- Leeds Cancer Centre, Leeds Teaching Hospitals National Health Service (NHS) Trust, Leeds, United Kingdom
In the last five years, the advent of combination immune checkpoint inhibitor atezolizumab and anti-angiogenic agent bevacizumab has transformed treatment of unresectable hepatocellular carcinoma. As patient outcomes improve, healthcare professionals will more frequently encounter patients with concomitant hepatocellular cancer and end stage kidney disease on haemodialysis. We present the first case in the literature of a 58-year-old male with multifocal hepatocellular carcinoma undertaking regular haemodialysis who was successfully treated with atezolizumab and bevacizumab with a partial response and stable disease for two years, who suffered grade 1 fatigue, grade 2 hypertension and eventually grade 3 wound infection leading to cessation of bevacizumab. After disease progression on atezolizumab monotherapy, all chemotherapy was stopped. We embed this case in a review of the current literature of atezolizumab and bevacizumab use in patients undertaking haemodialysis and conclude that both targeted therapies may be safely used in these patients. We recommend joint close management of these patients between oncology and nephrology teams, with initial cardiovascular risk stratification before commencing atezolizumab and bevacizumab therapy. During therapy, there should be regular monitoring of blood pressure, or proteinuria if the patient is oliguric under guidance of the dialysis team if preservation of residual renal function is required.
PART 1 – Treatment of multifocal HCC with atezolizumab and bevacizumab in patient on haemodialysis
We report the case of a 58-year-old male diagnosed with hepatocellular carcinoma (HCC) in 2015 with a background of chronic liver disease and multinodular CP-A5 liver cirrhosis secondary to alcohol excess and chronic Hepatitis B (undetectable DNA) and Hepatitis C infection, which was cured with direct-acting antivirals in 2015. He had a medical history of type 2 diabetes mellitus with retinopathy and a previous diagnosis of glomerulonephritis. He was a smoker of 10 cigarettes a day with no routine activities beyond walking short distances with a stick. He underwent a liver resection in May 2015 of the 30 mm HCC lesion in segment 6 of the liver. Histopathological analysis of the lesion revealed a moderately differentiated hepatocellular carcinoma with no microvascular invasion. Unfortunately, in 2017 the patient developed a new 21 mm lesion in segment 6 and a new 4 mm lesion in segment 4 of the liver. Microwave ablation of the segment 6 lesion took place in June 2017 and follow up computed tomography scan in July 2017 showed a satisfactory response with no active disease or new nodules. Unfortunately, in 2018, there was further disease recurrence with lesions in segment 8 (15mm and 13mm) and segment 3 (8mm). At this point blood tests revealed declining renal function with an EGFR of 42 ml/min and significant proteinuria of 3.7 g/L. Serum alpha-fetoprotein levels remained within normal limits throughout.
In January 2020, the patient was found to have end stage renal failure with refractory fluid retention and biopsy-proven diabetic nephropathy and commenced 3 times a week haemodialysis via an arteriovenous fistula. A further MRI scan of the liver in November 2020 revealed bi-lobar multifocal HCC with over 10 LR5 tumours. Marker lesions in segment 2 (26mm and 13mm) and segment 8 (19mm) were identified. In January 2021 after a year of haemodialysis, the patient commenced treatment with 1200mg atezolizumab and 1200mg bevacizumab (15mg/kg) every 3 weeks. The patient’s medication list included losartan, simvastatin, alfacalcidol, bumetanide, clopidogrel and Humulin M3 insulin. Prior to commencing treatment with atezolizumab and bevacizumab, the patient’s blood pressure was 132/76mmHg. At this point the patient was self-caring and could walk a few hundred yards with a stick, giving him a performance status of 1. Remarkably, the patient tolerated the infusions with grade 1 fatigue according to Common Terminology Criteria for Adverse Events (1). CT scan after 4 cycles showed stable disease with a trend towards response. Figure 1 shows the trend of the segment 2 lesion and the segment 8 lesion over the time on combination atezolizumab and bevacizumab therapy. The segment 8 lesion responded particularly well, demonstrating a 40% decrease in size from 20 mm to 12 mm between April 2021 and April 2022, with stable disease following this. Blood pressure measurements were made prior to each cycle and as part of haemodialysis monitoring. Figure 2 shows the blood pressure readings against time throughout the patient’s course. The patient’s antihypertensive regime was managed by both the renal and oncology teams. In 2020, bumetanide and bisoprolol was added by the nephrology team to control fluid overload and blood pressure, however these were ceased in January 2021 due to intradialytic hypotension. In February 2021, amlodipine was restarted by the dialysis team and then doubled by the oncology team in March due to a pre-cycle 4 grade 2 hypertension (1). In March 2022, the patient’s systolic blood pressure again rose above 160mmHg, however after the patient provided home blood pressure readings, no changes were made to the antihypertensive regime. Proteinuria monitoring during bevacizumab treatment was not possible as the patient was anuric. In September 2022 after 27 cycles, bevacizumab was permanently ceased due to grade 3 right toe diabetic foot infection requiring a prolonged course of antibiotics and right hallux and second toe amputation, as it was felt to be contributing to the patient’s peripheral vascular disease and hindrance of wound healing (1). Following cessation of bevacizumab unfortunately the patient experienced progression of disease. Subsequently, atezolizumab has been stopped and the focus of care has shifted to best supportive care. Figure 3 is a summary infographic of the patient’s clinical timeline.
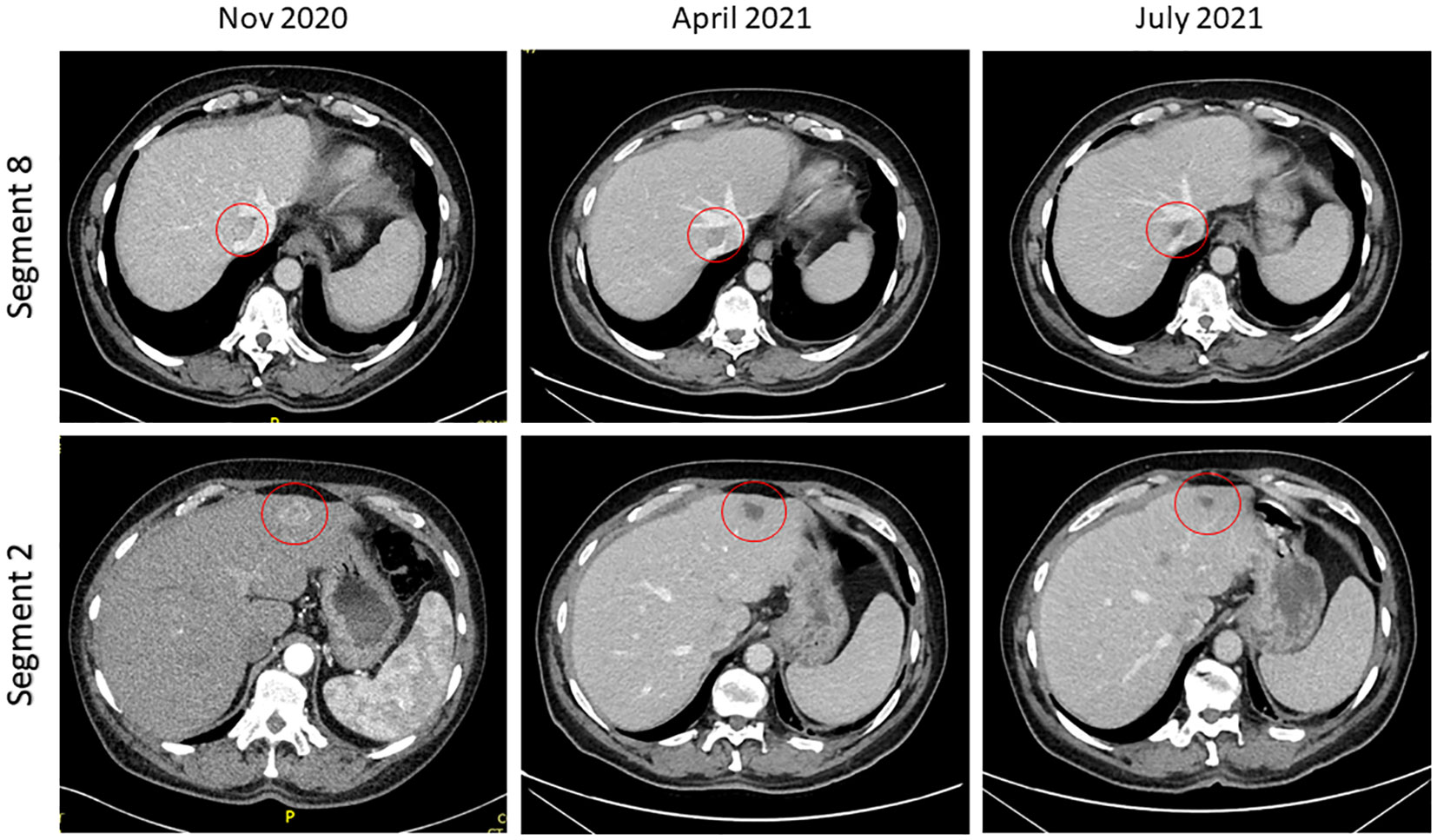
Figure 1 Computed tomography imaging demonstrating response in segment 2 and segment 8 lesions post cycle 4 and post cycle 8 of combination atezolizumab/bevacizumab.
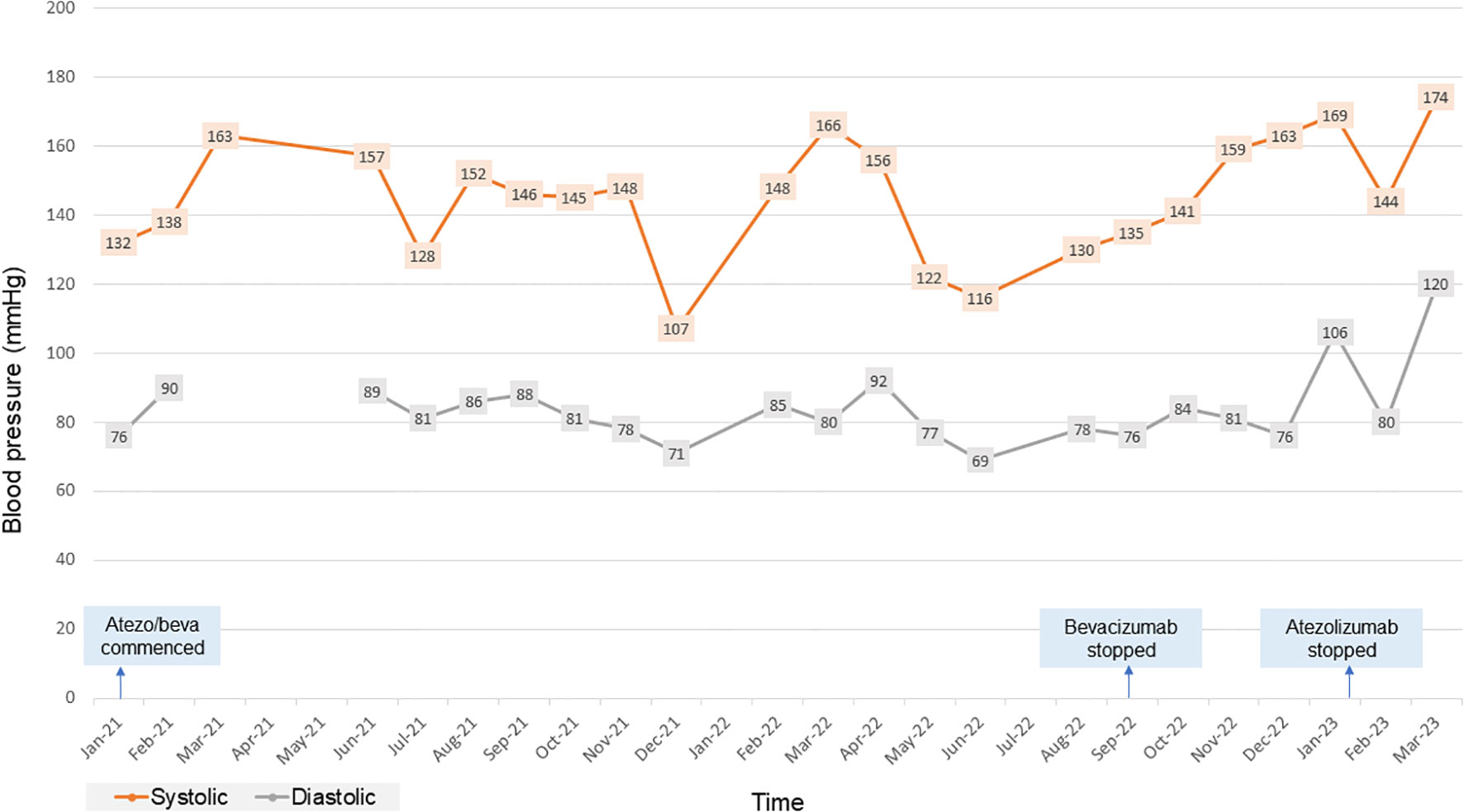
Figure 2 Chart showing the patient’s blood pressure readings over time for the duration of combination atezolizumab/bevacizumab treatment. atezo/beva, atezolizumab/bevacizumab.
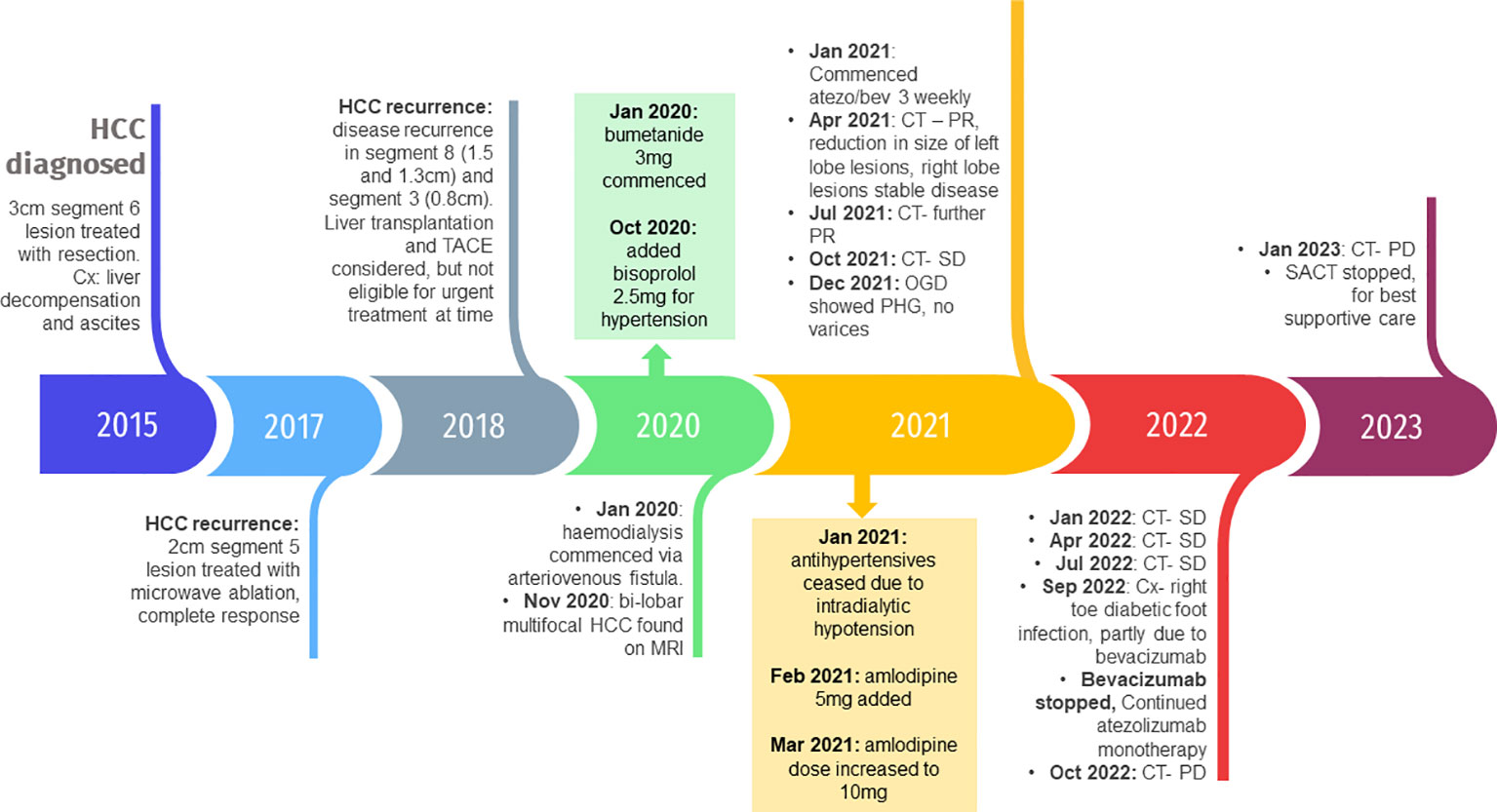
Figure 3 Timeline of the patient’s clinical course with key events, complications and medication changes. HCC, hepatocellular carcinoma; Cx, complications; TACE, transarterial chemoembolization; MRI, magnetic resonance image; CT, computed tomography; PR, partial response; SD, stable disease; OGD, oesophagogastroduodenoscopy; PHG, portal hypertensive gastropathy; PD, disease progression; SACT, systemic anticancer treatment.
Part 2
Introduction
HCC is the third leading cause of cancer-related deaths worldwide and is an increasing global health challenge as the number of new cases of primary liver cancer is predicted to increase by over 55% by 2040 (2). The systemic treatment of unresectable HCC has transformed over the last 5 years. The pivotal phase 3 trial IMBrave150, published in 2020, showed that the programmed cell death ligand 1 (PD-L1) inhibitor atezolizumab plus vascular endothelial growth factor (VEGF) inhibitor bevacizumab resulted in superior progression-free and overall survival compared to the multikinase inhibitor sorafenib (3).
UK National Institute of Clinical Excellence (NICE) and National Comprehensive Cancer Network (NCCN) guidelines have been updated to reflect this, now recommending atezolizumab plus bevacizumab to treat unresectable HCC in adults who have not had previous systemic treatment in patients with Child- Pugh (CP) grade A liver impairment and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 (4, 5). There is a complex relationship between HCC and end stage kidney disease (ESKD). ESKD is a risk factor for HCC development and vice versa, HCC can cause renal dysfunction, for example through hepatorenal syndrome or direct tumour invasion. Furthermore, there are common risk factors for HCC and ESKD development, and ESKD with dialysis negatively affects HCC prognosis. As patients with advanced HCC experience improved survival with new systemic therapies, clinicians will more frequently encounter HCC patients with co-existing ESKD receiving haemodialysis (6). It is therefore paramount to understand the impact of renal function and dialysis on HCC patients treated with new systemic therapies. We present the first case, to our knowledge, of a 58-year-old male with multifocal HCC and end stage renal failure receiving haemodialysis, who is being successfully treated with combination atezolizumab and bevacizumab. We embed the case within a brief literature review and discuss further important clinical considerations when treating patients with HCC and ESKD on haemodialysis (7).
Discussion
Atezolizumab and bevacizumab are humanised immunoglobulin antibodies of the IgG1 isotype, with a large molecular size (around 145 kDa). Pharmacokinetically, they have a small volume of distribution, mostly in the vascular compartment. The main route of elimination is intracellular catabolism following receptor-mediated endocytosis and hepatic or renal elimination is negligible. They are too large to be secreted through the glomerular filtration barrier, except possibly in high-grade non-selective proteinuria. Furthermore, the molecular size of these immunoglobulins is larger than dialysis pores, meaning they will not be filtered out of the bloodstream during haemodialysis (8–11). As a result, there is no reason to alter the dosage of either drug or no reason to believe there would be an increase in adverse events due to accumulation. As neither atezolizumab or bevacizumab dialyse, both drugs can be administered before or after haemodialysis (12–14).
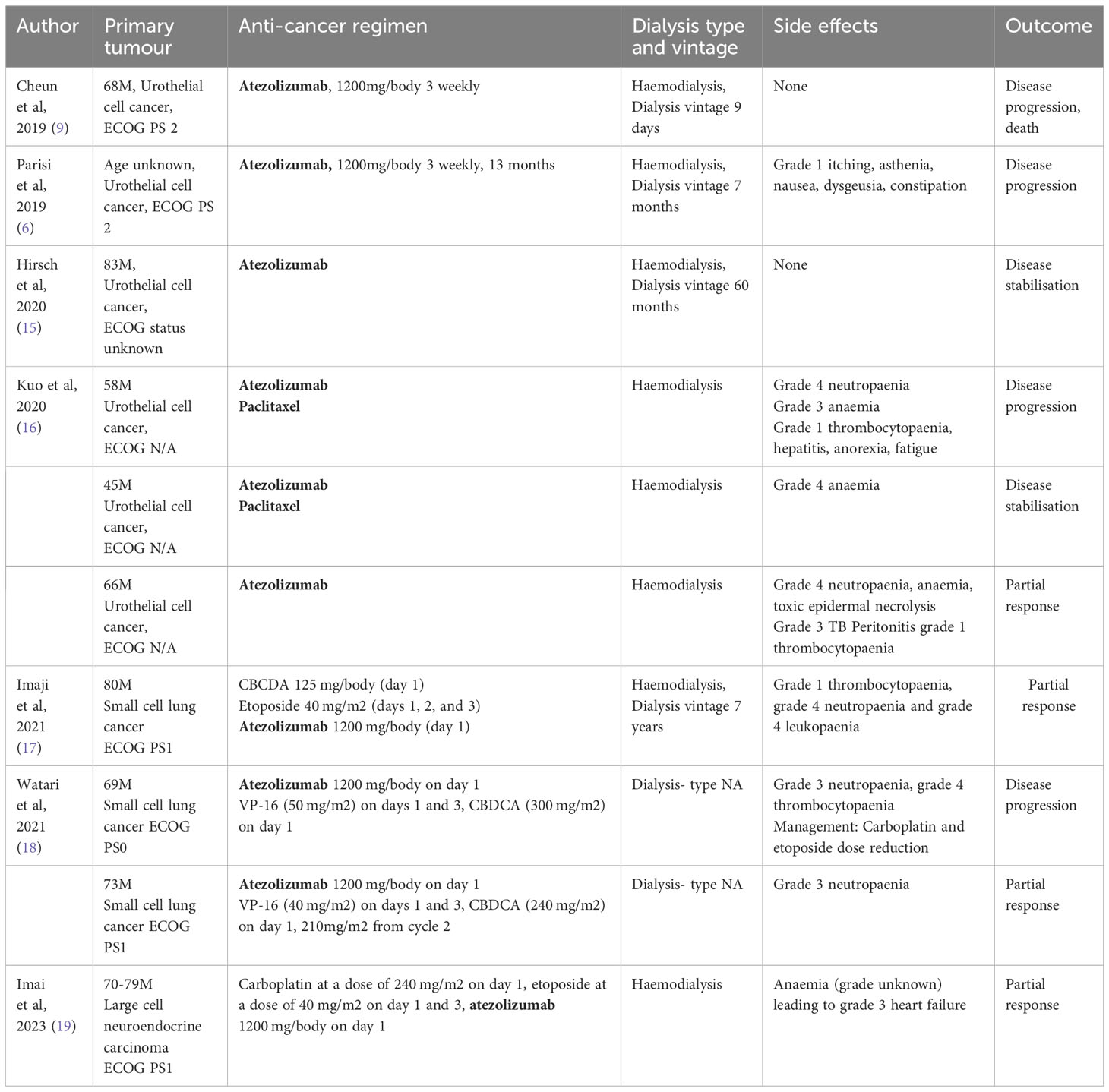
Table 1 shows a summary of current literature of atezolizumab use in patients with ESKD. Atezolizumab at full dose has been used successfully in multiple case reports of patients with dialysis. Data from a large real-world database of patients receiving PD-1 inhibitors for lung, renal, bladder, head and neck or melanoma, showed no increased rate of immune-related adverse events in patients with ESKD versus those without (20). Whilst immune checkpoint inhibitors can be given in patients with ESKD receiving dialysis, important consideration must be given to patients with previous kidney transplants as immune checkpoint inhibition can precipitate transplant rejection and allograft failure (15). Furthermore, in all patients there is a need to monitor renal function as immune checkpoint inhibition can cause a decline in renal function, a phenomenon referred to as immune checkpoint inhibitor associated acute kidney injury (ICP-I AKI). A real-world study of 1843 patients estimated incidence of this to be 3% (21).
Table 2 shows a summary of current literature of bevacizumab use in patients with ESKD. Renal toxicity, particularly proteinuria and hypertension are seen in use of these agents. VEGF and its receptors are expressed in abundance in podocytes, glomerular and tubular cells in the kidney and are crucial in the survival of mesangial and endothelial cells and therefore to the integrity of endothelial fenestrations in the glomerular filtration barrier. Proteinuria is a known dose-dependent adverse effect of bevacizumab and is due to VEGF-inhibition induced structural changes in glomerular cells; dysregulation of the kidney repair process and increased glomerulosclerosis; and reduction in endothelial fenestrations and loss of selective glomerular permeability (29–31). As therapeutic monoclonal antibodies are large molecules; they are not typically cleared by renal or dialysis filtration. However, it is possible that high-grade non-selective proteinuria can result in renal clearance of monoclonal antibodies (32). Current guidelines recommend suspending bevacizumab administration if 24-hour urine-protein collection is >2g and treatment discontinuation in cases of nephrotic-range proteinuria (>3.5g), which reduces the risk of any possible renal clearance and altered pharmacokinetics of either atezolizumab or bevacizumab (33). In our patient, proteinuria was not routinely monitored as he was anuric. In patients who are oliguric while on haemodialysis, whether strict proteinuria monitoring is needed is unclear. Proteinuria is a marker of ESKD progression and an independent risk factor for cardiovascular mortality (34, 35). Higher degrees of proteinuria in chronic haemodialysis patients are associated with inflammatory and cardiovascular markers of disease (36). Preservation of residual renal function in a longitudinal study of over 6000 patients was associated with better patient survival (37). Therefore, in oliguric patients undergoing haemodialysis, we recommend proteinuria monitoring in partnership with the nephrology team, to minimise the impact that bevacizumab-induced proteinuria has on ESKD progression and to help preserve residual renal function.
Bevacizumab induced hypertension of all grades has been observed in up to 36% of patients treated with bevacizumab. It is frequently observed in the first cycle of therapy and, like proteinuria, appears to be dose dependent (38, 39). Current recommendation is that bevacizumab can be started if blood pressure is <160/100 mmHg. If during therapy, blood pressure rises by >20 mmHg systolic or 10 mmHg diastolic or rises to >160/100 mmHg, it is recommended to omit a dose and reassess. If blood pressure remains above >150/95 mmHg with ambulatory or home blood pressure monitoring, antihypertensive treatment should commence (40). Hypertension is caused by VEGF-inhibition induced apoptosis and altered rarefaction of vascular endothelial cells and reduced production of vasodilators such as nitric oxide and prostacyclin (29–31). VEGF is also expressed in renal endothelial cells and podocytes, where it maintains normal glomerular filtration rate. VEGF blockade in the kidneys leads to renal injury, activation of the renin-angiotensin system, inadequate renal sodium excretion and volume overload (41, 42). Pre-existing hypertension, increased age, BMI, diabetes and dyslipidaemia are risk factors for developing treatment related hypertension (38, 43). The mechanisms of hypertension in dialysis patients are complex but include: an increase in extracellular body water which can be corrected by increasing ultrafiltration or more frequent dialysis sessions, as well as activation of the renin-angiotensin-aldosterone system; sympathetic over-activity; increased arterial stiffness related to altered calcium and phosphate metabolism; and endothelial dysfuction (44). Hypertension can affect up to 80-90% of dialysis patients (45). Heerspink et al. (2009) showed that blood pressure reduction in dialysis patients with antihypertensive treatment led to a 29% decreased risk of cardiovascular events (relative risk [RR], 0.71, 95% confidence interval 0.55–0.92), and a 20% decreased risk for all-cause mortality (RR, 0.80, 95% confidence interval 0.66–0.96) (46). Interdialytic blood pressure monitoring is gold standard for diagnosing hypertension in haemodialysis patients which can be obtained through ambulatory or home blood pressure monitoring. Strategies for lowering blood pressure include adjusting target dialytic weight and dietary advice regarding salt and fluid restriction in high volume states. In terms of pharmacological treatment, beta-blockers are effective in dialysis patients with left ventricular hypertrophy due to sympathetic overactivity. Dihydropyridine calcium-channel blockers are also effective in lowering blood pressure in high volume states and have great potency in reducing arterial smooth muscle cell contractility in the blood vessels, which is one of the mechanisms associated with VEGF induced hypertension. ACE-inhibitors or angiotensin receptor blockers are also effective; however no study has demonstrated superiority of these agents as anti-hypertensive agents in dialysis patients (47, 48). In a case series by Shetty et al, 2 of 3 of the patients taking 10mg/kg bevacizumab experienced grade 1 and 2 exacerbation of hypertension (25). In our case, the patient was at high risk of experiencing treatment related hypertension due to high BMI and diabetes. He experienced stage 2 hypertension (blood pressure >160/100mmHg), however this was not clearly due to bevacizumab therapy. As seen by Figure 2, blood pressure was highly variable and may have been due to variations in total body fluid with dialysis. Nevertheless, blood pressure was jointly managed between oncology and nephrology and when blood pressure rose above 160/100mmHg, home blood pressure monitoring was encouraged, and consequently there was titration of the dose of amlodipine, dietary advice was given and the dialysis regime was altered by the nephrology team.
Unfortunately, the patient in our case progressed while on single agent atezolizumab after bevacizumab was ceased. Currently there are no approved second-line treatments after failure of atezolizumab and bevacizumab. Sorafenib or lenvatinib may be used, but should be used with caution in dialysis patients due to possibility of greater incidence of adverse effects (49). A meta-analysis of possible second line treatments in 2022 analysed 14 phase two or three trials and showed that multikinase inhibitors regorafenib (hazard ratio 0.63, 95% confidence interval 0.50-0.79) and cabozatanib (hazard ratio 0.76, 95% confidence interval 0.63-0.92) significantly prolonged overall survival compared to placebo, after failure of sorafenib therapy (50). It is clear that head-to-head trials of multikinase inhibitors in the second line setting after failure of atezolizumab/bevacizumab therapy are urgently needed.
Conclusion
We present the first case in the literature of atezolizumab and bevacizumab used together in a patient with multifocal hepatocellular carcinoma and ESKD on haemodialysis. The case report uses doses of both agents comparative to or greater than other cases in the current literature. The patient in our case experienced grade 1 fatigue and grade 2 hypertension, neither of which were dose-limiting toxicities. Unfortunately, bevacizumab was ceased due to a grade 3 wound infection. When bevacizumab was ceased, there was disease progression, clearly demonstrating the synergistic efficacy of combination PD-L1 and VEGF inhibition (51). Haemodialysis should not be a contraindication to commencing these therapies as the literature supports normal pharmacokinetics in haemodialysis patients with both agents. Whilst both targeted therapies are associated with direct renal toxicities, they are less relevant in patients on haemodialysis. Of more relevance are toxicities that could be magnified by use of these agents in ESKD, such as bevacizumab-associated hypertension. We would recommend close joint management of these patients between oncology and nephrology teams and risk stratification of patients for the development of toxicities before commencement. We recommend monitoring of proteinuria if the patient is oliguric and monitoring of hypertension with ambulatory or home blood pressure monitoring, so that they can be appropriately managed to avoid dose-limiting toxicity or periods of cessation that may impact on cancer outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SA: Data curation, Software, Writing – original draft. AS: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Competing interests Dr AS has received consultancy fees from Roche Funding Dr SA is a National institute of Health Research (NIHR) Academic Clinical Fellow Dr AS is a Cancer Research UK (CRUK) Clinician Scientist Fellow.
Acknowledgments
We thank the patient for their consent and participation in this case report. We thank Leeds Teaching Hospitals NHS Trust.
Conflict of interest
AS has received consultancy fees from Roche.
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. (2017) (United States: Department of health and human services).
2. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol (2022) 77(6):1598–606. doi: 10.1016/j.jhep.2022.08.021
3. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
4. NICE. 1 Recommendations | Atezolizumab with bevacizumab for treating advanced or unresectable hepatocellular carcinoma | Guidance | NICE. (2020) UK: NICE. Available at: https://www.nice.org.uk/guidance/ta666/chapter/1-Recommendations. cited 2022 Aug 17.
5. NCCN. National Comprehensive Cancer Network Clinical Practice Guidelines: Hepatocellular Carcinoma Version 2.2023 (2023). Available at: https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf.
6. Yeh H, Chiang CC, Yen TH. Hepatocellular carcinoma in patients with renal dysfunction: Pathophysiology, prognosis, and treatment challenges. World J Gastroenterol (2021) 27(26):4104–42. doi: 10.3748/wjg.v27.i26.4104
7. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
8. Zweigart C, Boschetti-de-Fierro A, Hulko M, Nilsson LG, Beck W, Storr M, et al. Medium cut-off membranes - closer to the natural kidney removal function. Int J Artif Organs (2017) 40(7):328–34. doi: 10.5301/ijao.5000603
9. Cheun H, Kim M, Lee H, Oh KH, Keam B. Safety and efficacy of immune checkpoint inhibitors for end-stage renal disease patients undergoing dialysis: a retrospective case series and literature review. Invest New Drugs (2019) 37(3):579–83. doi: 10.1007/s10637-018-0673-y
10. National Cancer Institute. Atezolizumab (MPDL3280A, RO5541267, TECENTRIQ®) | Available Agents | NCI Formulary. Available at: https://nciformulary.cancer.gov/available_agents/Atezolizumab.htm.
11. Kamath AV. Translational pharmacokinetics and pharmacodynamics of monoclonal antibodies. Drug Discovery Today: Technologies (2016) 21–22:75–83. doi: 10.1016/j.ddtec.2016.09.004
12. Garnier-Viougeat N, Rixe O, Paintaud G, Ternant D, Degenne D, Mouawad R, et al. Pharmacokinetics of bevacizumab in haemodialysis. Nephrol Dialysis Transplantation. (2007) 22(3):975. doi: 10.1093/ndt/gfl664
13. Izzedine H, Etienne-Grimaldi MC, Renée N, Vignot S, Milano G. Pharmacokinetics of Sunitinib in hemodialysis. Ann Oncol (2009) 20(1):190–2. doi: 10.1093/annonc/mdn626
14. Centanni M, Moes DJAR, Trocóniz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet (2019) 58(7):835–57. doi: 10.1007/s40262-019-00748-2
15. Hirsch JS, Wanchoo R, Ng JH, Khanin Y, Jhaveri KD. Use of immune checkpoint inhibitors in end stage kidney disease patients, single center experience and review of the literature. Kidney360 (2020) 1(5):399–402. doi: 10.34067/KID.0000422020
16. Kuo MC, Su PJ, Huang CC, Luo HL, Chiu TJ, Li SH, et al. Safety and efficacy of immune checkpoint inhibitors for patients with metastatic urothelial carcinoma and end-stage renal disease: experiences from real-world practice. Front Oncol (2020) 10:584834. doi: 10.3389/fonc.2020.584834
17. Imaji M, Fujimoto D, Kato M, Tanaka M, Furuta K, Yamamoto N. Chemotherapy plus atezolizumab for a patient with small cell lung cancer undergoing haemodialysis: a case report and review of literature. Respirol Case Rep (2021) 9(5):e00741. doi: 10.1002/rcr2.741
18. Watari N, Yamaguchi K, Masuda T, Ito N, Sakamoto S, Horimasu Y, et al. Tolerability and efficacy of IMpower133 regimen modified for dialysis patients with extensive-stage small cell lung cancer: Two case reports. Thorac Cancer (2021) 12(21):2956–60. doi: 10.1111/1759-7714.14166
19. Imai R, Kitamura A. Successful treatment with atezolizumab in a haemodialysis patient with large cell neuroendocrine carcinoma. Respirol Case Rep (2023) 11(8):e01193. doi: 10.1002/rcr2.1193
20. Wang J, Dasari S, Elantably D, Alkrekshi A, Kim YD. Use of PD-1 inhibitors in patients with end-stage renal disease: safety and clinical outcomes from real-world data. Acta Oncologica (2022) 61(9):1157–61. doi: 10.1080/0284186X.2022.2127121
21. Seethapathy H, Zhao S, Chute DF, Zubiri L, Oppong Y, Strohbehn I, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. CJASN (2019) 14(12):1692–700. doi: 10.2215/CJN.00990119
22. Inauen R, Cathomas R, Boehm T, Koeberle D, Pestalozzi BC, Gillessen S, et al. Feasibility of using cetuximab and bevacizumab in a patient with colorectal cancer and terminal renal failure. OCL (2007) 72(3–4):209–10. doi: 10.1159/000112828
23. Horimatsu T, Miyamoto S, Morita S, Mashimo Y, Ezoe Y, Muto M, et al. Pharmacokinetics of oxaliplatin in a hemodialytic patient treated with modified FOLFOX-6 plus bevacizumab therapy. Cancer Chemother Pharmacol (2011) 68(1):263–6. doi: 10.1007/s00280-011-1633-9
24. Syrios J, Kechagias G, Tsavaris N. Treatment of patients with metastatic renal cell carcinoma undergoing hemodialysis: case report of two patients and short literature review. BMC Nephrol (2013) 14(1):84. doi: 10.1186/1471-2369-14-84
25. Shetty AV, Matrana MR, Atkinson BJ, Flaherty AL, Jonasch E, Tannir NM. Outcomes of patients with metastatic renal cell carcinoma and end-stage renal disease receiving dialysis and targeted therapies: a single institution experience. Clin Genitourin Cancer (2014) 12(5):348–53. doi: 10.1016/j.clgc.2014.01.004
26. van Berlo – van de Laar IRF, Brummelhuis WJ, Imholz ALT, Schellens JH, Huitema ADR, Jansman FGA. Dosing oxaliplatin in a haemodialysis patient with metastatic rectum cancer monitored by free platinum concentrations. J Clin Pharm Ther (2018) 43(4):574–7. doi: 10.1111/jcpt.12661
27. Funasaka C, Kanemasa Y, Shimoyama T, Ohta A, Omuro Y. Modified FOLFOX-6 plus bevacizumab chemotherapy for metastatic colorectal cancer in patients receiving hemodialysis: A report of three cases and review of the literature. Case Rep Oncol (2019) 12(2):657–65. doi: 10.1159/000502512
28. Tanaka T, Suzuki H, Ushijima T, Nagasu S, Akagi Y, Kawaguchi T, et al. Case report: Changes in serum bevacizumab concentration in a hemodialysis patient with unresectable colorectal cancer treated with FOLFIRI plus bevacizumab. Front Oncol (2022) 12:947013. doi: 10.3389/fonc.2022.947013
29. Cosmai L, Gallieni M, Liguigli W, Porta C. Renal toxicity of anticancer agents targeting vascular endothelial growth factor (VEGF) and its receptors (VEGFRs). J Nephrol (2017) 30(2):171–80. doi: 10.1007/s40620-016-0311-8
30. Porta C, Cosmai L, Gallieni M, Pedrazzoli P, Malberti F. Renal effects of targeted anticancer therapies. Nat Rev Nephrol (2015) 11(6):354–70. doi: 10.1038/nrneph.2015.15
31. Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med (2009) 122(4):322–8. doi: 10.1016/j.amjmed.2008.11.025
32. Fogueri U, Cheungapasitporn W, Bourne D, Fervenza FC, Joy MS. Rituximab exhibits altered pharmacokinetics in patients with membranous nephropathy. Ann Pharmacother (2019) 53(4):357–63. doi: 10.1177/1060028018803587
33. Brandes AA, Bartolotti M, Tosoni A, Poggi R, Franceschi E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist (2015) 20(2):166–75. doi: 10.1634/theoncologist.2014-0330
34. Hillege HL, Fidler V, Diercks GFH, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation (2002) 106(14):1777–82. doi: 10.1161/01.CIR.0000031732.78052.81
35. Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis (2013) 7:13–24. doi: 10.2147/IJNRD.S40522
36. Trimarchi H, Muryan A, Dicugno M, Young P, Forrester M, Lombi F, et al. Proteinuria: an ignored marker of inflammation and cardiovascular disease in chronic hemodialysis. Int J Nephrol Renovasc Dis (2012) 5:1–7. doi: 10.2147/IJNRD.S27675
37. Obi Y, Rhee CM, Mathew AT, Shah G, Streja E, Brunelli SM, et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol (2016) 27(12):3758–68. doi: 10.1681/ASN.2015101142
38. Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, et al. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst (2010) 102(9):596–604. doi: 10.1093/jnci/djq091
39. Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis (2007) 49(2):186–93. doi: 10.1053/j.ajkd.2006.11.039
40. Plummer C, Michael A, Shaikh G, Stewart M, Buckley L, Miles T, et al. Expert recommendations on the management of hypertension in patients with ovarian and cervical cancer receiving bevacizumab in the UK. Br J Cancer (2019) 121(2):109–16. doi: 10.1038/s41416-019-0481-y
41. Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature (2005) 438(7070):937–45. doi: 10.1038/nature04479
42. Li M, Kroetz DL. Bevacizumab-induced hypertension: clinical presentation and molecular understanding. Pharmacol Ther (2018) 182:152–60. doi: 10.1016/j.pharmthera.2017.08.012
43. Hamnvik OPR, Choueiri TK, Turchin A, McKay RR, Goyal L, Davis M, et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer (2015) 121(2):311–9. doi: 10.1002/cncr.28972
44. Bucharles SGE, Wallbach KKS, de Moraes TP, Pecoits-Filho R. Hypertension in patients on dialysis: diagnosis, mechanisms, and management. J Bras Nefrol (2019) 41(3):400–11. doi: 10.1590/2175-8239-jbn-2018-0155
45. Sarafidis PA, Li S, Chen SC, Collins AJ, Brown WW, Klag MJ, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med (2008) 121(4):332–40. doi: 10.1016/j.amjmed.2007.11.025
46. Heerspink HJL, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet (2009) 373(9668):1009–15. doi: 10.1016/S0140-6736(09)60212-9
47. Kim IS, Kim S, Yoo TH, Kim JK. Diagnosis and treatment of hypertension in dialysis patients: a systematic review. Clin Hypertens (2023) 29(1):24. doi: 10.1186/s40885-022-00226-1
48. de Jesus-Gonzalez N, Robinson E, Moslehi J, Humphreys BD. Management of antiangiogenic therapy-induced hypertension. Hypertension (2012) 60(3):607–15. doi: 10.1161/HYPERTENSIONAHA.112.196774
49. Leonetti A, Bersanelli M, Castagneto B, Masini C, Di Meglio G, Pellegrino B, et al. Outcome and safety of sorafenib in metastatic renal cell carcinoma dialysis patients: A systematic review. Clin Genitourinary Cancer (2016) 14(4):277–83. doi: 10.1016/j.clgc.2016.01.010
50. Solimando AG, Susca N, Argentiero A, Brunetti O, Leone P, De Re V, et al. Second-line treatments for advanced hepatocellular carcinoma: A systematic review and Bayesian network meta-analysis. Clin Exp Med (2022) 22(1):65–74. doi: 10.1007/s10238-021-00727-7
Keywords: hepatocellar carcinoma (HCC), immune check inhibitor (ICI), anti-angiogeneic therapy, atezolizumab, bevacizumab, haemodialysis (HD), end stage kidney disease (ESKD), patient outcome
Citation: Abraham S and Samson A (2024) Case report: Successful treatment of a patient undergoing haemodialysis with multifocal hepatocellular carcinoma using atezolizumab and bevacizumab. Front. Oncol. 13:1279501. doi: 10.3389/fonc.2023.1279501
Received: 18 August 2023; Accepted: 09 November 2023;
Published: 04 January 2024.
Edited by:
Maen Abdelrahim, Houston Methodist Research Institute, United StatesReviewed by:
Antonio Giovanni Solimando, University of Bari Aldo Moro, ItalyAlice Bexon, Independent Researcher, Montclair, United States
Copyright © 2024 Abraham and Samson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adel Samson, YS5zYW1zb25AbGVlZHMuYWMudWs=
 Shalin Abraham
Shalin Abraham Adel Samson
Adel Samson