
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 October 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1276976
This article is part of the Research Topic Radioresistance in Breast Cancer View all 9 articles
 Xiujuan Gui
Xiujuan Gui Jianli Zhao
Jianli Zhao Linxiaoxiao Ding
Linxiaoxiao Ding Jie Chai
Jie Chai Hongna Lai
Hongna Lai Yangyang Cai
Yangyang Cai Simin Luo
Simin Luo Yinduo Zeng
Yinduo Zeng Wenjing Wu
Wenjing Wu Haizhu Chen
Haizhu Chen Herui Yao*
Herui Yao* Ying Wang*
Ying Wang*Aim: The aim of this study was to identify potential safety concerns associated with Sacituzumab Govitecan (SG), an antibody-drug conjugate targeting trophoblastic cell-surface antigen-2, by analyzing real-world safety data from the largest publicly available worldwide pharmacovigilance database.
Methods: All data obtained from the FDA Adverse Event Reporting System (FAERS) database from the second quarter of 2020 to the fourth quarter of 2022 underwent disproportionality analysis and Bayesian analysis to detect and assess the adverse event signals of SG, considering statistical significance when the lower limit of the 95% CI >1, based on at least 3 reports.
Results: Total of 1072 cases were included. The main safety signals were blood and lymphatic system disorders [ROR(95CI)=7.23 (6.43-8.14)], gastrointestinal disorders [ROR(95CI)=2.01 (1.81-2.22)], and relative infection adverse events, such as neutropenic sepsis [ROR(95CI)=46.02 (27.15-77.99)] and neutropenic colitis [ROR(95CI)=188.02 (120.09-294.37)]. We also noted unexpected serious safety signals, including large intestine perforation [ROR(95CI)=10.77 (3.47-33.45)] and hepatic failure [ROR(95CI)=3.87 (1.45-10.31)], as well as a high signal for pneumonitis [ROR(95CI)=9.93 (5.75-17.12)]. Additionally, age sub-group analysis revealed that geriatric patients (>65 years old) were at an increased risk of neutropenic colitis [ROR(95CI)=282.05 (116.36-683.66)], neutropenic sepsis [ROR(95CI)=101.11 (41.83-244.43)], acute kidney injury [ROR(95CI)=3.29 (1.36-7.94)], and atrial fibrillation [ROR(95CI)=6.91 (2.86-16.69)].
Conclusion: This study provides crucial real-world safety data on SG, complementing existing clinical trial information. Practitioners should identify contributing factors, employ monitoring and intervention strategies, and focus on adverse events like neutropenic sepsis, large intestine perforation, and hepatic failure. Further prospective studies are needed to address these safety concerns for a comprehensive understanding and effective management of associated risks.
The rapid development of antibody-drug conjugates (ADCs) has been accompanied by advancements in cancer biomarkers, individualized therapy, and pharmaceutical technology in recent years (1, 2). ADCs have demonstrated remarkable selectivity and efficacy, thereby revolutionizing cancer therapy pattern (2). Sacituzumab govitecan (SG) is a novel ADC, which exhibits promising results for the treatment of solid tumors, including breast cancer and urothelial cancer (3).
SG is a novel ADC that is a trophoblastic cell surface antigen-2 (TROP-2) directed antibody conjugated to the active metabolite of irinotecan, SN-38 (govitecan) (4). TROP-2 is highly expression in breast cancer, particularly in triple negative breast cancer. SG binds TROP-2 and delivers SN-38 to the tumor upon cleavage of the CL2A linker (5). SN-38 then interacts with topoisomerase I, preventing the re-ligation of topoisomerase I-induced single-strand breaks, ultimately leading to apoptosis and cell death (5). The ASCENT trial demonstrated the impressive efficacy of SG in patients with metastatic triple-negative breast cancer, with significantly longer progression-free survival and overall survival compared to single-agent chemotherapy (6).
Despite SG is a novel promising ADC, the potential safety risks have not yet been fully studied. To date, evidence on the safety profile of SG is primarily derived from its development programs and approval trials. The most frequently reported adverse events in these trials include neutropenia, nausea, diarrhea, fatigue, anemia, vomiting, rash, and constipation (6, 7). However, it is important to note that randomized controlled trials, despite their rigorous design, may not always capture unexpected or rare adverse events. This can be due to limitations such as short follow-up periods, variations in dosages, and differences between the enrolled study populations and the real-world population (8–10). Therefore, it becomes crucial to identify and assess potential safety concerns associated with SG in real-world settings. This will help clinicians gain a comprehensive understanding of the side effects of SG and subsequently improve the quality of life for patients.
Spontaneous reporting systems are critical in collecting and documenting adverse events related to pharmacotherapy in real-world settings and serve as a valuable resource for early identification of potential safety issues (11, 12). Pharmacovigilance and post-marketing analysis of spontaneous reporting databases have proven to be effective methods for detecting unexpected or rare adverse events. These databases serve as valuable sources of data that aid in the identification of safety concerns associated with pharmacotherapy, and they play a crucial role in improving drug safety in real-world settings. The FDA Adverse Event Reporting System (FAERS) is the largest publicly available pharmacovigilance database globally, making it an invaluable resource for accessing credible information on adverse events.
Therefore, the aim of this study was to analyze the real-world safety data of SG using the FAERS as an important complement of clinical trials.
The study collected and analyzed adverse events reports from the publicly available version of the FAERS database, covering the period from the second quarter of 2020 to the fourth quarter of 2022. Adverse events were categorized according to the Preferred Terms and classified into System Organ Classes (SOC) using the Medical Dictionary for Regulatory Activities version 25.0. The FAERS data files consist of seven databases, including demographic and administrative information, information on adverse drug reactions, drug information, drug therapy start and end dates, report source information, and indications for use/diagnosis.
Initially, the raw data underwent a deduplication process. Specifically, the latest FDA_DT (reported date) was selected among cases with the same CASEID (case ID), while the higher PRIMARYID (reported record ID) was chosen when both the CASEID and FDA_DT were identical. Next, we selected only adverse events where SG was assigned as either primary suspected. Lastly, to eliminate any potential interference from product-related issues, we excluded events related to product temperature excursion, device complaints, product container quality, product reconstitution quality, device dislocation, device occlusion, and device infusion issues from further analysis.
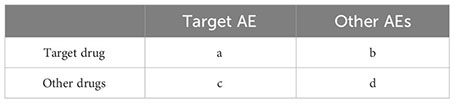
As shown in Figure 1, we adopted the case/non-case approach, which is akin to the case-control study design (13, 14). Cases were defined as adverse event reports where “SACITUZUMAB GOVITECAN,” “TRODELVY,” or “IMMU-132” was recorded, whereas non-cases represented adverse event reports for all other drugs included in the FAERS database (10, 14). Disproportionality analysis represents a key tool of analytical methods in pharmacovigilance research, enabling the identification of drug-associated adverse events as signals by comparing the proportion of adverse events occurring between a specific drug and all other drugs (15). Within this cohort, we tested for disproportionality: if the proportion of adverse events of interest was found to be higher in patients exposed to SG (cases) than those not exposed (non-cases), an association could be hypothesized between the drug and the adverse event and considered a disproportionality signal (16). We performed reporting odds ratio (ROR) (17), and Bayesian confidence propagation neural network (BCPNN) analyses using information component (IC) as the signal index (18) to investigate the association between SG and adverse events. Traditional thresholds for significance were used (lower limit of the 95% CI >1 and >0 for ROR and IC, respectively), and a safety signal was considered if both approaches were statistically significant. Meanwhile, we compared the medicine specifications of SG in order to identify any unexpected adverse events. The selection of severe adverse events was based on the Designated Medical Event list provided by the European Medicines Agency.
The onset time was defined as the interval between EVENT_DT (date of adverse event occurrence) and START_DT (start date for SG use) (19). Moreover, input errors reports or inaccurate date entries were excluded. SAS v.9.4 and SPSS v.21 were used to data process and statistical analyses.
A total 13,811,274 reports of adverse events were registered in FAERS, containing 2,917 SG-related adverse events in 1072 cases. Table 1 provides a breakdown of the cases included in this study, with 905 female cases (84.4%), 78 male cases (7.3%), and 89 cases (8.3%) where gender information was not reported. The United States, France, and Canada were the top three countries reporting adverse events. Among the cases, 221 had a weight of less than 80 kg, 74 had a weight between 80-100 kg, 35 had a weight exceeding 100 kg, and 742 were of unknown weight. Additionally, 136 cases were older than 65 years, 398 cases were between 18-64 years old, and 538 cases did not report age information. 85.8% of the reporters were medical professionals. Lastly, the number of reported cases for the years 2020, 2021, and 2022 were 81, 384, and 607, respectively.
We conducted a disproportionality analysis of the SOC to assess signal strengths and reports of SG. The results are presented in Figure 2. Our findings indicate that several SOCs exhibited strong signals, including hepatobiliary disorders [ROR(95CI)=1.78 (1.31-2.43)], metabolism and nutrition disorders [ROR(95CI)=1.28 (1.01-1.62)], investigations [ROR(95CI)=1.35 (1.18-1.54)], blood and lymphatic system disorders [ROR(95CI)=7.23 (6.43-8.14)], gastrointestinal disorders [ROR(95CI)=2.01 (1.81-2.22)], and general disorders and administration site conditions [ROR(95CI)=1.53 (1.41-1.67)]. Furthermore, the ROR (95CI) of infections and infestations was 1.13 (0.98-1.32); however, these findings did not reach statistical significance.
Table 2 presents a total of 75 adverse events with significant safety signals. Among these, febrile neutropenia [ROR(95CI)=20.23 (15.75-25.98)], neutropenic sepsis [ROR(95CI)=46.02 (27.15-77.99)], and neutropenic colitis [ROR(95CI)=188.02 (120.09-294.37)] were identified as three serious adverse events with high safety signals, excluding death and disease progression. Furthermore, we classified all significant adverse events in Table 2 according to the SOC, which is presented in Figures 3–5, 6. Specifically, for blood and lymphatic system-related adverse events (Figure 3), neutrophil count abnormal [ROR(95CI)= 73.20 (40.31-132.93)], febrile bone marrow aplasia [ROR(95CI)=39.42 (17.64-88.10)], and febrile neutropenia [ROR(95CI)=20.23 (15.75-25.98)] exhibited the highest ROR values. In terms of gastrointestinal and liver disorders (Figure 4), neutropenic colitis [ROR(95CI)=188.02 (120.09-294.37)], enteritis [ROR(95CI)=30.72 (15.93-59.23)], and gastrointestinal toxicity [ROR(95CI)=17.89 (6.70-47.78)] were identified as the top three ROR adverse events. Unexpected adverse events, including large intestine perforation [ROR(95CI)=10.77 (3.47-33.45)], hepatic failure [ROR(95CI)=3.87 (1.45-10.31)], cholestasis [ROR(95CI)=4.92 (1.84-13.12)], and hepatic cytolysis [ROR(95CI)=4.70 (1.51-14.58)], were also observed. For metabolism, nutrition, or general disorders (Figure 5), we found that disease progression [ROR(95CI)=60.09 (53.12-67.97)], weight fluctuation [ROR(95CI)=15.62 (8.63-28.27)], and general physical condition abnormal [ROR(95CI)=11.44 (4.75-27.54)] were the top three ROR adverse events. Lastly, Figure 6 displays other adverse events, including skin, nervous system, or infection-related events such as pneumonitis [ROR(95CI)=9.93 (5.75-17.12)] and neutropenic sepsis[ROR(95CI)=46.02(27.15-77.99)], periorbital oedema [ROR(95CI)=21.45 (8.03-57.31)], and skin tightness [ROR(95CI)=13.80 (4.44-42.89)], which exhibited the highest ROR values. Although the pneumonitis showed high signal, interstitial lung disease didn’t show a significant signal [ROR(95CI)=1.35 (0.44-4.20), n=3].
In the cohort of patients aged between 18 and 65 years, a total of 53 adverse events were identified with significant safety signals (Table 3), whereas in patients aged older than 65 years, 43 adverse events were reported (Table 4). Neutropenic colitis [ROR(95CI)=176.55 (91.15-341.97)], neutrophil count abnormal [ROR(95CI)=56.17 (20.99-150.28)], and neutropenic sepsis [ROR(95CI)=34.79 (14.44-83.85)] displayed the highest reporting odds ratios (RORs) among the top three adverse events in the 18-65 years old group. On the other hand, neutropenic colitis [ROR(95CI)=282.05 (116.36-683.66)], neutropenic sepsis [ROR(95CI)=101.11 (41.83-244.43)], and febrile bone marrow aplasia [ROR(95CI)=121.33 (38.9-378.43)] exhibited the highest ROR values among the top three adverse events in the >65 years old group. Notably, acute kidney injury [ROR(95CI)=3.29 (1.36-7.94)] and atrial fibrillation [ROR(95CI)=6.91 (2.86-16.69)] were identified as specific serious adverse events in patients aged over 65 years.
After excluding the reports with inaccurate, missing, or unknown onset time, a total of 526 adverse events were analyzed for their respective onset times. As shown in Figure 7, 360 reports (68.4%) indicated that the adverse events occurred within 30 days of drug administration. Over 80% of the adverse events were observed within 60 days of drug administration, while only a minor proportion of 2.1% reported onset times longer than 360 days.
To our knowledge, this pharmacovigilance study, based on the FAERS database, presents the first real-world safety profile of SG. The study highlights various safety concerns related to SG use and suggests a potential for serious and unexpected adverse events. Four main findings emerged from this study.
First, blood and lymphatic system disorders, along with gastrointestinal disorders, were the most frequently reported adverse events associated with SG use and showed significant high safety signals (Figure 2). Neutropenia, febrile neutropenia, and anemia were the most frequently reported adverse events in this SOC category. Consistent with the clinical trial findings, SG showed a high number of reports of neutropenia and febrile neutropenia (6, 20–22). Neutropenia is a common reason for treatment interruption (6), and it is a known toxicity associated with irinotecan and SN-38 (23). Notably, our analysis revealed a high safety signal for neutropenia-related adverse events, including neutropenic colitis and neutropenic sepsis. Several infection-related adverse events, such as sepsis, septic shock, and skin infection, also showed a high safety signal. Considering that neutropenia or myelosuppression increases the risk of infection, it is important to consider the use of granulocyte colony-stimulating factor, which helps stimulate the production of white blood cells in the bone marrow (24, 25). Additionally, it is crucial to address contributing factors that can mitigate the risk of infection-related complications, particularly neutropenic sepsis and sepsis. In such cases, appropriate anti-infective therapy should be considered. Furthermore, it is worth noting that neutropenic colitis, although rare, has shown significant signals. To manage neutropenic colitis effectively, strategies such as bowel rest, intravenous rehydration therapy, and the administration of broad-spectrum antibiotics may be beneficial. These interventions can help alleviate symptoms and promote recovery from neutropenic colitis (26).
Regarding gastrointestinal disorders, diarrhea was the most frequently reported adverse event associated with SG, which is consistent with regulatory trials (6). SG-related diarrhea was also associated with other adverse events, such as electrolyte imbalance, hypokalemia, hypocalcemia, and dehydration, all of which showed a high safety signal. Approximately 9% of patients experienced severe diarrhea, for which loperamide and supportive measures are recommended (6, 27). Additionally, nausea, vomiting, and constipation, as well as enteritis, colitis, and enterocolitis, were associated with high safety signals. Importantly, we identified an increased risk of large intestine perforation. It is hypothesized that SG-induced diarrhea may lead to weakened mucosal integrity and injury (28), which could potentially result in this serious adverse event. However, it is important to approach this unexpected finding with caution, as it is based on a limited number of reported cases (only three cases were reported). Given the small sample size, further investigation and analysis are necessary to gain a clearer understanding of the implications and significance of this finding.
Second, the potential hepatobiliary toxicity associated with SG warrants increased attention. Our analysis identified unexpected adverse events such as hepatic failure, cholestasis, and hepatic cytolysis. The liver and kidneys are most at risk due to systemic exposure to the unconjugated cytotoxic payload (29). Studies have reported that irinotecan-induced hepatotoxicity is mainly characterized by an increase in transaminase levels and the development of steatosis. Several significant risks and predisposing factors for this condition have been identified, including high body mass index or obesity, diabetes, and a high-fat diet (30). Furthermore, the potential mechanisms underlying hepatic injury induced by the cytotoxic payload of irinotecan include the expression of TROP-2 by normal cells, internalization of the payload by liver sinusoidal endothelial cells and Kupffer cells through endocytosis, and impairment of disposition pathways. To better understand the underlying mechanisms of drug-induced liver injury, further research is needed at the cellular and molecular levels.
Third, our analysis identified a high incidence of pneumonitis associated with SG. It is noteworthy, however, that only two cases of pneumonitis were reported in the Phase I-III clinical trials of SG (6, 7, 22, 31). The signal could potentially be due to a variety of factors, such as COVID-19 infection risk, increased risk of infection due to SG treatment, or interstitial lung disease induced by ADC. ADCs have been shown to induce interstitial lung disease, particularly trastuzumab deruxtecan, which resulted in 10.5% of patients experiencing interstitial lung disease or pneumonitis (32). A meta-analysis evaluated the incidence of ADC-associated pneumonitis and found that the total incidence of all-grade drug-associated pneumonitis was higher in ADCs (33). In our study, we did not observe significant safety concerns related to interstitial lung disease, which may indicate a potential advantage of SG compared to other ADCs. However, it is important to note that the number of reported cases of interstitial lung disease was limited in our study (n=3), which hinders a robust assessment of the risk. Although we did not observe significant safety concerns related to interstitial lung disease in our study, it is essential to consider the inherent limitations of our sample size. Therefore, further long-term monitoring of interstitial lung disease should be conducted to better understand the potential advantage of SG compared to other ADCs in terms of this particular adverse event
Fourth, it is widely recognized that older age is a risk factor for chemotherapy toxicity (23), which not only affects treatment decisions but also increases the risk of adverse events. In this study, there were 50% of the reports lack of age data, this may lead to the bias of the results. Compared with other studies, our findings were consistent with those of a study conducted by Rugo et al., which suggested that the safety profile of SG in patients aged ≥65 was generally similar to that of patients aged <65 and the overall study population (27). However, our analysis also revealed that geriatric patients (>65 years old) exhibited high signals of sepsis, febrile bone marrow aplasia, and unexpected adverse events, including acute kidney injury and atrial fibrillation. These signals indicated that the potential risk of infection, kidney injury, and cardiotoxicity was increased in geriatric patients. There are several factors likely to increase the risk of ADC-related adverse events, such as age-related changes in homeostasis and physiological conditions (34–36), as well as age-related pharmacokinetic changes (37, 38). It is crucial to monitor the safety of SG in geriatric patients, particularly those with renal lesions or cardiac diseases.
There are several limitations to this study that should be acknowledged. First, it is not possible to confirm a causal relationship between adverse events and SG administration based on data from FAERS. Future clinical studies may utilize the Naranjo Adverse Drug Reaction Probability Scale to assess whether there is a causal relationship between an adverse event and the drug (39). Second, it is important to note that the unavoidable bias in this study cannot be eliminated. In general, safety signals may be biased by unmeasured confounding factors, such as height, weight, age, drug co-administration, or the knowledge and attitudes of healthcare professionals (10). For example, certain demographic or clinical characteristics (such as age, gender, or pre-existing medical conditions) might influence the reporting patterns and frequencies of adverse events, potentially leading to biased results when using the signal algorithm. Third, the absence of age data in the database can introduce bias when conducting age subgroup analysis. Fourth, the safety signals identified in this study may have been affected by cancer symptoms or other treatments. Inclusion of such symptoms in the analysis can affect the interpretation and analysis of the data, potentially impacting the overall findings and conclusions of the study. Fifth, as SG is a newly approved drug (April 2020), the quantity of reports in special populations was not sufficient. Longer-term and continuous monitoring is necessary to explore the potential risks and benefits of SG.
This analysis, based on real-world pharmacovigilance data from the FAERS database, revealed significant safety signals associated with SG. The main safety concerns of SG align with those observed in clinical trials and involve blood and lymphatic system disorders, gastrointestinal disorders, and associated infections. Notably, rare, unexpected, and serious safety signals such as large intestine perforation and hepatic failure were also identified. To mitigate these adverse events, practitioners should identify potential contributing factors and implement appropriate monitoring and intervention strategies. This study provides important real-world safety data for SG, complementing existing findings from clinical trials. The identified safety concerns warrant further prospective investigation to ensure comprehensive understanding and management of these risks.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
XG: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. JZ: Data curation, Investigation, Writing – review & editing. LD: Data curation, Investigation, Writing – review & editing. JC: Data curation, Writing – review & editing. HL: Data curation, Writing – review & editing. YC: Investigation, Writing – review & editing. SL: Investigation, Writing – review & editing. YZ: Investigation, Writing – review & editing. WW: Investigation, Writing – review & editing. HC: Investigation, Supervision, Writing – review & editing. HY: Formal Analysis, Project administration, Resources, Supervision, Writing – review & editing. YW: Data curation, Formal Analysis, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (No. 82271793 and 82071754).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tarantino P, Carmagnani PR, Corti C, Modi S, Bardia A, Tolaney SM, et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J Clin (2022) 72:165–82. doi: 10.3322/caac.21705
2. Wu Q, Qian W, Sun X, Jiang S. Small-molecule inhibitors, immune checkpoint inhibitors, and more: fda-approved novel therapeutic drugs for solid tumors from 1991 to 2021. J Hematol Oncol (2022) 15:143. doi: 10.1186/s13045-022-01362-9
3. Syed YY. Sacituzumab govitecan: first approval. Drugs (2020) 80:1019–25. doi: 10.1007/s40265-020-01337-5
4. Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-trop-2 igg-sn-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res (2011) 17:3157–69. doi: 10.1158/1078-0432.CCR-10-2939
5. Wahby S, Fashoyin-Aje L, Osgood CL, Cheng J, Fiero MH, Zhang L, et al. Fda approval summary: accelerated approval of sacituzumab govitecan-hziy for third-line treatment of metastatic triple-negative breast cancer. Clin Cancer Res (2021) 27:1850–4. doi: 10.1158/1078-0432.CCR-20-3119
6. Bardia A, Hurvitz SA, Tolaney SM, Zelnak AB, Weaver R, Traina T, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med (2021) 16:1529–41. doi: 10.1056/NEJMoa2028485
7. Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, et al. Sacituzumab govitecan, a trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase i/ii immu-132-01 basket trial. Ann Oncol (2021) 32:746–56. doi: 10.1016/j.annonc.2021.03.005
9. Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis (1967) 20:637–48. doi: 10.1016/0021-9681(67)90041-0
10. Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the fda adverse event reporting system. Int J Med Sci (2013) 10:796–803. doi: 10.7150/ijms.6048
11. Hauben M, Bate A. Decision support methods for the detection of adverse events in post-marketing data. Drug Discovery Today (2009) 14:343–57. doi: 10.1016/j.drudis.2008.12.012
12. Gastaldon C, Raschi E, Kane JM, Barbui C, Schoretsanitis G. Post-marketing safety concerns with esketamine: a disproportionality analysis of spontaneous reports submitted to the fda adverse event reporting system. Psychother Psychosom. (2021) 90:41–8. doi: 10.1159/000510703
13. Chavant F, Favreliere S, Lafay-Chebassier C, Plazanet C, Perault-Pochat MC. Memory disorders associated with consumption of drugs: updating through a case/noncase study in the french pharmacovigilance database. Br J Clin Pharmacol (2011) 72:898–904. doi: 10.1111/j.1365-2125.2011.04009.x
14. Capella D, Pedros C, Vidal X, Laporte JR. Case-population studies in pharmacoepidemiology. Drug Saf. (2002) 25:7–19. doi: 10.2165/00002018-200225010-00002
15. Noguchi Y, Tachi T, Teramachi H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief Bioinform (2021) 22:1–14. doi: 10.1093/bib/bbab347
16. Pace ND, Multani JK. On the reporting of odds ratios and risk ratios. Nutrients. (2018) Nutrients 10:1512. doi: 10.3390/nu10101512
17. van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R. Egberts AC. A comparison measures disproportionality Signal detection spontaneous reporting Syst adverse Drug reactions. Pharmacoepidemiol Drug Saf. (2002) 11:3–10. doi: 10.1002/pds.668
18. Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol (1998) 54:315–21. doi: 10.1007/s002280050466
19. Van Holle L, Zeinoun Z, Bauchau V, Verstraeten T. Using time-to-onset for detecting safety signals in spontaneous reports of adverse events following immunization: a proof of concept study. Pharmacoepidemiol Drug Saf. (2012) 21:603–10. doi: 10.1002/pds.3226
20. Ocean AJ, Starodub AN, Bardia A, Vahdat LT, Isakoff SJ, Guarino M, et al. Sacituzumab govitecan (immu-132), an anti-trop-2-sn-38 antibody-drug conjugate for the treatment of diverse epithelial cancers: safety and pharmacokinetics. Cancer. (2017) 123:3843–54. doi: 10.1002/cncr.30789
21. Starodub AN, Ocean AJ, Shah MA, Guarino MJ, Picozzi VJ, Vahdat LT, et al. First-in-human trial of a novel anti-trop-2 antibody-sn-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res (2015) 21:3870–8. doi: 10.1158/1078-0432.CCR-14-3321
22. Kalinsky K, Diamond JR, Vahdat LT, Tolaney SM, Juric D, O'Shaughnessy J, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/her2-negative metastatic breast cancer: final results from a phase i/ii, single-arm, basket trial. Ann Oncol (2020) 31:1709–18. doi: 10.1016/j.annonc.2020.09.004
23. Dean L. Irinotecan Therapy and UGT1A1 Genotype. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, eds. Medical Genetics Summaries. Bethesda (MD): National Center for Biotechnology Information (US) (2012).
24. Rugo HS, Bianchini G, Cortes J, Henning JW, Untch M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. Esmo Open (2022) 7:100553. doi: 10.1016/j.esmoop.2022.100553
25. Ba Y, Shi Y, Jiang W, Feng J, Cheng Y, Xiao L, et al. Current management of chemotherapy-induced neutropenia in adults: key points and new challenges: committee of neoplastic supportive-care (cons), China anti-cancer association committee of clinical chemotherapy, China anti-cancer association. Cancer Biol Med (2020) 17:896–909. doi: 10.20892/j.issn.2095-3941.2020.0069
26. Prescott AE, Ravindra A, Javed A. Neutropenic enterocolitis: a rare complication of sacituzumab govitecan. Case Rep Oncol (2022) 15:687–93. doi: 10.1159/000525351
27. Rugo HS, Tolaney SM, Loirat D, Punie K, Bardia A, Hurvitz SA, et al. Safety analyses from the phase 3 ascent trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer. (2022) 8:98. doi: 10.1038/s41523-022-00467-1
28. Pusztaszeri MP, Genta RM, Cryer BL. Drug-induced injury in the gastrointestinal tract: clinical and pathologic considerations. Nat Clin Pract Gastroenterol Hepatol (2007) 4:442–53. doi: 10.1038/ncpgasthep0896
29. Zuo P. Capturing the magic bullet: pharmacokinetic principles and modeling of antibody-drug conjugates. AAPS J (2020) 22:105. doi: 10.1208/s12248-020-00475-8
30. Han J, Zhang J, Zhang C. Irinotecan-induced steatohepatitis: current insights. Front Oncol (2021) 11:754891. doi: 10.3389/fonc.2021.754891
31. Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Flechon A, et al. Trophy-u-01: a phase ii open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol (2021) 39:2474–85. doi: 10.1200/JCO.20.03489
32. Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med (2022) 386:1143–54. doi: 10.1056/NEJMoa2115022
33. Zhu Z, Shen G, Li J, Qiu T, Fang Q, Zheng Y, et al. Incidence of antibody-drug conjugates-related pneumonitis in patients with solid tumors: a systematic review and meta-analysis. Crit Rev Oncol Hematol (2023) 184:103960. doi: 10.1016/j.critrevonc.2023.103960
34. Stevenson JM, Davies JG, Martin FC. Medication-related harm: a geriatric syndrome. Age Ageing. (2019) 49:7–11. doi: 10.1093/ageing/afz121
35. Lattanzio F, Landi F, Bustacchini S, Abbatecola AM, Corica F, Pranno L, et al. Geriatric conditions and the risk of adverse drug reactions in older adults: a review. Drug Saf. (2012) 35 Suppl 1:55–61. doi: 10.1007/BF03319103
36. Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol (2004) 57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x
37. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev (2009) 41:67–76. doi: 10.1080/03602530902722679
38. Davies EA, O'Mahony MS. Adverse drug reactions in special populations - the elderly. Br J Clin Pharmacol (2015) 80:796–807. doi: 10.1111/bcp.12596
Keywords: Sacituzumab govitecan, FAERS, safety, pharmacovigilance, pneumonitis, sepsis
Citation: Gui X, Zhao J, Ding L, Chai J, Lai H, Cai Y, Luo S, Zeng Y, Wu W, Chen H, Yao H and Wang Y (2023) Assessing real-world safety concerns of Sacituzumab govitecan: a disproportionality analysis using spontaneous reports in the FDA adverse event reporting system. Front. Oncol. 13:1276976. doi: 10.3389/fonc.2023.1276976
Received: 13 August 2023; Accepted: 20 September 2023;
Published: 06 October 2023.
Edited by:
San-Gang Wu, First Affiliated Hospital of Xiamen University, ChinaReviewed by:
Chengguang Zhao, Wenzhou Medical University, ChinaCopyright © 2023 Gui, Zhao, Ding, Chai, Lai, Cai, Luo, Zeng, Wu, Chen, Yao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Wang, d2FuZ3k1NTZAbWFpbC5zeXN1LmVkdS5jbg==; Herui Yao, eWFvaGVydWlAbWFpbC5zeXN1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.