
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 28 November 2023
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1280208
This article is part of the Research Topic Monoclonal Antibodies and Immune Checkpoint Inhibitors in the treatment of Cancer View all 28 articles
Camrelizumab, a monoclonal antibody, blocks programmed cell death protein-1 from binding to T cells and programmed cell death ligand 1 on tumor cells, thereby ensuring sustained T cell activation and blocking immune escape of various types of cancer, including nasopharyngeal carcinoma. Reactive cutaneous capillary endothelial hyperplasia (RCCEP) is the most common immune-related adverse event in patients treated with camrelizumab. We report a case nasopharyngeal carcinoma in a patient with camrelizumab-induced RCCEP. A 68-year-old man diagnosed with nasopharyngeal carcinoma developed RCCEP at multiple locations after 3 months of camrelizumab treatment. RCCEP of the right lower eyelid affected closure of the right eye. In this report, we also reviewed previous literature on camrelizumab-induced RCCEP. In summary, the mechanism underlying camrelizumab-induced RCCEP remains unclear. RCCEP typically gradually subsides after discontinuing camrelizumab treatment. Larger nodules can be treated with lasers, ligation, or surgery. Although surgical excision is effective, RCCEP may recur in patients undergoing camrelizumab treatment. RCCEP management may not be required in the absence of adverse effects on the patient’s daily life.
In recent years, immune checkpoint blockade therapy has demonstrated remarkable efficacy in the treatment of various malignant tumors (1, 2). Previous studies have shown that programmed cell death ligand 1 is highly expressed in patients with nasopharyngeal carcinoma (NPC) (3–5). Therefore, the combination of programmed cell death protein 1 and programmed cell death ligand 1, which are expressed by T cells and tumor cells, respectively, can block signal transduction and enhance immune system activity, thereby destroying cancer cells (6, 7). Camrelizumab is a monoclonal antibody against programmed cell death protein 1 that was developed by Jiangsu Hengrui Medicine Co (8).
Immune checkpoint inhibitors are associated with a range of immune-related adverse events (irAEs) (9, 10), which are often associated with immune system overactivation. Reactive cutaneous capillary endothelial hyperplasia (RCCEP) is the most common adverse event associated with camrelizumab use and usually occurs in the skin of the head, face, and trunk (11). However, the mechanism underlying camrelizumab-induced RCCEP remains unclear. We herein present a case in which a patient with NPC developed RCCEP, a “tumor-like” nodule, on the right lower eyelid after undergoing camrelizumab and chemotherapy. RCCEP uncommonly occurs in this location, and the nodule interfered with the patient’s ability to close the right eye owing to the thinness of the skin in this area. The nodule was surgically removed, and the patient’s prognosis was good.
In October 12, 2021, a 68-year-old man was diagnosed with T3N2M1 NPC, based on the American Joint Committee on Cancer’s Cancer Staging Manual, Eight Edition (12). The patient received chemotherapy (capecitabine, 625 mg/m2 twice daily, orally) and immunotherapy (camrelizumab injection, 200 mg every 21 days). On February 17, 2022, the patient underwent the seventh cycle of camrelizumab injection therapy. The timeline of the patient’s entire treatment progress is shown in Figure 1. Approximately 6 days later, the patient developed scattered bright red spots on the head, face, and trunk. Some spots gradually developed into pea-sized nodules (Figure 2). Two months later, the nodule on the right lower eyelid had grown to the size of a peanut (Figure 2). Head computed tomography revealed a nodule that protruded outward and squeezed the normal eye tissue inward (Figure 3). In addition, the nodule pulled the lower eyelid downwards, which affected eyelid closure owing to the thin skin and soft tissue of the lower eyelid (Figures 2A, C). After considering the patient’s condition and preferences, we resected the right lower eyelid nodule. The patient recovered well postoperatively and was able to close the right eye without difficulty (Figures 2B, D).
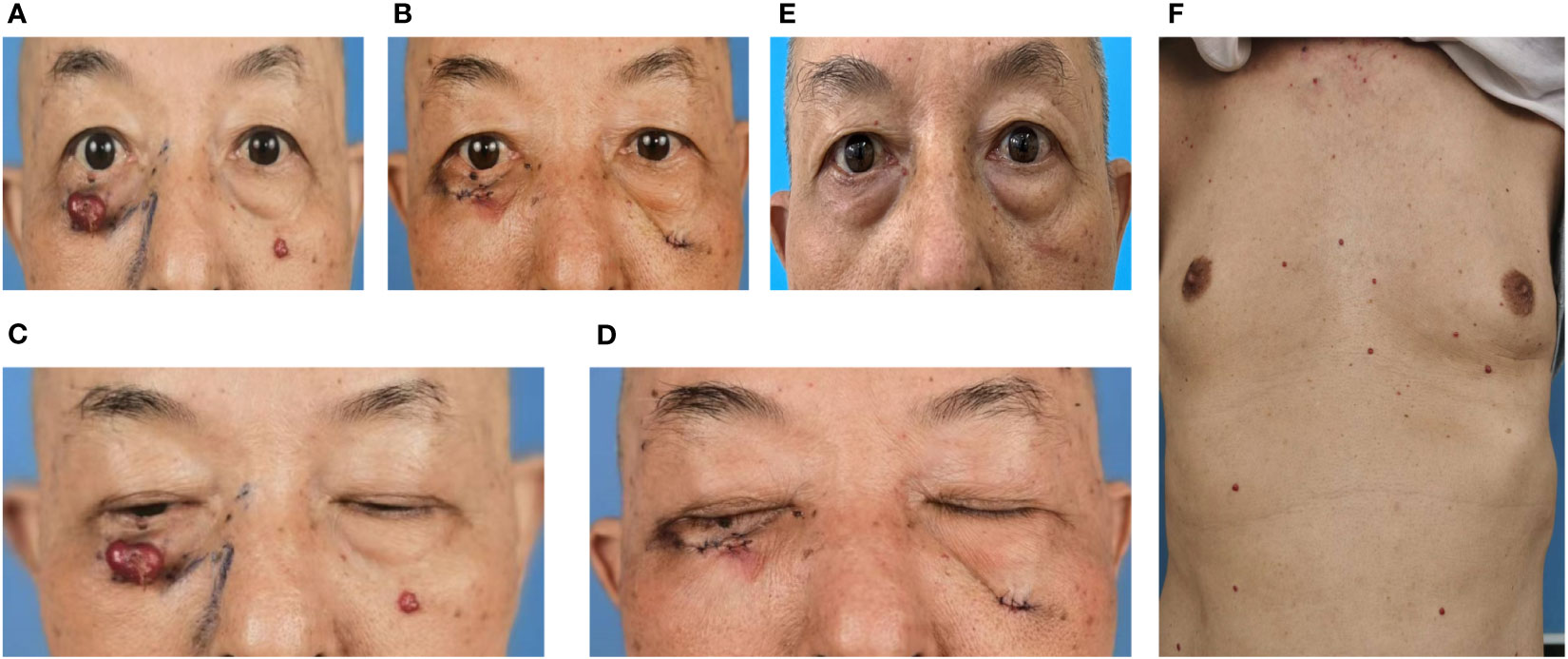
Figure 2 Dark red nodules were distributed over the patient’s head, face, and trunk. (A, C) Before surgery. (B, D) After surgery. (C) Before surgery, the nodule pulled on the right lower eyelid, resulting in incomplete closure of the right eye. (D) After surgery, the patient could close the right eye normally. (E) Six months after the operation, no recurrence of the right lower eyelid nodule was observed, although new nodules were noted in other parts of the face. (F) Dark red nodules distributed over the patient’s trunk before surgery.
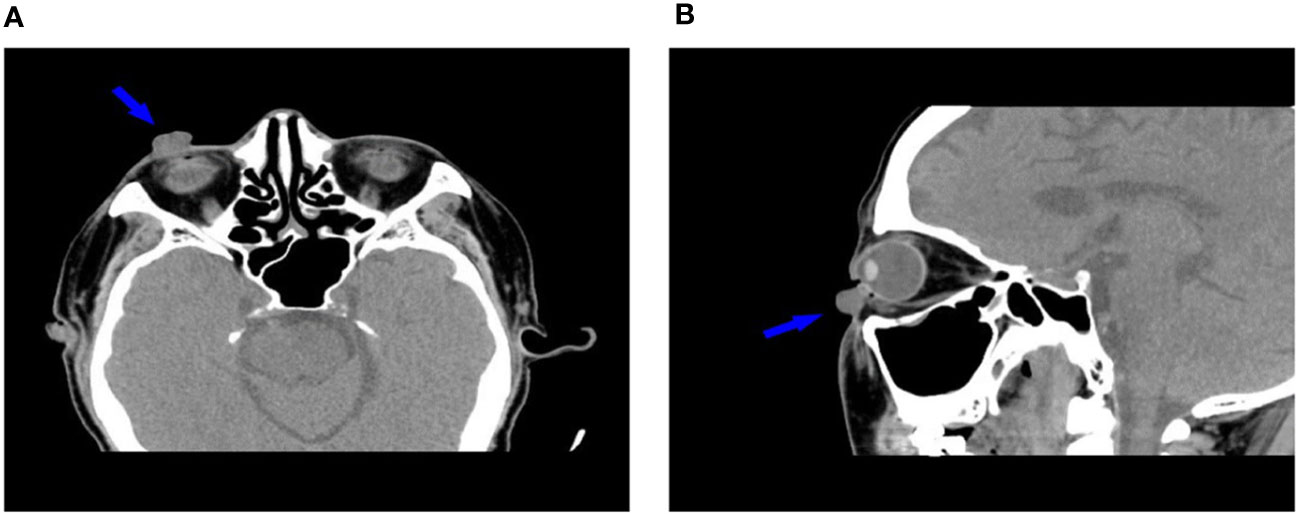
Figure 3 Head computed tomography (A: transverse plane, B: sagittal plane) shows the relationship between the nodule and surrounding soft tissues (arrows).
The removed nodule is shown in (Figure 4A). Histopathological examination revealed that the lesions comprised proliferated capillaries, which were distributed in nodular and lobulated forms. Large vessels were surrounded by small vessels; lumens varied in size and contained red blood cells (Figure 4B). Vascular endothelial cells were densely arranged. The nuclei were oval or short and spindle-shaped; mitotic figures were easily observed (Figure 4C). These pathological results supported a diagnosis of RCCEP. During the 6-month follow-up period, the surgical area of the right lower eyelid had recovered well, without RCCEP recurrence (Figure 2E). Because the patient continued to receive camrelizumab postoperatively, dark red nodules remained on other parts of the body (Figure 2E); however, these lesions resolved spontaneously after the patient completed 1 year of immunotherapy. According to Naranjo’s adverse drug reaction probability scale (Table 1), RCCEP was most likely caused by camrelzumab.
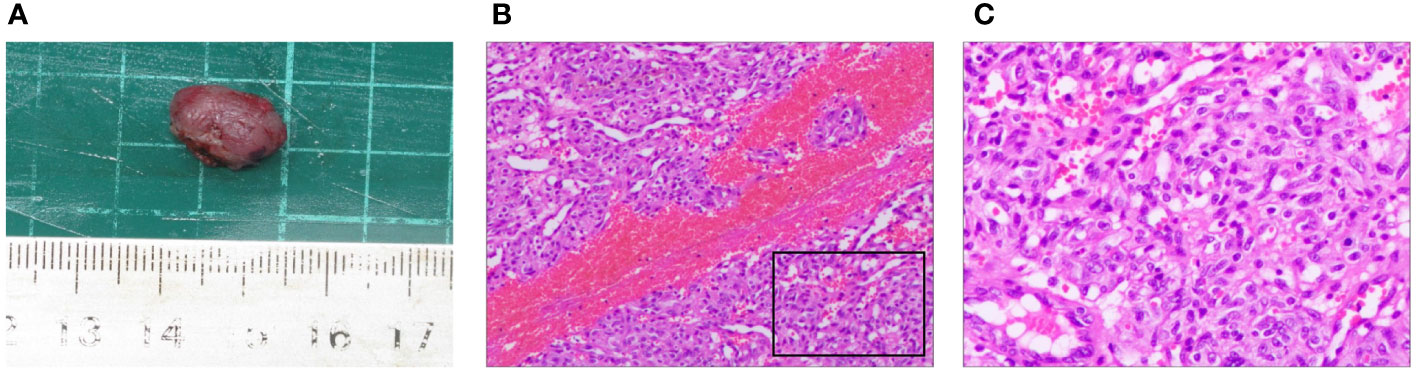
Figure 4 Pathological characteristics of the right lower eyelid nodule. (A) The size of the surgically resected nodule was about 1.5 cm × 1.0 cm × 0.9 cm. (B, (C) Hematoxylin and eosin staining showed extensive capillary proliferation. (B) × 200; (C) × 400.
Skin reactions in various organs are the most frequent irAEs, which are triggered by immune checkpoint inhibitors (13). The most frequent side effect of camrelizumab is RCCEP (8, 11). RCCEP appears primarily in the skin of the head, face, and torso (11). RCCEP in these regions is not typically fatal, although may affect function and coordination in the affected regions. The present case involved a patient who received camrelizumab therapy and subsequently developed right lower eyelid RCCEP that affected the patient’s ability to close the eye. According to current RCCEP grading criteria, this patient was classified as having a grade 2 lesion (single or multiple nodules, with the greatest nodule diameter being >10 mm, with or without rupture and bleeding) (14). Surgery was performed to restore the patient’s ability to close the right eye. Postoperative histopathological examination confirmed the RCCEP diagnosis. Since treatment with camrelizumab and capecitabine was effective, this regimen was continued postoperatively. Although the unresected nodules persisted and new nodules appeared, the patient’s daily life was unaffected. The RCCEP spontaneously resolved after the patient completed 1 year of treatment.
The mechanism by which camrelizumab triggers RCCEP is currently unclear. The predominant theory is that skin capillary endothelial cells exhibit overly active immune responses. RCCEP is histopathologically characterized by enhanced capillary endothelial cell proliferation and numerous mitotic figures. The molecular mechanism of camrelizumab may be that it activates CD4+ T lymphocytes, thereby increasing interleukin-4 levels in T helper 2 cytokines. This subsequently stimulates CD163+ M2 macrophage differentiation and promotes capillary endothelial cell proliferation by releasing vascular endothelial growth factor (VEGF) A (11, 15). Camrelizumab may also induce RCCEP by causing VEGF receptor-2-induced activation of vascular endothelial cell proliferation (16). These proposed mechanisms offer several potential targets for RCCEP prevention.
RCCEP can be classified as a cherry hemangioma (CH), both grossly and histopathologically (17, 18). CHs are the most prevalent form of acquired cutaneous vascular hyperplasia and are more common in older adults; the most frequently affected sites are the trunk and upper extremities (19, 20). CH etiology is attributed to gene mutations, chemical exposure, and viral infection (19). Gene mutation studies have focused on GNAQ, GNA11, and GNA14 (21, 22). Moreover, evidence suggests that VEGFR2 mutations can cause CHs, although the specific mechanism has not been elucidated (23, 24). CH has distinct clinical and histopathological features, although it is not included in the most recent edition of the International Society for the Study of Vascular Anomalies classification of vascular anomalies (25). The early stage of a CH is usually characterized by a flat red spot that gradually enlarges and becomes a red, blue, or purple papule. Histopathological investigations have revealed that CHs consist of lobulated, small-to-mildly dilated, thin-walled vessels with various sized lumens lined with a single layer of endothelial cells (21). These lesions are typically asymptomatic and do not require specific management. Effective treatment methods for CHs can also be used to treat RCCEP.
Infantile hemangioma (IH), also known as infantile capillary hemangioma or strawberry hemangioma, is a benign lesion commonly found on the head, neck, trunk, and extremities. Most of these lesions resolve spontaneously (26). IH growth can be divided into three stages: rapid vascular endothelial cell proliferation, decreased vascular endothelial cell proliferation, and replacement of vascular tissue with fibrofatty tissue (27). Oral propranolol administration, laser therapy, and surgery are the most common clinical treatment options for IH (28). However, the pathogenesis of IH is unclear. The current mainstream view is that pluripotent stem cells respond abnormally to stimuli, such as hypoxia and the renin-angiotensin system (27). As with CH, GNAQ, GNA11, and GNA14 mutations may also cause IH (29–31). Furthermore, gene mutations may interfere with the VEGF A signaling pathway (32, 33). VEGF receptor-2 is the receptor for VEGF A, and some patients with IH have VEGFR2 mutations (34, 35).
Pyogenic granuloma (PG), which is more accurately termed lobular capillary hemangioma, is an acquired benign lesion that occurs in tissues such as the skin and mucous membranes (36, 37). Chronic mild irritation, hormonal imbalances, and drug influences are considered the main PG etiologies (38–40). Cutaneous PG manifests as painless, red, and fleshy nodules that closely resemble RCCEP. Histologically, PG consists of clusters of proliferating capillaries arranged in a lobular structure (41, 42). Current evidence attributes its pathogenesis to effects on the upstream mediator gene, BRAF, on the mitogen-activated protein kinase pathway (43, 44). Although some PGs resolve spontaneously, most require treatment. Treatments include surgical resection, cryotherapy, laser therapy, and imiquimod cream. Among these treatments, surgical resection is the most effective and results in the lowest recurrence rate (37, 45).
Apatinib has successfully lowered the incidence of RCCEP (46–49). Apatinib is a tyrosine kinase inhibitor that selectively inhibits VEGF receptor-2 (50, 51) and inhibits VEGF-induced endothelial cell migration and proliferation, thereby preventing new blood vessel formation in the tumor tissue. Therefore, the combination of apatinib and camrelizumab may prevent RCCEP development by inhibiting capillary endothelial cell proliferation.
Many studies have shown that patients receiving camrelizumab combined with chemotherapy have better progression-free and overall survival rates than those of patients receiving chemotherapy alone (52–57). Camrelizumab and chemotherapy combined can achieve greater clinical benefits in patients with advanced NPC (58, 59). Camrelizumab combined with chemotherapy can also reduce the risk of RCCEP. Fang et al. reported that camrelizumab administration alone in patients with NPC resulted in a RCCEP incidence of 88% (82/93), compared with only 22% (5/23) when camrelizumab was administered in combination with gemcitabine and cisplatin (58).
By preserving immune activity, anti-programmed cell death protein 1 therapy suppresses tumors over the long term. An overactivated immune system may cause irAEs. Improvements in illness prognosis and the emergence of irAEs represent two sides of the same coin. The clinician is responsible for adjusting the medication regimen according to the clinical situation and intervening if adverse reactions occur. As previously stated, most patients with RCCEP do not require special treatment. RCCEP may gradually resolve if camrelizumab is ineffective and subsequently discontinued. If rupture and bleeding occur, the wound surface should be promptly disinfected, and antibacterial drugs should be administered externally if necessary. Therapeutic measures can be taken when RCCEP adversely affects the patients’ daily life. Traditional treatment methods include cryotherapy, electrosurgery, ligation, and surgical resection (19, 60). With the recent development of light therapy, safer and more effective options have become available for the treatment of capillary hemangiomas; nevertheless, these treatments are expensive (61–63). Several types of lasers can be used to treat capillary hemangiomas, including pulsed dye, alexandrite, neodymium-doped yttrium aluminum garnet, copper bromide, krypton, 532-nm diode, and potassium-titanyl-phosphate lasers (63–67). Intense pulsed light therapy can also be used to treat capillary hemangiomas (68). In cases of grade 3 or higher RCCEP, drug therapy should be immediately discontinued to reduce the mortality risk.
The development of immunotherapeutic drugs has increased the possibilities for cancer treatment. Meanwhile, cancer diagnosis and treatment require ever-increasing levels of cooperation among multiple disciplines, and increased focus on safe and rational drug administration is required by clinicians. Herein, we described a patient with camrelizumab-induced RCCEP in the right lower eyelid. Although this lesion affected the patient’s ability to close the right eye, their prognosis was good after surgery. This case illustrates the importance of considering the therapeutic efficacy versus the risk to maximize the benefit during cancer treatment. In addition, the data obtained in our clinical practice and provided herein can be used as a reference for improved medication regimen guidance.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of the Second Affiliated Hospital of Shantou University Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. YXL: Data curation, Writing – original draft. XZ: Methodology, Supervision, Writing – review & editing. QC: Supervision, Writing – review & editing. ST: Methodology, Supervision, Writing – review & editing. JC: Data curation, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Medical Scientific Research Foundation of Guangdong Province (A2022192 and B2023166); Shantou Science and Technology project (220811205271404) and Guangdong Provincial Bureau of Traditional Chinese Medicine research project (20232082) supported this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol (Toronto Ont) (2022) 29(5):3044–60. doi: 10.3390/curroncol29050247
2. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun (2020) 11(1):3801. doi: 10.1038/s41467-020-17670-y
3. Fang W, Zhang J, Hong S, Zhan J, Chen N, Qin T, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget (2014) 5(23):12189–202. doi: 10.18632/oncotarget.2608
4. Lee VH, Lo AW, Leung CY, Shek WH, Kwong DL, Lam KO, et al. Correlation of PD-L1 expression of tumor cells with survival outcomes after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. PloS One (2016) 11(6):e0157969. doi: 10.1371/journal.pone.0157969
5. Zhu Q, Cai MY, Chen CL, Hu H, Lin HX, Li M, et al. Tumor cells PD-L1 expression as a favorable prognosis factor in nasopharyngeal carcinoma patients with pre-existing intratumor-infiltrating lymphocytes. Oncoimmunology (2017) 6(5):e1312240. doi: 10.1080/2162402x.2017.1312240
6. Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer (2018) 6(1):8. doi: 10.1186/s40425-018-0316-z
7. Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, et al. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer Res (2015) 75(3):508–18. doi: 10.1158/0008-5472.Can-14-1215
8. Markham A, Keam SJ. Camrelizumab: first global approval. Drugs (2019) 79(12):1355–61. doi: 10.1007/s40265-019-01167-0
9. Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell (2021) 184(21):5309–37. doi: 10.1016/j.cell.2021.09.020
10. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol (2022) 19(4):254–67. doi: 10.1038/s41571-022-00600-w
11. Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen Z, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol (2020) 13(1):47. doi: 10.1186/s13045-020-00886-2
12. Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA: Cancer J Clin (2017) 67(2):122–37. doi: 10.3322/caac.21389
13. Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer (Oxford Engl 1990) (2016) 60:12–25. doi: 10.1016/j.ejca.2016.02.010
14. Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol (2012) 67(5):1025–39. doi: 10.1016/j.jaad.2012.02.010
15. Kuske M, Haist M, Jung T, Grabbe S, Bros M. Immunomodulatory properties of immune checkpoint inhibitors-more than boosting T-cell responses? Cancers (2022) 14(7). doi: 10.3390/cancers14071710
16. Finlay WJJ, Coleman JE, Edwards JS, Johnson KS. Anti-PD1 'SHR-1210' aberrantly targets pro-angiogenic receptors and this polyspecificity can be ablated by paratope refinement. mAbs (2019) 11(1):26–44. doi: 10.1080/19420862.2018.1550321
17. Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, et al. Addition of low-dose decitabine to anti-PD-1 antibody camrelizumab in relapsed/refractory classical hodgkin lymphoma. J Clin Oncol (2019) 37(17):1479–89. doi: 10.1200/jco.18.02151
18. Teng Y, Guo R, Sun J, Jiang Y, Liu Y. Reactive capillary hemangiomas induced by camrelizumab (SHR-1210), an anti-PD-1 agent. Acta Oncol (Stockholm Sweden). (2019) 58(3):388–9. doi: 10.1080/0284186x.2019.1567935
19. Qadeer HA, Singal A, Patel BC. Cherry Hemangioma. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Ankur Singal declares no relevant financial relationships with ineligible companies. Disclosure: Bhupendra Patel declares no relevant financial relationships with ineligible companies. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC (2023). Available at: https://pubmed.ncbi.nlm.nih.gov/33085354/.
20. Kim JH, Park HY, Ahn SK. Cherry angiomas on the scalp. Case Rep Dermatol (2009) 1(1):82–6. doi: 10.1159/000251395
21. Liau JY, Lee JC, Tsai JH, Chen CC, Chung YC, Wang YH. High frequency of GNA14, GNAQ, and GNA11 mutations in cherry hemangioma: a histopathological and molecular study of 85 cases indicating GNA14 as the most commonly mutated gene in vascular neoplasms. Modern Pathol (2019) 32(11):1657–65. doi: 10.1038/s41379-019-0284-y
22. Klebanov N, Lin WM, Artomov M, Shaughnessy M, Njauw CN, Bloom R, et al. Use of targeted next-generation sequencing to identify activating hot spot mutations in cherry angiomas. JAMA Dermatol (2019) 155(2):211–5. doi: 10.1001/jamadermatol.2018.4231
23. Lim YH, Odell ID, Ko CJ, Choate KA. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol (2015) 151(11):1240–3. doi: 10.1001/jamadermatol.2015.1925
24. Espinosa Lara P, Medina-Puente C, Riquelme Oliveira A, Jiménez-Reyes J. Eruptive cherry angiomas developing in a patient treated with ramucirumab. Acta Oncol (Stockholm Sweden) (2018) 57(5):709–11. doi: 10.1080/0284186x.2017.1410287
25. Kunimoto K, Yamamoto Y, Jinnin M. ISSVA classification of vascular anomalies and molecular biology. Int J Mol Sci (2022) 23(4). doi: 10.3390/ijms23042358
26. Satterfield KR, Chambers CB. Current treatment and management of infantile hemangiomas. Survey Ophthalmol (2019) 64(5):608–18. doi: 10.1016/j.survophthal.2019.02.005
27. Rodríguez Bandera AI, Sebaratnam DF, Wargon O, Wong LF. Infantile hemangioma. Part 1: Epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol (2021) 85(6):1379–92. doi: 10.1016/j.jaad.2021.08.019
28. Sebaratnam DF, Rodríguez Bandera AL, Wong LF, Wargon O. Infantile hemangioma. Part 2: management. J Am Acad Dermatol (2021) 85(6):1395–404. doi: 10.1016/j.jaad.2021.08.020
29. Lim YH, Bacchiocchi A, Qiu J, Straub R, Bruckner A, Bercovitch L, et al. GNA14 somatic mutation causes congenital and sporadic vascular tumors by MAPK activation. Am J Hum Genet (2016) 99(2):443–50. doi: 10.1016/j.ajhg.2016.06.010
30. Couto JA, Ayturk UM, Konczyk DJ, Goss JA, Huang AY, Hann S, et al. A somatic GNA11 mutation is associated with extremity capillary malformation and overgrowth. Angiogenesis (2017) 20(3):303–6. doi: 10.1007/s10456-016-9538-1
31. Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. New Engl J Med (2013) 368(21):1971–9. doi: 10.1056/NEJMoa1213507
32. Ye X, Abou-Rayyah Y, Bischoff J, Ritchie A, Sebire NJ, Watts P, et al. Altered ratios of pro- and anti-angiogenic VEGF-A variants and pericyte expression of DLL4 disrupt vascular maturation in infantile haemangioma. J Pathol (2016) 239(2):139–51. doi: 10.1002/path.4715
33. Greenberger S, Bischoff J. Pathogenesis of infantile haemangioma. Br J Dermatol (2013) 169(1):12–9. doi: 10.1111/bjd.12435
34. Castrén E, Salminen P, Vikkula M, Pitkäranta A, Klockars T. Inheritance patterns of infantile hemangioma. Pediatrics (2016) 138(5). doi: 10.1542/peds.2016-1623
35. Butnariu LI, Gorduza EV, Florea L, Ţarcă E, Moisă ŞM, Trandafir LM, et al. The genetic architecture of vascular anomalies: current data and future therapeutic perspectives correlated with molecular mechanisms. Int J Mol Sci (2022) 23(20). doi: 10.3390/ijms232012199
36. Mills SE, Cooper PH, Fechner RE. Lobular capillary hemangioma: the underlying lesion of pyogenic granuloma. A study of 73 cases from the oral and nasal mucous membranes. Am J Surg Pathol (1980) 4(5):470–9. doi: 10.1097/00000478-198010000-00007
37. Giblin AV, Clover AJ, Athanassopoulos A, Budny PG. Pyogenic granuloma - the quest for optimum treatment: audit of treatment of 408 cases. J Plastic Reconstruct Aesthetic Surg JPRAS (2007) 60(9):1030–5. doi: 10.1016/j.bjps.2006.10.018
38. Harris MN, Desai R, Chuang TY, Hood AF, Mirowski GW. Lobular capillary hemangiomas: An epidemiologic report, with emphasis on cutaneous lesions. J Am Acad Dermatol (2000) 42(6):1012–6. doi: 10.1067/mjd.2000.104520
39. Gomes SR, Shakir QJ, Thaker PV, Tavadia JK. Pyogenic granuloma of the gingiva: A misnomer? - A case report and review of literature. J Indian Soc Periodontol (2013) 17(4):514–9. doi: 10.4103/0972-124x.118327
40. Piguet V, Borradori L. Pyogenic granuloma-like lesions during capecitabine therapy. Br J Dermatol (2002) 147(6):1270–2. doi: 10.1046/j.1365-2133.2002.050006.x
41. Gupta S, Radotra BD, Kumar B. Multiple, genital lobular capillary haemangioma (pyogenic granuloma) in a young woman: a diagnostic puzzle. Sexually Transmit Infect (2000) 76(1):51–2. doi: 10.1136/sti.76.1.51
42. Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol (2007) 29(4):408–11. doi: 10.1097/DAD.0b013e31812f5342
43. Henning B, Stieger P, Kamarachev J, Dummer R, Goldinger SM. Pyogenic granuloma in patients treated with selective BRAF inhibitors: another manifestation of paradoxical pathway activation. Melanoma Res (2016) 26(3):304–7. doi: 10.1097/cmr.0000000000000248
44. Groesser L, Peterhof E, Evert M, Landthaler M, Berneburg M, Hafner C. BRAF and RAS mutations in sporadic and secondary pyogenic granuloma. J Invest Dermatol (2016) 136(2):481–6. doi: 10.1038/jid.2015.376
45. Lee J, Sinno H, Tahiri Y, Gilardino MS. Treatment options for cutaneous pyogenic granulomas: a review. J Plastic Reconstruct Aesthetic Surg JPRAS (2011) 64(9):1216–20. doi: 10.1016/j.bjps.2010.12.021
46. Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res (2019) 25(2):515–23. doi: 10.1158/1078-0432.Ccr-18-2484
47. Zhou C, Wang Y, Zhao J, Chen G, Liu Z, Gu K, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy. Clin Cancer Res (2021) 27(5):1296–304. doi: 10.1158/1078-0432.Ccr-20-3136
48. Lan C, Shen J, Wang Y, Li J, Liu Z, He M, et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): A multicenter, open-label, single-arm, phase II trial. J Clin Oncol (2020) 38(34):4095–106. doi: 10.1200/jco.20.01920
49. Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer (2022) 10(4). doi: 10.1136/jitc-2022-004656
50. Roviello G, Ravelli A, Polom K, Petrioli R, Marano L, Marrelli D, et al. Apatinib: A novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Letters (2016) 372(2):187–91. doi: 10.1016/j.canlet.2016.01.014
51. Fathi Maroufi N, Rashidi MR, Vahedian V, Akbarzadeh M, Fattahi A, Nouri M. Therapeutic potentials of Apatinib in cancer treatment: Possible mechanisms and clinical relevance. Life Sci (2020) 241:117106. doi: 10.1016/j.lfs.2019.117106
52. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9(3):305–14. doi: 10.1016/s2213-2600(20)30365-9
53. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-sq): A phase 3 trial. J Thorac Oncol (2022) 17(4):544–57. doi: 10.1016/j.jtho.2021.11.018
54. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. Jama (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836
55. Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res (2021) 27(11):3069–78. doi: 10.1158/1078-0432.Ccr-20-4691
56. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed as first-line treatment for advanced nonsquamous NSCLC: extended follow-up of cameL phase 3 trial. J Thorac Oncol (2023) 18(5):628–39. doi: 10.1016/j.jtho.2022.12.017
57. da Silva LL, Aguiar PN Jr., Park R, Edelman Saul E, Haaland B, de Lima Lopes G. Comparative efficacy and safety of programmed death-1 pathway inhibitors in advanced gastroesophageal cancers: A systematic review and network meta-analysis of phase III clinical trials. Cancers (2021) 13(11). doi: 10.3390/cancers13112614
58. Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol (2018) 19(10):1338–50. doi: 10.1016/s1470-2045(18)30495-9
59. Masterson L, Howard J, Gonzalez-Cruz J, Jackson C, Barnett C, Overton L, et al. Immune checkpoint inhibitors in advanced nasopharyngeal carcinoma: Beyond an era of chemoradiation? Int J Cancer (2020) 146(8):2305–14. doi: 10.1002/ijc.32869
60. Robati RM, Ghasemi-Pour M. Efficacy and safety of cryotherapy vs. electrosurgery in the treatment of cherry angioma. J Eur Acad Dermatol Venereol JEADV (2018) 32(9):e361–e3. doi: 10.1111/jdv.14936
61. Valdebran M, Martin B, Kelly KM. State-of-the-art lasers and light treatments for vascular lesions: from red faces to vascular malformations. Semin Cutaneous Med Surg (2017) 36(4):207–12. doi: 10.12788/j.sder.2017.044
62. Rothfleisch JE, Kosann MK, Levine VJ, Ashinoff R. Laser treatment of congenital and acquired vascular lesions. A review. Dermatol Clinics (2002) 20(1):1–18. doi: 10.1016/s0733-8635(03)00043-3
63. Stier MF, Glick SA, Hirsch RJ. Laser treatment of pediatric vascular lesions: Port wine stains and hemangiomas. J Am Acad Dermatol (2008) 58(2):261–85. doi: 10.1016/j.jaad.2007.10.492
64. Tanzi EL, Lupton JR, Alster TS. Lasers in dermatology: four decades of progress. J Am Acad Dermatol (2003) 49(1):1–31. doi: 10.1067/mjd.2003.582
65. Pancar GS, Aydin F, Senturk N, Bek Y, Canturk MT, Turanli AY. Comparison of the 532-nm KTP and 1064-nm Nd:YAG lasers for the treatment of cherry angiomas. J Cosmetic Laser Ther (2011) 13(4):138–41. doi: 10.3109/14764172.2011.594058
66. Remlova E, Dostalová T, Michalusová I, Vránová J, Navrátil L, Rosina J. Hemangioma curative effect of PDL, alexandrite, Er:YAG and CO(2) lasers. Photomed Laser Surg (2011) 29(12):815–25. doi: 10.1089/pho.2011.3058
67. Harst K, Welzel J, Schuh S. How efficient is laser therapy for telangiectasias, spider veins, and cherry angiomas?-A study using dynamic optical coherence tomography. Lasers Surg Med (2023). doi: 10.1002/lsm.23676
Keywords: reactive cutaneous capillary endothelial proliferation, camrelizumab, nasopharyngeal carcinoma, case report, literature review
Citation: Lin Y, Lin Y, Zhong X, Chen Q, Tang S and Chen J (2023) A case report and literature review on reactive cutaneous capillary endothelial proliferation induced by camrelizumab in a nasopharyngeal carcinoma patient. Front. Oncol. 13:1280208. doi: 10.3389/fonc.2023.1280208
Received: 19 August 2023; Accepted: 08 November 2023;
Published: 28 November 2023.
Edited by:
Prakash Radhakrishnan, University of Nebraska Medical Center, United StatesReviewed by:
Jian Zhu, General Hospital of Central Theater Command, ChinaCopyright © 2023 Lin, Lin, Zhong, Chen, Tang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiasheng Chen, Y2hlbmppYXNoZW5nLTM2QGZveG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.