90%), warm ischemia time (I2 = 97%), and eGFR decline (I2 = 95%).
3.5 Sensitivity analysis
In this study, the identification of conspicuous heterogeneity in aspects such as duration of hospital stay, blood loss, warm ischemia time, and eGFR decline prompted the implementation of a sensitivity analysis. The aim was to precisely determine the fundamental origin of this heterogeneity and to assess the robustness and coherence of the study’s conclusions. The outcomes derived from this analysis revealed the absence of noteworthy fluctuations in the extent of heterogeneity, thus implying a consistent source of heterogeneity in terms of the duration of hospital stay, blood loss, warm ischemia time, and eGFR decline (as depicted in Supplementary Table 2).
3.6 Publication bias
To evaluate the possible existence of publication bias in the examined research, we performed an analysis centered on variables including operative time, length of hospital stay, warm ischemia time, and overall complications. Our results revealed that the distribution across the studies exhibited an almost symmetrical trend, suggesting a low probability of publication bias (as depicted in Supplementary Figure 1).
4 Discussion
This study presents an all-encompassing summary of the available evidence regarding perioperative outcomes, renal function, and oncological outcomes connected with the utilization of LPN and OPN for addressing intricate renal tumors. Additionally, certain essential discoveries unearthed in this study underscore the requirement for further thorough exploration.
Laparoscopic surgery is employed in clinical practice; however, LPN demands the surgeon’s precise procedural expertise, encompassing tasks like tumor excision, reconstruction of the renal pelvis and calices, kidney hemostasis, and the intricate laparoscopic suture technique within confined spaces (22). As a result, OPN is favored for managing complex renal tumors due to its expansive surgical field of vision and broad spectrum of indications. The analysis indicated that LPN was associated with a longer operative time compared to OPN. This occurrence may be attributed to the following factors. Certain studies have employed Retroperitoneal LPN for complex renal tumors. The execution of retroperitoneal LPN necessitates meticulous surgical skills from the surgeon, encompassing tasks such as tumor excision, restoration of the renal pelvis, achieving kidney hemostasis, and executing precise suture techniques within confined spaces. Importantly, it should be noted that the learning curve for retroperitoneal LPN is extended and requires a heightened level of proficiency from the surgeon. Therefore, despite the array of advantages associated with retroperitoneal LPN, it continues to exhibit certain drawbacks, including an extended learning curve, elevated prerequisites for surgical proficiency among medical practitioners, and prolonged operative durations. Hence, further research is warranted to validate this conclusion. However, LPN demonstrated a shortened hospitalization period in comparison to OPN, primarily due to its reduced occurrence of postoperative complications, considering that complications can significantly prolong the duration of hospitalization (23). At the same time, the use of ultrasonic scalpel during operation can quickly remove the lesion, reduce intraoperative blood loss, and is more conducive to the recovery of intestinal function, thereby shortening the length of hospital stay (24, 25). However, the length of hospitalization is also influenced by institutional capacity and the proficiency of the surgeon. The rapid advancements in anesthesiology and the management of perioperative care might potentially contribute to a reduction in hospital stay. Thus, additional studies are imperative to validate this outcome.
Blood loss during surgery is a critical factor for assessing surgical quality. An important benefit of minimally invasive surgery is the reduction in operative blood loss. Our study revealed a noteworthy observation: LPN demonstrated reduced blood loss compared to OPN. This discrepancy can be attributed to the utilization of a laparoscopic vision imaging system, which provides an expansive surgical field of vision, enabling surgeons to promptly identify and address bleeding sites using electric coagulation forceps (26). These reasons could account for the lower blood loss observed in the LPN group compared to the OPN group. However, despite this finding, an analysis of cumulative outcomes indicated no statistically significant difference in transfusion rates between LPN and OPN. This phenomenon could be linked to the expertise of physicians and the established hospital transfusion protocols. However, a significant difference in the prevalence of transfusion rates between LPN and OPN was not observed. This lack of distinction could be attributed to the level of experience of the medical practitioners and the institution’s guidelines pertaining to blood transfusion, both of which have a potential impact on the transfusion rate.
The combined results suggested the absence of a statistically significant distinction in warm ischemia time between LPN and OPN. Moreover, certain elements deserve our focus. Some studies suggest that keeping warm ischemia time under 25 or 30 minutes is advisable to minimize the potential risk of detrimental effects on renal function (27, 28). In a recent study, Buffi et al. (29) presented the perioperative outcomes of 255 patients who underwent robot-assisted PN across four high-volume medical centers. The study revealed that 33.7% of patients experienced a warm ischemia time surpassing 20 minutes, while 7.8% had a warm ischemia time exceeding 30 minutes. In summary, the ischemic duration associated with LPN proves to be well-tolerated in the context of intricate renal tumors. Recent research has highlighted that warm ischemia time holds a relatively minor influence on long-term renal functional outcomes. However, preoperative renal function and the preservation of kidney count emerge as notably interconnected with the key determinants of long-term renal results (30). Furthermore, Fergany et al. (31) highlighted the significant role of age in the recovery of long-term renal function post-surgery. Thus, further research is essential to validate the influence of warm ischemia time on postoperative renal function. It is important to highlight that the results revealed no significant difference in the decline of estimated glomerular filtration rate (eGFR) between the LPN and OPN groups.
In terms of complications, we utilized the Clavien classification to assess surgical complications. LPN demonstrated a lower incidence of overall complications compared to OPN. For patients undergoing minimally invasive surgery, there is a notable reduction in both intraoperative blood loss and postoperative pain, which significantly benefits the recovery process. In the OPN group, the incision made between the 11th or 12th rib for partial nephrectomy is more invasive, exerting considerable stress on the body and increasing the likelihood of severe complications that can impede postoperative recovery (32). Kızılay et al. (33) emphasized that when comparing LPN with OPN for renal cancer treatment, they discovered noteworthy variations in postoperative C-reactive protein levels among patients undergoing LPN and OPN. This finding underscores that open surgery can trigger a heightened stress response in the body, potentially impacting postoperative recovery. Additionally, minimally invasive surgery offers several advantages, such as smaller incisions and limited anatomical exposure. These aspects contribute to a reduced risk of adjacent organ damage and, consequently, fewer postoperative complications.
The assessment of oncologic outcomes is of utmost importance. In fact, heightened tumor complexity appears to be linked with invasive traits and a lower survival rate. During partial nephrectomy, the presence of dense and adherent “sticky” perirenal fat surrounding the kidney can complicate the procedure and impede the precise demarcation of the surgical margin between the renal tumor and healthy tissue (34). This could potentially result in positive surgical margins and significant perioperative complications. However, A meta-analysis of PSM and OS revealed no statistically significant difference between LPN and OPN. However, there are several crucial considerations to be mindful of. Firstly, PSM might not necessarily serve as a definitive predictor of recurrence (35). Secondly, numerous factors could potentially influence the occurrence of PSM, including tumor diameter, surgical approach, and tumor stage (36). Consequently, additional research is imperative to validate the findings we have presented. Thirdly, due to a shortage of available literature, it is currently challenging to ascertain the potential differences in metastatic recurrence between the two groups, as well as their potential implications on recurrence-free survival.
Several other significant issues necessitate thorough discussion. The studies included in this analysis employed varying surgical methods for performing PN. Notably, the choice between retroperitoneal and intraperitoneal approaches introduced some heterogeneity across the studies. The retroperitoneal approach offers numerous advantages, including simplified ligation of the renal artery, thereby reducing blood loss during the isolation of renal tumors. Simultaneously, it minimizes interference with the intestines, subsequently lowering the risk of complications (37). However, it’s important to note that this approach does come with certain disadvantages, such as limited surgical space. Consequently, further investigations of high quality are imperative to ascertain the more suitable surgical approach for complex renal tumors. Secondly, the procedures encompassed within this study were executed by surgical teams possessing substantial expertise in laparoscopic surgery, and the data were sourced from large institutions. Moreover, it’s noteworthy that the selection of the surgical approach was not randomized; rather, it was guided by clinical and radiological evaluations. Consequently, the outcomes may not readily generalize to low-volume centers characterized by limited familiarity with laparoscopic techniques. Thus, further studies are imperative to substantiate our findings.
The study was conducted following the rigorous guidelines of PRISMA (13). Nonetheless, certain limitations influence the analysis. Firstly, all the studies were retrospective or prospective, ranging from low to moderate quality. Furthermore, no randomized controlled trials have been included in our study. Consequently, these studies are undoubtedly prone to inherent selection bias. Secondly, no subgroup analyses were conducted based on the surgical method (transperitoneal or retroperitoneal), potentially introducing some heterogeneity into the results. Furthermore, we conducted a pooled analysis of studies that employed kidney and PADUA scores ≥ 7, which might unveil subtle distinctions. However, the scarcity of adequate literature hindered our capacity to perform subgroup analyses. Finally, the included studies offered limited insights into outcomes in other oncology domains (e.g., free survival and cancer-specific survival), thus impeding a comparison of the two groups in terms of these outcomes.
5 Conclusions
LPN stands out as a more favorable choice than OPN for the management of intricate renal tumors. LPN brings forth benefits including a decreased hospitalization period, diminished blood loss, and a lower occurrence of complications. Moreover, LPN shows comparable oncological results when contrasted with OPN. However, it’s important to acknowledge that the majority of the included studies were of moderate quality and held a retrospective nature. Thus, additional research that integrates studies of superior quality and extended follow-up durations is imperative for a thorough comparison of the outcomes between these two approaches.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
FZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. J-SH: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. K-YZ: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. X-HL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1283935/full#supplementary-material
Supplementary Figure 1 | Funnel plot (A) operative time, (B) length of hospital stay, (C) warm ischemia time, (D) overall complication.
References
1. Grange R, Tradi F, Izaaryene J, Daidj N, Brunelle S, Walz J, et al. Computed tomography-guided percutaneous cryoablation of T1b renal tumors: safety, functional and oncological outcomes. Int J Hyperthermia (2019) 36(1):1065–71. doi: 10.1080/02656736.2019.1675913
2. Grünwald V, Ivanyi P, Zschäbitz S, Wirth M, Staib P, Schostak M, et al. Nivolumab switch maintenance therapy after tyrosine kinase inhibitor induction in metastatic renal cell carcinoma: A randomized clinical trial by the interdisciplinary working group on renal tumors of the german cancer society (NIVOSWITCH; AIO-NZK-0116ass). Eur Urol (2023) 84(6):571–8. doi: 10.1016/j.eururo.2023.09.004
3. Xia L, Wang X, Xu T, Guzzo TJ. Systematic review and meta-analysis of comparative studies reporting perioperative outcomes of robot-assisted partial nephrectomy versus open partial nephrectomy. J Endourol (2017) 31(9):893–909. doi: 10.1089/end.2016.0351
4. Huang J, Su R, Zhang C, Bao Y, Hu X, Ye X, et al. Comparative analysis of salvage partial nephrectomy versus radical nephrectomy after the failure of initial partial nephrectomy. Urol Oncol (2023) 41(10):434.e17–434.e25. doi: 10.1016/j.urolonc.2023.07.010
5. Kutikov A, Uzzo RG. , The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol (2009) 182(3):844–53. doi: 10.1016/j.juro.2009.05.035
6. Ficarra V, Novara G, Secco S, Macchi V, Porzionato A, De Caro R, et al. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol (2009) 56(5):786–93. doi: 10.1016/j.eururo.2009.07.040
7. Meyer A, Woldu SL, Weinberg AC, Thoreson GR, Pierorazio P, Matulay JT, et al. Predicting renal parenchymal loss after nephron sparing surgery. J Urol (2015) 194(3):658–63. doi: 10.1016/j.juro.2015.03.098
8. Laganosky DD, Filson CP, Master VA. Surgical margins in nephron-sparing surgery for renal cell carcinoma. Curr Urol Rep (2017) 18(1):8. doi: 10.1007/s11934-017-0651-5
9. Abdelhafez M, Bastian A, Rausch S, Stenzl A, Bedke J, Kruck S. Laparoscopic versus open partial nephrectomy: comparison of overall and subgroup outcomes. Anticancer Res (2017) 37(1):261–5. doi: 10.21873/anticanres.11316
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. doi: 10.1136/bmj.n71
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
12. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
13. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj (2016) 355:i4919. doi: 10.1136/bmj.i4919
14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
15. Giulioni C, Castellani D, Di Biase M, Ferrara V, Benedetto Galosi A. Laparoscopic and open nephron-sparing surgery for radius exophytic/endophytic nearness anterior/posterior location nephrometry score 7 and higher kidney tumors: A comparison of oncological and functional outcomes using the Pentafecta score. Urol Res Pract (2023) 49(3):178–83. doi: 10.5152/tud.2023.22233
16. Liu Z, Zhang X, Lv P, Wu B, Bai S. Functional, oncological outcomes and safety of laparoscopic partial nephrectomy versus open partial nephrectomy in localized renal cell carcinoma patients with high anatomical complexity. Surg Endosc (2022) 36(10):7629–37. doi: 10.1007/s00464-022-09225-7
17. Guo Y, Xu Q, Chen B, Liu L, Wang Y, Zhu A, et al. Clinical outcomes and effect on intraoperative blood loss and postoperative pain of patients undergoing retroperitoneal laparoscopic partial nephrectomy for complex renal tumors. World J Surg Oncol (2021) 19(1):282. doi: 10.1186/s12957-021-02397-x
18. Li TT, Feng J, Li YL, Sun Q. A retrospective study of open and endoscopic nephron sparing surgery in the treatment of complex renal tumors. Pak J Med Sci (2021) 37(4):1031–5. doi: 10.12669/pjms.37.4.3457
19. Yu F, Xu Q, Liu XG. Impact of laparoscopic partial nephrectomy and open partial nephrectomy on outcomes of clear cell renal cell carcinoma. Front Surg (2021) 8:681835. doi: 10.3389/fsurg.2021.681835
20. Chiancone F, Fabiano M, Meccariello C, Fedelini M, Persico F, Fedelini P. Laparoscopic versus open partial nephrectomy for the management of highly complex renal tumors with PADUA score ≧̸10: A single center analysis. Urologia (2021) 88(4):343–7. doi: 10.1177/03915603211001677
21. Mari A, Tellini R, Porpiglia F, Antonelli A, Schiavina R, Amparore D, et al. Perioperative and mid-term oncological and functional outcomes after partial nephrectomy for complex (PADUA score ≥10) renal tumors: A prospective multicenter observational study (the RECORD2 project). Eur Urol Focus (2021) 7(6):1371–9. doi: 10.1016/j.euf.2020.07.004
22. Würnschimmel C, Di Pierro GB, Moschini M, Grande P, Baumeister P, Roth M, et al. Robot-assisted laparoscopic partial nephrectomy vs conventional laparoscopic partial nephrectomy: functional and surgical outcomes of a prospective single surgeon randomized study. J Endourol (2020) 34(8):847–55. doi: 10.1089/end.2020.0143
23. Qian J, Jiang J, Li P, Zhang S, Bao M, Qin C, et al. Factors influencing the feasibility of segmental artery clamping during retroperitoneal laparoscopic partial nephrectomy. Urology (2019) 129:92–7. doi: 10.1016/j.urology.2019.03.024
24. Calpin GG, Ryan FR, McHugh FT, McGuire BB. Comparing the outcomes of open, laparoscopic and robot-assisted partial nephrectomy: a network meta-analysis. BJU Int (2023) 132(4):353–64. doi: 10.1111/bju.16093
25. Wang L, Li KP, Liu Y, Yin S, Zhu PY. Perioperative and oncologic outcomes of transperitoneal versus retroperitoneal laparoscopic radical nephrectomy for large-volume renal carcinoma (> 7 cm): a systematic review and pooled analysis of comparative outcomes. World J Surg Oncol (2023) 21(1):86. doi: 10.1186/s12957-023-02967-1
26. Li KP, Chen SY, Wang CY, Yang L. Comparison between minimally invasive partial nephrectomy and open partial nephrectomy for complex renal tumors: a systematic review and meta-analysis. Int J Surg (2023) 109(6):1769–82. doi: 10.1097/js9.0000000000000397
27. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol (2010) 58(3):340–5. doi: 10.1016/j.eururo.2010.05.047
28. Zargar H, Akca O, Autorino R, Brandao LF, Laydner H, Krishnan J, et al. Ipsilateral renal function preservation after robot-assisted partial nephrectomy (RAPN): an objective analysis using mercapto-acetyltriglycine (MAG3) renal scan data and volumetric assessment. BJU Int (2015) 115(5):787–95. doi: 10.1111/bju.12825
29. Buffi NM, Saita A, Lughezzani G, Porter J, Dell’Oglio P, Amparore D, et al. Robot-assisted partial nephrectomy for complex (PADUA score ≥10) tumors: techniques and results from a multicenter experience at four high-volume centers. Eur Urol (2020) 77(1):95–100. doi: 10.1016/j.eururo.2019.03.006
30. Lane BR, Russo P, Uzzo RG, Hernandez AV, Boorjian SA, Thompson RH, et al. Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol (2011) 185(2):421–7. doi: 10.1016/j.juro.2010.09.131
31. Fergany AF, Saad IR, Woo L, Novick AC. Open partial nephrectomy for tumor in a solitary kidney: experience with 400 cases. J Urol (2006) 175(5):1630–3; discussion 1633. doi: 10.1016/s0022-5347(05)00991-2
32. Fan G, Meng Y, Zhu S, Ye M, Li M, Li F, et al. Three-dimensional printing for laparoscopic partial nephrectomy in patients with renal tumors. J Int Med Res (2019) 47(9):4324–32. doi: 10.1177/0300060519862058
33. Kızılay F, Turna B, Apaydın E, Semerci B. Comparison of long-term outcomes of laparoscopic and robot-assisted laparoscopic partial nephrectomy. Kaohsiung J Med Sci (2019) 35(4):238–43. doi: 10.1002/kjm2.12038
34. Zheng Y, Espiritu P, Hakky T, Jutras K, Spiess PE. Predicting ease of perinephric fat dissection at time of open partial nephrectomy using preoperative fat density characteristics. BJU Int (2014) 114(6):872–80. doi: 10.1111/bju.12579
35. Marszalek M, Carini M, Chlosta P, Jeschke K, Kirkali Z, Knüchel R, et al. Positive surgical margins after nephron-sparing surgery. Eur Urol (2012) 61(4):757–63. doi: 10.1016/j.eururo.2011.11.028
36. Malkoç E, Maurice MJ, Kara Ö, Ramirez D, Nelson RJ, Dagenais J, et al. Predictors of positive surgical margins in patients undergoing partial nephrectomy: A large single-center experience. Turk J Urol (2019) 45(1):17–21. doi: 10.5152/tud.2018.57767
Keywords: laparoscopic partial nephrectomy, open partial nephrectomy, complex renal tumors, meta-analysis, outcomes
Citation: Zhang F, Hu J-s, Zhang K-y and Liu X-h (2024) Perioperative, functional, and oncologic outcomes of laparoscopic partial nephrectomy versus open partial nephrectomy for complex renal tumors: a systematic review and meta-analysis. Front. Oncol. 13:1283935. doi: 10.3389/fonc.2023.1283935
Received: 27 August 2023; Accepted: 19 December 2023;
Published: 10 January 2024.
Edited by:
Angelo Naselli, MultiMedica Holding SpA (IRCCS), ItalyReviewed by:
Giacomo Maria Pirola, IRCCS MultiMedica, ItalyDaniele Castellani, Polytechnic University of Le Marche, Italy
Copyright © 2024 Zhang, Hu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-hua Liu, ZXNodWFAMTYzLmNvbQ==
 Fan Zhang
Fan Zhang Xiao-hua Liu
Xiao-hua Liu