- 1West China University Hospital of Sichuan University, Chengdu, Sichuan, China
- 2The Department of Obstetrics and Gynecology, West China Second University Hospital of Sichuan University, Chengdu, Sichuan, China
- 3Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, Sichuan, China
Objective: The efficacy of the first-line monodrug chemotherapy has been generally established for low-risk GTN. Most patients can achieve a complete response after the first-line monodrug chemotherapy. However, which monodrug chemotherapy regimen is better for individual patients with GTN is not yet certain. This study aimed to assess the efficacy of first-line monodrug chemotherapy in low-risk gestational trophoblastic neoplasia (GTN).
Method: Databases, including PubMed, Embase, Web of Science, and Cochrane Library, were searched from inception to November 1, 2022, for case–control studies on first-line monodrug chemotherapy in GTN. Network meta-analysis was performed to compare the efficacy outcome of six monodrug chemotherapy regimens in GTN, with a complete response rate as the endpoint.
Result: Twenty-four studies were considered eligible, including 9 randomized controlled trials (RCTs) and 15 non-RCTs. A total of 3344 patients with low-risk GTN were involved. Six monodrug chemotherapy regimens were included and analyzed. In descending order of efficacy, these six regimens were VP-16 (5 days), ACT-D (5 days), MTX (5 days), ACT-D (1.25 mg/m2), MTX (8 days), and MTX (30–50 mg/m2) in all study, and five regimens were ACT-D (5 days), MTX (5 days), ACT-D (1.25 mg/m2), MTX (8 days), and MTX (30–50 mg/m2) in RCT.
Conclusion: Among the six first-line monodrug chemotherapy regimens for low-risk GTN in all study, VP-16 (5 days) was the best in terms of efficacy. And five regimens in RCT, ACT-D was the best. However, the finding needs to be validated through more high-quality clinical studies.
1 Introduction
Gestational trophoblastic neoplasia (GTN) is a rare malignancy originating from placental trophoblasts. Despite the high metastatic potential and lethal risk, GTN is associated with a response rate as high as 90% under most situations (1, 2). The International Federation of Gynecology and Obstetrics (FIGO)/World Health Organization (WHO) prognosis scoring system (2000) classifies GTN into low risk (≤6 points), high risk (>6–12 points), and ultra high risk (≥13 points), for which stratified treatment is recommended.
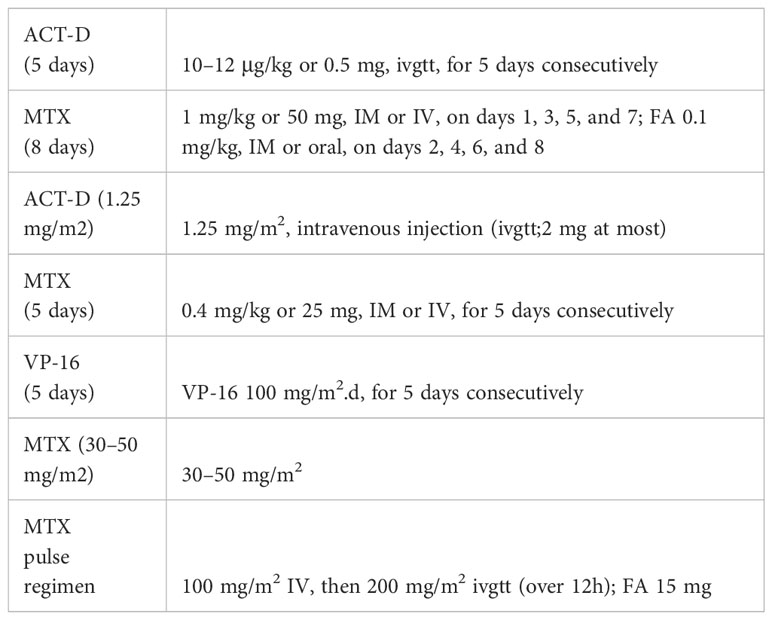
The FIGO 2021 guidelines (3) recommend monodrug chemotherapy for low-risk GTN and combination chemotherapy for high-risk GTN. For the former, the commonly used first-line agents are methotrexate (MTX) and actinomycin D (ACT-D). However, which monodrug or chemotherapy regimen is the best for individual patients has not yet been established. An intramuscular injection of MTX is a convenient and widely used MTX dosing regimen due to the prevalence of day care wards and family doctors in foreign countries. In China, textbooks recommend the 5-fluorouracil (5-FU)/floxuridine regimen. Even today, some Chinese grassroots-level hospitals, or even grade-3 first-class hospitals, are still using this regimen, although it has already been removed from the 2015 FIGO guidelines (4). The reason is that 5-FU is usually given for a long period, causing obvious adverse and toxic effects, for example, severe bone marrow suppression and ulceration of the intestinal mucosa, further leading to diarrhea. In some serious cases, pseudomembranous colitis induced by Staphylococcus aureus may even occur, leading to death. Seven monodrug chemotherapy regimens are more commonly used in low-risk GTN: (1) MTX (8 days) regimen: MTX 1 mg/kg or 50 mg, intramuscular (IM) or intravenous (IV), on days 1, 3, 5, and 7; FA 0.1 mg/kg, IM or oral, on days 2, 4, 6, and 8; (2) MTX (5 days) regimen: MTX 0.4 mg/kg or 25 mg, IM or IV, for 5 days consecutively; (3) ACT-D (1.25 mg/m2) regimen: ACT-D 1.25 mg/m2, intravenous injection (ivgtt;2 mg at most); (4) ACT-D (5 days) regimen: ACT-D 10–12 μg/kg or 0.5 mg, ivgtt, for 5 days consecutively; (5) MTX (30–50 mg/m2) regimen: MTX 30–50 mg/m2, IV; (6)VP-16 (5 days) regimen:VP-16 100 mg/m2.d;(7) MTX pulse regimen: MTX 100 mg/m2 IV, then 200 mg/m2 ivgtt (over 12h); FA 15 mg. Six monodrug chemotherapy regimens are shown in Table 1.
The efficacy of the first-line monodrug chemotherapy has been generally established for low-risk GTN. Most patients can achieve a complete response after the first-line monodrug chemotherapy. However, which monodrug chemotherapy regimen is better for individual patients with GTN is not yet certain. A meta-analysis (5) comparing several first-line chemotherapy regimens included 7 RCTs, involving 667 patients with low-risk GTN. The results showed that ACT-D was significantly better than MTX in terms of effectiveness. The pulsed chemotherapy regimens using ACT-D and MTX did not differ significantly in side effects. Nevertheless, more high-quality evidence is needed to treat low-risk GTN. Given the diversity of the treatment regimens, a network meta-analysis can inform the choice of the optimal regimen for this condition. We performed a network meta-analysis with a complete response rate after the first-line monodrug chemotherapy to offer clues for the clinical choice of the chemotherapy regimen.
2 Data and method
Network meta-analysis was performed according to the PRISMA guidelines (6, 7).
2.1 Literature retrieval
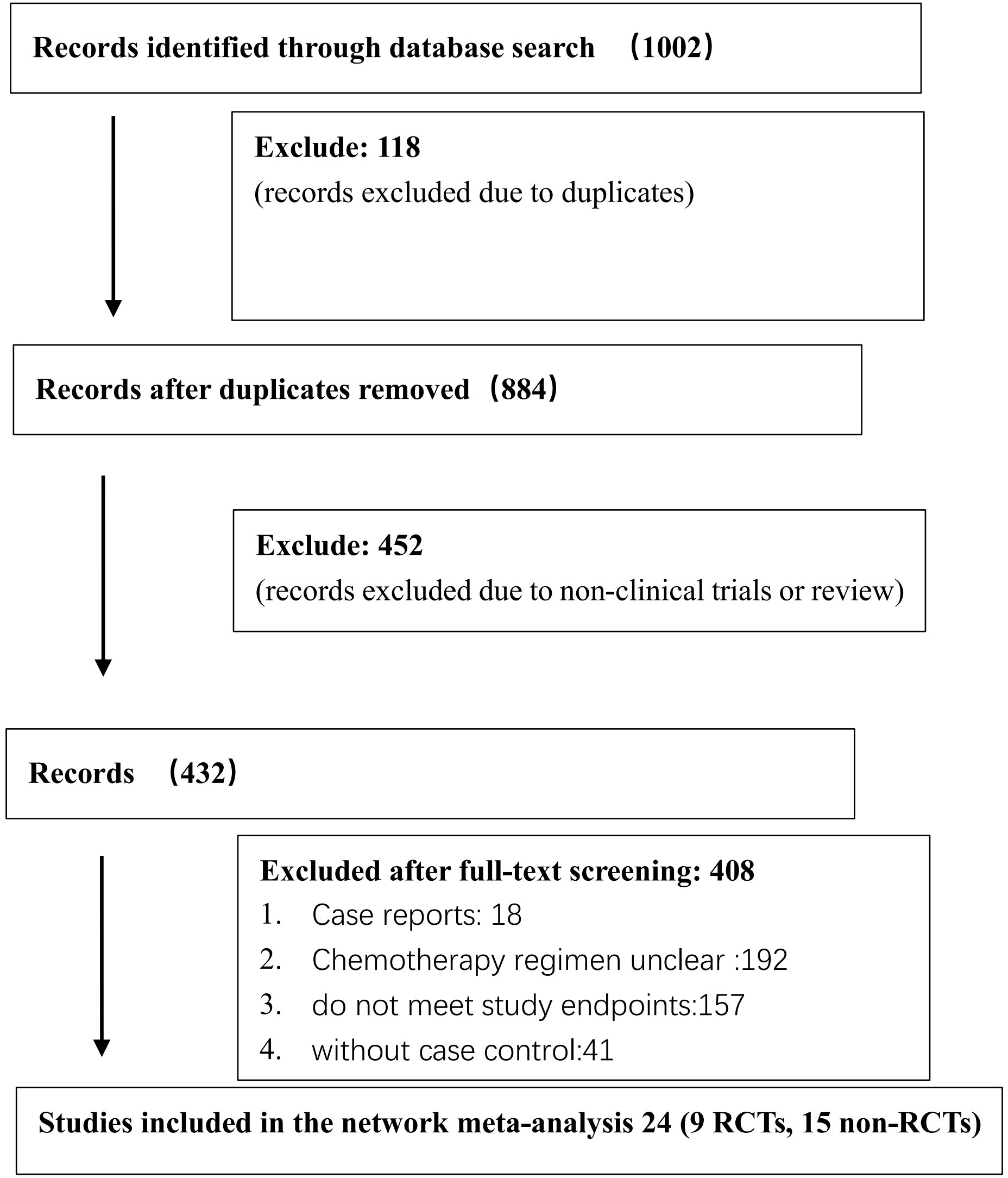
The Cochrane Library, PubMed, Embase, and Web of Science databases were searched using the Medical Subject Heading (MeSH)-term search strategy from inception to November 1, 2022. Literature search and screening were conducted by two researchers independently. The divergence of opinions was settled through a discussion between the two researchers. If the problem still persisted, a third researcher specialized in methodology was invited. The search words included the following: low-risk or low risk, gestational trophoblastic neoplasia, or gestational trophoblastic tumor. The flowchart of the literature search and screening is shown in Figure 1.
2.2 Inclusion and exclusion criteria
The inclusion and exclusion criteria were developed based on the Population, Intervention, Comparison, Outcomes, and Studies principles. The participants were confirmed with low-risk GTN, with a prognostic score ≤6 according to the FIGO/WHO prognostic scoring system; the studies were RCTs or non-RCTs; and the interventional treatments were first-line monodrug chemotherapy regimens, which included but were not confined to the following: MTX (8 days) regimen, MTX (5 days) regimen, ACT-D (1.25 mg/m2) regimen, ACT-D (5 days) regimen, MTX (30–50 mg/m2) regimen, and MTX pulsed regimen. The primary endpoint was the complete response rate after the first-line monodrug chemotherapy. Published studies written in English were included.
Studies without controls, studies containing an unclear description of the chemotherapy regimens, studies using oral chemotherapy regimens as interventional treatment, and studies from which important endpoint data could not be extracted were excluded.
2.3 Data extraction
Two researchers independently extracted the following data from the included studies: authors, publication time, study site, study type, interventional treatment, subjects, sample size, and age. The complete response rate after the first-line monodrug chemotherapy was the outcome measure. Randomization scheme, blinding, and reporting were used as methodological indicators. The divergence of opinions was settled through a discussion between two researchers. If the problem still persisted, a third researcher specialized in methodology was invited.
2.4 Bias and quality assessment of the included studies
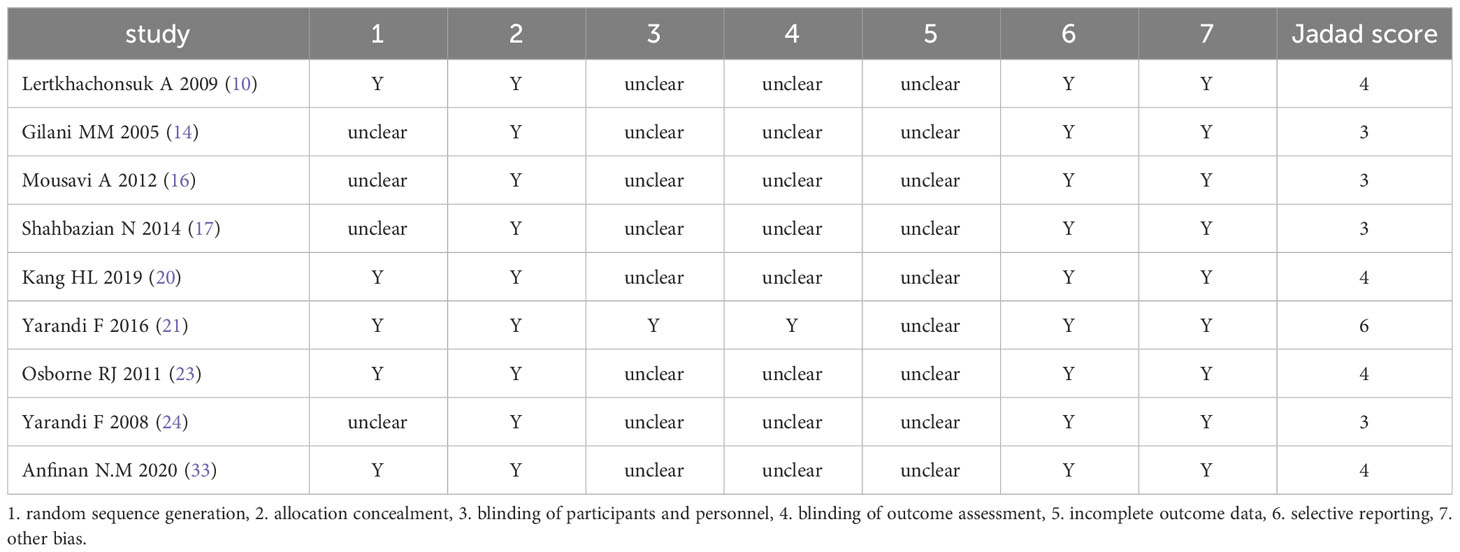
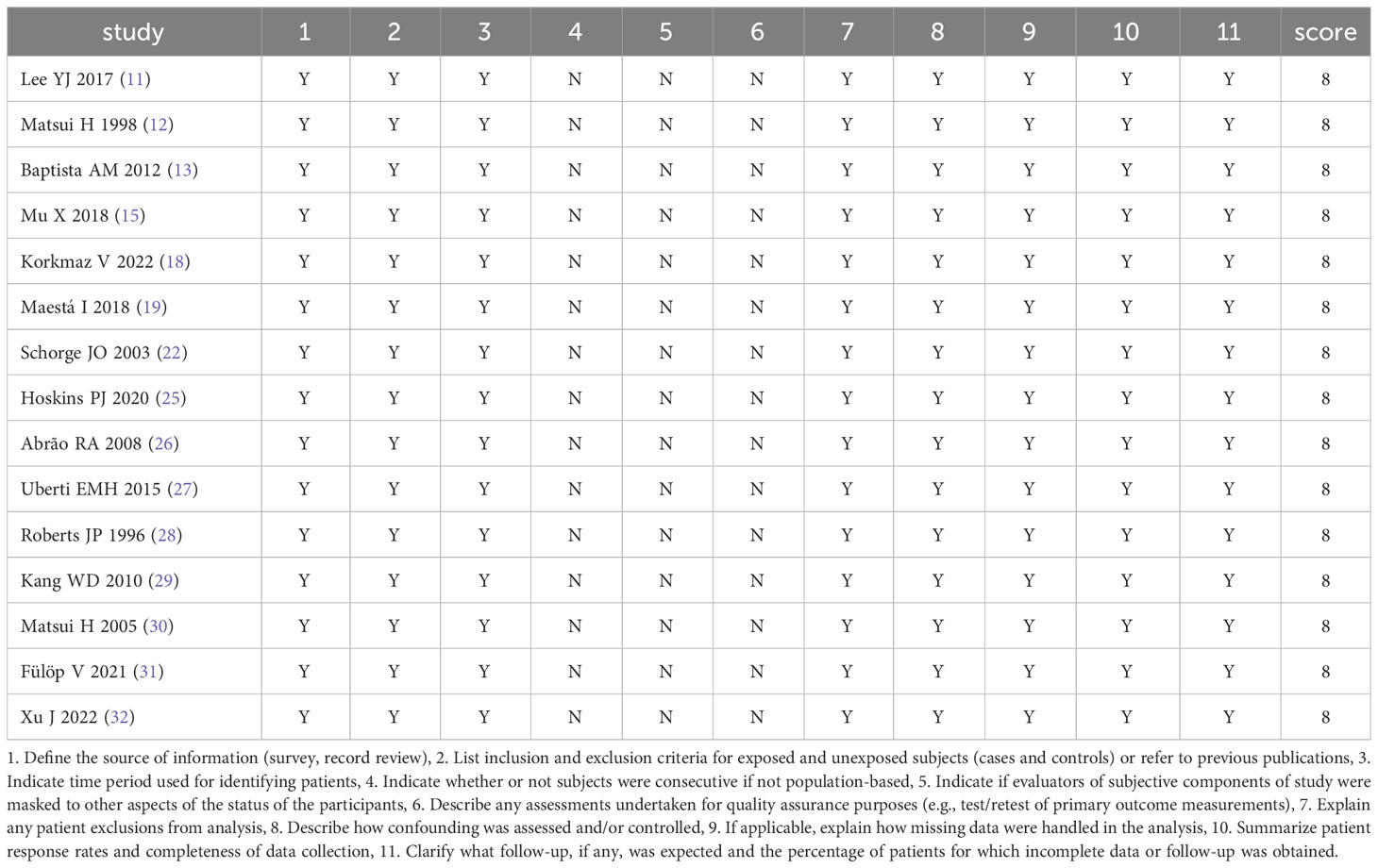
Bias and quality assessment was conducted for RCTs using Cochrane collaboration’s tool for assessing the risk of bias (8). The following seven categories of indicator data were included for bias and quality assessment: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias); and (7) other bias. Bias and quality assessment was conducted for the included non-RCTs using the quality assessment tools for observational studies (9). The following 11 categories of indicator data were included for bias and quality assessment: (1) the source of information was defined (survey and record review), (2) inclusion and exclusion criteria were listed for exposed and unexposed participants (cases and controls) or previous publications were referred to; (3) time period used for identifying patients was indicated; (4) whether participants were consecutive if not population based was indicated; (5) whether evaluators of subjective components of study were masked to other aspects of the status of the participants was indicated; (6) any assessments undertaken for quality assurance purposes were described (e.g., test/retest of primary outcome measurements); (7) any patient exclusions from analysis were explained; (8) how confounding was assessed and/or controlled was described; (9) if applicable, how missing data were handled in the analysis were explained; (10) patient response rates and completeness of data collection were summarized; and (11) what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained were clarified. Bias and quality assessment was conducted for each study based on the aforementioned criteria.
2.5 Data analysis
The data on the sample size and complete response rate under different interventional treatments were extracted from the included studies. Then, network meta-analysis was performed using R 4.2.2. The odds ratio was calculated for the enumeration data, and the measurements were expressed as mean ± standard deviation. First, the chi-square test for homogeneity was performed. I2 ≤50% indicated small heterogeneity, and the study was considered eligible for meta-analysis. I2 >50% indicated large heterogeneity. Thus, the sources of heterogeneity were identified and removed before the meta-analysis. Network analysis was performed by running the Markov Chain Monte Carlo algorithm.
3 Results
3.1 Basic features of the included studies
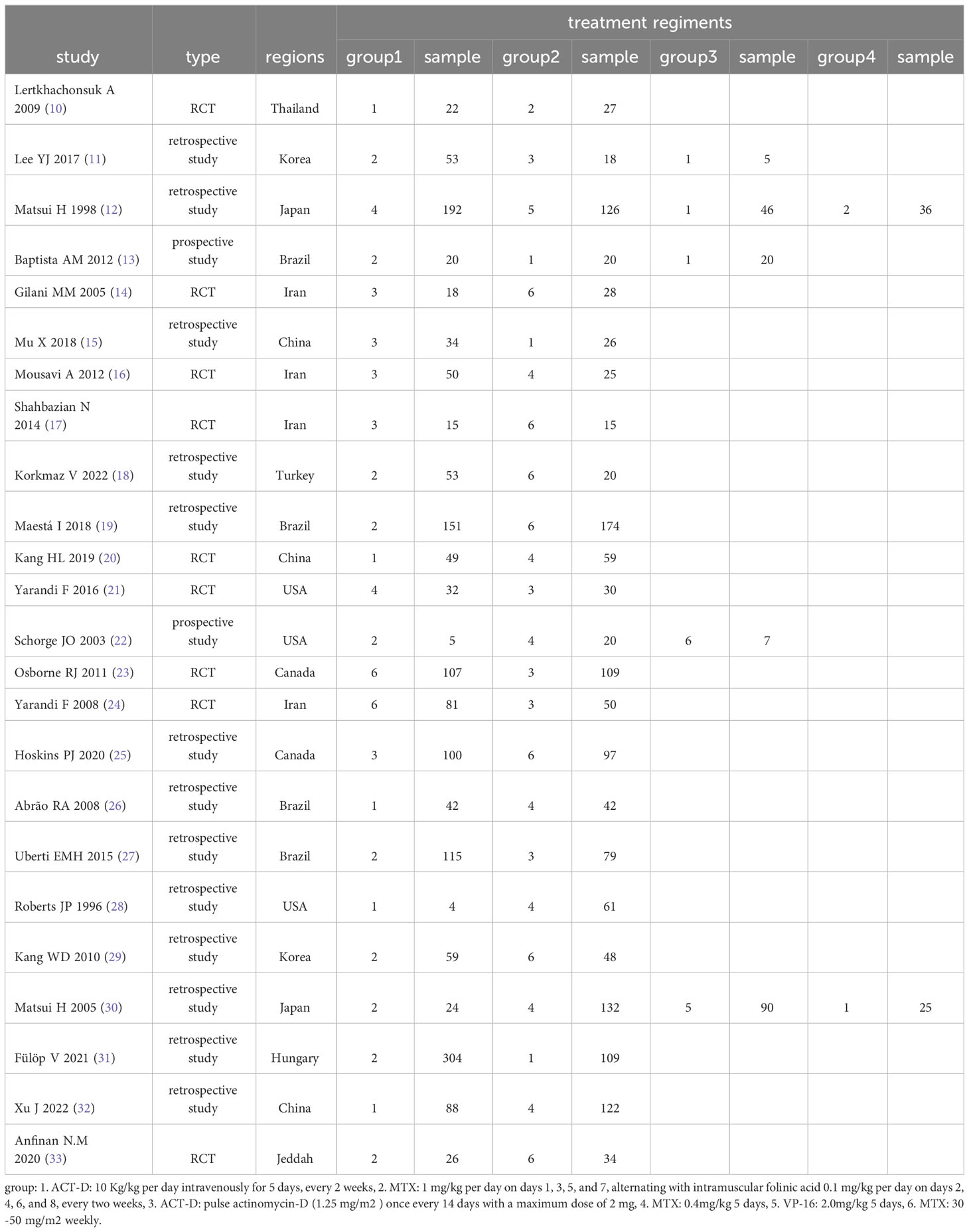
The search strategy was developed using the MeSH terms. The preliminary screening yielded 1002 studies, among which repeated studies and those not eligible for the meta-analysis were excluded, resulting in 24 eligible ones (10–33). Specifically, 9 RCTs (10, 14, 16, 17, 20, 21, 23, 24, 33) and 15 non-RCTs (11–13, 15, 18, 19, 22, 25–32) were finally included. A total of 3344 low-risk patients were involved. The following six first-line monodrug chemotherapy regimens were involved in studies: MTX (8 days) regimen, MTX (5 days) regimen, ACT-D (1.25 mg/m2) regimen, ACT-D (5 days) regimen, MTX(30–50 mg/m2) regimen, and VP-16 (5 days) regimen. The basic features of the included studies are presented in Table 2.
3.2 Bias and quality assessment of the included studies
The results of bias and quality assessment of the 9 RCTs are shown in Table 3, and those of the 15 non-RCTs are shown in Table 4. The included studies were mostly clinical trials of medium quality.
3.3 Complete response rate of first-line monodrug chemotherapy regimens in low-risk GTN
Six studies compared the ACT-D (5 days) regimen and the MTX (8 days) regimen; two studies compared the ACT-D (5 days) regimen and ACT-D (1.25 mg/m2) regimen; two studies compared the MTX (8 days) regimen and the ACT-D (1.25 mg/m2) regimen; five studies compared the ACT-D (5 days) regimen and the MTX (5 days) regimen; two studies compared the ACT-D (5 days) regimen and the VP-16 (5 days) regimen; two studies compared the MTX (5 days) regimen and the VP-16 (5 days) regimen; five studies compared the ACT-D (1.25 mg/m2) regimen and the MTX (30–50 mg/m2) regimen; three studies compared the ACT-D (1.25 mg/m2) regimen and the MTX (5 days) regimen; five studies compared the MTX (8 days) regimen and the MTX (30–50 mg/m2) regimen; two studies compared the MTX (8 days) regimen and the MTX (5 days) regimen; and one study compared the MTX (5 days) regimen and the MTX (30–50 mg/m2) regimen.
Six monodrug chemotherapy regimens were included and analyzed. The probability ranking results show that VP-16 (5 days) is most likely to be the most effective treatment option. The probability is about 99%, followed by ACT-D (5 days)(78%), MTX (5 days)(45%), ACT-D (1.25 mg/m2)(43%). Subgroup analysis found ACT-D (5 days) is most likely to be the most effective treatment option, MTX (5 days), ACT-D (1.25 mg/m2), MTX (8 days), and MTX (30–50 mg/m2) in RCT(there are no RCTs on VP-16). The evidence graph and the results of network meta-analysis in all study are shown in Figure 2. The evidence graph and the results of network meta-analysis in RCT are shown in Figure 3.
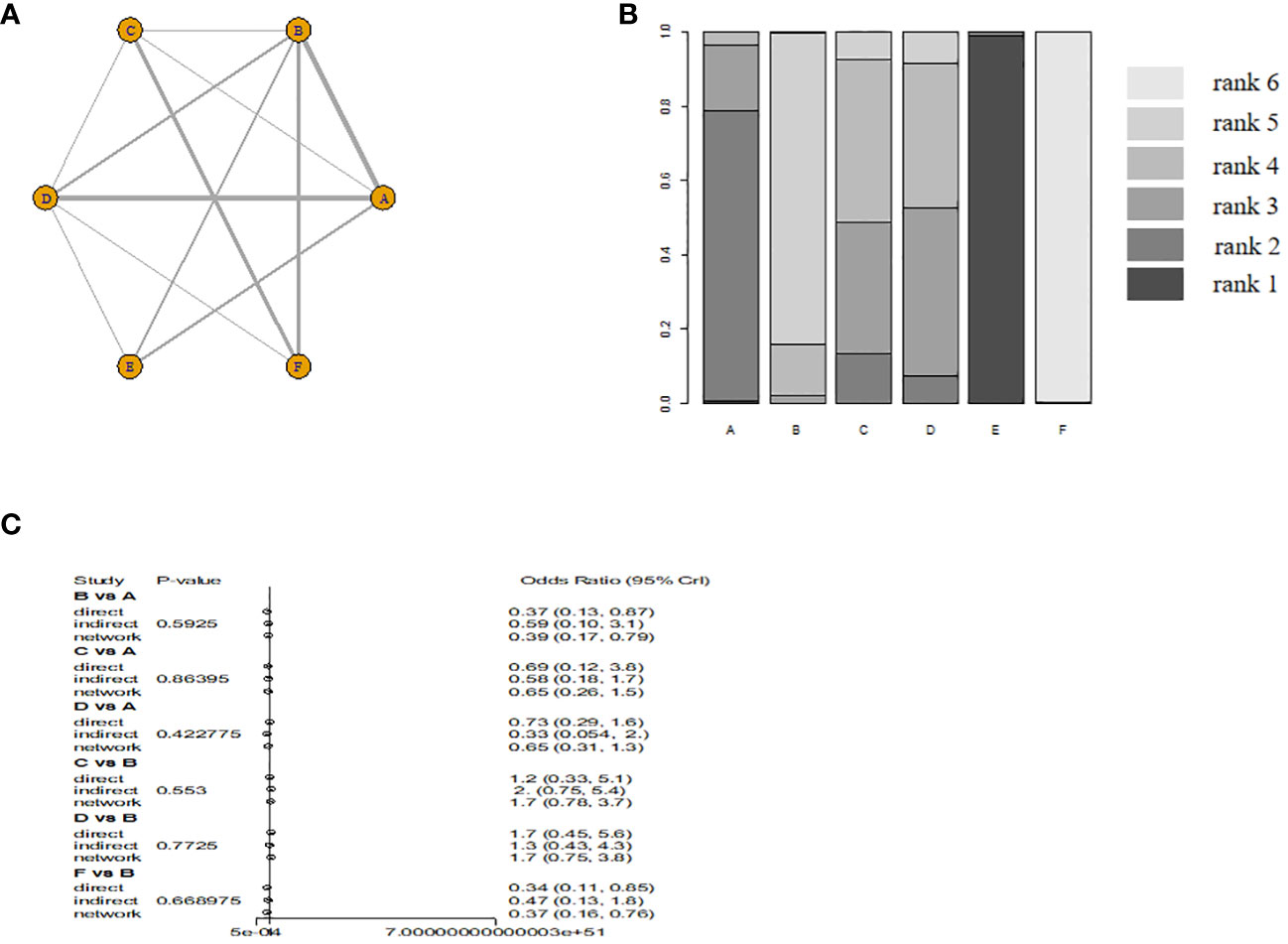
Figure 2 The evidence graph and the results of network meta-analysis in all study (A) The evidence graph, (B) ranks of treatments, (C) forest of network meta-analysis). A: ACT-D (10 ug/kg per day intravenously for 5 days,every 2 weeks), B: MTX(1 mg/kg per day on days 1, 3, 5, and 7, alternating with intramuscular folinic acid 0.1 mg/kg per day on days 2, 4, 6, and 8, every two weeks), C: pulse Act-D(pulse actinomycin-D (1.25 mg/m2) once every 14 days with a maximum dose of 2 mg), D: MTX(0.4mg/kg 5 day), E: VP-16(2.0mg/kg 5 day), F: MTX(30 mg/m2/weekly).
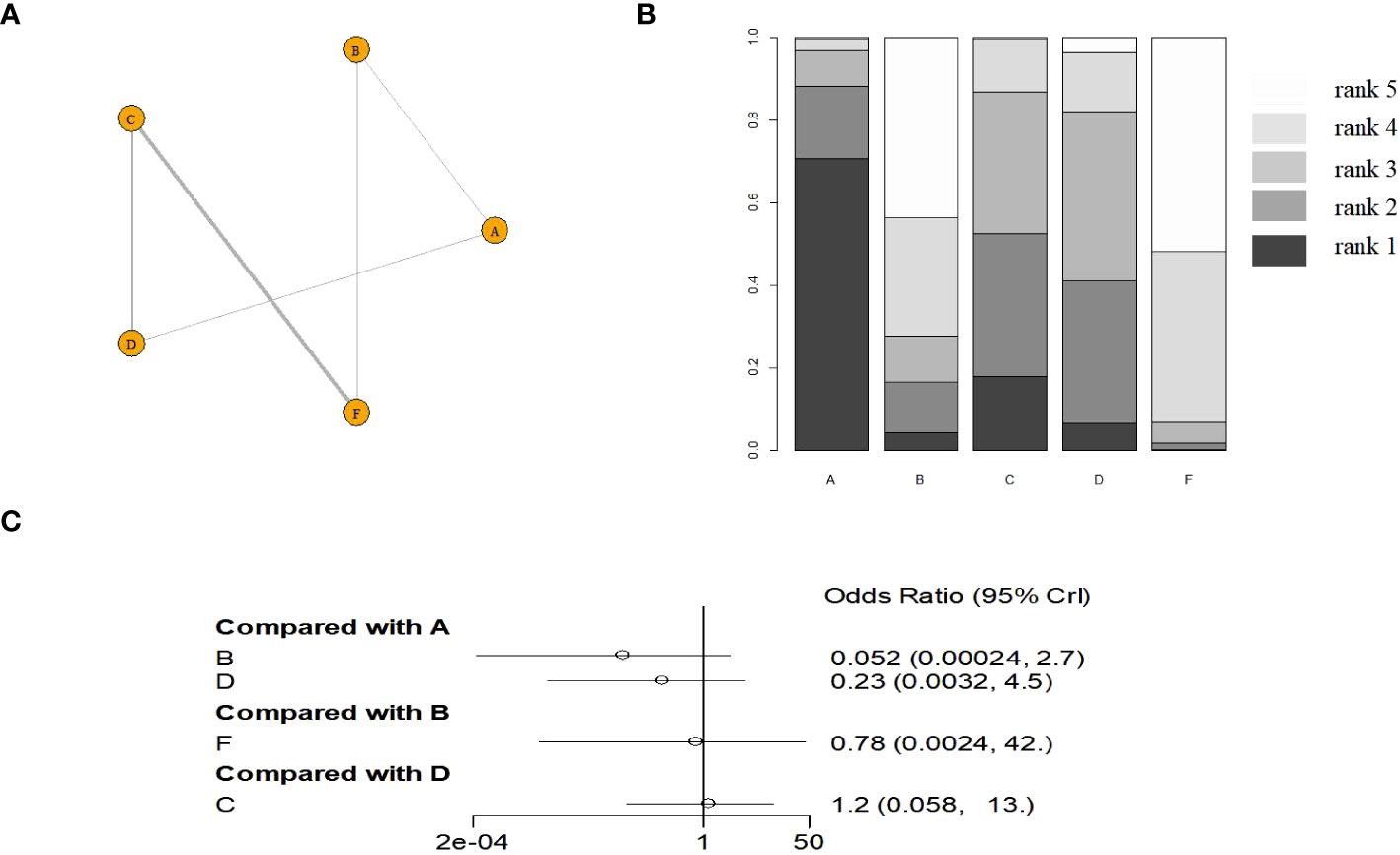
Figure 3 The evidence graph and the results of network meta-analysis in RCT (A) The evidence graph, (B) ranks of treatments, (C) forest of network meta-analysis). A: ACT-D (10 ug/kg per day intravenously for 5 days,every 2 weeks), B: MTX(1 mg/kg per day on days 1, 3, 5, and 7, alternating with intramuscular folinic acid 0.1 mg/kg per day on days 2, 4, 6, and 8, every two weeks), C: pulse Act-D(pulse actinomycin-D (1.25 mg/m2) once every 14 days with a maximum dose of 2 mg), D: MTX(0.4mg/kg 5 day), E: VP-16(2.0mg/kg 5 day), F: MTX(30 mg/m2/weekly).
Network meta-analysis has good consistency with traditional meta-analysis, the efficacy value and ranking probability do not change significantly, and the results are stable. GTN-node splitting analysis of inconsistency are presented in Supplementary Figure S1 and Supplementary Table S1. The analysis of heterogeneity are shown in Supplementary Figure S2 and Supplementary Table S2.
4 Discussion
The FIGO guidelines (3) recommend monodrug chemotherapy for low-risk GTN, and the options include MTX and ACT-D. The hCG level should be monitored once every 2 weeks before each cycle to guide the subsequent treatment. If the hCG level drops to normal after chemotherapy, two to three cycles of chemotherapy should be given before discontinuation. If a satisfactory initial response to chemotherapy is followed by a reduction in the hCG level to the plateau (down by <10% after three cycles of chemotherapy) or first a decrease and then an increase (hCG < 1000 µ/L), the regimen should be changed to the one different from the initial treatment. If MTX has been previously used, the monodrug therapy should be changed to ACT-D and vice versa.
Although MTX and ACT-D monodrug chemotherapy regimens are recommended as the preferred treatments by international guidelines, which one is better for individual patients is not yet certain. Studies have been conducted on combination therapies or other first-line monodrug chemotherapy regimens, but the controversy regarding the optimal dosing regimen for chemotherapy continues. Matsui et al. (12) compared the efficacy of MTX, VP-16, and ACT-D in 247 patients with low-risk GTN. The result showed that the complete response rate of the MTX (5 days) regimen, VP-16 (5 days) regimen, ACT-D (5 days) regimen, and MTX (8 days) regimen was 73.6%, 90.1%, 84.0%, and 60.0%, respectively. The complete response rate was significantly higher for the VP-16 and ACT-D regimens than for the other two conventional regimens. The complete response rate was significantly higher for the VP-16 regimen than for the other three regimens. Maestá et al. (19) analyzed the efficacy of different MTX dosing regimens in 325 patients with low-risk GTN, namely, MTX (30–50 mg/m2) and MTX (8 days) regimens. Compared with the MTX (30–50 mg/m2) regimen, the MTX (8 days) regimen was found to have a higher sustained response rate (84% vs 62%, P < 0.001). Although the latter also had a higher incidence of adverse reactions, nearly all of these reactions were controllable. The MTX (8 days) regimen was superior to the MTX (30–50 mg/m2) regimen. Xu et al. (32) evaluated the efficacy of the ACT-D (5 days) regimen against the MTX (5 days) regimen in low-risk GTN. The results showed that the complete response rate was 72.73% in the ACT-D (5 days) regimen and 75.41% in the MTX (5 days) regimen, indicating no significant difference. Compared with the ACT-D group, the MTX monodrug group significantly reduced in the total number of chemotherapy cycles and average hospitalization cost (P < 0.05). No serious adverse reactions were reported in any group. However, the ACT-D monodrug group had a higher incidence of leukopenia (grade 1 or 2) (59.38% vs 17.39%). The MTX regimen (5 days) might be the preferred treatment option. This study compared six monodrug chemotherapy regimens involving three common agents using network meta-analysis. We intended to settle the controversy regarding the chemotherapy regimen most suited for individual patients with low-risk GTN. We found that VP-16 (5 days) regimen might be the preferred option in terms of efficacy when the complete response is used as the endpoint of the study, the probability ranking is about 99%, which is significantly higher than the other five chemotherapy options.
Network meta-analysis was performed to compare the efficacy outcome of six monodrug chemotherapy regimens in GTN, with a complete response rate as the endpoint. There may be other single-drug regimens or different ways of using the same drug regimen, which were not included in the analysis. For example, the MITO study compared clinical outcomes of patients diagnosed with low-risk gestational trophoblastic neoplasia (GTN) receiving intramuscular methotrexate 50 mg total dose/day versus 1 mg/kg/day in a 8-day methotrexate/folinic acid (MTX/FA) regimen. Because the both regimens in this study are MTX (8 days) regimen, the study was excluded (34). At the same time, we should also consider the feasibility of the treatment plan, applicability and disturbance to patients. Need to investigate patients’ personal feelings. Choose a treatment plan based on multiple factors.
This study had certain limitations despite the clinical guidance it might provide. First, the included studies were mostly retrospective and of moderate quality. Considerable heterogeneity was found in the sample size across the included studies, which were published over a long period, leading to the risk of bias. Besides, we only included studies written in English at the expense of the loss of studies written in other languages. The conclusions drawn from the limited number of studies might contain some biases. The only endpoint discussed in the present study was the complete response rate. We did not analyze the incidence and severity of adverse reactions across the chemotherapeutic agents and dosing regimens, which might have affected the accuracy of the conclusions. The first-line monodrug chemotherapy regimens for low-risk GTN might differ in the incidence and severity of side effects. However, nearly all patients tolerated the associated adverse reactions. Very few cases of intolerance to adverse reactions were reported. The aforementioned results indicated that a complete response rate might be a more useful efficacy outcome compared with the incidence and severity of adverse reactions. These findings need to be further confirmed using high-quality evidence.
To conclude, we performed a network meta-analysis to compare the efficacy of six monodrug chemotherapy regimens in low-risk GTN. The evidence suggested that the VP-16 (5 days) regimen might be the preferred option in terms of efficacy, followed by ACT-D (5 days), MTX (5 days), ACT-D (1.25 mg/m2), MTX (8 days), and MTX (30–50 mg/m2). However, our conclusions should be verified through high-quality RCTs involving a large sample size. There are currently multiple registered clinical studies in progress, and the results of these studies will give us more clinical guidance (35).
Author contributions
FZ: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing. LK: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1276771/full#supplementary-material
Supplementary Figure 1 | GTN-node splitting analysis of inconsistency (A: in all study, B: in RCT). (A) ACT-D (10 ug/kg per day intravenously for 5 days,every 2 weeks), (B) MTX(1 mg/kg per day on days 1, 3, 5, and 7, alternating with intramuscular folinic acid 0.1 mg/kg per day on days 2, 4, 6, and 8, every two weeks), (C) pulse Act-D(pulse actinomycin-D (1.25 mg/m2) once every 14 days with a maximum dose of 2 mg), (D) MTX(0.4mg/kg 5 day), (E) VP-16(2.0mg/kg 5 day), (F) MTX(30 mg/m2/weekly).
Supplementary Figure 2 | analysis of heterogeneity(A: in all study, B: in RCT). (A) ACT-D (10 ug/kg per day intravenously for 5 days,every 2 weeks), (B) MTX(1 mg/kg per day on days 1, 3, 5, and 7, alternating with intramuscular folinic acid 0.1 mg/kg per day on days 2, 4, 6, and 8, every two weeks), (C) pulse Act-D(pulse actinomycin-D (1.25 mg/m2) once every 14 days with a maximum dose of 2 mg), (D) MTX(0.4mg/kg 5 day), (E) VP-16(2.0mg/kg 5 day), (F) MTX(30 mg/m2/weekly).
References
1. Newlands ES, Bagshawe KD, Begent RH, Rustin GJ, Holden L. Results with the EMA/CO (etoposide, methotrexate, actinomycin D, cyclophos- phamide, vincristine) regimen in high risk gestational tropho- blastic tumours, 1979 to 1989. Br J Obstet Gynaecol (1991) 98(6):550–7. doi: 10.1111/j.1471-0528.1991.tb10369.x
2. Berkowitz RS, Goldstein DP. Current advances in the manage- ment of gestational trophoblastic disease. Gynecol Oncol (2013) 128(1):3–5. doi: 10.1016/j.ygyno.2012.07.116
3. Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Diagnosis and man- agement of gestational trophoblastic disease:2021 update. Int J Gynaecol Obstet (2021) 155(Suppl 1):86–93. doi: 10.1002/ijgo.13877
4. Ngan HY, Seckl MJ, Berkowitz RS, Hancock BW, Osborne R. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet (2015) 131 Suppl 2:S123–6. doi: 10.1016/j.ijgo.2015.06.008
5. Lawrie TA, Alazzam M, Tidy J, Hancock BW, Osborne R. First-line chemotherapy in low-risk gestational trophoblastic neoplasia. Cochrane Database Syst Rev (2016) 2016(6):CD007102. doi: 10.1002/14651858.CD007102.pub4
6. Hutton B, Salanti G, Caldwell DM, Schmid Ch, loannidis JP. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med (2015) 162(11):777–84. doi: 10.7326/M14-2385
7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2012) 29(372):n71. doi: 10.1136/bmj.n71
8. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
9. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2011). Ottawa Hospital Research Institute. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed November 1, 2022).
10. Lertkhachonsuk A, Israngura N, Wilailak S, Tangtrakul S. Actinomycin d versus methotrexate-folinic acid as the treatment of stage I, low-risk gestational trophoblastic neoplasia: a randomized controlled trial. Int J Gynecol Cancer (2009) 19(5):985–8. doi: 10.1111/IGC.0b013e3181a8333d
11. Lee YJ, Park JY, Kim DY, Suh DS, Kim JH, Kim YM, et al. Comparing and evaluating the efficacy of methotrexate and actinomycin D as first-line single chemotherapy agents in low risk gestational trophoblastic disease. J Gynecol Oncol (2017) 28(2):e8. doi: 10.3802/jgo.2017.28.e8
12. Matsui H, Iitsuka Y, Seki K, Sekiya S. Comparison of chemotherapies with methotrexate, VP-16 and actinomycin-D in low-risk gestational trophoblastic disease. Remission rates Drug toxicities Gynecol Obstet Invest (1998) 46(1):5–8. doi: 10.1159/000009987
13. Baptista AM, Belfort P. Comparison of methotrexate, actinomycin D, and etoposide for treating low-risk gestational trophoblastic neoplasia. Int J Gynaecol Obstet (2012) 119(1):35–8. doi: 10.1016/j.ijgo.2012.04.027
14. Gilani MM, Yarandi F, Eftekhar Z, Hanjani P. Comparison of pulse methotrexate and pulse dactinomycin in the treatment of low-risk gestational trophoblastic neoplasia. Aust N Z J Obstet Gynaecol (2005) 45(2):161–4. doi: 10.1111/j.1479-828X.2005.00366.x
15. Mu X, Song L, Li Q, Yin R, Zhao X, Wang D. Comparison of pulsed actinomycin D and 5-day actinomycin D as first-line chemotherapy for low-risk gestational trophoblastic neoplasia. Int J Gynaecol Obstet (2018) 143(2):225–31. doi: 10.1002/ijgo.12629
16. Mousavi A, Cheraghi F, Yarandi F, Gilani MM, Shojaei H. Comparison of pulsed actinomycin D versus 5-day methotrexate for the treatment of low-risk gestational trophoblastic disease. Int J Gynaecol Obstet (2012) 116(1):39–42. doi: 10.1016/j.ijgo.2011.08.003
17. Shahbazian N, Razi T, Razi S, Yazdanpanah L. Comparison of the efficacy of methotrexate and actinomycin D in the treatment of patients with stage I low risk gestational trophoblastic neoplasia (GTN). Med J Islam Repub Iran (2014) 22:28:78.
18. Korkmaz V, Sunar V, Akar S, Alinca CM, Arik Z, Boran N, et al. Comparison of weekly methotrexate regimen versus methotrexate folinic acid 8-day regimen for treatment of low-risk gestational trophoblastic neoplasia. Asia Pac J Clin Oncol (2022) 18(3):326–32. doi: 10.1111/ajco.13623
19. Maestá I, Nitecki R, Horowitz NS, Goldstein DP, de Freitas Segalla Moreira M, Elias KM, et al. Effectiveness and toxicity of first-line methotrexate chemotherapy in low-risk postmolar gestational trophoblastic neoplasia: The New England Trophoblastic Disease Center experience. Gynecol Oncol (2018) 148(1):161–7. doi: 10.1016/j.ygyno.2017.10.028
20. Kang HL, Zhao Q, Yang SL, Duan W. Efficacy of combination therapy with actinomycin D and methotrexate in the treatment of low-risk gestational trophoblastic neoplasia. Chemotherapy (2019) 64(1):42–7. doi: 10.1159/000500165
21. Yarandi F, Mousavi A, Abbaslu F, Aminimoghaddam S, Nekuie S, Adabi K, et al. Five-day intravascular methotrexate versus biweekly actinomycin-D in the treatment of low-risk gestational trophoblastic neoplasia: A clinical randomized trial. Int J Gynecol Cancer (2016) 26(5):971–6. doi: 10.1097/IGC.0000000000000687
22. Schorge JO, Lea JS, Farrar DF, King MR, Coleman RL, Miller DS. Management of low-risk gestational trophoblastic neoplasia in indigent women. J Reprod Med (2003) 48(10):780–4.
23. Osborne RJ, Filiaci V, Schink JC, Mannel RS, Alvarez Secord A, Kelley JL, et al. Phase III trial of weekly methotrexate or pulsed dactinomycin for low-risk gestational trophoblastic neoplasia: a gynecologic oncology group study. J Clin Oncol (2011) 29(7):825–31. doi: 10.1200/JCO.2010.30.4386
24. Yarandi F, Eftekhar Z, Shojaei H, Kanani S, Sharifi A, Hanjani P. Pulse methotrexate versus pulse actinomycin D in the treatment of low-risk gestational trophoblastic neoplasia. Int J Gynaecol Obstet (2008) 103(1):33–7. doi: 10.1016/j.ijgo.2008.05.013
25. Hoskins PJ, Le N, Kumar A, Pina A, Sabourin JN, Kim H, et al. Single or two drug combination therapy as initial treatment for low risk, gestational trophoblastic neoplasia. A Can analysis Gynecol Oncol (2020) 157(2):367–71. doi: 10.1016/j.ygyno.2020.02.005
26. Abrão RA, de Andrade JM, Tiezzi DG, Marana HR, Candido dos Reis FJ, Clagnan WS. Treatment for low-risk gestational trophoblastic disease: comparison of single-agent methotrexate, dactinomycin and combination regimens. Gynecol Oncol (2008) 108(1):149–53. doi: 10.1016/j.ygyno.2007.09.006
27. Uberti EMH, do Carmo Fajardo M, da Cunha AGV, Frota SS, Braga A, Ayub ACK. Treatment of low-risk gestational trophoblastic neoplasia comparing biweekly eight-day Methotrexate with folinic acid versus bolus-dose Actinomycin-D, among Brazilian women. Rev Bras Ginecol Obstet (2015) 37(6):258–65. doi: 10.1590/SO100-720320150005366
28. Roberts JP, Lurain JR. Treatment of low-risk metastatic gestational trophoblastic tumors with single-agent chemotherapy. Am J Obstet Gynecol (1996) 174(6):1917–23. doi: 10.1016/S0002-9378(96)70229-6
29. Kang WD, Choi HS, Kim SM. Weekly methotrexate (50mg/m(2)) without dose escalation as a primary regimen for low-risk gestational trophoblastic neoplasia. Gynecol Oncol (2010) 117(3):477–80. doi: 10.1016/j.ygyno.2010.02.029
30. Matsui H, Suzuka K, Yamazawa K, Tanaka N, Mitsuhashi A, Seki K, et al. Relapse rate of patients with low-risk gestational trophoblastic tumor initially treated with single-agent chemotherapy. Gynecol Oncol (2005) 96(3):616–20. doi: 10.1016/j.ygyno.2004.11.011
31. Fülöp V, Szigetvári I, Szepesi J, Lahm E, Végh G, Demeter J, et al. The diagnostics and treatment of low-risk gestational trophoblastic neoplasia (GTN): 42-year experience. Eur J Gynaecological Oncol (2021) 42(6):1159–65.
32. Xu J, Wang X, Qu P. Comparing and evaluating five-day chemotherapy agents actinomycin D and methotrexate in low-risk post-molar gestational trophoblastic neoplasia: A retrospective analysis. Clin Exp Obstetrics Gynecol (2022) 49:5. doi: 10.31083/j.ceog4905106
33. Anfinan NM, Al Khatieb MT, Sait HK, Sait MK, Baghlaf OA, Mousa AA, et al. Eight days versus weekly intramuscular methotrexate for the treatment of low-risk gestational trophoblastic neoplasia. Eur J Gynaecological Oncol (2020) 41(2):227–32. doi: 10.1007/s00404-009-1014-3
34. Mangili G, Cioffi R, Danese S, Frigerio L, Fettandina G, Cormio G, et al. Does methotrexate (MTX) dosing in a 8-day MTX/FA regimen for the treatment of low-risk gestational trophoblastic neoplasia affect outcomes? The MITO-9 study. Gynecol Oncol (2018) 151(3):449–52. doi: 10.1016/j.ygyno.2018.09.025
35. Jiang F, Mao M, Xiang Y, Lu X, Guan CL, Jiao LZ, et al. Comparing biweekly single-dose actinomycin D with multiday methotrexate therapy for low-risk gestational trophoblastic neoplasia (FIGO Score 0-4): study protocol for a prospective, multicentre, randomized trial. BMC Cancer (2023) 23(1):784. doi: 10.1186/s12885-023-11225-2
Keywords: first-line chemotherapy, low-risk gestational trophoblastic neoplasia, monodrug, network meta-analysis, GTN
Citation: Zhou F and Kemin L (2024) First-line monodrug chemotherapy in low-risk gestational trophoblastic neoplasia: a network meta-analysis. Front. Oncol. 13:1276771. doi: 10.3389/fonc.2023.1276771
Received: 13 August 2023; Accepted: 11 December 2023;
Published: 05 January 2024.
Edited by:
Anthony Taylor, University of Leicester, United KingdomReviewed by:
Stefano Restaino, Ospedale Santa Maria della Misericordia di Udine, ItalyKomsun Suwannarurk, Thammasat University, Thailand
Copyright © 2024 Zhou and Kemin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Kemin, bGlrZW1pbjIwMDQwOUAxNjMuY29t
 Fang Zhou1
Fang Zhou1 Li Kemin
Li Kemin