
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 November 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1282066
This article is part of the Research Topic Emerging Mutations in Colorectal Cancer Development and Progression View all 13 articles
Background: Colorectal cancer (CRC) is a globally significant health concern, necessitating effective preventive strategies through identifying modifiable risk factors. Constipation, characterized by infrequent bowel movements or difficulty passing stools, has been proposed as a potential CRC risk factor. However, establishing causal links between constipation and CRC remains challenging due to observational study limitations.
Methods: Mendelian randomization (MR) utilizes genetic variants as instrumental variables, capitalizing on genetically determined variation to assess causal relationships. In this dual-sample bidirectional MR study, we extracted genetic data from independent cohorts with CRC (Include colon cancer and rectal cancer) and constipation cases. Genome-wide association studies (GWAS) identified constipation and CRC-associated genetic variants used as instruments to infer causality. The bidirectional MR analysis evaluated constipation’s impact on CRC risk and the possibility of reverse causation.
Results: Employing bidirectional MR, we explored the causal relationship between constipation and CRC using publicly available GWAS data. Analysis of constipation’s effect on CRC identified 26 significant SNPs, all with strong instrumental validity. IVW-random effect analysis suggested a potential causal link [OR = 1.002(1.000, 1.004); P = 0.023], although alternative MR approaches were inconclusive. Investigating CRC’s impact on constipation, 28 significant SNPs were identified, yet IVW analyses found no causal effect [OR = 0.137(0.007, 2.824); P = 0.198]. Other MR methods also yielded no significant causal association. We analyzed constipation separately from colon and rectal cancer using the same methodology in both directions, and no causal relationship was obtained.
Conclusion: Our bidirectional MR study suggests a potential constipation-CRC link, with mixed MR approach outcomes. Limited evidence supports constipation causing CRC. Reliable instruments, minimal heterogeneity, and robust analyses bolster these findings, enriching understanding. Future research should explore additional factors to enhance comprehension and clinical implications.
Colorectal cancer (CRC) stands as a global health concern, contributing significantly to morbidity and mortality rates worldwide (1, 2). The quest to identify modifiable risk factors for CRC is of paramount importance to develop effective preventive strategies (3, 4). Among the potential risk factors, constipation, characterized by infrequent bowel movements or difficulty passing stools, has garnered attention for its potential association with CRC risk (5, 6). Previous observational studies have generated substantial controversy regarding the causal relationship between constipation and CRC (7–11). Establishing a definitive causal relationship between constipation and CRC remains challenging due to inherent limitations within observational studies (12).
To address these challenges, we present a pioneering approach that employs Mendelian randomization (MR) analysis to investigate the potential causal link between constipation and CRC risk (13). MR leverages genetic variants, often using single nucleotide polymorphisms(SNPs) as instrumental variables, utilizing their strong associations with constipation to infer causality (14). This approach offers a unique advantage by minimizing biases arising from confounding and reverse causation, which often impede the accuracy of observational studies (13, 15).
Our research framework involves the use of large-scale genetic and epidemiological datasets to perform a bidirectional MR analysis. This analysis investigates both the effect of constipation on CRC risk and the reciprocal influence of CRC risk on constipation occurrence. By elucidating these bidirectional relationships, we aim to provide robust evidence that contributes to our understanding of the interplay between constipation and CRC.
We searched the Open Genome-Wide Association Studies (OpenGWAS) database for curated GWAS summary datasets. Using publicly available data, we conducted a two-sample MR study, using genetic variants linked to constipation and colorectal cancer as instrumental variables (IVs) with a P-value threshold of 1×10-5 to get enough SNPs, and pruned IVs by linkage disequilibrium (LD) (r2≥0.01, kb>10,000) (16). In addition, palindromic SNPs were removed by using minor allele frequencies to prevent strand ambiguity issues (17). We computed the R2 statistic and F statistic for the instrumental variables in the exposure. The R2 statistic signifies the variance explained by the instrumental variables. Each individual variant demonstrated an F statistic equal to or exceeding 10, indicating strong instrumental variables. An F statistic below 10 is generally deemed a ‘weak IV’. Hence, the potential for weak instrument bias in our analysis was notably low. Constipation data from European individuals (n = 218,810), colorectal cancer data from Europeans (n = 377,673), colon cancer (CC) data from Europeans (n = 462,933) and rectal cancer (RC) data from Europeans (n = 456,276) were used. Data details can be found at https://gwas.mrcieu.ac.uk/. See Figure 1 for the analysis flow.
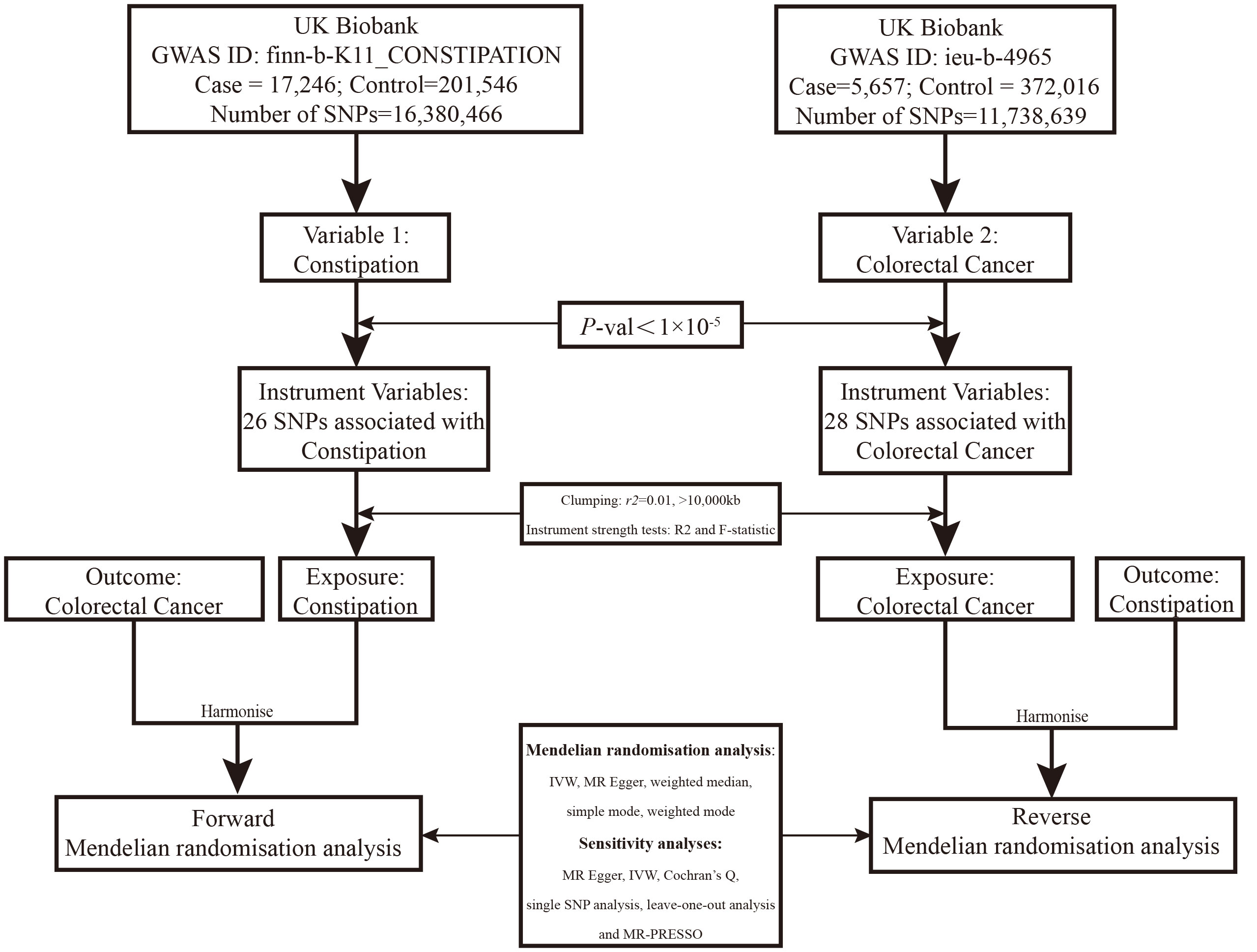
Figure 1 Overview of the two-sample MR study design used to investigate the causal association between constipation and CRC.
Moreover, we used PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk/) to check if instruments used were associated with potential confounders for the effect of constipation on CRC, and of CRC on constipation. We performed a leave-one-out analysis to check if any individual SNP was driving the observed association for both constipation on CRC, and for CRC on constipation.
This study utilized the Inverse Variance Weighted (IVW) - Random Effects as the primary MR method. Four additional MR approaches were employed: Weighted Median, Weighted Mode, Simple Mode, and MR-Egger. IVW involves meta-analyzing SNP exposure and outcome effects, adjusting for heterogeneity. The Weighted Median calculates the median causal estimate, Weighted Mode identifies the mode, and Simple Mode estimates causality without weights. MR-Egger addresses pleiotropy. Combining methods enhances robustness, offering varied insights. IVW assumes valid instrumental variables; deviations impact precision. These techniques provide a comprehensive view of the causal relationship, considering different assumptions and biases. All Mendelian randomization analyses were conducted using the RStudio Software (Version: 2023.06.0 Build 421) and R Software (Version: 4.3.1).
We examined the heterogeneity between SNPs using Cochran’s Q-statistics (18) and I2 statistic (19, 20). Additionally, we conducted a “leave-one-out” analysis to explore the potential influence of individual SNPs on the causal association (21).
Effect of Constipation on CRC: The Constipation GWAS identified 26 independent genome-wide significant SNPs. All SNPs utilized in the MR analysis were considered “strong” instruments, each possessing an F statistic greater than 10. The F statistic takes into account the SNP’s effect magnitude and precision on Constipation. Individual F statistics ranged from 20 to 30. While IVW-random effect analysis indicated a potential causal link between Constipation and CRC odds [OR = 1.002 (1.000, 1.004); P = 0.023], the other four approaches did not provide substantial evidence of a causal association (Table 1, Figure 2, Figure 3).
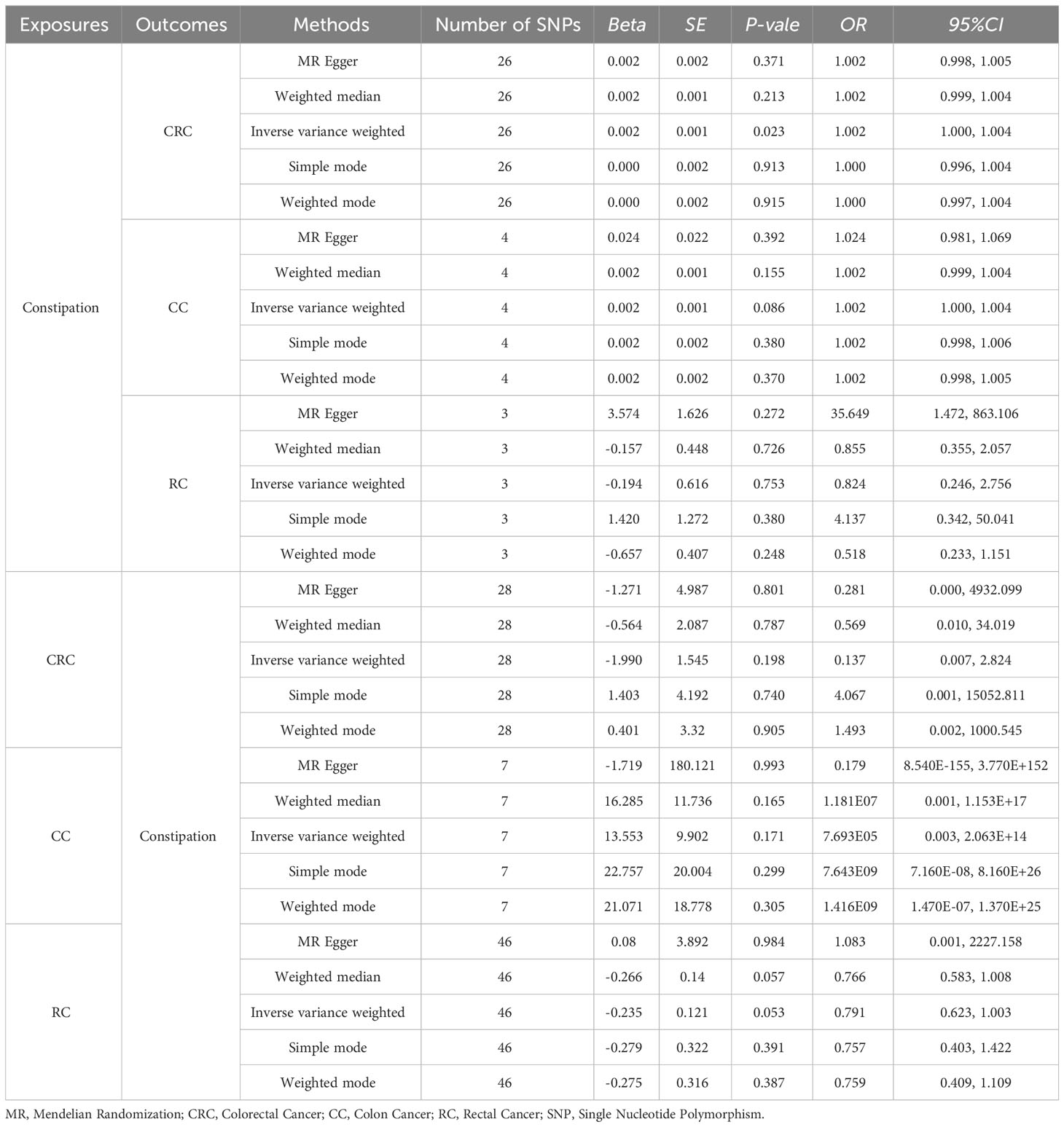
Table 1 Results of two-sample bidirectional MR analysis of the causal effects between Constipation and CRC (include CC and RC).
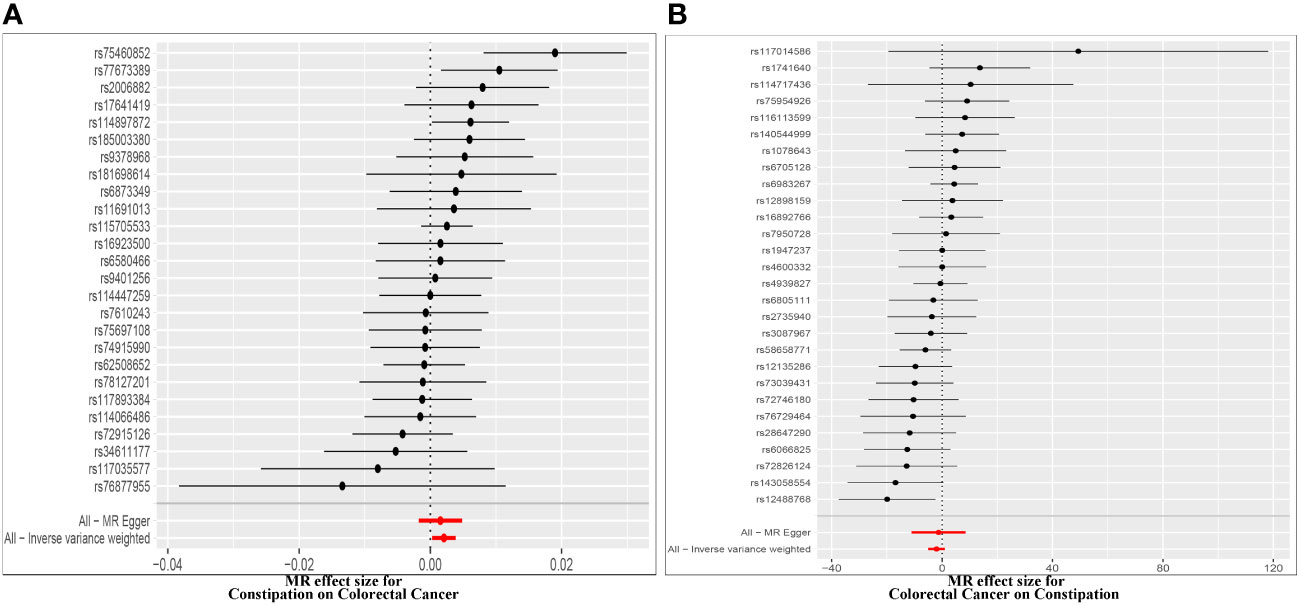
Figure 2 Forest plot of MR effect of the causal relationship between constipation and CRC (A). Effect of Constipation on CRC; (B) Effect of CRC on Constipation.
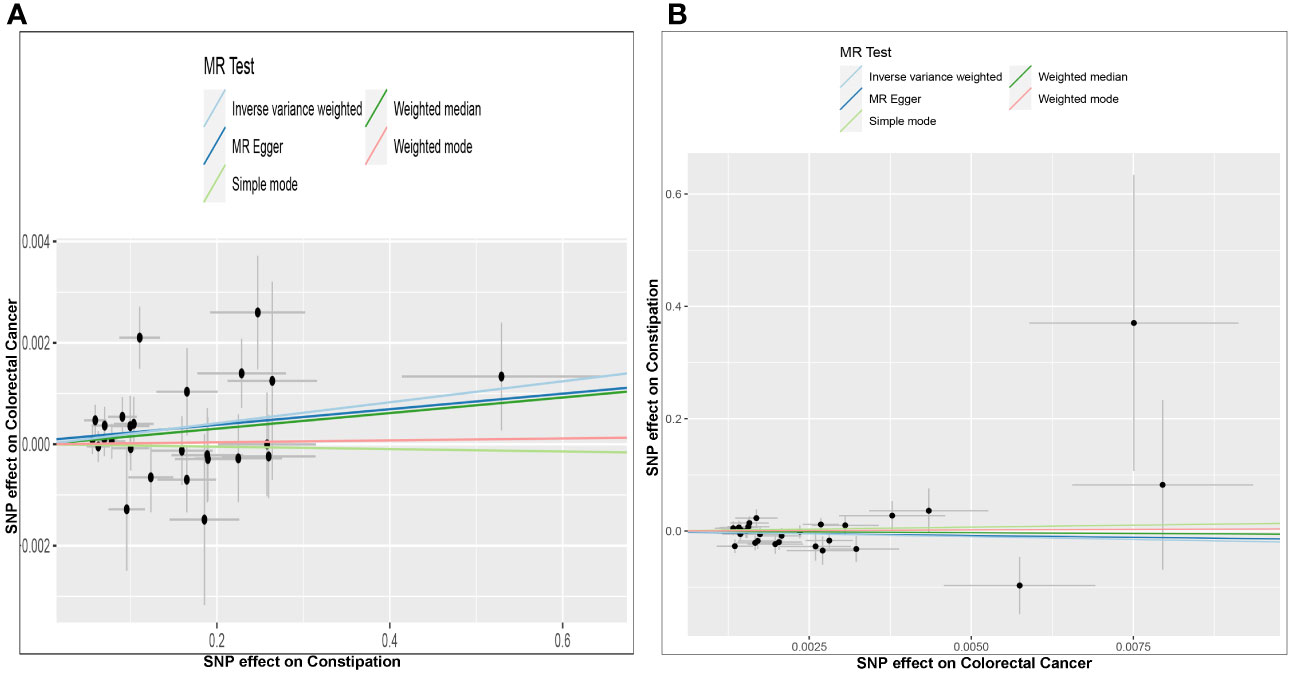
Figure 3 Scatter plots of genetic associations between constipation and CRC. The slopes of each line represent the causal association for each method. The light blue line represents the inverse‐variance weighted estimate, the green line represents the weighted median estimate, the dark blue line represents the Mendelian randomization‐Egger estimate, the red line represents the weighted mode estimate, and the light green line represents the simple mode estimate (A). Effect of Constipation on CRC; (B) Effect of CRC on Constipation.
Effect of Constipation on CC: The Constipation GWAS identified 4 independent genome-wide significant SNPs. All SNPs utilized in the MR analysis were considered “strong” instruments, each possessing an F statistic greater than 10. The F statistic takes into account the SNP’s effect magnitude and precision on Constipation. All the five approaches did not provide substantial evidence of a causal association (All results in Table 1).
Effect of Constipation on RC: The Constipation GWAS identified 3 independent genome-wide significant SNPs. All SNPs utilized in the MR analysis were considered “strong” instruments, each possessing an F statistic greater than 10. The F statistic takes into account the SNP’s effect magnitude and precision on Constipation. All the five approaches did not provide substantial evidence of a causal association (All results in Table 1).
Effect of CRC on Constipation: The GWAS on CRC identified 28 independent genome-wide significant SNPs. All SNPs used in the MR analysis were “strong” instruments with an F statistic >10, where the F statistic is a function of both magnitude and precision of the SNP’s effect on Constipation. Individual F statistics ranged from 21 to 90. The IVW-random effect analyses showed no evidence of a causal effect of CRC on the odds of Constipation [OR =0.137 (0.007, 2.824); P = 0.198]. In addition, other four approaches did not yield evidence of a causal association of CRC on the odds of Constipation (Table 1, Figure 2, Figure 3).
Effect of CC on Constipation: The GWAS on CC identified 28 independent genome-wide significant SNPs. All SNPs used in the MR analysis were “strong” instruments with an F statistic >10, where the F statistic is a function of both magnitude and precision of the SNP’s effect on Constipation. All the five approaches did not yield evidence of a causal association of CC on the odds of Constipation (Table 1).
Effect of RC on Constipation: The GWAS on RC identified 46 independent genome-wide significant SNPs. All SNPs used in the MR analysis were “strong” instruments with an F statistic >10, where the F statistic is a function of both magnitude and precision of the SNP’s effect on Constipation. All the five approaches did not yield evidence of a causal association of RC on the odds of Constipation (Table 1).
Cochran’s Q test assessed heterogeneity among instrumental variable estimates from individual genetic variants. The results showed no significant evidence of heterogeneity (Table 2, Figure 4). Low heterogeneity suggests more reliable Mendelian randomization (MR) estimates. I2 values also indicated low heterogeneity, reinforcing MR estimate reliability (Table 2). “Leave-one-out” analysis, where each SNP was removed to assess its impact on the IVW point estimate (Figure 4), indicated no single SNP significantly influenced the overall result. The funnel plot and MR Egger regression test displayed no significant asymmetry, indicating minimal publication bias and directional horizontal pleiotropy (Figure 5). Overall, minimal heterogeneity, low I2 values, stable “leave-one-out” results, and absence of asymmetry confirm MR estimate reliability and mitigate bias concerns.
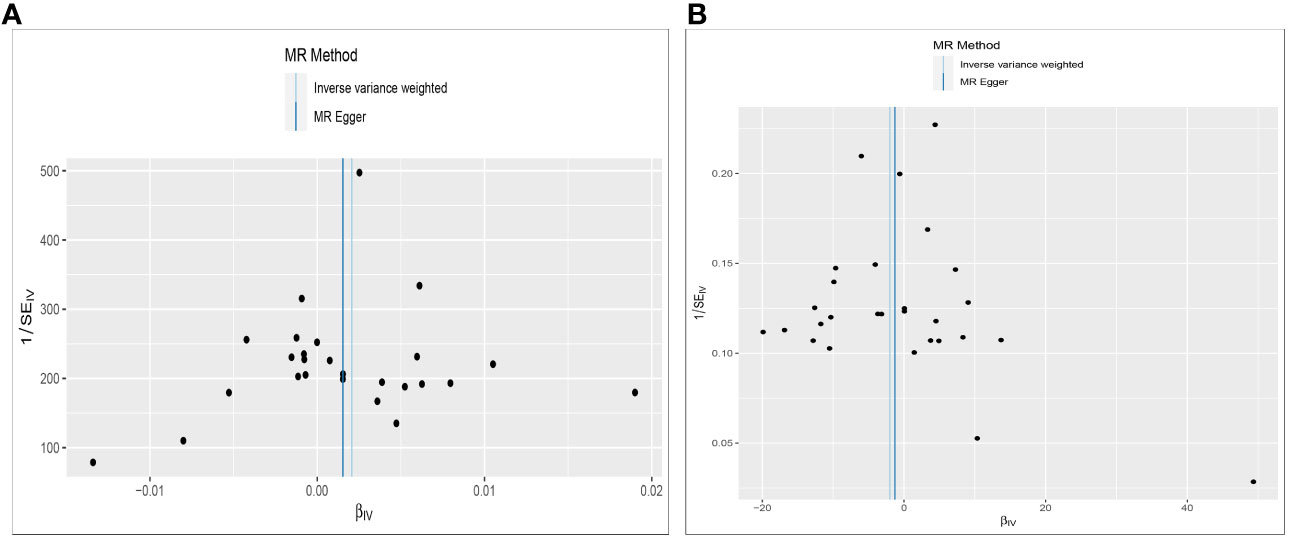
Figure 4 Funnel plot to assess heterogeneity. The light blue line represents the inverse‐variance weighted estimate, and the dark blue line represents the Mendelian randomization‐Egger estimate (A). Effect of Constipation on CRC; (B) Effect of CRC on Constipation.
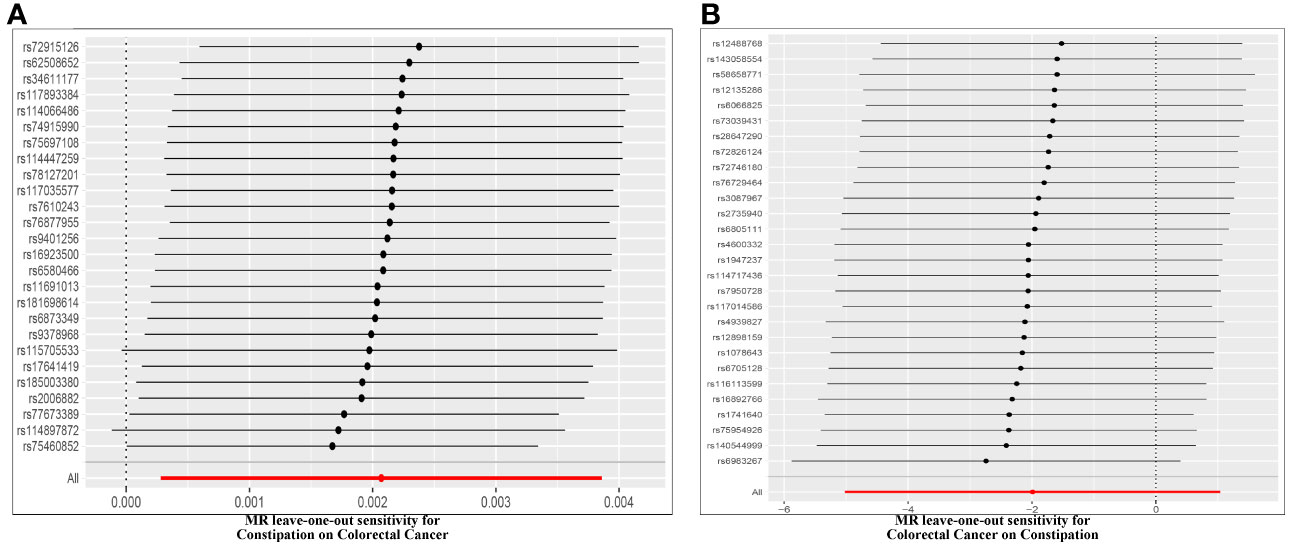
Figure 5 Leave-one-out of SNPs associated with constipation and CRC. Each black point represents result of the IVW MR method applied to estimate the causal effect between constipation and CRC excluding particular SNP (A). Effect of Constipation on CRC; (B) Effect of CRC on Constipation.
The findings of our bidirectional MR study provide valuable insights into the relationship between constipation and the risk of developing CRC. Our results suggest that constipation is associated with an increased risk of CRC, indicating a potential role for constipation as a modifiable risk factor for CRC. The observed link between constipation and an increased risk of CRC aligns with previous epidemiological and clinical studies (22), but not with the results of systematic review (7). Chronic constipation may lead to prolonged exposure to potential carcinogens in the colon, such as bile acids, which can promote tumor growth and initiate colorectal cancer (23). Additionally, constipation can disrupt the gut microbiota composition and function, leading to dysbiosis, increased inflammation, and altered metabolism of dietary components, all of which have been implicated in CRC development (12). Although our analyses found a causal relationship between constipation and colorectal cancer, this relationship was not statistically strong, and when constipation was analyzed separately from colon and rectal cancer, no such relationship was found. The possible reason for this may be that there are other potential risk factors that may interact with constipation and CRC.
The lack of evidence supporting a reverse causation relationship, where CRC would increase the risk of constipation, is an interesting finding. Some previous observational studies have suggested that there is no bidirectional relationship between CRC and constipation (7), our bidirectional MR analysis did not support this view. This finding suggests that CRC development may not directly contribute to the occurrence of constipation, but rather highlights the potential impact of constipation as a risk factor for CRC.
The strengths of our study lie in its utilization of a bidirectional MR approach, which provides stronger evidence for causal relationships compared to traditional observational studies. By leveraging SNPs as instrumental variables, we effectively address the issue of reverse causation and minimize the impact of confounding factors (15). Additionally, the use of two large, independent cohorts enhances the robustness and generalizability of our findings (13). However, several limitations should be considered when interpreting our results. Firstly, the bidirectional MR approach assumes that the instrumental variables are valid and accurately represent the exposure of interest. Although we carefully selected genetic variants associated with constipation and CRC from GWAS, the possibility of pleiotropy, where the genetic variants influence other pathways apart from constipation or CRC, cannot be entirely ruled out. Secondly, our study primarily focuses on the genetic predisposition to constipation and CRC and does not account for potential environmental or lifestyle factors that may mediate the observed associations. Future research should explore the potential mechanisms underlying the association between constipation and CRC to better understand the biological pathways involved. Additionally, efforts should be directed toward investigating other potential risk factors that may interact with constipation and CRC, such as dietary habits, physical activity, and medication use.
In conclusion, our bidirectional MR study provides evidence supporting the hypothesis that constipation increases the risk of developing CRC. These findings highlight the importance of managing constipation as a potential modifiable risk factor for CRC. Public health interventions should focus on promoting regular bowel movements, healthy dietary habits, and maintaining a balanced gut microbiota to reduce the risk of CRC in individuals with a history of constipation. Further research is warranted to validate our findings and explore additional factors associated with constipation and CRC risk.
The datasets supporting the conclusions of this article are available in OpenGWAS database website (https://gwas.mrcieu.ac.uk/). The other data generated or analyzed during this study are available in this published article and its supplementary information files.
LW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. HW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. FH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. X-YL: Investigation, Methodology, Writing – original draft, Writing – review & editing. Y-HZ: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. B-FZ: Funding acquisition, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. H-YL: Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. 1. Science and Technology Fund Project of Guizhou Health Commission in 2023, No. gzwkj2023-042. 2. National Natural Science Foundation of China, No. 8206100697; 82060114. 3. Doctoral research startup fund in 2021, No. gyfybsjy-2021-57.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Zheng RS, Zhang SW, Sun KX, Chen R, Wang SM, Li L, et al. Cancer statistics in China 2016. Zhonghua Zhong Liu Za Zhi (2023) 45:212–20. doi: 10.3760/cma.j.cn112152-20220922-006
3. Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev (2019) 9:CD002200. doi: 10.1002/14651858.CD002200.pub4
4. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States 2022: profiles, trends, and determinants. Chin Med J (Engl) (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
5. Bharucha AE. Constipation. Best Pract Res Clin Gastroenterol (2007) 21:709–31. doi: 10.1016/j.bpg.2007.07.001
6. Bisht P, Dagar N, Kumar N, Velayutham R, Arumugam S. Potential targets in constipation research: A review. Curr Drug Targets (2023) 24:247–60. doi: 10.2174/1389450124666221209123541
7. Power AM, Talley NJ, Ford AC. Association between constipation and colorectal cancer: systematic review and meta-analysis of observational studies. Am J Gastroenterol (2013) 108:894–903. doi: 10.1038/ajg.2013.52
8. Citronberg J, Kantor ED, Potter JD, White E. A prospective study of the effect of bowel movement frequency, constipation, and laxative use on colorectal cancer risk. Am J Gastroenterol (2014) 109:1640–9. doi: 10.1038/ajg.2014.233
9. Dzierzanowski T, Mercadante S. Constipation in cancer patients - an update of clinical evidence. Curr Treat Options Oncol (2022) 23:936–50. doi: 10.1007/s11864-022-00976-y
10. Staller K, Olen O, Soderling J, Roelstraete B, Tornblom H, Song M, et al. Chronic constipation as a risk factor for colorectal cancer: results from a nationwide, case-control study. Clin Gastroenterol Hepatol (2022) 20:1867–1876.e1862. doi: 10.1016/j.cgh.2021.10.024
11. Wang W, Liu Y, Yang X, Sun J, Yue Z, Lu D, et al. Effects of electroacupuncture for opioid-induced constipation in patients with cancer in China: A randomized clinical trial. JAMA Netw Open (2023) 6:e230310. doi: 10.1001/jamanetworkopen.2023.0310
12. Wang LW, Ruan H, Wang BM, Qin Y, Zhong WL. Microbiota regulation in constipation and colorectal cancer. World J Gastrointest Oncol (2023) 15:776–86. doi: 10.4251/wjgo.v15.i5.776
13. Sekula P, Del Greco MF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
14. Birney E. Mendelian randomization. Cold Spring Harb Perspect Med (2022) 12(4):a041302. doi: 10.1101/cshperspect.a041302
15. Burgess S, Daniel RM, Butterworth AS, Thompson SG, Consortium EP-I. Network Mendelian randomization: using genetic variants as instrumental variables to investigate mediation in causal pathways. Int J Epidemiol (2015) 44:484–95. doi: 10.1093/ije/dyu176
16. Pritchard JK, Przeworski M. Linkage disequilibrium in humans: models and data. Am J Hum Genet (2001) 69:1–14. doi: 10.1086/321275
17. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the h uman phenome. eLife (2018) 7:e34408. doi: 10.7554/eLife.34408
18. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ (1997) 315:1533–7. doi: 10.1136/bmj.315.7121.1533
19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
20. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol (2016) 45:1961–74. doi: 10.1093/ije/dyw220
21. Mikshowsky AA, Gianola D, Weigel KA. Assessing genomic prediction accuracy for Holstein sires using bootstrap aggregation sampling and leave-one-out cross validation. J Dairy Sci (2017) 100:453–64. doi: 10.3168/jds.2016-11496
22. Peng Y, Liu F, Qiao Y, Wang P, Ma B, Li L, et al. Association of abnormal bowel health with major chronic diseases and risk of mortality. Ann Epidemiol (2022) 75:39–46. doi: 10.1016/j.annepidem.2022.09.002
Keywords: constipation, colon cancer, rectal cancer, colorectal cancer, Mendelian randomization
Citation: Wu L, Wu H, Huang F, Li X-y, Zhen Y-h, Zhang B-f and Li H-y (2023) Causal association between constipation and risk of colorectal cancer: a bidirectional two-sample Mendelian randomization study. Front. Oncol. 13:1282066. doi: 10.3389/fonc.2023.1282066
Received: 23 August 2023; Accepted: 30 October 2023;
Published: 16 November 2023.
Edited by:
Paul Ruff, University of the Witwatersrand, South AfricaReviewed by:
Ali Reza Safarpour, Gastroenterohepatology Research Center, IranCopyright © 2023 Wu, Wu, Huang, Li, Zhen, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-yang Li, bGloYWl5YW5nQGdtYy5lZHUuY24=; Bao-fang Zhang, emhhbmdiYW9mYW5nQGdtYy5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.