
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 20 November 2023
Sec. Gynecological Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1276310
This article is part of the Research Topic Fertility Preservation View all 5 articles
Cisplatin is a common anticancer drug able to kill tumor cells, but it causes adverse reactions in the kidney, digestive tract, and other systems. The antitumor effects of cisplatin are mainly due to its ability to bind to the DNA in tumor cells to prevent replication, thereby reducing RNA and protein syntheses, leading to cell damage and death. Cisplatin has a wide range of applications; it can be used to treat cervical, thyroid, ovarian, and other cancers. Cisplatin has a beneficial therapeutic effect, but its therapeutic selectivity is poor. In addition to eliminating diseased target cells, cisplatin can damage normal cells; in women of reproductive age being treated for cancer, cisplatin can lead to ovarian function impairment, premature ovarian failure (POF), and/or infertility. Therefore, reducing the adverse effects of cisplatin on ovarian function is an important topic in clinical research. In this paper, we explore the research progress on the POF caused by cisplatin treatment.
Premature ovarian failure (POF), the premature aging of the ovaries, manifests itself by secondary amenorrhea before the age of 40, with follicle-stimulating hormone levels >40 u/L (1), estradiol levels <20 pg/mL, and low anti-Mullerian hormone values close to 0 ng/mL (2). In addition, ultrasound examinations show no follicles in the ovary and often an atrophic shrunken uterus with a thin endometrium. The etiology of POF is complex and unclear in most patients, but may be related to autoimmune factors, infections, or drugs. Common treatment drugs causing POF include platinum compounds, antimetabolic drugs, and antibiotics. The widely used platinum drugs bind covalently to ovarian DNA, form cross-links between DNA chains, and lead to follicle DNA cleavage during replication, thereby inhibiting follicle DNA transcription and synthesis; however, these toxic effects are not specific to primordial follicles (3). Researchers have suggested that high cisplatin doses can induce hyperactivation of primordial follicles during quiescent states, resulting in loss of ovarian reserve function (4). 2 Cytotoxic mechanisms of cisplatin.
Cisplatin is a common tumor therapeutic agent available only with a medical prescription due to its toxicity and side effects. Studies have suggested that platinum compounds can be used in cancer therapy, and that these drugs have cytotoxic effects (5). Cisplatin has strong cytotoxic effects that can destroy cancer cells, through its effects on DNA metabolism (Figure 1). The drug hydrolyzes in the cell mainly according to the concentration state and the carrier protein channel on the surface of the cell membrane, and then produces binding reaction with other substances. For example, adenosine triphosphate is transported through the endoplasmic reticulum along with cisplatin, glutathione (GSH), and L-cysteine. The fusion of cisplatin with GSH, cysteine (L(+)-Cysteine), and other proteins leads to the depletion of antioxidant reserves in the cytoplasm and causes cellular oxidative stress, which is the major mode of action of cisplatin toxicity. Oxidative stress affects antioxidant enzymes and other molecules, reduces GSH, and increases cisplatin toxicity (6). When cisplatin enters cancer cells, it activates reactive oxygen species pathways, resulting in apoptosis. Cisplatin hydrolysates fuse with DNA bases in the nucleus, and these complexes get identified by the nucleotide excision repair protein (NER), and the mismatch repair (MMR) and Pt-DNA complexes. HMG1 (High Mobility Group 1) and HMG2 (High Mobility Group 2) are two highly conserved nuclear proteins, which play an important role in a variety of biological processes such as DNA repair and apoptosis, and can improve the effect of cisplatin therapy, which includes the following aspects (7): First, the differentiation of in-strand DNA complexes between similar guanines can change the cell cycle activity and induce death. Second, these proteins accelerate the binding of p53 and DNA to induce trans-activation of target genes involved in cell cycle development, DNA repair, and cell apoptosis. Third, they inhibit Pt-DNA complex repair enhancing cisplatin efficacy. NER is an effective enzyme clearing Pt-DNA complexes. If NER is defective, cells become hypersensitive to cisplatin. MMR protein complexes repair DNA damage, but not cisplatin–DNA complexes, and unsuccessful repair initiates cell death. In summary, DNA damage activates multiple cellular signaling pathways that cause cell death. Recent studies have elucidated potential mechanisms of chemotherapy-induced ovarian damage, including DNA damage, oxidative stress, mitochondrial dysfunction, and apoptotic signalling abnormalities induced by cisplatin and other chemotherapeutic agents (8, 9).
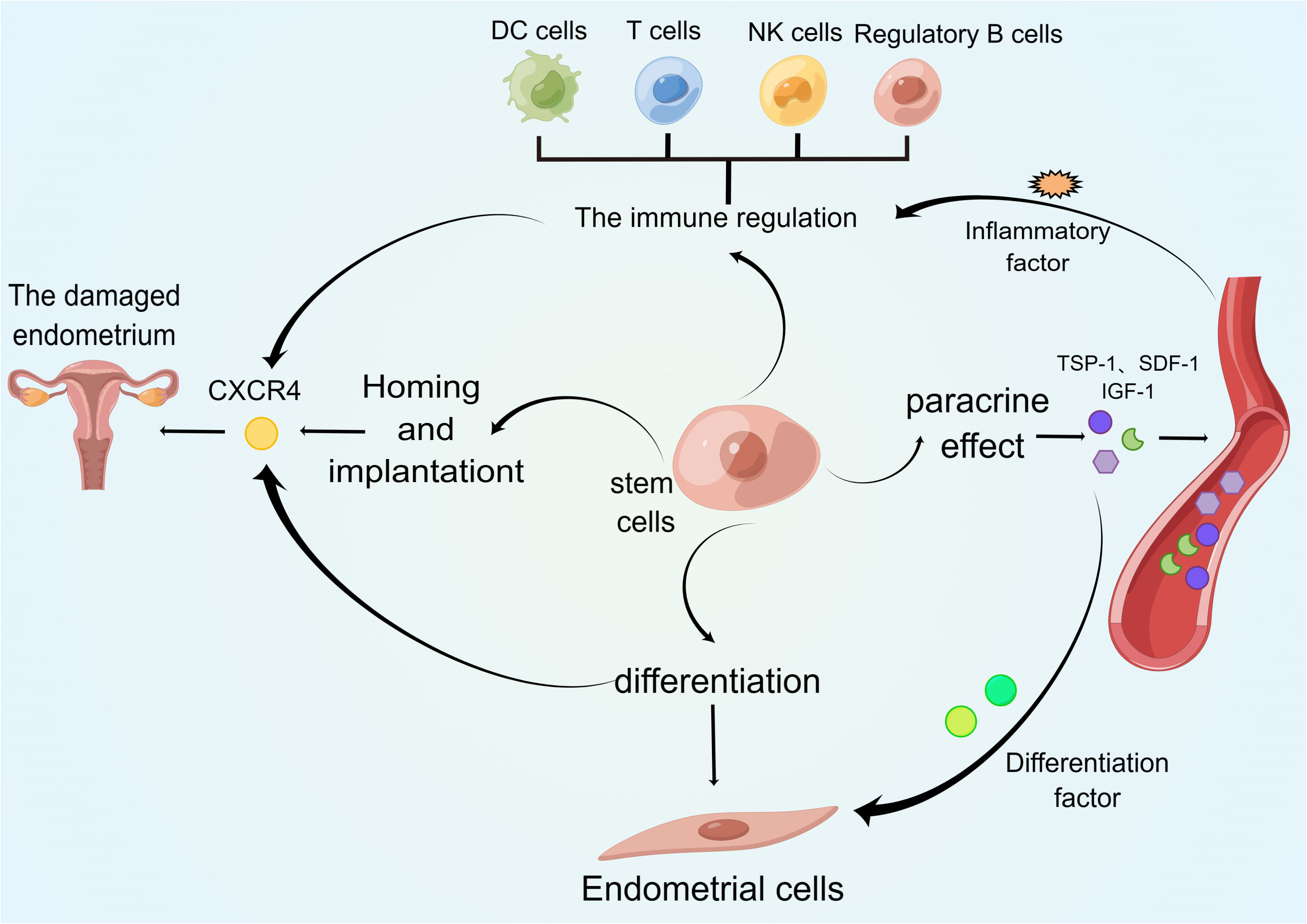
Figure 1 Regulatory cells in premature ovarian failure. This figure shows the major immune cell types and cytokines involved in the pathogenesis of premature ovarian failure.
The average age of menopause for women is between 50 and 52 years, at which point the ovarian function declines. The main POF inducer is the reproductive system (10). The clinical manifestations of POF include menstrual cycle disorders and infertility. The complex etiology of POF can be divided into two main classes: 1) Idiopathic POF, caused by unknown factors (11); and, 2) POF with clear causes such as genetic and immunological factors. The specific POF mechanisms have remained unclear, and clinical studies have focused on iatrogenic, genetic, and immune system factors.
Patients with POF have been found to harbor chromosome abnormalities and genetic polymorphisms associated with the development of POF, and among them, 50%–90% of patients present the idiopathic type (12) and show multiple POF-associated genes. The pathogenesis of idiopathic POF includes multiple gene deletions and environmental factors. X chromosome gene mutations have been associated with the occurrence of POF (13), they include mutations in DACH2, FMR1, and BMP15. In addition, some autosomal genes such as FSHR, NR5A1, and BMP15 are associated with POF (14). An association between meiosis gene mutations and ovarian decline has also been demonstrated, underscoring the role of meiosis genes for regulating ovarian function (15).
The ovary is a common autoimmune target in organ-specific and systemic autoimmune diseases, and these can lead to POF, polycystic ovarian syndrome, unexplained infertility, or endometriosis (Figure 2). In the case of POF, the autoimmune cause is associated with lymphocytic ovaritis, other autoimmune diseases, and autoantibodies against ovarian antigens. Approximately 20% of patients with primary ovarian insufficiency (POI) have been diagnosed with other autoimmune diseases, often thyroid, adrenal, and pancreatic diseases, and approximately 10% of patients with Addison’s disease develop POI (16). Autoimmune ovarian injury may be associated with a variety of causes, including changes in T cell subsets, T-cell-mediated damage (17), and increased numbers of B cells producing autoantibodies.
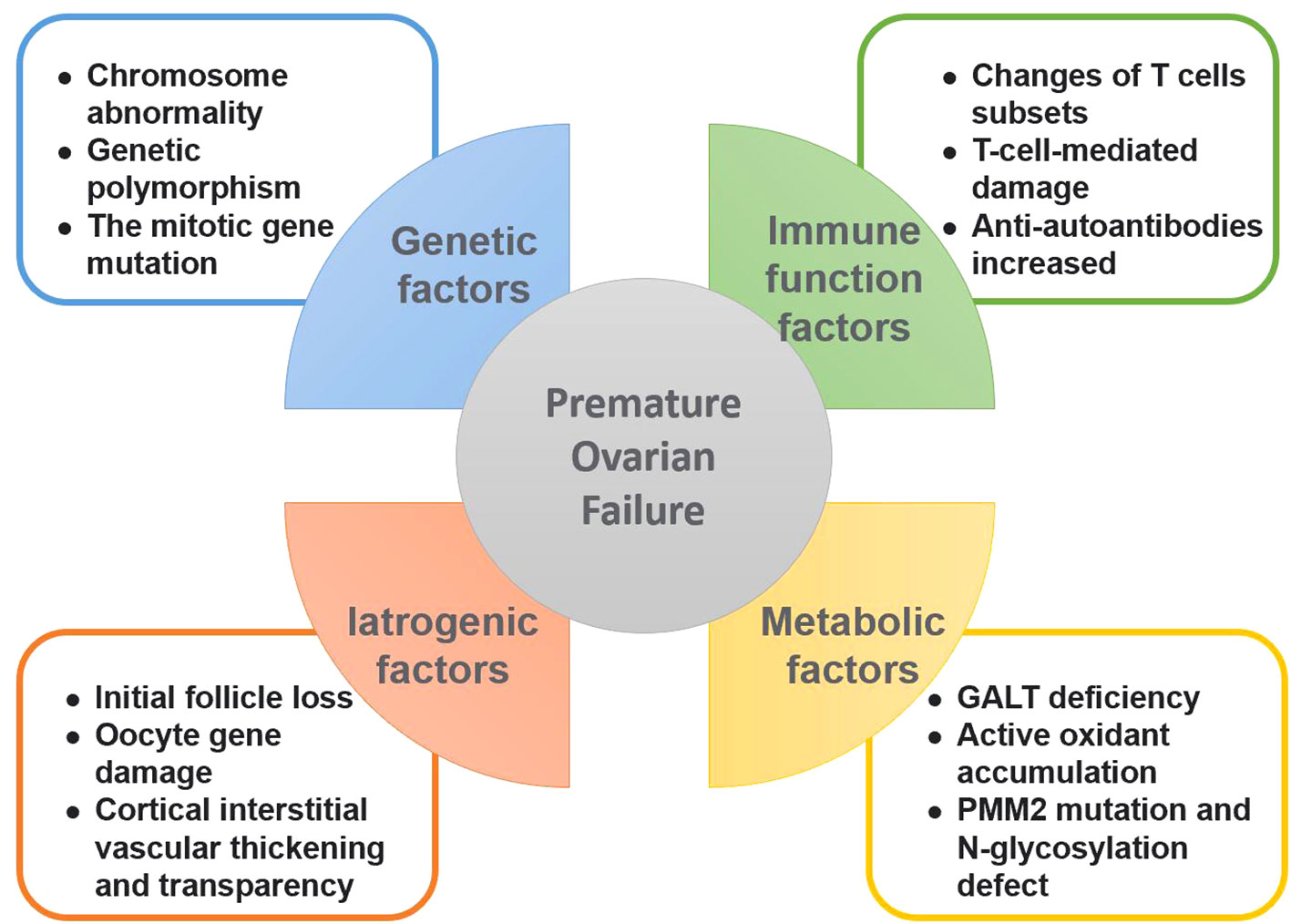
Figure 2 Causative factors of premature ovarian failure. This figure summarizes the various factors leading to premature ovarian failure, including genetic factors, autoimmune factors, iatrogenic factors and metabolic abnormalities.
Chemotherapy has multiple effects on ovarian function (18). Initially, during the clinical treatment, transiently induced amenorrhea can induce the growth of follicle groups, and ovarian function decline leads to POF. Once chemotherapy is stopped, the follicular cisterna provides the initial follicles to replenish the growing follicle group that promotes menstruation. However, some initial follicles are absent and lead to premature menopause with the passing of time and ultimately POF. In addition, the drug causes the double-stranded DNA of the oocyte to break causing genetic damage (19) and resulting in oocyte death. Laparoscopic surgery for ovarian cyst causes less tissue trauma, and the postoperative ovarian reserve function indexes and ovarian status are better than those after open surgery (20).
Ovarian function is decreased in patients with galactosemia, but the mechanisms responsible for this remain unclear. Patients with galactosemia present a galactose-1-phosphate uridyl transferase deficiency manifested by accumulation of galactose and its metabolites in the body, resulting in ovarian cell swelling and dysfunction (21). In addition, galactose can lead to the accumulation of active oxidants in the body (21), such as methyl glycol, which can block the REDOX cycle of glutathione, thereby destroying ovarian cells. Patients with congenital disorders of glycosylation present PMM2 and N-glycosylation defects that affect the production of follicles and lead to POF (22).
Cisplatin produces toxicity and side effects, which affect the clinical outcome of patients. Researchers administering cisplatin (5 mg/kg) to rats found marked toxic effects related to the dosage and timing in relation to their sexual cycle (23). The concentration of the medication, the administration route, and the age of patients also affect the actions of cisplatin. During treatment, cisplatin kills many rapidly dividing cells, the endocrine system is greatly affected, and the resulting ovarian dysfunction may be irreversible. Cisplatin combines with DNA to form complexes between and within the chain, affecting cell transcription and causing DNA damage. If left untreated, the drug activates cell-death signaling pathways. Studies have confirmed that the drug can induce ovarian cell death and tissue necrosis (24). In addition, cisplatin activates the non-receptor tyrosine kinase (Abl) and downstream p53 homologous protein in mouse ovarian blast cells, which continuously aggregate to cause oocyte death. Oocyte apoptosis can be reduced by treatment with an Abl activity blocker (18). Cisplatin also activates mitochondrial pathways that release cytochrome C into the cytoplasm leading to caspase pathway activation, which causes a stress response and cell death (25).
Due to the significant renal toxicity of cisplatin therapy, pre-treatment hydration therapy must be initiated before the treatment (26): A large infusion needs to be given 2–16 hours before and at least 6 hours after the cisplatin administration. In addition, patients need to drink plenty water and undergo diuretic treatments to ensure a daily urine volume within 2000–3000 mL. Changes in blood potassium and magnesium concentrations also need monitoring to avoid kidney function injury and cardiac burden. The discussion on side effects mitigation strategies was removed to align with the focus on POF.
For gastrointestinal toxicity causing nausea and vomiting, antiemesis drugs can be used before the medication, a four-drug combination regimen consisting of NK1 receptor antagonist, 5-HT3 receptor antagonist, dexamethasone, and olanzapine has been recommended by the American Society of Clinical Oncology guidelines (27).
For hematological toxicity, granulocyte colony stimulating factor (G-CSF), recombinant human platelet growth factor, erythropoietin (EPO) and other drugs can be used to treat the symptoms, the chemotherapy doses can be adjusted, or blood transfusions may be required (28).
The neurotoxicity caused by cisplatin treatment cannot be avoided. Some neuroprotectants may be effective, such as α-lipoic acid (29), but their value needs to be validated by clinical studies.
An efficient hormone therapy would allow for completion of adolescent development and alleviation of hormone deficiencies and sequelae. Clinical treatment for individual patients should consider the advantages and disadvantages of drugs (Table 1). Common secondary characteristics of primary POF are lacking and the bone mineral density is decreased. A common regimen includes estradiol treatment, with small initial doses and gradual progression, and the development of secondary sex characteristics can be observed (35). Progesterone protects the endometrium. The purpose of a subsequent treatment is to ensure continual effects and prevent infertility. Hormonal replacement therapy is used in patients with secondary amenorrhea and complete development to decrease cardiovascular problems.
Oocytes divide and proliferate often and are thereby prone to genetic damage. Cisplatin drugs damage mature follicles and deplete the primordial follicles. GnRH analogues can block ovarian activity, maintaining the tissues and making them less sensitive to cisplatin cytotoxicity. In addition, GnRH agonists can reduce blood flow and the concentration of local chemotherapeutic agent and alleviate ovarian function injuries. GnRH agonists can stimulate gonadotropin bursts, causing the primordial follicles to develop into immature follicles. Chemotherapy drugs act on actively dividing cells, and as a result, the ovaries become natural targets for these drugs. For this reason, GnRH agonists are clinically recommended 2 weeks before treatment. Studies have validated the protective effect of GnRH to preserve the ovarian function. In a study, premenopausal women aged between 20 and 40 years who received adjuvant chemotherapy for breast cancer from January 2002 to April 2012 were classified into two groups: Women in one group were treated with GnRH agonists for ovarian protection during chemotherapy, and those in the other group were not administered GnRH. The survival analysis using stratified Cox regression showed that women treated with concurrent GnRH agonists had better recurrence-free survival (adjusted hazard ratio, 0.21; P = 0.009; unadjusted hazard ratio, 0.33; P = 0.034) (32). Thus, GnRH agonists during chemotherapy maintain the therapeutic effect of the chemotherapeutic agent and protect the ovarian function. However, recent studies have questioned the protective effect of GnRH agonists on ovarian function during chemotherapy, indicating that GnRH agonists may not prevent chemotherapy-induced ovarian damage (36) Another study by Turan et al. suggested that there is insufficient evidence to support the biologic basis and clinical utility of GnRH agonists for fertility preservation in cancer patients (37). The utility of GnRH agonists for fertility preservation remains controversial and requires further research.
Traditional Chinese medical treatments for kidney can reduce the side effects of chemotherapeutic drugs, and the selection of drugs that promote adequate blood circulation in patients is done mainly to improve the function of the ovaries and promote the correct functioning of the uterus (38). Other Chinese remedies include deer fetal ointment to relieve the symptoms of maladaptation and to regulate the endocrine system. However, the research in this area is ongoing and in the animal experimental stages; therefore, clinical application is not available.
Nierestrol can reduce the end-metabolism of lipid peroxides in vivo (39). The decrease in the hydroxyl radical levels and the increase in SOD activity can help protect the ovarian function and reduce the toxic effect of chemotherapeutic drugs.
Platinum anticancer drugs such of cisplatin are cell cycle non-specific drugs that act on the cellular DNA. During clinical treatments, these drugs affect ovarian function and cause ovarian impairment that can lead to POF. POF is the main factor affecting female infertility. However, POF can progress without clear manifestations, and clinical diagnoses are mostly made during later stages of the disease. POF diversity and the genetic heterogeneity in women may contribute to the regulation of ovarian function. Therefore, understanding the genetic mechanisms of POF may lead to early detection and improvements in treatments for the condition. Knowledge of the chemotherapy-related effects can be used to prevent disease occurrences and provide a reliable basis for clinical treatments.
ZL: Writing – original draft, Writing – review & editing. HQ: Writing – original draft. ZL: Writing – original draft. YB: Writing – review & editing. KY: Writing – review & editing. QM: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Jiangxi Provincial Chemotherapy Toxicity Research Fund (NO.20220605A).
The figures were created with figdraw software (www.figdraw.com).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder–a plea for universal definitions. J Assist Reprod Genet (2015) 32(12):1709–12. doi: 10.1007/s10815-015-0595-y
2. Ceccarelli F, Orefice V, Perrone G, Pirone C, Perricone C, Truglia S, et al. Premature ovarian failure in patients affected by systemic lupus erythematosus: a cross-sectional study. Clin Exp Rheumatol (2020) 38(3):450–4.
3. Massacesi C, Bascioni R, Cellerino R, Scartozzi M, Bracci R, Alessandroni P, et al. Cisplatin, epirubicin and cyclophosphamide (PEC) in the treatment of advanced ovarian cancer. J Exp Clin Cancer Res (2000) 19(1):13–6.
4. Xie Y, Li S, Zhou L, Lin H, Jiao X, Qiu Q, et al. Rapamycin preserves the primordial follicle pool during cisplatin treatment in vitro and in vivo. Mol Reprod Dev (2020) 87(4):442–53. doi: 10.1002/mrd.23330
5. Song M, Cui M, Liu K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur J Med Chem (2022) 232:114205. doi: 10.1016/j.ejmech.2022.114205
6. Ahmed EA, Omar HM, Aelghaffar S Kh Abd, Ragb SMM, Nasser AY. The antioxidant activity of vitamin C, DPPD and L-cysteine against Cisplatin-induced testicular oxidative damage in rats. Food Chem Toxicol (2011) 49(5):1115–21. doi: 10.1016/j.fct.2011.02.002
7. Dilruba S, Kalayda GV. Platinum-based drugs: past, present and future. Cancer Chemother Pharmacol (2016) 77(6):1103–24. doi: 10.1007/s00280-016-2976-z
8. Bedoschi GM, Navarro PA, Oktay KH. Novel insights into the pathophysiology of chemotherapy-induced damage to the ovary. Panminerva Med (2019) 61(1). doi: 10.23736/S0031-0808.18.03494-8
9. Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol (2016) 12(20):2333–44. doi: 10.2217/fon-2016-0176
10. Davis SR, Baber RJ. Treating menopause - MHT and beyond. Nat Rev Endocrinol (2022) 18(8):490–502. doi: 10.1038/s41574-022-00685-4
11. Shah D, Nagarajan N. Premature menopause - Meeting the needs. Post Reprod Health (2014) 20(2):62–8. doi: 10.1177/2053369114531909
12. Cordts EB, Christofolini DM, Santos AAD, Bianco B, Barbosa CP. Genetic aspects of premature ovarian failure: a literature review. Arch Gynecol Obstet (2011) 283(3):635–43. doi: 10.1007/s00404-010-1815-4
13. Beke A, Piko H, Haltrich I, Csomor J, Matolcsy A, Fekete G, et al. Molecular cytogenetic analysis of Xq critical regions in premature ovarian failure. Mol Cytogenet (2013) 6(1):62. doi: 10.1186/1755-8166-6-62
14. Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J Mol Endocrinol (2010) 45(5):257–79. doi: 10.1677/JME-10-0070
15. Caburet S, Arboleda VA, Llano E, Overbeek PA, Barbero JL, Oka K, et al. Mutant cohesin in premature ovarian failure. N Engl J Med (2014) 370(10):943–9. doi: 10.1056/NEJMoa1309635
16. Ayesha, Jha V, Goswami D. Premature ovarian failure: an association with autoimmune diseases. J Clin Diagn Res (2016) 10(10):Qc10–qc12.
17. Gao H, Gao L, Wang W. Advances in the cellular immunological pathogenesis and related treatment of primary ovarian insufficiency. Am J Reprod Immunol (2022) 88(5):e13622. doi: 10.1111/aji.13622
18. Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update (2012) 18(5):525–35. doi: 10.1093/humupd/dms022
19. Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol Reprod (2015) 93(3):67.
20. Leetanaporn R, Tintara H. A comparative study of outcome of laparoscopic salpingo-oophorectomy versus open salpingo-oophorectomy. J Obstet Gynaecol Res (1996) 22(1):79–83. doi: 10.1111/j.1447-0756.1996.tb00941.x
21. Thakur M, Feldman G, Puscheck EE. Primary ovarian insufficiency in classic galactosemia: current understanding and future research opportunities. J Assist Reprod Genet (2018) 35(1):3–16. doi: 10.1007/s10815-017-1039-7
22. Wolthuis DF, Janssen MC, Cassiman D, Lefeber DJ, Morava E. Defining the phenotype and diagnostic considerations in adults with congenital disorders of N-linked glycosylation. Expert Rev Mol Diagn (2014) 14(2):217–24. doi: 10.1586/14737159.2014.890052
23. Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E, et al. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol (2004) 44(1):6–9. doi: 10.1111/j.1479-828X.2004.00143.x
24. Rossi V, Lispi M, Longobardi S, Mattei M, Di Rella F, Salustri A, et al. LH prevents cisplatin-induced apoptosis in oocytes and preserves female fertility in mouse. Cell Death Differ (2017) 24(1):72–82. doi: 10.1038/cdd.2016.97
25. Clark JS, Faisal A, Baliga R, Nagamine Y, Arany I. Cisplatin induces apoptosis through the ERK-p66shc pathway in renal proximal tubule cells. Cancer Lett (2010) 297(2):165–70. doi: 10.1016/j.canlet.2010.05.007
26. Oka T, Kimura T, Suzumura T, Yoshimoto N, Nakai T, Yamamoto N, et al. Magnesium supplementation and high volume hydration reduce the renal toxicity caused by cisplatin-based chemotherapy in patients with lung cancer: a toxicity study. BMC Pharmacol Toxicol (2014) 15:70. doi: 10.1186/2050-6511-15-70
27. Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol (2006) 24(18):2932–47. doi: 10.1200/JCO.2006.06.9591
28. Koinis F, Nintos G, Georgoulias V, Kotsakis A. Therapeutic strategies for chemotherapy-induced neutropenia in patients with solid tumors. Expert Opin Pharmacother (2015) 16(10):1505–19. doi: 10.1517/14656566.2015.1055248
29. Kim KH, Lee B, Kim Y-R, Kim M-A, Ryu N, Da Jung J, et al. Evaluating protective and therapeutic effects of alpha-lipoic acid on cisplatin-induced ototoxicity. Cell Death Dis (2018) 9(8):827. doi: 10.1038/s41419-018-0888-z
30. Cartwright B, Robinson J, Seed PT, Fogelman I, Rymer J. Hormone replacement therapy versus the combined oral contraceptive pill in premature ovarian failure: A randomized controlled trial of the effects on bone mineral density. J Clin Endocrinol Metab (2016) 101(9):3497–505. doi: 10.1210/jc.2015-4063
31. Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab (2004) 89(8):3907–13. doi: 10.1210/jc.2004-0015
32. Kim J, Kim M, Lee JH, Lee H, Lee SK, Bae SY, et al. Ovarian function preservation with GnRH agonist in young breast cancer patients: does it impede the effect of adjuvant chemotherapy? Breast (2014) 23(5):670–5. doi: 10.1016/j.breast.2014.07.005
33. Pinelli S, Artini PG, Basile S, Obino MER, Sergiampietri C, Giannarelli D, et al. Estrogen treatment in infertile women with premature ovarian insufficiency in transitional phase: a retrospective analysis. J Assisted Reprod Genet (2017) 35(3):475–82.
34. Leonard RCF, Adamson DJA, Bertelli G, Mansi J, Yellowlees A, Dunlop J, et al. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol (2017) 28(8):1811–6. doi: 10.1093/annonc/mdx184
35. Costa GPO, Ferreira-Filho ES, Simoes RDS, Soares-Junior JM, Baracat EC, Maciel GAR, et al. Impact of hormone therapy on the bone density of women with premature ovarian insufficiency: A systematic review. Maturitas (2023) 167:105–12. doi: 10.1016/j.maturitas.2022.09.011
36. Adams HJA, Kwee TC. The predictive value of interim FDG-PET in early-stage Hodgkin lymphoma is not well established. Ann Oncol (2018) 29(2):510–2. doi: 10.1093/annonc/mdx644
37. Turan V, Bedoschi G, Rodriguez-Wallberg K, Sonmezer M, Pacheco FS, Oktem O, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation: lack of biologic basis and the need to prioritize proven methods. J Clin Oncol (2019) 37(1):84–6. doi: 10.1200/JCO.18.00420
38. Li M, Xiao Y-B, Wei L, Liu Q, Liu P-Y, Yao J-F. Beneficial effects of traditional Chinese medicine in the treatment of premature ovarian failure. Evid Based Complement Alternat Med (2022) 2022:5413504. doi: 10.1155/2022/5413504
Keywords: cisplatin, premature ovarian failure, apoptosis, protection strategies, research and analysis
Citation: Li Z, Qi H, Li Z, Bao Y, Yang K and Min Q (2023) Research progress on the premature ovarian failure caused by cisplatin therapy. Front. Oncol. 13:1276310. doi: 10.3389/fonc.2023.1276310
Received: 11 August 2023; Accepted: 06 November 2023;
Published: 20 November 2023.
Edited by:
Berna Dilbaz, University of Health Sciences (Turkey), TürkiyeReviewed by:
Giuliano Marchetti Bedoschi, University of Sao Paulo, BrazilCopyright © 2023 Li, Qi, Li, Bao, Yang and Min. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Min, MTU2OTU2MzQxN0BxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.