- Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China
Background: The molecular classification of endometrial cancer has previously been shown to be associated with clinical outcomes. However, there are insufficient data to support the routine use of molecular classification for the treatment of patients seeking fertility preservation.
Methods: Here, we retrospectively investigated 90 patients received fertility-sparing treatment. We used a next generation sequencing (NGS) panel to classify these patients into four subtypes. All patients received hormonal therapy combined with hysteroscopy. Therapeutic effects were evaluated by hysteroscopy every three months during the treatment.
Results: Patients with POLE mutations had the highest disease progression rate (50.0%, P=0.013), while the microsatellite instability-high (MSI-H) group had the highest recurrence rate (50.0%, P=0.042). PIK3CA mutation (hazard ratio (HR): 0.61; 95% confidence interval (CI): 0.37–0.99; P=0.046), overweight (HR: 0.56; 95% CI: 0.32–0.96; P=0.033) and obesity (HR: 0.44; 95% CI: 0.20–0.95; P=0.036) were associated with a significantly lower cumulative complete response (CR) rate. The combination of gonadotropin-releasing hormone analogues (GnRH-a) and letrozole (HR: 3.43; 95% CI: 1.81–6.52; P< 0.001) was associated with a significantly higher cumulative CR rate. KRAS mutation was significantly associated with disease progression (P=0.002). In wild-type TP53 patients, PTEN and PIK3CA mutations significantly prolonged the duration of treatment to achieve CR (log rank P=0.034; P=0.018).
Conclusion: The implementation of molecular classification for EC patients undergoing fertility-sparing treatment is promising and can facilitate the selection of appropriate medical regimes to achieve better outcomes in patients with EC who require fertility preservation treatment.
1 Introduction
The incidence rates of endometrial cancer (EC) have been rising over recent years, with an estimated 65,950 new cases and 12,550 deaths in the U.S.A in 2022 (1). Early-onset endometrial cancer (EOEC), diagnosed in patients under 50 years-of-age, is relatively uncommon, while recent studies have indicated that the incidence of EC is rising continually among young patients, particularly in women under the age of 45 (2, 3). According to the National Cancer Institute, the incidence rates of EC among women aged 20-34 years and 35-44 years were 1.8% and 5.3%, respectively (4). This implied that the proportion of cases managed by fertility-sparing treatment (FST) is increasing when compared to hysterectomy in young patients with early-stage endometrial cancer. Currently, the majority of FST studies related to EC focus mostly on assessing the treatment effects of various therapies and identifying clinical factors that impact FST outcomes (5–7). However, as research advances, studies of EC are transitioning toward a molecular-based approach.
The molecular classification of EC was first proposed by The Cancer Genome Atlas (TCGA) in 2013, which classified EC into four subtypes based on array and sequence technologies: (1) POLE ultramutated, (2) microsatellite instability hypermutated, (3) copy-number low, and (4) copy-number high (8). Subsequently, clinically applicable molecular classification systems were developed based on immunohistochemistry (IHC) or next generation sequencing (NGS) (9–12). NGS has been established to represent an effective method for the molecular classification of EC and shows high concordance with the final hysterectomy specimens when applied to curettage samples (13). According to NGS molecular classification, EC can be divided into four subtypes: (1) POLE mutated (POLE mut), (2) microsatellite instability hypermutated (MSI-H), 3. TP53 wild-type (TP53 wt), and (4) TP53 abnormal (TP53 abn). Both National Comprehensive Cancer Network (NCCN) and ESGO-ESMO-ESTRO have included molecular classification as a consideration to guide treatment strategies for EC patients undergoing surgery (14, 15). In addition, molecular classification has been encouraged in the newest ESGO/ESHRE/ESGE guidelines for EC patients receiving FST (15).
There were only a limited number of studies exploring the relationship between molecular classification and FST for patients with EC. However, these studies reported different outcomes. Some studies suggested that different molecular subtypes responded differently to conservative treatment, and that mismatch repair deficiency (MMR-D) may be associated with a longer treatment duration and a higher risk of recurrence than other subtypes (16, 17). However, another study indicated that molecular classification might not exert prognostic significance for EC patients undergoing FST (18). Consequently, there is an urgent need for further clinical research to confirm the significance of molecular classification for patients with EC undergoing FST.
In this single-center retrospective study, we aimed to evaluate the efficacy of FST among different molecular subtypes in patients with EC. Furthermore, we aimed to identify novel molecular prognostic factors for FST by comprehensively analyzing genomic changes in patients with EC by NGS testing.
2 Materials and methods
2.1 Study population
In this retrospective study, we investigated all EC patients receiving FST in the Obstetrics and Gynecology Hospital of Fudan University between January 2021 and January 2022. These patients include those who were initially diagnosed with EC and those who progressed to EC during the course of treatment. The study was approved by Ethics Committees of Obstetrics and Gynecology Hospital of Fudan University.
The diagnosis of EC was confirmed by at least two experienced gynecological pathologists according to the World Health Organization (WHO) Pathological Classification of EC (2014). Tissue specimens were obtained by dilation and curettage (D&C) with or without hysteroscopy.
The criteria for inclusion were as follows: (1) aged between 18 and 45 years, (2) a strong desire to preserve fertility, (3) histologically proven endometrial endometrioid carcinoma (EEC) upon initial diagnosis or progressed from atypical endometrial hyperplasia (AEH) during FST, (4) disease limited to the endometrium as observed and no suspicious or metastatic lesions by enhanced magnetic resonance imaging (MRI) or transvaginal ultrasound, (5) non-pregnant state, (6) no contraindication for progestin treatment, and (7) molecular classification of an endometrial lesion obtained prior to the initiation of treatment. The criteria for exclusion were as follows: (1) a history of local or systemic hormone treatment for more than one month prior to initial evaluation and treatment in our center, (2) specimens had insufficient DNA quality for NGS, and (3) patients transferred to another hospital during the treatment.
2.2 Diagnosis and assessment
General information (including age, weight, height) and serum samples were collected prior to any form of treatment. All serum samples were collected and examined in the laboratory at the Obstetrics and Gynecology Hospital, Fudan University.
Body mass index (BMI) was calculated as weight (kg)/height (m2); a BMI ≥25 kg/m2 was considered as overweight while a BMI ≥30 kg/m2 was considered as obesity. According to our previous study (19), we considered a patient to be insulin resistant (IR) when the homeostasis model assessment-insulin resistance (HOMA-IR) was ≥2.95.
2.3 Treatment and evaluation
Patients who met the inclusion criteria received comprehensive evaluation, and a multidisciplinary team decided the therapeutic regimens for each patient. All patients received one of the following therapies: (1) oral megestrol acetate (MA) at a dose of 160 mg/d; (2) oral medroxyprogesterone acetate (MPA) at a dose of 250 mg/d; (3) levonorgestrel intrauterine system (LNG-IUS) insertion; (4) oral MA at a dose of 160 mg/d combined with LNG-IUS, or (5) gonadotropin-releasing hormone analogues (GnRH-a) at a dose of 3.75 mg/4w (intra-muscular [i.m.]) combined with oral letrozole at a dose of 2.5 mg/d. Some patients also received oral metformin at a dose of 1500 mg/d or rosuvastatin at a dose of 10 mg/d, depending on their medical complications.
Complete hysteroscopic evaluation was performed every three months during medical treatment to evaluate the efficacy of FST. Endometrial lesions were removed under hysteroscopy, and endometrium biopsy was performed if no obvious lesion was found. The cut-off points for analysis were extended to the 16th and 32nd weeks to account for slight variations in the timing of hysteroscopic evaluations.
The response to hormone treatment was assessed histologically using specimens obtained during each hysteroscopic evaluation. Complete response (CR) was defined as the absence of hyperplasia or cancer. Partial response (PR) was defined as pathological improvement. No response (NR) was defined as the persistence of EC. Progressive disease (PD) was defined as any appearance of disease with a higher degree, such as a higher histological grade, deep myometrial invasion, and/or extrauterine lesions. Recurrence was defined as atypical hyperplasia or carcinoma developing after CR was achieved. Time to CR was measured from the time point at which treatment was initiated to the time point at which CR was diagnosed pathologically by hysteroscopy.
Patients ceased or changed FST if unacceptable side effects occurred any time. Definitive hysterectomy was suggested if NR was evident after 6 months, PR was evident after 9 months, or disease progression occurred at any time during the treatment. For patients who refused hysterectomy, a multiple disciplinary discussion was held, and alternative treatments were considered. Once a patient achieved CR, the same regimen was continued for another 2–3 months for consolidation. Hysteroscopy was performed three months after the first CR. If CR was confirmed, patients were told to prepare for pregnancy or assisted reproductive technology as soon as possible.
2.4 Maintenance and follow-up
Low-dose cyclic progestin, oral contraceptives, or the LNG-IUS, were administered to patients without a recent pregnancy plan or after delivery to prevent recurrence. The patient was followed-up every 3 to 6 months. Ultrasound and endometrial biopsy was performed with a Pipelle to allow evaluation of the endometrium. All patients were followed-up until December 2022.
2.5 Molecular classification
Using the NGS classification panel, patients were classified into one of four molecular subtypes: (1) POLE mut, (2) MSI-H, (3) TP53 wt, and (4) TP53 abn (13).
2.6 Gene sequencing
Genomic DNA (tumor cell content ≥30%) from paraffin-embedded (FFPE) tissue samples was extracted, purified, and quantified using an Endometrial Cancer Molecular Classification Gene Mutation Detection Kit (Xiamen SpaceGen Co., Xiamen, China). DNA sequencing was performed on the NextSeq500 Illumina platform (Illumina Trading (Shanghai) Co., Ltd., Shanghai, China). The sequencing depth was up to 5000X, with an appropriate sensitivity to identify variants with a mutation frequency as low as 1%. We detected a range of genes related to the molecular classification of EC, including POLE, TP53, MLH1, MSH2, PMS2, MSH6, EPCAM, PIK3CA, PTEN, and KRAS. The Promega MSI Analysis System (version 1.2) was deployed on Biosystems 3500 and 3500xL Genetic Analyzers (Thermo Fisher Scientific) to identify microsatellite status. This sequencing strategy screened for mutations with a frequency > 1%, and pathological (P)/likely pathological (LP)/uncertain significant (VUS) variants were defined based on the current knowledge of relevant genes and clinical data (20, 21).
2.7 IHC analysis
IHC staining was performed on FFPE tissue specimens using a range of monoclonal antibodies: MLH1 (DAKO-ES05), PMS2 (DAKO-EPS1), MSH2 (DAKO-FE11), MSH6 (DAKO-EP49), p53 (DAKO-DO-7), and PTEN (DAKO-6H2.1), and utilizing a Leica Bond Max detection system. We also used two additional antibodies: estrogen receptor (ER) (Novocastra, NCL-ER-6F11) and progesterone receptor (PgR) (Novocastra, NCL-L-PGR-312). To analyze MMR (mismatch repair) proteins, the nuclear positivity of MMR proteins in more than 5% of cancer cells was used as a criterion for intact expression. Normal lymphocytes and/or stromal cells were used as internal positive controls. The overexpression pattern of p53 was defined as diffuse and strong nuclear staining in more than 80% of tumor cell nuclei; when no staining was observed, then we defined a sample as having a complete absence pattern. weak focal positive staining was defined as a wild-type pattern.
2.8 Statistical analysis
Continuous variables are given as medians and ranges. Categorical variables are presented as frequencies and percentages. Differences in the descriptive variables between the two groups were analyzed by the Student’s t-test or the Mann–Whitney U test, and differences between than two groups were detected by one-way analysis of variance (ANOVA) or the Kruskal–Wallis H test where appropriate. Kaplan-Meier curves were used to estimate and present therapeutic durations and the differences between groups were compared by log-rank tests. The Cox regression model was used to estimate the hazard ratios (HRs) for CR. Statistical significance was considered as a P-value < 0.05 (two-tailed). All statistical analyses were performed in SPSS (version 25.0, IBM, Armonk, NY, USA).
3 Results
A study flow chart is presented in Figure 1. A total of 115 EEC patients receiving FST at the Obstetrics and Gynecology Hospital of Fudan University between January 2021 and January 2022 were retrospectively investigated. Overall, 25 cases were excluded, including eight patients who had a history of local or systemic progestin treatment for more than one month, four patients who transferred to another hospital, and 13 patients whose specimens could not be sequences or had insufficient tumor tissue for DNA extraction. Ultimately, 90 cases were included in this study. Six (6.7%) patients had POLE mutation, five (5.6%) patients were classified as MSI-H, 84 (86.7%) patients were classified as TP53 wt, and one patient (1.1%) was classified as TP53 abn.
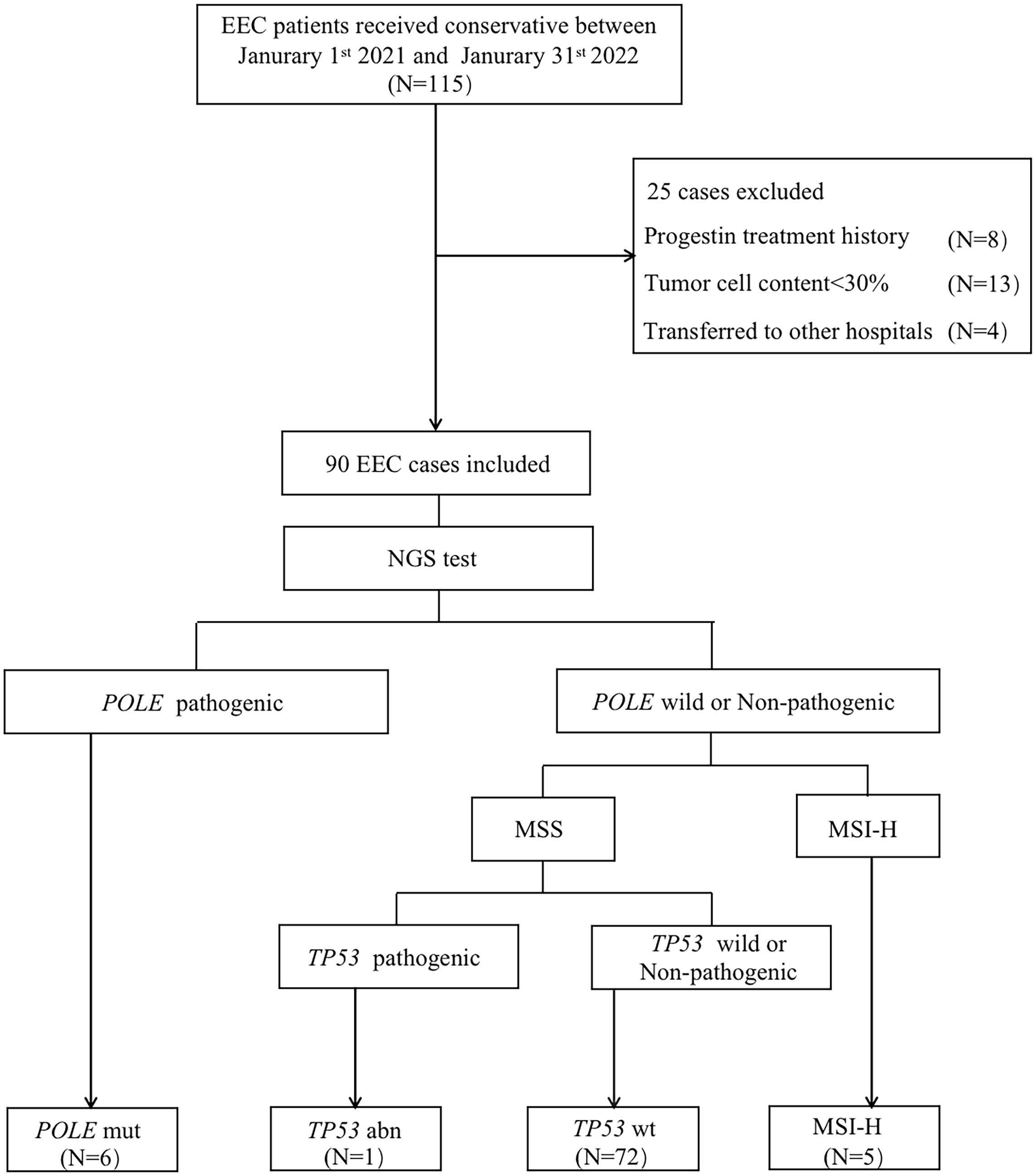
Figure 1 Flow chart showing the process used for patient selection. EEC, endometrioid endometrial cancer; NGS, next generation sequencing; POLE mut, DNA polymerase epsilon mutation; MSI-H, high microsatellite instability; MSS, microsatellite stable; TP53 abn, TP53 abnormal; TP53 WT, TP53 wildtype.
3.1 Patient clinical characteristics
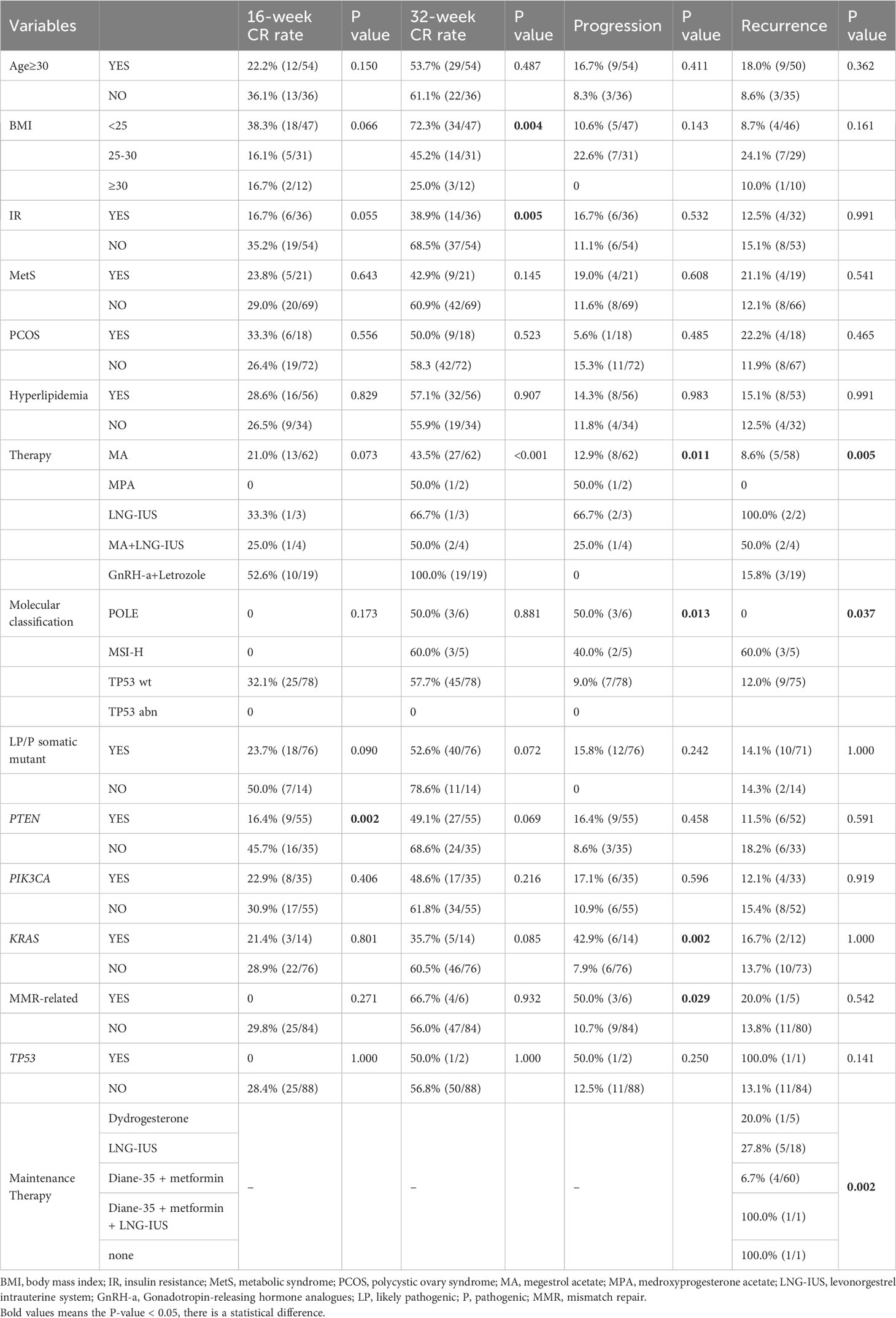
The characteristics of the patients are presented in Table 1. In our study cohort, 36 patients were over 30 years-of-age at the time of treatment. Overweight patients accounted for 47.8% of the cohort, while obese patients accounted for 13.3% of the cohort. In total, 40.0% of patients had insulin resistance, and 60.0% had hyperlipidemia. All patients had positive estrogen and progesterone receptor expression prior to the first administration of hormone therapy. Overall, 70.0% of patients in our study cohort received MA as the main FST, while another 20.0% received a combination therapy of GnRH-a and letrozole. When considering the four subgroups, a significant difference was observed for the initial treatment regimens, with the highest proportion of patients in the TP53 wt group receiving MA or GnRH-a combined with letrozole as the initial treatment (P=0.005). After 16 weeks of treatment, the TP53 wt group featured 32.0% of patients achieving CR, although no statistically significant difference was observed compared to other subgroups. After 32 weeks of treatment, the CR rates for the four subgroups were as follows: POLE mut vs. MSI-H vs. TP53 wt vs. TP53 abn: 50.0% (3/6) vs. 60.0% (3/5) vs. 57.7% (45/78) vs. 0 (P=0.881). During follow-up, one patient in the TP53 abn group did not achieve CR; in contrast, the rates of CR in the other three subgroups all exceeded 80.0% (P=0.035). A total of 12 patients experienced disease recurrence after achieving CR, with a recurrence rate of 60.0% observed in the MSI-H group; this was significantly higher than that in the POLE mut and TP53 wt subgroups (P=0.037). During the treatment process, disease progression occurred in 12 patients, featuring 50.0% and 40.0% of patients in the POLE mut and MSI-H subgroups, respectively; this compared to only 9% in the TP53 wt subgroup (P=0.013). The median follow-up period for all patients was 59.8 weeks (range: 19.1-104.0 weeks).

Table 1 Patients’ clinical characteristics in fertility-preserving patients (N=90) cohort according to NGS-based molecular classification.
3.2 Molecular and tppathological characteristics
The somatic mutation results for all patients in the study cohort are shown in Figure 2. Patients in the MSI-H group were all 30 years-of-age or older. One patient in the MSI-H group experienced recurrence and progression during FST, with the pathological type progressing to grade 3. Two patients were grade 2, both were TP53 wt. During follow-up, a total of five patients failed to achieve CR, one was classified as POLE mut, three were classified as TP53 wt, and one was classified as TP53 abn. In terms of IHC results, five patients showed a loss of MMR-related protein. All patients exhibited wild-type p53 expression.
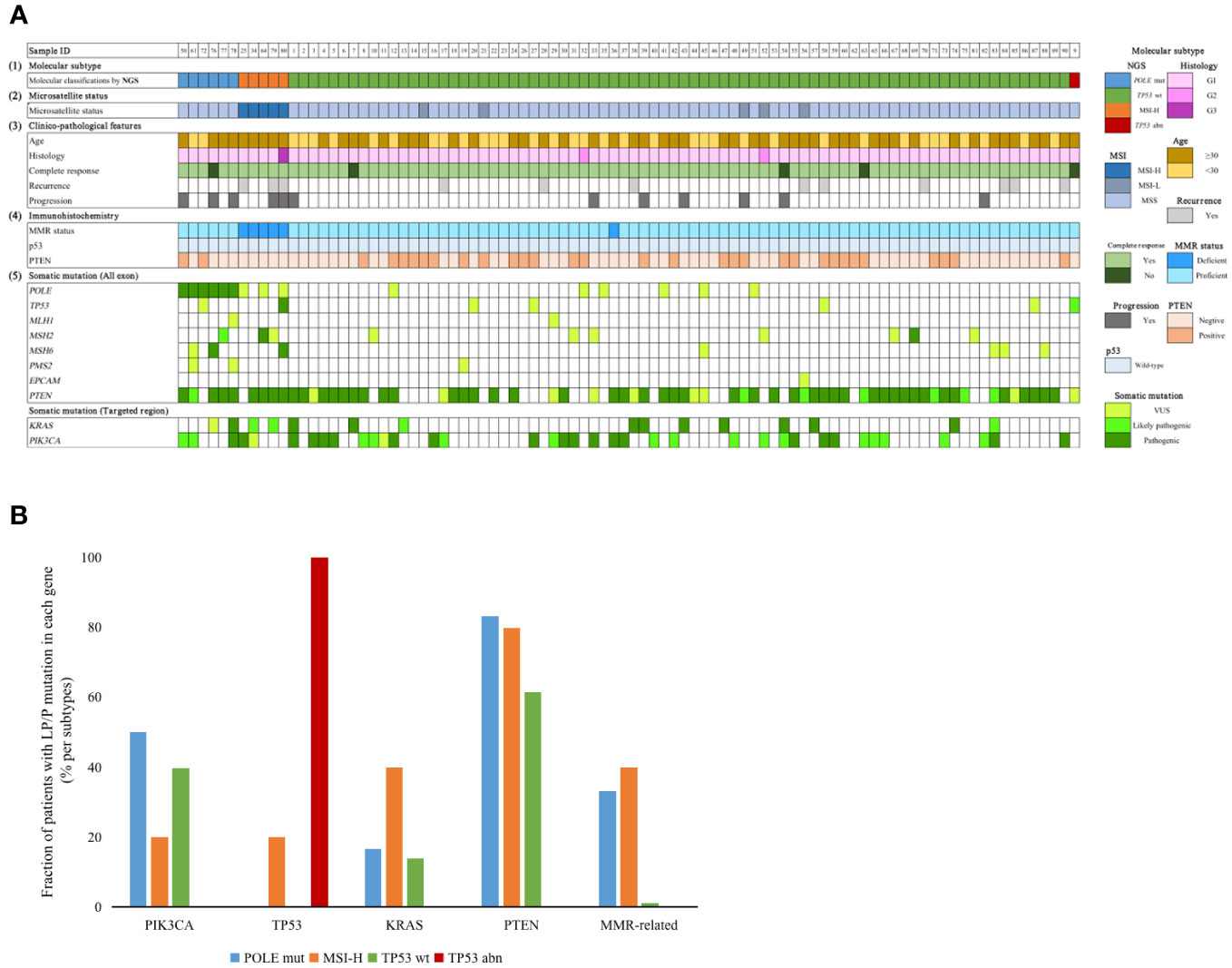
Figure 2 (A) Clinicopathological factors and mutation profiles in our cohort. (1) Molecular subtype by NGS. (2) Microsatellite status. (3) Clinical factors, CR status, recurrence status and progression status. (4) IHC staining. (5) Mutation profiles of the 90 EEC patients. (B) Distribution of different genes with P/LP mutations in the four subgroups. NGS, next generation sequencing; MMR, mismatch repair; POLE mut, DNA polymerase epsilon mutation; MSI-H, high microsatellite instability; MSI-L, low microsatellite instability; MSS, microsatellite stable; TP53 abn, TP53 abnormal; TP53 WT, TP53 wildtype.
When considering our target genes, PTEN was the gene with the highest frequency of P/LP mutations; this was detected in 57/90 (63.3%) patients; this was followed by PIK3CA, KRAS, POLE, MMR-related, and TP53, detected in 35/90 (38.9%), 14/90 (15.6%), 6/90 (6.7%), 5/90 (5.6%) and 2/90 (2.2%) patients, respectively. Fourteen patients did not have any P/LP mutations according to our gene panel. PIK3CA and PTEN mutations were more frequent in the POLE mut group; however, this was not statistically significant. It appeared that the presence of TP53 P/LP mutations was an unfavorable factor for the outcome of FST. One MSI-H patient, with a concurrent TP53 pathogenic mutation, experienced disease recurrence and progression after achieving CR, while another patient classified as TP53abn did not achieve CR during the follow-up period.
3.3 The effects of related factors on treatment outcomes
Table 2 shows the associations between variables and FST outcomes. LP/P PTEN mutations significantly reduced the CR rate at 16 weeks of treatment (P=0.002). The CR rate at 32 weeks of treatment decreased significantly with increasing BMI (P=0.004) and insulin resistance (P=0.005). Surprisingly, the combination of GnRH-a and letrozole as the initial treatment resulted in a 100.0% CR rate at 32 weeks (P<0.001); this was significantly higher than any of the other therapies. In our cohort, all patients who progressed had pathological progression, but no evidence of metastasis was found by enhanced MRI. There was a significant difference in disease progression rates among different initial therapies (P=0.011), with lower rates observed in the MA and GnRH-a combined with letrozole groups. At the molecular level, LP/P mutations in POLE, KRAS, and MMR-related genes were significantly associated with disease progression (P=0.013, P=0.002, P=0.029, respectively). In addition, MSI-H patients had a higher recurrence rate after CR (60.0%, P=0.005). There were significant differences in recurrence rates among different initial treatment and maintenance therapies; patients who were treated with MA as the initial treatment and Diane-35 plus metformin as the maintenance treatment after CR had a significantly lower recurrence rate (P=0.005; P=0.008, respectively).
Univariate Cox regression analysis demonstrated that overweight (HR: 0.48; 95% CI: 0.29-0.78; P=0.003), obesity (HR: 0.34; 95% CI: 0.17-0.70; P=0.003) and IR (HR: 0.56; 95% CI: 0.36-0.87; P=0.010) were associated with a lower cumulative CR rate. While GnRH-a combined with letrozole was significantly associated with a higher cumulative CR rate (HR: 4.81; 95% CI: 2.60-8.93; P<0.001) (Figure 3).
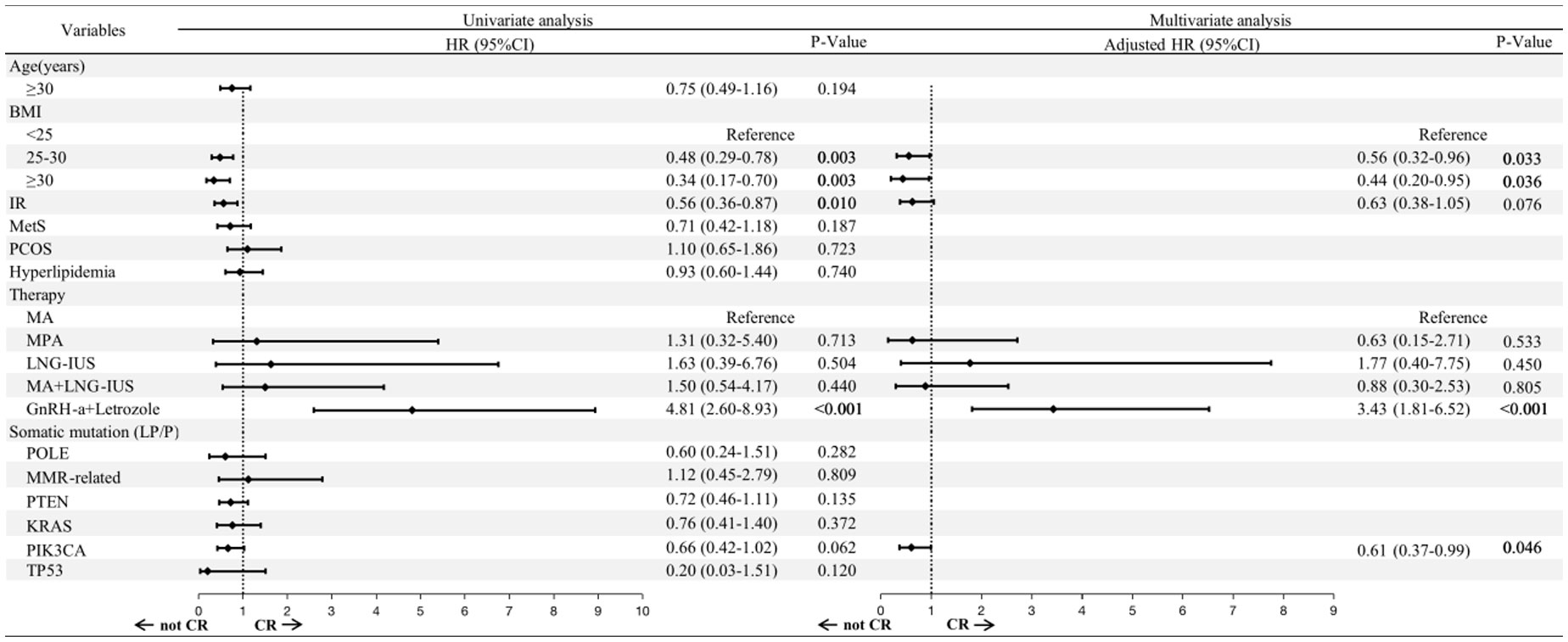
Figure 3 Risk factors associated with the FST outcomes, as determined by Cox regression. BMI, body mass index; IR, insulin resistance; MetS, metabolic syndrome; PCOS, polycystic ovary syndrome; MA, megestrol acetate; MPA, medroxyprogesterone acetate; LNG-IUS, levonorgestrel intrauterine system; GnRH-a, Gonadotropin-releasing hormone analogues; LP, likely pathogenic; P, pathogenic; HR, hazard ratio; CI, confidence interval.
We selected factors with P<0.01 in the univariate Cox regression for further multivariate analysis. The adverse effects of overweight (HR: 0.56; 95% CI: 0.32-0.96; P=0.033) and obesity (HR: 0.44; 95% CI: 0.20-0.95; P =0.036) remained significant. It is worth noting that PIK3CA mutation was associated with a lower cumulative CR rate after multivariate Cox regression (HR: 0.61; 95% CI: 0.37-0.99; P =0.046). GnRH-a combined with letrozole was still considered a favorable factor for CR (HR: 3.43; 95% CI: 1.81-6.52; P<0.001).
3.4 The effects of different molecular mutants on the outcomes of oncological treatment
The treatment durations of the four molecular subtypes are presented in Figure 4A. Following multivariate Cox regression analysis, we found that the initial treatment significantly influenced the CR rate. Thus, our analysis excluded patients who received GnRH-a combined with letrozole as the initial therapy. No significant difference was found in the time to achieve CR when compared across the four subtypes (log rank test; P=0.086); however, we did find that only patients in the TP53 wt group achieved CR at 24 weeks of treatment. Further analysis of the molecular characteristics of the TP53 wt group (Figure 4B) showed that patients without targeted gene mutations had a shorter duration of treatment (log rank P=0.014), while PTEN and PIK3CA mutations prolonged the duration of treatment when conservative therapy was administered (log rank test: P=0.034; log rank test: P=0.018).
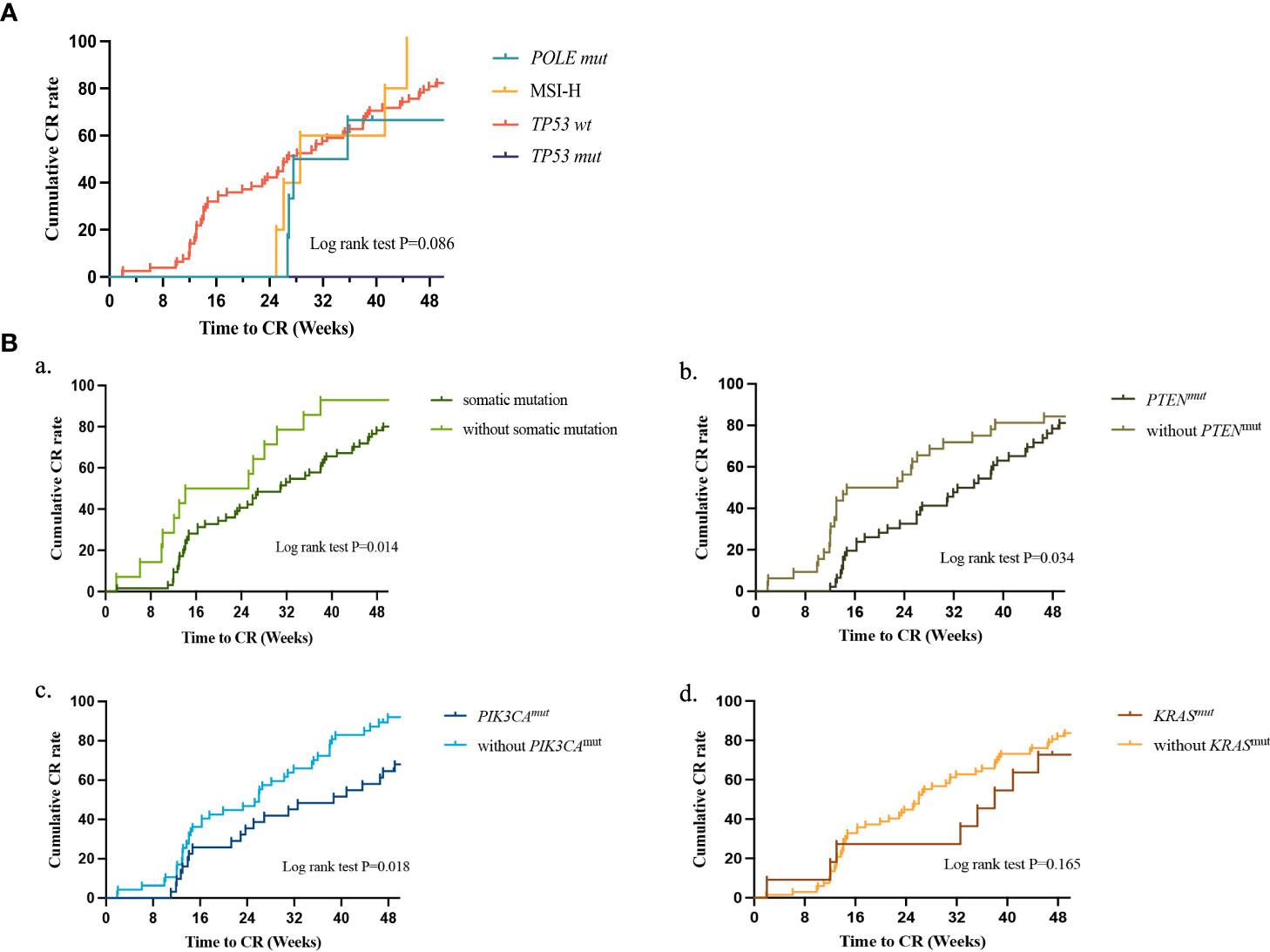
Figure 4 (A) Treatment duration to CR between different molecular classifications as determined by Kaplan-Meier analysis and compared by the log-rank test. POLE mut, DNA polymerase epsilon mutation; MSI-H, high microsatellite instability; TP53 abn, TP53 abnormal; TP53 WT, TP53 wildtype; CR, complete response. (B) Treatment duration to CR between different somatic mutants as determined by Kaplan-Meier analysis and compared by the log-rank test in TP53 WT. (a) patients with or without somatic mutation; (b) patients with or without PTEN mutation; (c) patients with or without PIK3CA mutation; (d) patients with or without KRAS mutation. CR, complete response.
3.5 Patients with POLE mut, MSI-H, and TP53 abn
Supplementary Table 1 presents the treatment details of patients with POLE mut, MSI-H, and TP53 abn in our study. In our cohort, the P286R mutation accounted for 66.6% of all POLE mut cases, with M444K and V411L mutations in the remaining two cases. All five MSI-H patients underwent germline genetic testing, and one patient was diagnosed with Lynch syndrome (LS). Three POLE mut and two MSI-H patients changed therapy during treatment. One POLE mut patient received hysterectomy due to a failure to respond after nine months of treatment, while another LS patient underwent surgery due to disease recurrence and progression. Histopathological examination showed no deep myometrial invasion, lymphovascular space invasion (LVSI), or metastasis to the ovaries or lymph nodes.
3.6 Details of patients who underwent a change in therapeutic regimen
Next, we analyzed patients who changed their therapeutic regimen during treatment following multidisciplinary discussion. A total of 19 patients changed their therapy, with three (50%) in the POLE mut subgroup, two (40%) in the MSI-H subgroup, and 14 (17.9%) in the TP53 wt subgroup, as shown in Supplementary Figure 1. At the final follow-up, 89.5% of patients achieved CR following a change in therapy. Two patients each in the POLE mut and TP53 WT subgroups changed their treatment due to disease progression. Sixteen patients switched to GnRH-a combined with letrozole, while three patients switched to a combination therapy featuring Diane-35 and metformin. We noticed that two patients still did not achieve CR within the follow-up period after changing therapeutic regime.
4 Discussion
4.1 Molecular characteristics and the outcomes of conservative treatment
In this study, we demonstrated that molecular classification can be used to predict the prognoses of patients with EC when treated conservatively. Our findings are different from those of previous studies in that patients with POLE mutation did not show a better response to progesterone therapy and were instead found to be insensitive to such treatment. Kaplan-Meier survival curve analysis did not reveal a significant difference among the four subgroups; this may have been related to a change of treatment and the small size of the patient cohort. In our study cohort, only one patient in the POLE mut group benefited from high-dose progesterone therapy. Furthermore, in the POLE mut group, patients with AEH were more likely to progress to EC during treatment; this was in stark contrast to the patients undergoing surgery. By analyzing existing reports, we only found three cases of conservatively treated EC patients with POLE mutations, two of whom received oral progesterone therapy but eventually underwent surgical treatment due to disease recurrence or progression; furthermore, only one patient achieved CR without recurrence after six months of treatment with LNG-IUS (16, 22). These data suggested that POLE mutation may be one of the unfavorable factors of FST in EC patients. Similar to surgical patients, those with TP53 mutation were found to be associated with a poor outcome. One patient with EC was classified as a TP53 abn in our study and did not achieve CR even after 90 weeks of treatment; this patient did not change her therapeutic regime due to irregular follow-up. Another patient with MSI-H and TP53 mutation experienced disease recurrence and progression to high-grade EEC during treatment. In addition, we found that 60.0% (3/5) of patients in the MSI-H group experienced disease recurrence; this finding was consistent with previously reported research findings (17).
We found that patients in the POLE mut and MSI-H subgroups had a high tumor mutation burden (TMB), with the former showing a higher burden. In a recent study, Riggs et al. reported that the high TMB was associated with an increased frequency of DACH1 gene mutation (23). The DACH1 gene was positively correlated with progesterone receptor expression (24), thus suggesting that the insensitivity of patients in the POLE mut and MSI-H subgroups to progesterone may be related to mutation of the DACH1 gene. Moreover, the detection of DACH1 gene mutations can facilitate the identification of appropriate patients for FST. The lower TMB in the MSI-H subgroup when compared to the POLE mut subgroup may be correlated with the difference in long-term treatment efficacy between the two groups. Recently, Hu et al. reported that the expression of PDGFC, DIO2, SOX9, and BCL11A was upregulated in progesterone-insensitive endometrial lesions when compared with progesterone-sensitive endometrial lesions, while the expression of FOXO1, IRS2, APOE, FYN, and KLF4 was downregulated, as based on the integrated analysis of ATAC-Seq and RNA-Seq (25). By conducting differential gene enrichment analysis of TCGA data (the analytical data were not presented in this article), we found that the expression of IRS2 was downregulated in POLE mut and MSI-H groups of EC patients compared with the TP53 wt group. IRS2 acts downstream of insulin receptor, activating the MAPK and PI3K/AKT pathways and inducing glucose uptake and membrane marker expression (26). Our study suggested that the downregulation of IRS2 may be one of the reasons for the poor response to progesterone conservation therapy in the POLE mut and MSI-H groups.
Further analysis based on NGS results showed that patients with likely pathogenic/pathogenic gene mutations had longer treatment duration to achieve CR compared to those without any mutations, and patients with PTEN mutation had significantly lower response rates compared to those without PTEN mutation, consistent with our previous study (27). Additionally, our study found that PIK3CA mutation is also one of the factors that prolong the treatment duration.
In addition, AEH, a precursor to endometrial cancer, was found concurrent EC in approximately 32.6% of patients (28). However, studies on the molecular characterization of these patients are still limited. In a retrospective analysis conducted by Puechl AM and colleagues, of 37 patients with AEH, one of two (50%) patients with MMR-D demonstrated disease progression, one of four (25%) patients with POLE mutations experienced disease progression, and only two of 27 (7.4%) patients with p53wt demonstrated disease progression (29). Our study confirms these findings, suggesting that AEH patients with POLE mutations and MSI-H are more likely to experience disease progression. However, due to the limited sample size, further studies are essential to explore this patient subgroup in depth, potentially for providing valuable prognostic insights and facilitating the development of more personalized treatment and follow-up strategies based on molecular features.
4.2 Molecular characteristics and disease prognoses
For patients undergoing surgery, different molecular subtypes were associated with different prognoses, the POLE mut group was associated with a higher 10-year recurrence-free survival (RFS) rate, whereas the p53mut EC patients presented with a higher rate of distant recurrence and lower overall survival (30). In our study, LP/P mutations in POLE, KRAS, and MMR-related genes were associated with higher rates of disease progression. Following Cox multivariate regression analysis, PIK3CA was identified as a risk factor for achieving CR in endometrial cancer. Of the four subtypes, the TP53 wt group had the largest number of patients. A previous study showed that patients with mutant KRAS and wild-type ARID1A were associated with a poorer 5-year recurrence-free survival in a TP53 wt group (31). And our study identified PTEN and PIK3CA mutations as new molecular markers affecting the outcome of FST in patients with EC. PTEN, a tumor suppressor gene, was reported to be mutated in 57–83% of all cases of EC and is the most common molecular event in early endometrial cancer (32). PTEN is also a known negative regulator of the PI3K/AKT/mTOR pathway, and studies have reported a significant association between the loss of PTEN expression and metastatic disease (33). PIK3CA is also associated with disease invasion. For example, Hayes et al. proposed that EC with PIK3CA mutation should be considered as having invasive cancer, whereas those without this gene mutation would be candidates for a more conservative approach (34). In our present study, we found a correlation between PIK3CA mutation and poorer outcomes following conservative therapy. It has been reported that KRAS plays an early role in the progression of EC (30); similarly, our study cohort showed a higher rate of KRAS pathogenic mutations in the disease progression group.
Some patients with EC exhibited multiple molecular characteristics; previous studies showed patients with MMR-D and POLE mut carrying TP53 mutations had better prognoses than single TP53 mutation in surgical patients (35). In our study, one MSI-H patient who carried a pathogenic TP53 mutation experienced disease progression during treatment; in this case, the histological type progressed to high-grade endometrioid carcinoma. This suggested that closer monitoring should be conducted for patients carrying TP53 mutation. Currently, the outcomes of conservative treatment for patients with multiple molecular characteristics remain unclear and more clinical data need to be acquired and analyzed.
4.3 Other possible factors affecting the efficacy of conservative therapy
Our analyses confirmed the correlation between weight and FST outcome in patients with EC. Overweight and obesity were identified as independent risk factors that affect the duration of treatment in patients undergoing conservative treatment. Previous studies demonstrated a significant correlation between different BMI categories and the outcomes of conservative treatment (7). In our study cohort, the overweight populations in the POLE mut and MSI-H groups were higher than that in the TP53 wt group, although no statistically significant difference was detected. The molecular classification of EC will allow us to focus on the correlation between different molecular characteristics and the outcomes of conservative treatment, and also considered the joint effects of molecular characteristics and metabolism on the outcomes of conservative treatment in patients with EC. The interaction between molecular characteristics and metabolism represents a significant research direction in the future.
4.4 The relationship between treatments and the outcomes of conservative therapy
GnRH-a, a gonadotropin-releasing hormone agonist, can suppress the pituitary secretion of gonadotropins, reduce the secretion of ovarian hormones, inhibit ovarian function, and reduce the circulating levels of estrogen. In recent years, GnRH-a has been reported as an effective FST for patients with endometrial cancer (36, 37). Our analysis demonstrated that in the POLE mut subgroup, the use of GnRH-a combined with letrozole as the initial treatment method yielded significantly greater benefits than progesterone. This indirectly confirmed the insensitivity of POLE mut to progesterone.
Our study analyzed the prognostic value of molecular classification and other molecular features for patients with EC receiving FST. However, this study also had certain limitations that need to be considered. First, our analysis was limited by its retrospective nature and the use of a single institution database; this may have induced possible bias in the selection of patients. However, detailed data recording and strict adherence to inclusion and exclusion criteria for every AEH or EEC patient were applied throughout the study to avoid selection bias. Therefore, further prospective studies are now required to validate the full impact of molecular classification for patients with EC undergoing FST.
In conclusion, our study demonstrated that the molecular classification of EC represents a useful classification system applicable to patients receiving conservative treatment. However, its guidance for prognoses should be distinguished from that of surgical patients. In addition, we found that somatic pathogenic mutations of some other genes were also associated with the prognoses of conservative treatment, including PTEN, KRAS, and PIK3CA. The findings of our study indicated that molecular classification has the potential to differentiate EC patients with similar histological features but different prognoses, consequently providing direction for personalized therapeutic and monitoring regimens for patients with unique molecular profiles.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committees of Obstetrics and Gynecology Hospital of Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YXu: Writing – original draft, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – review & editing. MZ: Data curation, Formal Analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing, Software. LZ: Data curation, Investigation, Writing – review & editing. BW: Writing – original draft. TW: Data curation, Formal Analysis, Writing – review & editing. YXue: Data curation, Supervision, Validation, Writing – review & editing. ZX: Formal Analysis, Software, Supervision, Writing – review & editing. WS: Methodology, Project administration, Writing – review & editing. XC: Conceptualization, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. CW: Conceptualization, Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. National Natural Science Foundation of China (General Program, 82273233), Natural Science Foundation of Shanghai (22ZR1408900), Clinical Research Plan of SHDC (No. SHDC2020CR4079), National Natural Science Foundation of China (General Program, 81772777), and Shanghai “Rising Stars of Medical Talent” Youth Development Program-Outstanding Youth Medical Talents (SHWJRS2021-99).
Acknowledgments
We would like to thank pathologists from the Obstetrics & Gynecology Hospital of Fudan University for their technological help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1282356/full#supplementary-material
Supplementary Figure 1 | Patients changed therapy in the study cohort. POLE mut, DNA polymerase epsilon mutation; MSI-H, high microsatellite instability; TP53 wt, TP53 wildtype; CR, complete response; PD, progressive disease; NR, No response; PR, partial response; GnRH-a, Gonadotropin-releasing hormone analogues.
References
1. American Cancer Society. Cancer facts and figures (2022). Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf (Accessed December 9, 2022).
2. Liu L, Habeshian TS, Zhang J, Peeri NC, Du M, De Vivo I, et al. Differential trends in rising endometrial cancer incidence by age, race, and ethnicity. JNCI Cancer Spectr (2023) 136:pkad001. doi: 10.1093/jncics/pkad001
3. Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health (2019) 4:e137–47. doi: 10.1016/S2468-2667(18)30267-6
4. National Cancer Institute. Surveillance, Epidemiology, and End Results Program Cancer Stat facts: Uterine Cancer. Available at: http://seer.cancer.gov/statfacts/html/corp.html (Accessed January 26, 2023).
5. Mutlu L, Manavella DD, Gullo G, McNamara B, Santin AD, Patrizio P. Endometrial cancer in reproductive age: fertility-sparing approach and reproductive outcomes. Cancers (Basel) (2022) 14:5187. doi: 10.3390/cancers14215187
6. Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, Shan WW, et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. BJOG (2020) 127:848–57. doi: 10.1111/1471-0528.16108
7. Liu S, Wang L, Wu P, Luo S, Shan W, Chen X, et al. Effects of weight status and related metabolic disorders on fertility-sparing treatment outcomes in endometrial atypical hyperplasia and endometrial cancer: A retrospective study. Cancers (Basel) (2022) 14:5024. doi: 10.3390/cancers14205024
8. Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497:67–73. doi: 10.1038/nature12113
9. Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, et al. A clinically applicable molecular-based classifification for endometrial cancers. Br J Cancer (2015) 113:299–310. doi: 10.1038/bjc.2015.190
10. Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer (2017) 123:802–13. doi: 10.1002/cncr.30496
11. López-Reig R, Fernández-Serra A, Romero I, Zorrero C, Illueca C, García-Casado Z, et al. Prognostic classification of endometrial cancer using a molecular approach based on a twelve-gene NGS panel. Sci Rep (2019) 9:18093. doi: 10.1038/s41598-019-54624-x
12. Li Y, Feng J, Zhao C, Meng L, Shi S, Liu K, et al. A new strategy in molecular typing: the accuracy of an NGS panel for the molecular classification of endometrial cancers. Ann Transl Med (2022) 10:870. doi: 10.21037/atm-22-3446
13. Rao Q, Liao J, Li Y, Zhang X, Xu G, Zhu C, et al. Application of NGS molecular classification in the diagnosis of endometrial carcinoma: a supplement to traditional pathological diagnosis. Cancer Med (2023) 12:5409–19. doi: 10.1002/cam4.5363
14. National Comprehensive Cancer Network. Uterine neoplasms, version 1.2023. NCCN clinical practice guidelines in oncology. Available at: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (Accessed February 26, 2023).
15. Rodolakis A, Scambia G, Planchamp F, Acien M, Di Spiezio Sardo A, Farrugia M, et al. ESGO/ESHRE/ESGE Guidelines for the fertility-sparing treatment of patients with endometrial carcinoma. Hum Reprod Open (2023) 2023:hoac057. doi: 10.1093/hropen/hoac057
16. Chung YS, Woo HY, Lee JY, Park E, Nam EJ, Kim S, et al. Mismatch repair status influences response to fertility-sparing treatment of endometrial cancer. Am J Obstet Gynecol (2021) 224:370.e1–370.e13. doi: 10.1016/j.ajog.2020.10.003
17. Raffone A, Catena U, Travaglino A, Masciullo V, Spadola S, Della Corte L, et al. Mismatch repair-deficiency specifically predicts recurrence of atypical endometrial hyperplasia and early endometrial carcinoma after conservative treatment: a multi-center study. Gynecol Oncol (2021) 161:795–801. doi: 10.1016/j.ygyno.2021.03.029
18. Ran X, Hu T, Li Z. Molecular classification in patients with endometrial cancer after fertility-preserving treatment: application of proMisE classifier and combination of prognostic evidence. Front Oncol (2022) 12:810631. doi: 10.3389/fonc.2022.810631
19. Shan W, Ning C, Luo X, Zhou Q, Gu C, Zhang Z, et al. Hyperinsulinemia is associated with endometrial hyperplasia and disordered proliferative endometrium: a prospective cross-sectional study. Gynecol Oncol (2014) 132:606–10. doi: 10.1016/j.ygyno.2014.01.004
20. Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res (2018) 46:D1062–7. doi: 10.1093/nar/gkx1153
21. Chakravarty D, Gao J, Phillips SM, Kundra R, Zhang H, Wang J, et al. OncoKB: A precision oncology knowledge base. JCO Precis Oncol (2017) 2017:PO.17.00011. doi: 10.1200/PO.17.00011
22. Falcone F, Normanno N, Losito NS, Scognamiglio G, Esposito Abate R, Chicchinelli N, et al. Application of the Proactive Molecular Risk Classifier for Endometrial Cancer (ProMisE) to patients conservatively treated: outcomes from an institutional series. Eur J Obstet Gynecol Reprod Biol (2019) 240:220–5. doi: 10.1016/j.ejogrb.2019.07.013
23. Riggs MJ, Lin N, Wang C, Piecoro DW, Miller RW, Hampton OA, et al. DACH1 mutation frequency in endometrial cancer is associated with high tumor mutation burden. PloS One (2020) 15:e0244558. doi: 10.1371/journal.pone.0244558
24. Li W, Wang S, Qiu C, Liu Z, Zhou Q, Kong D, et al. Comprehensive bioinformatics analysis of acquired progesterone resistance in endometrial cancer cell line. J Transl Med (2019) 17:58. doi: 10.1186/s12967-019-1814-6
25. Hu JL, Yierfulati G, Wang LL, Yang BY, Lv QY, Chen XJ. Identification of potential models for predicting progestin insensitivity in patients with endometrial atypical hyperplasia and endometrioid endometrial cancer based on ATAC-Seq and RNA-Seq integrated analysis. Front Genet (2022) 13:952083. doi: 10.3389/fgene.2022.952083
26. Neff AM, Yu J, Taylor RN, Bagchi IC, Bagchi MK. Insulin signaling via progesterone-regulated insulin receptor substrate 2 is critical for human uterine decidualization. Endocrinology (2020) 161:bqz021. doi: 10.1210/endocr/bqz021
27. Xue Y, Dong Y, Lou Y, Lv Q, Shan W, Wang C, et al. PTEN mutation predicts unfavorable fertility preserving treatment outcome in the young patients with endometrioid endometrial cancer and atypical hyperplasia. J Gynecol Oncol (2023) 34:e53. doi: 10.3802/jgo.2023.34.e53
28. Doherty MT, Sanni OB, Coleman HG, Cardwell CR, McCluggage WG, Quinn D, et al. Concurrent and future risk of endometrial cancer in women with endometrial hyperplasia: A systematic review and meta-analysis. PloS One (2020) 15:e0232231. doi: 10.1371/journal.pone.0232231
29. Puechl AM, Spinosa D, Berchuck A, Secord AA, Drury KE, Broadwater G, et al. Molecular classification to prognosticate response in medically managed endometrial cancers and endometrial intraepithelial neoplasia. Cancers (Basel) (2021) 13:2847. doi: 10.3390/cancers13112847
30. Vermij L, Smit V, Nout R, Bosse T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology (2020) 76:52–63. doi: 10.1111/his.14015
31. Asami Y, Kobayashi Kato M, Hiranuma K, Matsuda M, Shimada Y, Ishikawa M, et al. Utility of molecular subtypes and genetic alterations for evaluating clinical outcomes in 1029 patients with endometrial cancer. Br J Cancer (2023) 128:1582–91. doi: 10.1038/s41416-023-02203-3
32. Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst (2000) 92:924–30. doi: 10.1093/jnci/92.11.924
33. Salvesen HB, Stefansson I, Kretzschmar EI, Gruber P, MacDonald ND, Ryan A, et al. A population-based study of mutations, promoter methylation and PTEN protein expression. Int J Oncol (2004) 25:1615–23.
34. Hayes MP, Wang H, Espinal-Witter R, Douglas W, Solomon GJ, Baker SJ, et al. PIK3CA and PTEN mutations in uterine endometrioid carcinoma and complex atypical hyperplasia. Clin Cancer Res (2006) 12:5932–5. doi: 10.1158/1078-0432.CCR-06-1375
35. León-Castillo A, Gilvazquez E, Nout R, Smit VT, McAlpine JN, McConechy M, et al. Clinicopathological and molecular characterisation of 'multiple-classifier' endometrial carcinomas. J Pathol (2020) 250:312–22. doi: 10.1002/path.5373
36. Zhou H, Cao D, Yang J, Shen K, Lang J. Gonadotropin-releasing hormone agonist combined with a levonorgestrel-releasing intrauterine system or letrozole for fertility-preserving treatment of endometrial carcinoma and complex atypical hyperplasia in young women. Int J Gynecol Cancer (2017) 27:1178–82. doi: 10.1097/IGC.0000000000001008
Keywords: endometrial cancer, NGS - next generation sequencing, fertility preservation treatment, molecular classifcation, molecular features
Citation: Xu Y, Zhao M, Zhang L, Wang T, Wang B, Xue Y, Xu Z, Shao W, Chen X and Wang C (2023) Outcomes of fertility preservation treatments in patients with endometrial cancer with different molecular classifications based on an NGS panel. Front. Oncol. 13:1282356. doi: 10.3389/fonc.2023.1282356
Received: 24 August 2023; Accepted: 23 October 2023;
Published: 09 November 2023.
Edited by:
Marcia Hall, Mount Vernon Cancer Centre, United KingdomReviewed by:
Diego Raimondo, University of Bologna, ItalyTae Hoon Kim, University of Missouri, United States
Copyright © 2023 Xu, Zhao, Zhang, Wang, Wang, Xue, Xu, Shao, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojun Chen, eGlhb2p1bmNoZW4yMDEzQHNpbmEuY29t; Chao Wang, d2FuZzE5ODAtNTVAMTYzLmNvbQ==
†These authors have contributed equally to this work
‡These authors have contributed equally to this work
§ ORCID: Xiaojun Chen, orcid.org/0000-0002-5493-1500
Chao Wang, orcid.org/0000-0003-4636-5104
 Yan Xu
Yan Xu Mingming Zhao†
Mingming Zhao† Bo Wang
Bo Wang Wenyu Shao
Wenyu Shao Xiaojun Chen
Xiaojun Chen Chao Wang
Chao Wang