- 1The Second Clinical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Guangzhou University of Chinese Medicine, Guangzhou, China
Introduction: Distant metastases of vulvar SCC most commonly involve the lung, liver, bone, skin, and lymph nodes. Metastasis from vulvar SCC to the kidneys is extremely rare, with only one case reported in the literature to date.
Case presentation: We report the case of a 53-year-old postmenopausal female patient was diagnosed with vulvar squamous cell carcinoma in an external hospital and following the diagnosis, she had been performed a vulvectomy for squamous cell carcinoma of the vulva, at that time, the patient had not undergone inguinal lymphadenectomy. In July 2019, she was admitted to our hospital due to upper right quadrant pain. An enhanced whole-body CT scan showed a mixed-density tumor of the right kidney with invasion into the right renal portal vein and multiple enlarged retroperitoneal lymph nodes. Positron emission tomography-computed tomography (PET - CT) scan showed a significantly increased radioactivity uptake in the tumor and enlarged lymph nodes, but PET-CT did not show abnormal enlargement of bilateral inguinal lymph nodes and no abnormal increase in radioactivity uptake. PET-CT examination did not show recurrence in terms of local of vulvar. These results led us to be gravely worried about possible renal carcinoma, so it was agreed upon to perform laparoscopic nephrectomy of the right kidney in the same month. Histology of the resected tumor confirmed it to be poorly differentiated squamous cell carcinoma with invasion consistent with metastatic vulvar carcinoma. Based on clinical history, radiological and histological facts, the patient was diagnosed with kidney metastasis from vulvar squamous cell carcinoma. Recovery from surgery went well and the patient was transferred to the oncology department and underwent a chemotherapy regimen consisting of paclitaxel and nedaplatin for further treatment. After 6 courses of chemotherapy. For a year after treatment, the patient had lived progression-free. Unfortunately, she died of tumor progression in July 2022.
Conclusion: Although renal metastasis from vulvar SCC is rare, renal metastasis should be considered for the patient with a history of vulvar cancer, whenever a mass is identified in the kidney. Timely surgical removal of renal metastasis may prolong the survival time.
Introduction
According to previous studies, vulvar cancer represents 3-5% of malignancies among in the gynecologic genital tract (1). The most common histological type of vulvar cancer is squamous cell carcinoma (SCC). This disease is most commonly diagnosed in postmenopausal women between the ages of 65 to 80, amongst which the occurrence of distant metastases is very rare. The prognosis for vulvar SCC is related to its size, location, degree of cell differentiation, whether lymph node metastasis occurred and the therapeutic measures taken (2). Distant metastases of vulvar SCC most commonly involve the lung, liver, bone, skin, and lymph nodes. Metastasis from vulvar SCC to the kidneys is extremely rare, with only one case reported in the literature to date (3). In this report, we shall discuss a rare case of renal metastasis from a primary vulvar SCC.
Case presentation
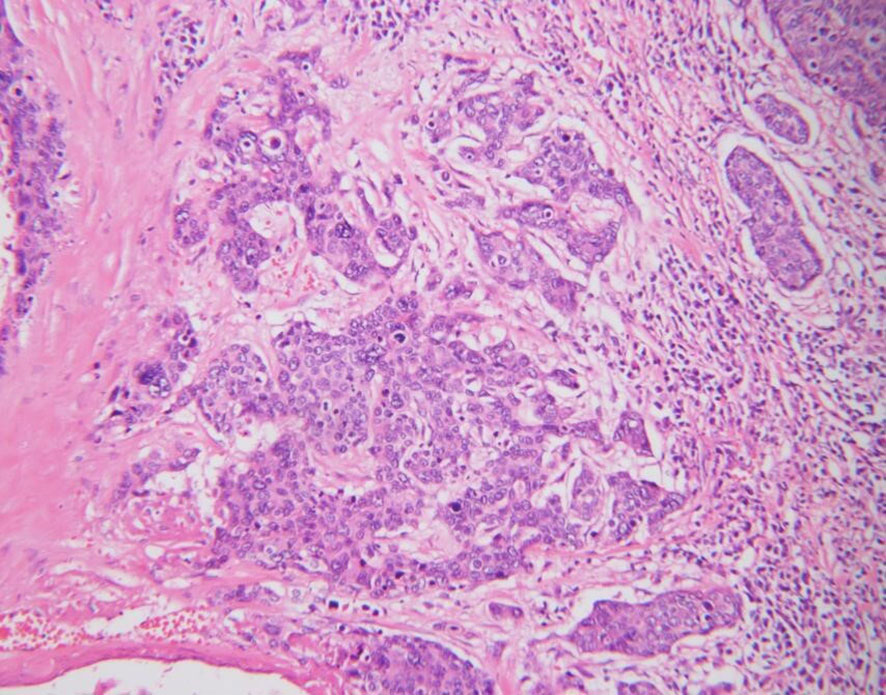
In 2016, a 53-year-old postmenopausal female patient was diagnosed with vulvar squamous cell carcinoma in an external hospital and following the diagnosis, she had been performed a vulvectomy for squamous cell carcinoma of the vulva, at that time, the patient had not undergone inguinal lymphadenectomy. During postoperative pathological examination, the vulvar tumor has been confirmed to be poorly differentiated squamous cell carcinoma (Figure 1), the pathological stage was Ib. The patient also received postoperative adjuvant radiotherapy and chemotherapy, in which the radiotherapy regimen was 50 Gy, while the concurrent chemotherapy drug was cisplatin weekly regimen, 40 mg/m2. She remained in remission for 3 years after surgery.
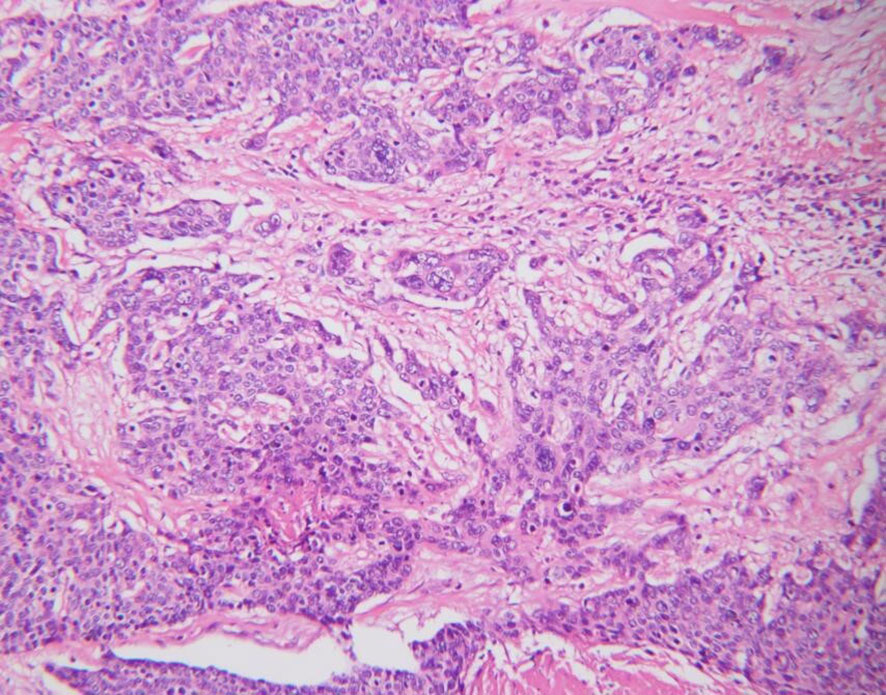
In July 2019, she was admitted to our hospital due to upper right quadrant pain. Physical examination of the patient at that time showed no superficial enlargement of the systemic lymph nodes, especially the inguinal lymph nodes. An enhanced whole-body CT scan showed a mixed-density tumor of the right kidney with invasion into the right renal portal vein and multiple enlarged retroperitoneal lymph nodes (Figure 2). Positron emission tomography-computed tomography (PET - CT) scan showed a significantly increased radioactivity uptake in the tumor and enlarged lymph nodes (Figure 3), but PET-CT did not show abnormal enlargement of bilateral inguinal lymph nodes and no abnormal increase in radioactivity uptake. PET-CT examination did not show recurrence in terms of local of vulvar. These results led us to be gravely worried about possible renal carcinoma, so it was agreed upon to perform laparoscopic nephrectomy of the right kidney in the same month. Histology of the resected tumor confirmed it to be poorly differentiated squamous cell carcinoma with invasion consistent with metastatic vulvar carcinoma (Figure 4). Based on clinical history, radiological and histological facts, the patient was diagnosed with kidney metastasis from vulvar squamous cell carcinoma. Recovery from surgery went well and the patient was transferred to the oncology department and underwent a chemotherapy regimen consisting of paclitaxel and nedaplatin for further treatment. After 6 courses of chemotherapy, CT showed that the enlarged intractraperitoneal lymph nodes had reduced in size. For a year after treatment, the patient had lived progression-free. Unfortunately, she died of tumor progression in July 2022. In the end, the patient lived for 3 years since the discovery of renal metastasis of vulvar SCC.
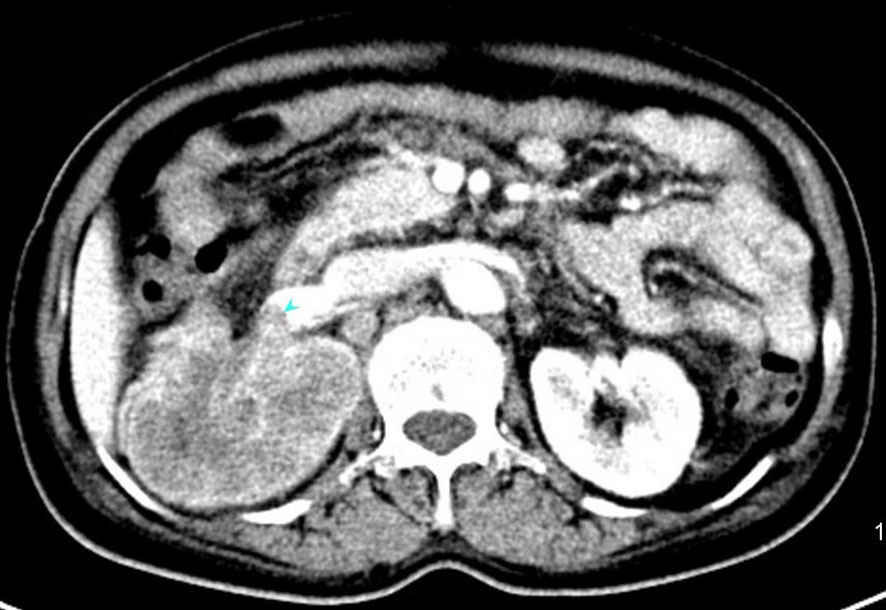
Figure 2 An enhanced computed tomography (CT) scan showing a mixed density tumor of the right kidney measuring 6.6×7.0×9.4cm with invasion into right renal portal vein.
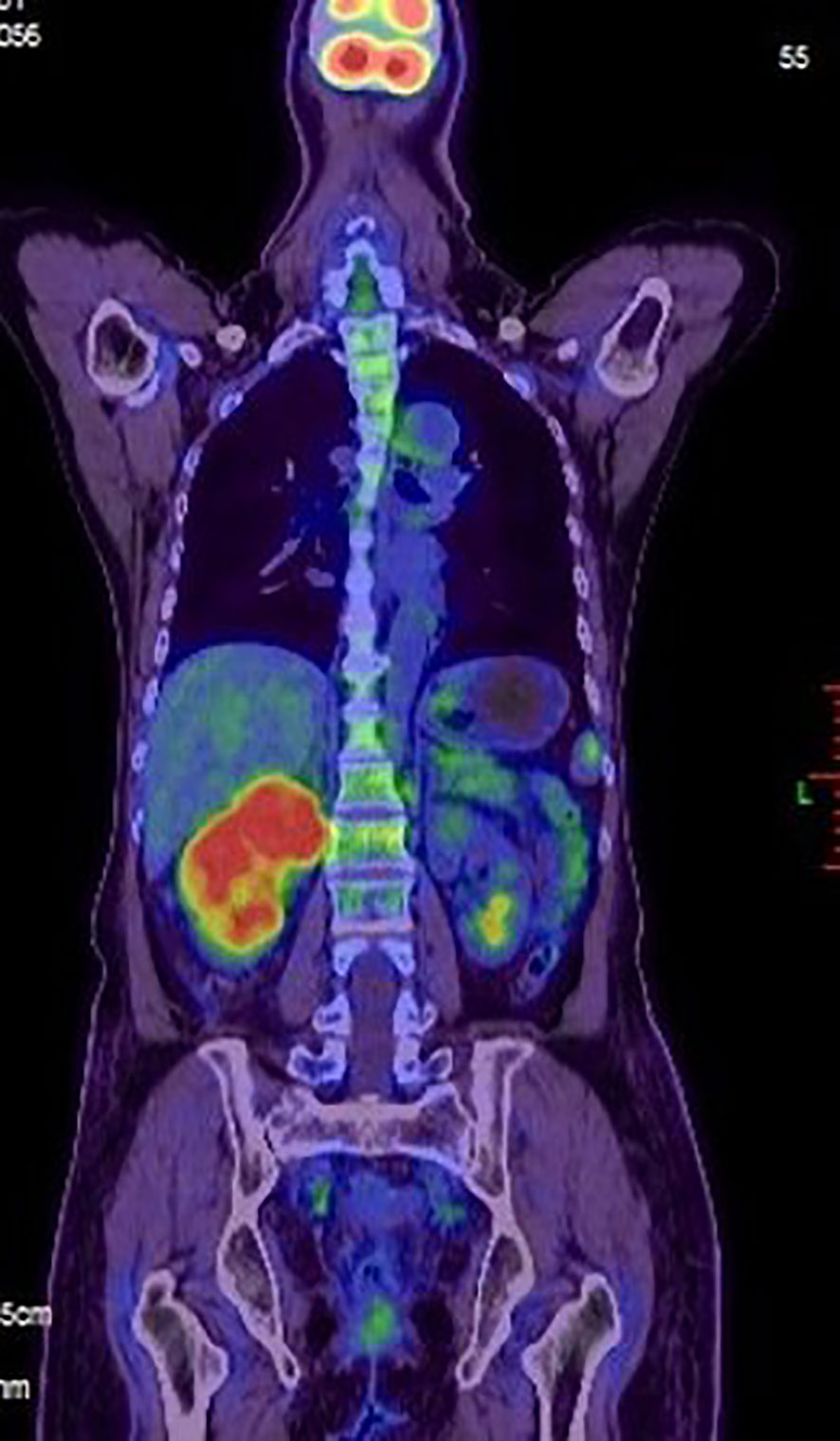
Figure 3 A positron emission tomography-computed tomography (PET-CT) scan was made. Evidence of intense FDG uptake in the tumor of the right kidney.
Discussion
Vulvar carcinoma represents about 5% of all female genital tract malignancies and the incidence increases with age and peaks at age 70. There are two etiologic pathways for vulvar squamous cell carcinomas namely the human papillomavirus (HPV) - mediated pathway and the HPV - independent pathway, while the HPV-independent pathway accounts for 60% of diagnosed cases (4). A retrospective study of vulvar cancer showed that the 2-year-OS rate of patients who had metastasized after the diagnosis of metastasis was 11.3%, and the median survival period from the first diagnosis of metastasis is 5.6 months (5). Prognosis depends on the stage at age, surgical pathological stage, histological differentiation, as well as the presence of lymph node metastases (6, 7). Another retrospective multicenter study evaluating 502 cases of vulvar cancer indicated that the 5-year survival rate was only 15% for distant recurrences (8). The most frequent distant metastases are pulmonary and hepatic metastases, but renal metastases are seldom. Distant metastases are rare in vulvar carcinoma with an incidence rate of 2-7% as shown in previous studies (8, 9). Due to a lack of prospective trial data, the treatment of vulvar cancer is still controversial, as there is no unified standard regimen. Extensive local excision is considered to be the best treatment for early-stage vulvar cancer, but there is still a risk of local recurrence if surgery is inappropriate (10). Treatment for advanced vulvar cancer includes multimodal therapies which consist of neoadjuvant radiotherapy and chemotherapy and are highly individualized. When radiotherapy or surgery is not feasible, the strategies for treatment usually involve platinum-based systemic combination therapy (11). According the literature, combination chemotherapy is considered more effective than monotherapy. With the use of combination regimens, chemotherapy patients achieved response rates of 40 - 56% (12, 13). With the development of molecular basic research on tumor cell immune recognition and immune regulation, immunotherapy in vulvar cancer has aroused great interest in recent years. Immunotherapy may be a viable alternative to the current treatment for vulvar squamous cell carcinomas (14). Vulvar cancer is characterized by a series of immune evasion mechanisms which are aggressive and lead to a range of symptoms in the organism (15). Due to limited relevant pathological reports, data on the role of immune checkpoint inhibitors in vulvar cancer are often obtained from trials involving other types of tumors. However, treatment with single immune checkpoint inhibitors indicates an overall lack of significant effectiveness based on the data (16, 17).
A study by the University of Texas MD Anderson Cancer Center, which indicated that there were only 151 cases of renal metastasis reported at their facilities from November 1985 to November 2013 (18). Kidney metastasis commonly occurs through a combination of lymphatic and venous routes. Renal metastases are rare, and most studies are only found in the form of case reports through literature search. With only having one reported case in the literature to date, renal metastases from vulvar SCC can be considered extremely rare. In the first case that was reported, the patient had a 24-month recurrence-free survival time after radical surgery and chemo-radiation of vulvar cancer relapse (3). She was treated with radical vulvectomy and chemo-radiation therapy due to vulvar SCC and remained in remission for 24 months. In 2010, she was diagnosed with metastatic squamous cell carcinoma from the vulva to the kidney and underwent nephrectomy. After nephrectomy and paraortic lymph node dissection, the patient’s health declined progressively, and, unfortunately, passed away 2 weeks later.
From current medical standards, treatment patterns for renal metastases are not clear and only appear in individual case reports. Adamy et al. stated in their article, that over the last two decades, 13 patients underwent nephrectomy for metastasis to the kidney, in which 5 cases of the primary tumors were lung cancer. They concluded that nephrectomy can be offered for highly selected patients as a therapeutic option, which positively affected the survival rate of selected patients with renal metastasis (19).
In our report, the patient had a 36-month recurrence-free survival time after the vulvectomy for vulvar SCC, but renal metastasis and retroperitoneal lymph nodes metastasis recurred 3 years later. The primary treatment was not perfect because no inguinal lymph node dissection was performed after the vulvectomy. Panici PB et al. reported nodal status represents a very important prognostic factor in patients of vulvar SCC (20). The recent research suggests that patients with early stage unifocal vulvar SCC(<4 cm) and no suspicious and/or enlarged lymph nodes at imaging should be considered for sentinel lymph node biopsy (21).
We determined the most appropriate treatment plan for the patient after performing a nephrectomy, which comprised a paclitaxel and nedaplatin combination chemotherapy. Following 6 courses of chemotherapy, the CT scan showed that the enlarged lymph nodes had reduced in size. The patient had an one-year progression-free survival. Unfortunately, she died of tumour progression in July 2022. In the end, the patient lived for 3 years since the discovery of renal metastasis of vulvar SCC.
The treatment outcome of a recurrent disease in vulvar SCC is disappointing, and the prognosis of distant metastasis is poor. Since the discovery of renal metastasis of vulvar SCC, the patient has survived for 3 years, which is significantly longer than what has been reported in the literature to date. It is suggested that our surgical resection of the right kidney and combination chemotherapy is still of great significance for these patients and are worthy of clinical application.
Conclusions
To the best of our knowledge, our report is the second case of vulvar SCC metastasis to the kidney. The diagnosis was based on clinical history, radiological and histological facts. Although renal metastasis from vulvar SCC is rare, renal metastasis should be considered for the patient with a history of vulvar cancer, whenever a mass is identified in the kidney. Timely surgical removal of renal metastasis may prolong the survival time.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Guangdong Provincial of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JH: Writing – original draft. YX: Writing – original draft. LW: Investigation, Resources. ZW: Supervision, Validation. JP: Formal analysis, Writing – review & editing. ZB: Validation, Project administration.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SCC, Squamous cell carcinoma; PET - CT, Positron emission tomography-computed tomography; CT, Computed tomography.
References
1. Carter JS, Downs LS Jr. Vulvar and vaginal cancer. Obstet Gynecol Clin North Am (2012) 39(2):213–31. doi: 10.1016/j.ogc.2012.04.002
2. Buttmann-Schweiger N, Klug SJ, Luyten A, Holleczek B, Heitz F, du Bois A, et al. Incidence patterns and temporal trends of invasive nonmelanotic vulvar tumors in Germany 1999-2011. A population-based cancer registry analysis. PloS One (2015) 10(5):e0128073. doi: 10.1371/journal.pone.0128073
3. Agrawal A, Wood KA, Giede CK, Chibbar R. A case of kidney metastasis in vulvar squamous cell carcinoma: a case report and review of literature. Case Rep Clin Med (2013) 02(5):306–9. doi: 10.4236/crcm.2013.25082
4. Bigby SM, Eva LJ, Fong KL, Jones RW. The natural history of vulvar intraepithelial neoplasia, differentiated type: evidence for progression and diagnostic challenges. Int J Gynecol Pathol (2016) 35(6):574–84. doi: 10.1097/PGP.0000000000000280
5. Prieske K, Haeringer N, Grimm D, Trillsch F, Eulenburg C, Burandt E, et al. Patterns of distant metastases in vulvar cancer. Gynecol Oncol (2016) 142(3):427–34. doi: 10.1016/j.ygyno.2016.07.009
6. Hacker NF, Barlow EL. Staging for vulvar cancer. Best Pract Res Clin Obstet Gynaecol (2015) 29(6):802–11. doi: 10.1016/j.bpobgyn.2015.01.004
7. Le ST, Karia PS, Vollenhoven BJ, Besaw RJ, Feltmate CM, Schmults CD. Evaluation of AJCC and an alternative tumor classification system for primary vulvar squamous cell carcinoma. J Natl Compr Canc Netw (2018) 16(1):42–9. doi: 10.6004/jnccn.2017.7022
8. Maggino T, Landoni F, Sartori E, Zola P, Gadducci A, Alessi C, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF Study. Cancer (2000) 89(1):116–22. doi: 10.1002/1097-0142(20000701)89:1<116::aid-cncr16>3.0.co;2-4
9. Witteveen PO, van der Velden J, Vergote I, Guerra C, Scarabeli C, Coens C, et al. Phase II study on paclitaxel in patients with recurrent, metastatic or locally advanced vulvar cancer not amenable to surgery or radiotherapy: a study of the EORTC-GCG (European Organisation for Research and Treatment of Cancer–Gynaecological Cancer Group). Ann Oncol (2009) 20(9):1511–6. doi: 10.1093/annonc/mdp043
10. Rottmann M, Beck T, Burges A, Dannecker C, Kiechle M, Mayr D, et al. Trends in surgery and outcomes of squamous cell vulvar cancer patients over a 16-year period (1998-2013): a population-based analysis. J Cancer Res Clin Oncol (2016) 142(6):1331–41. doi: 10.1007/s00432-016-2135-2
11. How JA, Jazaeri AA, Soliman PT, Fleming ND, Gong J, Piha-Paul SA, et al. Pembrolizumab in vaginal and vulvar squamous cell carcinoma: a case series from a phase II basket trial. Sci Rep (2021) 11(1):3667. doi: 10.1038/s41598-021-83317-7
12. Cormio G, Loizzi V, Gissi F, Serrati G, Panzarino M, Carriero C, et al. Cisplatin and vinorelbine chemotherapy in recurrent vulvar carcinoma. Oncology (2009) 77(5):281–4. doi: 10.1159/000259259
13. Wagenaar HC, Colombo N, Vergote I, Hoctin-Boes G, Zanetta G, Pecorelli S, et al. Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable, squamous-cell carcinoma of the vulva: an EORTC Gynaecological Cancer Cooperative Group Study. European Organization for Research and Treatment of Cancer. Gynecol Oncol (2001) 81(3):348–54. doi: 10.1006/gyno.2001.6180
14. Cocks M, Chaux A, Jenson EG, Miller JA, Rodriguez Pena MDC, Tregnago AC, et al. Immune checkpoint status and tumor microenvironment in vulvar squamous cell carcinoma. Virchows Arch (2020) 477(1):93–102. doi: 10.1007/s00428-020-02759-y
15. Borella F, Preti M, Bertero L, Collemi G, Castellano I, Cassoni P, et al. Is there a place for immune checkpoint inhibitors in vulvar neoplasms? A state of the art review. Int J Mol Sci (2020) 22(1):190. doi: 10.3390/ijms22010190
16. Lee L, Matulonis U. Immunotherapy and radiation combinatorial trials in gynecologic cancer: A potential synergy? Gynecol Oncol (2019) 154(1):236–45. doi: 10.1016/j.ygyno.2019.03.255
17. Lin LL, Lakomy DS, Ning MS, Simpkins F, Jhingran A. Combining novel agents with radiotherapy for gynecologic Malignancies: beyond the era of cisplatin. Int J Gynecol Cancer (2020) 30(4):409–23. doi: 10.1136/ijgc-2020-001227
18. Zhou C, Urbauer DL, Fellman BM, Tamboli P, Zhang M, Matin SF, et al. Metastases to the kidney: a comprehensive analysis of 151 patients from a tertiary referral centre. BJU Int (2016) 117(5):775–82. doi: 10.1111/bju.13194
19. Adamy A, Von Bodman C, Ghoneim T, Favaretto RL, Bernstein M, Russo P. Solitary, isolated metastatic disease to the kidney: Memorial Sloan-Kettering Cancer Center experience. BJU Int (2011) 108(3):338–42. doi: 10.1111/j.1464-410X.2010.09771.x
20. Panici PB, Tomao F, Domenici L, Giannini A, Giannarelli D, Palaia I, et al. Prognostic role of inguinal lymphadenectomy in vulvar squamous carcinoma: younger and older patients should be equally treated. A prospective study and literature review. Gynecol Oncol (2015) 137(3):373–9. doi: 10.1016/j.ygyno.2015.03.013
Keywords: vulvar carcinoma, squamous cell carcinoma, renal metastasis, nephrectomy, vulvectomy
Citation: He J, Xiao Y, Wang L, Wang Z, Pan J and Bai Z (2024) Case report: A kidney metastasis from vulvar squamous cell carcinoma. Front. Oncol. 13:1280531. doi: 10.3389/fonc.2023.1280531
Received: 20 August 2023; Accepted: 27 December 2023;
Published: 16 January 2024.
Edited by:
Stergios Boussios, Canterbury Christ Church University, United KingdomReviewed by:
Violante Di Donato, Unitelma Sapienza University, ItalyChiara Mazziotta, University of Ferrara, Italy
Copyright © 2024 He, Xiao, Wang, Wang, Pan and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zunguang Bai, enVuZ3VhbmdiYWlAMTYzLmNvbQ==
 Junwei He1,2
Junwei He1,2 Zunguang Bai
Zunguang Bai