
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 20 October 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1275800
This article is part of the Research TopicReviews in Hematologic Malignancies: 2023View all 15 articles
Nearly a billion people worldwide are infected with the hepatitis B Virus (HBV) and about a third of them have chronic infection. HBV is an important cause of morbidity and mortality, including acute and chronic hepatitis and hepatocellular carcinoma (HCC). Screening and control of primary HBV infection through vaccination represent a major advance in global public health, but large sections of the world population, in both developed and underdeveloped countries, remain unscreened and unvaccinated. In addition to being a global cause of liver disease, an important role of HBV in lymphoma has also emerged. First, the high risk of HBV reactivation in previously infected patients receiving chemo-immunotherapy necessitates the systematic evaluation of HBV serological status in all non-Hodgkin’s lymphoma (NHL) cases and preemptive antiviral therapy for those who may have chronic or occult HBV infection. Second, HBV has been shown to infect lymphocytes, namely B-cells, and has been associated with a higher risk of developing B-cell lymphoma, most clearly in countries where HBV is endemic. While the risk of HBV reactivation with chemoimmunotherapy in NHL is well known, the role and the impact of HBV as a global lymphoma risk factor and potential oncogenic driver in B-cells are very poorly understood. Here, we review the clinical and scientific evidence supporting an association between HBV and B-cell lymphoma, with a particular focus on diffuse large B-cell lymphoma (DLBCL) and provide an overview of the estimated impact of HBV infection on the biology and clinical course of DLBCL. We also discuss ways to gain a better insight into the unmet need posed by HBV in lymphoma and whether assessing immune responses to HBV, measuring viral loads, and detecting the presence of HBV-encoded proteins in tumor tissue could be integrated into the molecular and clinical risk stratification of patients with DLBCL.
Viral infections are associated with several types of cancer with widely different levels of risk in different populations and geographical areas and the global role of viruses as oncogenic drivers is widely recognized. Six viruses (HBV, Hepatitis C Virus [HCV], Epstein-Barr Virus [EBV], Human papillomavirus [HPV], Human T-Cell Lymphotropic Virus [HTLV-1], and Human Herpes Virus-8 [HHV-8]) are classified as class I carcinogenic agents by the International Agency for Research on Cancer (IARC) (1) and about 10% of all cancers worldwide can be attributed to viruses (2). Global vaccination campaigns for HPV and HBV have been implemented, and efforts to test the efficacy and safety of vaccines for EBV are ongoing in clinical trials (NCT04645147, NCT05164094) (3). In addition, ongoing studies show the potential of targeted therapies for virus-associated cancers. EBV-targeting adoptive cellular immunotherapies (4) and new treatments that use the presence of oncogenic viruses as an intrinsic tumor-specific vulnerability are being investigated. For example, a small molecule inhibiting the EBV protein EBNA-1 (VK-2019) has shown promising anti-tumor efficacy in preclinical models of EBV-positive cancers (5) and a Phase I clinical trial of VK-2019 in nasopharyngeal carcinoma (NPC) is ongoing (NCT04925544). Additionally, the combination of the histone deacetylase (HDAC) inhibitor Nanatinostat and the nucleoside analog valganciclovir (VGCV) was recently granted orphan drug designation (ODD) for EBV-positive lymphomas by the FDA, based on initial Phase 1/2 data (6), and an international multi-cohort Phase 2 clinical trial (Naval-1) is ongoing (NCT05011058) (7). These studies show the potential of developing targeted therapies for virus-associated cancers.
Awareness of the linkage between carcinogenic viruses and cancers remains inadequate in the public and in parts of the medical and advocacy communities. This is the case of the association between prior infection with HBV and risk of B-cell lymphoma, in particular diffuse large B-cell lymphoma (DLBCL), the most common type of aggressive B-cell lymphoma worldwide (1). While the importance of chronic HBV infection in the development of HCC is well established, its role as a risk factor for DLBCL is less known. Consequently, education efforts to increase awareness of symptoms of DLBCL among HBV seropositive patients are inadequate, potentially leading to a delay in diagnosis of DLBCL in this patient population. Likewise, the impact of HBV vaccination and antiviral therapy on the risk of developing DLBCL remains unknown.
The goal of this paper is to provide a background on HBV infection and DLBCL, and then critically review the evidence supporting a role for chronic HBV infection in DLBCL, outline the criteria currently defining HBV-associated DLBCL, provide available estimates of its frequency and distribution globally and in the U.S., and review the distinctive aspects of HBV-associated DLBCL that have been identified. We will also offer an assessment of the unmet need and opportunity in terms of scientific discovery and public health impact.
In the United States, 850,000 individuals are estimated to be living with HBV (8). The prevalence of past or present HBV infection amongst people in the U.S. is 4.3% (9). However, populations of foreign-born minorities, with a higher prevalence of HBV, are likely underrepresented in this calculation. This pathogen is thought to be responsible for over 296 million chronic infections worldwide (10). Further, in 2021, the World Health Organization (WHO) reported that only 30.4 million people living with HBV knew their HBV status, accounting for only 10% of the total people in the world living with chronic HBV infection (10).
HBV infection carries a heavy financial toll for patients and healthcare systems, with total HBV hospitalization charges in the U.S. increasing from $357 million in 1990 to $1.5 billion in 2003 (11). In 2019, the total mean all-cause annual healthcare costs for HBV patients with Medicare who had decompensated cirrhosis, HCC, received liver transplants, or had compensated liver disease was $479,595 (12). This financial burden is felt globally (13). The significant financial and health impact of HBV makes understanding this virus and its sequelae important.
HBV is an enveloped virus with a circular, partially double-stranded DNA genome, which belongs to the Hepadnaviridae family. The infectious virion consists of a lipid envelope containing the HBV surface antigen (HBsAg). This surrounds an inner nucleocapsid composed of the HBV core antigen (HBcAg) complexed with virally encoded polymerase (14). The viral genome contains 4 overlapping open reading frames that encode proteins essential for viral replication (14). HBV entry into host cells is mediated by low-affinity binding to heparan sulfate proteoglycans (HSPGs), followed by high-affinity binding to sodium taurocholate co-transporting polypeptides (NTCPs). Glypican 5 is an HSPG that preferentially binds HBV (15, 16). HSPGs are found at the surface and in the extracellular matrix of most human cells. At least some of the virus’ hepatotropic nature is thought to be due to the prevalence of glypican 5 in the liver.
Worldwide, the most common route of HBV transmission is perinatal transmission, especially in endemic areas (15). HBV is also transmitted through percutaneous and mucous membrane exposures and sexual intercourse with infected individuals (8). Once the infection is acquired, the host can experience an acute infection with complete recovery, a fulminant course with hepatic failure, or a chronic infection (17).
The infection is diagnosed by detecting HBsAg and anti-hepatitis B core IgM antibody (HBcAb) in plasma. Chronic HBV is characterized by the persistent presence of HBsAg in the serum for greater than 6 months, in addition to HBcAb (18). Chronic HBV infections are typically characterized by four phases, varying in length and defined by laboratory results and clinical symptoms.
Mortality among adults with chronic HBV far exceeds that of uninfected individuals. One study including 39,206 patients concluded that those with chronic HBV infection had a 1.9-fold (95% CI 1.1–3.3) increased hazard of all-cause mortality compared to uninfected people, and a 13.3-fold (95% CI 3.9–45.5) increased hazard of liver-related mortality (19). Hepatocellular carcinoma (HCC) is closely associated with HBV, with studies showing that chronically infected individuals have 100 times the risk of developing HCC than non-carriers (20). Unfortunately, deaths from chronic HBV infection are increasing, with a rising incidence of HCC of particular concern in endemic areas like Africa and the Western Pacific (21). Professional medical societies, such as World Health Organization (WHO) and the American Association for the Study of Liver Diseases (AASLD), vary in their recommendations for screening and treatment (22). There are significant barriers to effective HBV screening, prevention, and treatment, with an impact that may not be limited to chronic disease and HCC, but may include B-cell lymphoma, specifically diffuse large B-cell lymphoma (DLBCL).
Non-Hodgkin’s Lymphomas (NHLs) are hematologic malignancies of mature lymphocytes and one of the more common cancers in the United States, accounting for about 4% of all cancers (23). DLBCL is an aggressive NHL that comprises about 30% of all lymphoma cases. It is the most common subtype of NHL in the U.S (24) and worldwide (25). DLBCL is most prevalent in elderly patients, with a median age at diagnosis in the 7th decade of life. The incidence of DLBCL varies by race, with racial differences in age and gender distribution (26–28). However, the incidence of DLBCL increases with age for all races (27).
In clinical practice, DLBCL is frequently classified based on immunohistochemistry (IHC)-defined cell-of-origin (COO). There are two major subtypes, a germinal center B-cell (GCB) type and a non-GCB type, corresponding to the activated B-cell (ABC) type defined by gene expression profiling (29). The use of cytogenetics and fluorescence in situ hybridization (FISH) further classify DLBCL by identifying chromosomal translocations in tumor cells, in particular rearrangements involving MYC (8q24), BCL-2 (18q21), and BCL-6 (3q27). DLBCL carrying genetic rearrangements of both MYC and BCL-2 genes, with or without a rearranged BCL-6 gene, were formerly known as double-hit (or triple-hit if BCL-6 is also involved) lymphomas. Double hit and triple hit (DH/TH) DLBCL represent approximately 10% of all DLBCL and are particularly aggressive and chemotherapy-resistant. Data are emerging about the impact of deletions or inactivating mutations of TP53, a tumor suppressor gene involved in cell cycle arrest and apoptosis. TP53 mutations are present in about 10% of DLBCL cases and are an independent predictor of poor prognosis (30, 31). More recently, next generation sequencing (NGS) studies of whole genomes and transcriptomes in untreated DLBCL have identified several genetic clusters and molecular subgroups characterized by specific cancer-driving signatures and epigenetic pathways, with distinct outcomes (32–34).
The International Prognostic Index (IPI) is a risk stratification tool for DLBCL patients taking into account age, performance status, serum lactate dehydrogenase (LDH), number of extranodal sites, and stage (35). This scale, or one of its modifications, is the mainstay of risk stratification in DLBCL (36). While multiple studies report inferior outcomes in patients with EBV-positive (37), HCV-positive (38), and HBV-positive lymphomas (39), serological status and quantitative viral load measurements have not been included in any of the risk-stratification tools for lymphoma, except for plasma EBV DNA in extranodal NK/T-cell lymphoma (ENKTL) (40).
The standard first-line therapy for most DLBCL patients is the combination of the chemotherapy regimen CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), with rituximab, a monoclonal antibody that targets the pan-B cell surface antigen CD20. This regimen is referred to as R-CHOP. With this regimen, 60-65% of patients are cured. The prognosis is poor for 35-40% of patients who relapse following R-CHOP or have refractory disease. Current research is focused on finding better risk-stratification tools, to identify patients who will do well with R-CHOP versus those who may require more aggressive regimens up-front.
Patients with DH/TH DLBCL generally have more aggressive clinical courses, with advanced-stage presentation, extranodal involvement, higher serum LDH, and a high IPI score. DH/TH DLBCL carries a particularly poor prognosis, with a 5-year survival of <30% (41). TP53 is also commonly mutated in DH/TH DLBCL and Double-Hit Signature (DHITsig)-positive DLBCL, which adds an unfavorable feature to these patients (42). Therapeutic approaches more aggressive than R-CHOP are often used for DH DLBCL, but overall survival rates remain poor (43).
It is well known that HBV increases the risk of HCC. Studies show that chronically HBV-infected individuals have 100 times the risk of developing HCC than non-infected individuals (20). Epidemiological and seroprevalence studies have shown that HBV also increases the risk of other types of cancer, such as NHL. This association, particularly between HBV and B-cell lymphomas, has been documented in studies from both endemic and non-endemic areas. A 2007 case-control study conducted in the U.S. showed that patients (N=3,888) with chronic HBV infection were 2.8 times more likely to develop NHL than matched controls (N=205,203; HR = 2.80, 95% CI = 1.16-6.75). This study controlled for age, race, sex, income, Charlson comorbidity index, study site, and HCV infection (44). A recent 2018 meta-analysis of 58 published studies included data on 53,714 NHL cases and 1,778,591 controls. The studies were from Asia (N=46), Europe (N=8), North America (N=2), Africa (N=1) and Oceania (N=1). The meta-analysis found that HBV-infected individuals were 2.5 times more likely to develop NHL than non-infected individuals (45). These findings have been replicated (46, 47). A recent cohort study by Spradling (47) used data from the U.S. National Cancer Institute (NCI) and the National Program of Cancer Registries to assess the incidence of specific cancers in patients ≥20 years old with HBV compared to non-HBV-infected patients of the same age range. Patients with previous or current HCV or Human Immunodeficiency Virus (HIV) coinfection were excluded. Results showed, with a 95% confidence interval, that patients with HBV had over 2.5 times the risk of developing NHL compared to the general population. Thus, multiple case-control and cohort studies from endemic and non-endemic areas show that HBV infection is associated with a 2-3 fold higher risk of developing NHL, compared to controls, leading to the question of the mechanism behind this association (Table 1, Figure 1).
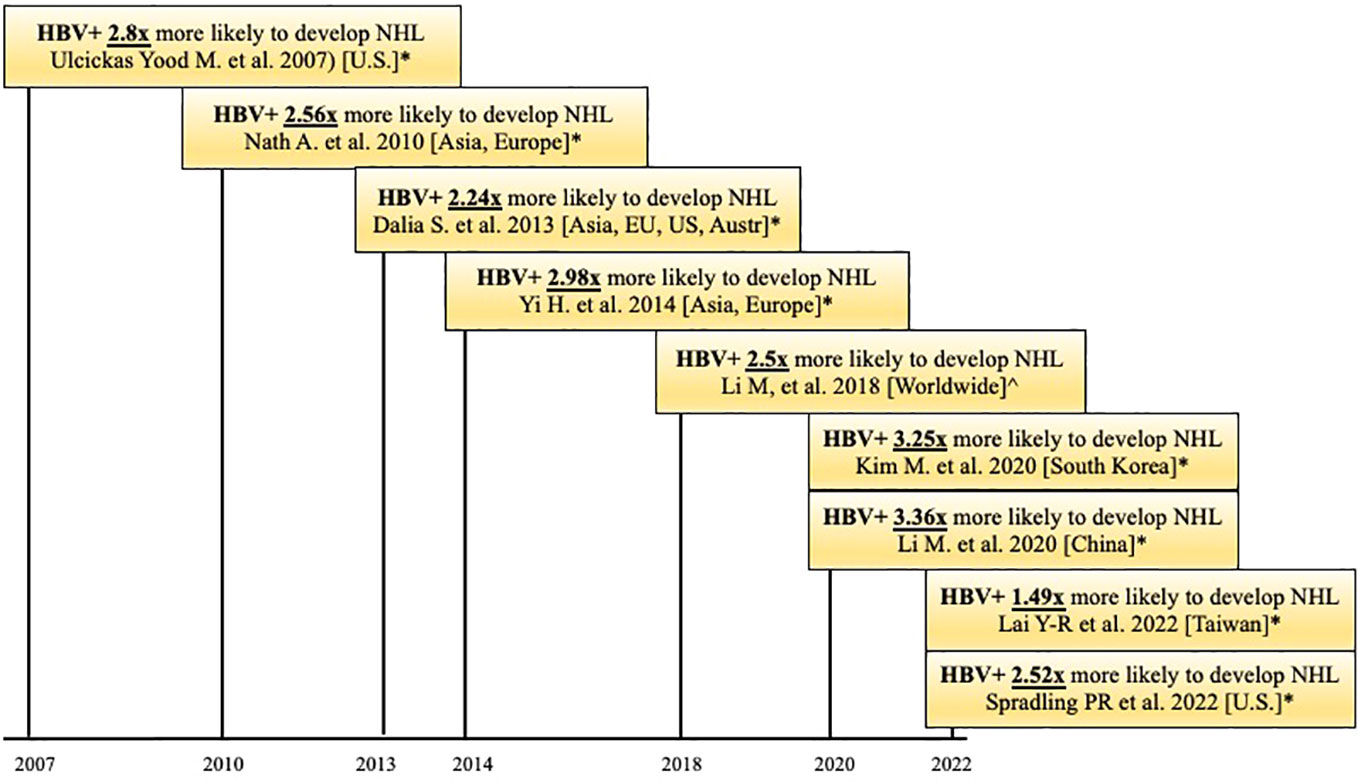
Figure 1 Timeline of published case-control and cohort studies (*) and meta-analyses (^) that observed that HBV patients are more likely to develop Non-Hodgkin Lymphoma (NHL). HBV+ = positive for HBsAg.
The ability of HBV to infect human lymphocytes has been reported in multiple studies but remains poorly understood (Table 1). A 2011 study used human bone marrow (BM) from the iliac crest of healthy volunteers aged 18-36 years, who had no serologic evidence of current or previous HBV infection. BM cells were exposed to HBV in vitro for 24 hours. Following a ten-week incubation period, the BM stem cells were harvested, and their DNA was extracted. HBsAg and HBeAg levels were then measured using electrochemiluminescence (56). This approach assessed the efficiency with which HBV productively infected bone marrow stem cells in vitro, based on the expression of HBV-encoded proteins. Results showed that the infection efficiency was comparable to its ability to infect primary human hepatocytes and human hepatoma cell lines (56). Later studies confirmed this finding (57). It was further shown that the HBV residing in a patient’s BM stem cells could infect HBV-naïve hepatocytes in transplanted livers. This finding was important as it presented a possible source of graft re-infection following stem cell transplantation in patients with chronic HBV (58, 59).
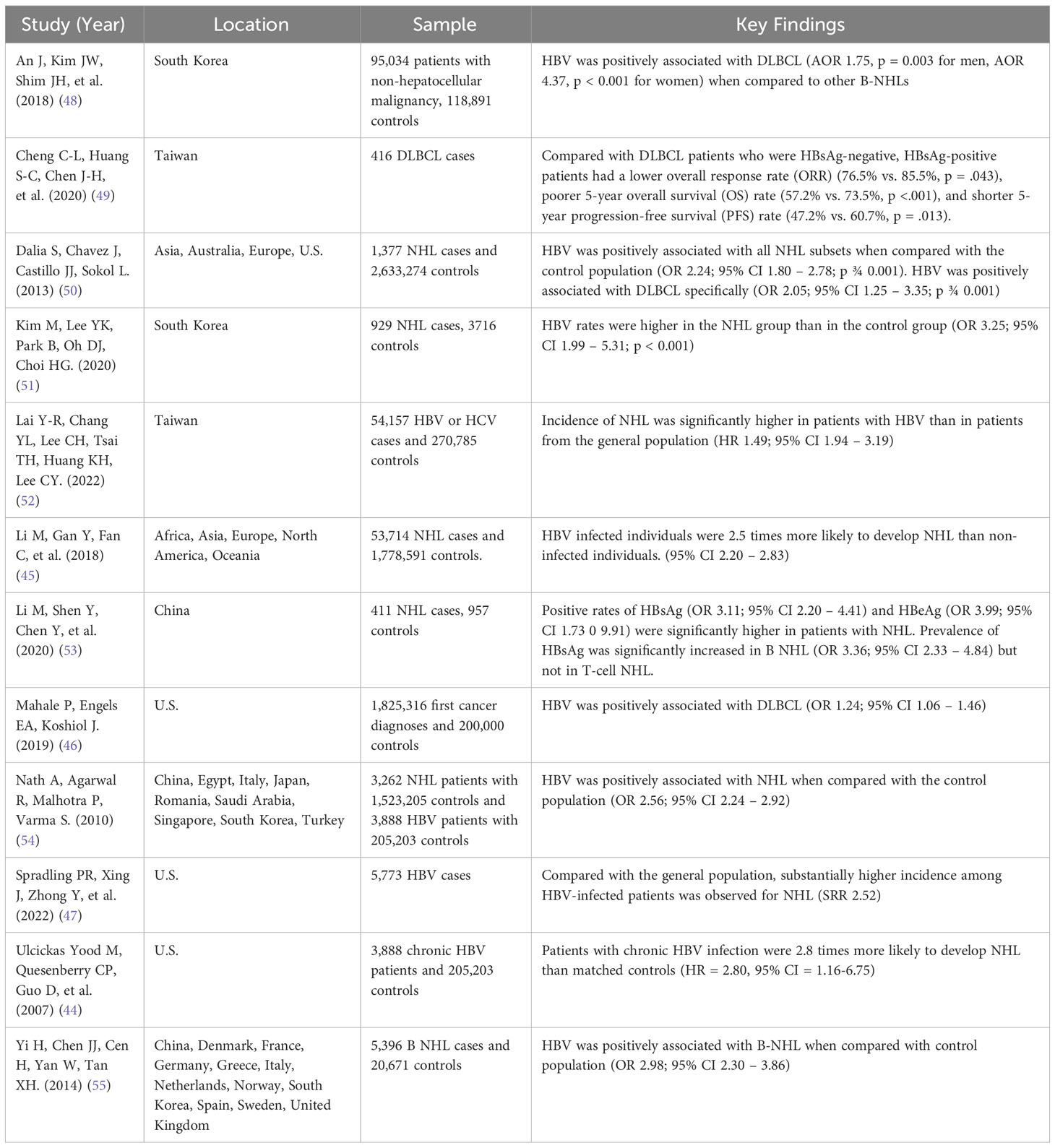
Table 1 Summary of studies analyzing HBV and its effect on Non-Hodgkin Lymphoma (NHL) and Diffuse Large B-Cell Lymphoma (DLBCL).
During HBV’s life cycle, viral DNA can be integrated into the genome of infected cells (60). Integrated genomes can serve as templates for RNAs coding for viral proteins. HBV genome integration is a primary driver of HCC. Known cancer genes such as the telomerase reverse transcriptase (TERT) (61), mixed-lineage leukemia 4 (MLL4) (62), and Cyclin E1 (CCNE1) (63) are preferential integration sites in HCC and about one-third of the genes recurrently targeted by HBV integration are cancer-related genes (64). Recently, Svicher et al. published an overview of the mechanisms of HBV DNA integration into immune cells, highlighting the hypothesis that the oncogenic effect of HBV in lymphoma is driven by the integration of HBV DNA into lymphocytes (65). It was noted that peripheral blood mononuclear cells (PBMCs) may act as a extrahepatic reservoir for HBV infection.
HBV DNA integration has been shown to affect multiple gene sites (65), as shown in Figure 2. A 2020 study identified the integration of HBV DNA in the lymphoma cells of 34 individuals with NHL (53). In total, 313 integration sites were identified. Half of the integration occurred in intergenic regions (49.5%), and the remaining took place in introns (44.7%), 3’-untranslated region (1.6%), gene upstream region (1.3%), and gene downstream region (1.3%). In the NHL samples, HBV integration had preferential targets. Seven genes, ANKS1B, CAPZB, CTNNA3, EGFLAM, FHOD3, HDAC4, and OPCML were found to be repeatedly targeted by HBV DNA integration. HBV DNA is regarded as a strong cis activator of flanking genes, so integration can influence the expression of target genes over a long distance (60). Six of the seven genes were found to be overexpressed in NHL, based on publicly available databases such as TCGA. KEGG (Kyoto Encyclopedia of Genes and Genomes) analysis of the HBV-targeted genes in NHL revealed that terms related to developmental process and cell differentiation, signal transduction, cell junction, and transcriptional regulation were significantly enriched (p<.05). Axon guidance was the most impacted pathway (p<0.0001), followed by Ras signaling, glycosaminoglycan biosynthesis, and cytokine-cytokine receptor interaction (p<.05). These pathways are also enriched in studies of HBV-targeted genes in HCC which implies that some pathways are commonly affected by HBV integration in HCC and NHL. This study is important, as it demonstrates that HBV DNA can integrate into the genome of NHL cells, affecting genes that have been reported to play an oncogenic role in other cancers.
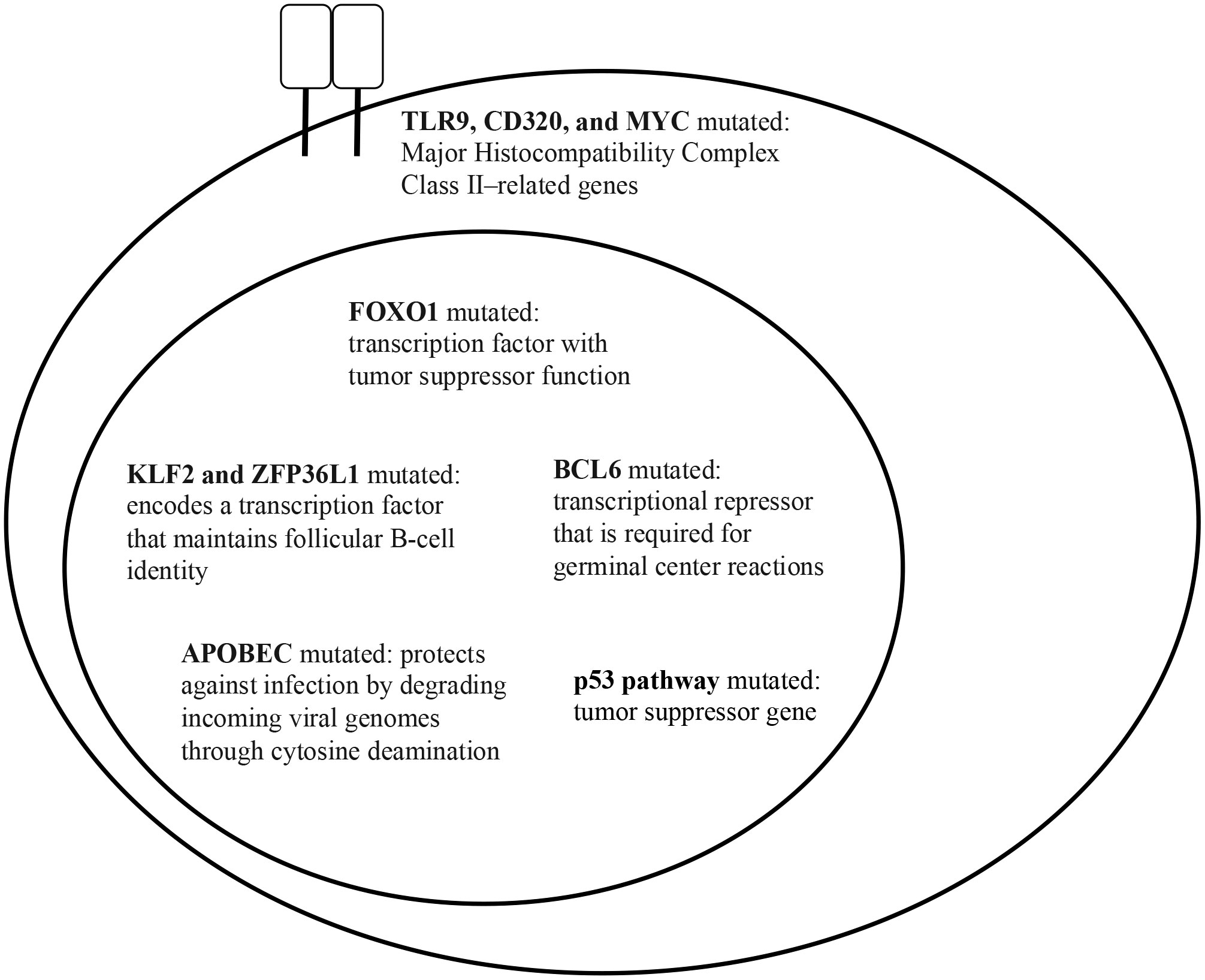
Figure 2 HBsAg+ DLBCL key gene mutations (data taken from Ren W. et al. (39)).
Numerous in vitro and in vivo studies have shown the lymphotropism of HBV. In vitro, HBV was shown to be able to infect bone marrow progenitor cells and inhibit their growth (66). Mature lymphocytes were also shown to be infected with HBV, with HBV mRNA found in B-cells and T-cells (67). In vivo studies, most notably with chimpanzees, showed that chimpanzees with chronic HBV infection showed HBV DNA in PMBCs (68). Svicher et. al. further discusses the evidence that HBV infection is persistent in hematopoietic and lymphoid cells, which may act as a site for HBV reactivation and genome changes (65).
Multiple retrospective studies and meta-analyses have shown that in patients chronically infected with HBV, the most common type of NHL is DLBCL (16, 39, 50, 54, 55). HBV-associated DLBCL has been shown to have an incidence of 14.3% in West Africa and, according to a meta-analysis, has poor prognosis (69, 70). A 2018 study by An et al. (48) revealed a significantly positive link between HBV infection and DLBCL (adjusted odds ratio [AOR] 1.75, p = 0.003 for men, and AOR 4.37, p < 0.001 for women) when compared to other B-NHL. A 2020 study by Cheng et al. (49), including 426 patients with DLBCL at the National Taiwan University Hospital in Taipei, Taiwan, found that 23.6% of the patients were positive for HBsAg. When compared to HBsAg- patients, HBsAg+ patients were younger, diagnosed more frequently with advanced-stage disease, had lower overall response rates to R-CHOP (57.2% vs 73.5%, p < 0.001), and had shorter 5-year progression-free survival rates (47.2% vs 60.7%, p=0.013) (49). Another 2020 study, including data from 929 patients with NHL and 3716 healthy subjects in Korea, found that HBV rates were higher in the NHL group than in the control group (p < 0.001). The adjusted OR of HBV infection in patients with NHL was 3.25 (95% CI, 1.99 – 5.31) (51). Lastly, a 2022 retrospective cohort study used the nationally representative database in Taiwan to investigate the correlation between HBV and NHL. The study showed that the incidence rate of NHL was significantly higher in patients with HBV than in patients from the general population (HR, 2.49; 95% CI, 1.94 – 3.19) (52) (Table 2).
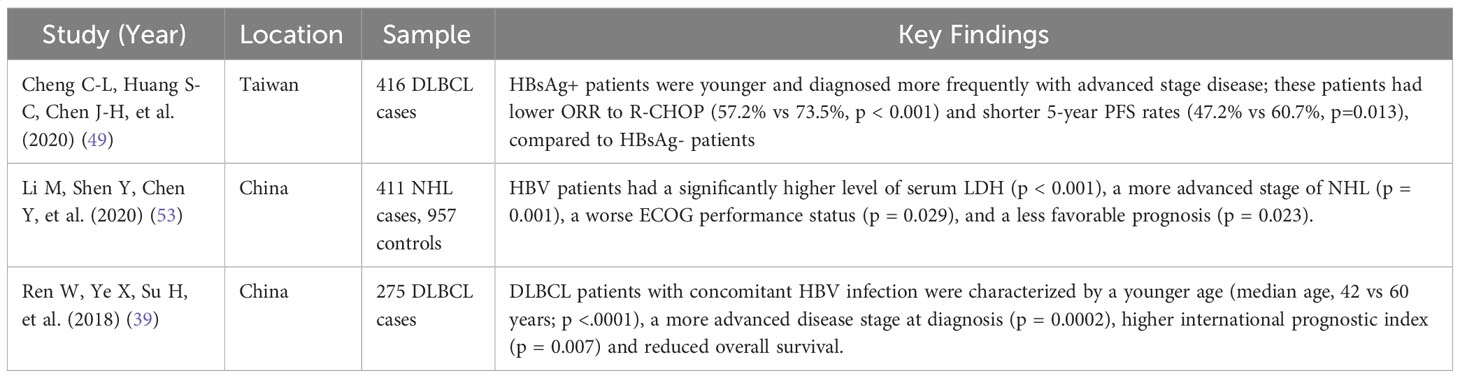
Table 2 Summary of studies showing differential outcomes in HBV-positive and HBV-negative DLBCL patients.
HBV proteins can be detected in tissue biopsies from patients with DLBCL and chronic HBV infection. Huang et al. assessed tumor biopsies from 96 HBsAg+ and 10 HBsAg- DLBCL patients treated at five Chinese centers (16). The HBV antigen HBx, a protein essential for viral replication, was present in the lymphoma cells in 48.9% of HBsAg+ DLBCL patients; additionally, the HBV antigen Pre-S2, a component of HBsAg, was detected in the lymphoma cells of 57.2% HBsAg+ DLBCL patients. The authors also showed that the presence of HBx antigen in DLBCL cells was associated with high MYC expression (Table 3).
The mutational landscape of HBV-associated DLBCL in a cohort of 275 Chinese patients was assessed in a landmark 2018 study by Ren and colleagues (39). This study showed that DLBCL patients with concomitant HBV infection were characterized by a younger age (median age, 42 vs. 60 years; p <.0001), more advanced disease stage at diagnosis, and shorter overall survival. GC-type and ABC-type DLBCL were equally frequent. An enhanced rate of mutagenesis and an increased total mutation load were observed in HBsAg+ DLBCL genomes (median, 15,036 vs. 9,902 mutations). In addition, more non-silent mutations were observed in HbsAg+ DLBCLs (median, 99 vs 66). The genome-wide mutational signatures of 60 DLBCL cases were characterized based on the 96 possible mutation types. Seven mutational signatures were extracted from the cohort and three were significantly enriched in HBsAg+ tumors. One of the signatures was linked to APOBEC enzymes, a family of proteins with anti-viral functions (71), suggesting that HBV-associated DLBCLs are associated with distinct mutational signatures. Additionally, TMSB4X, FAS, UBE2A, DDX3X, CXCR4, KLF2, and SGK1, were significantly more mutated in the HBsAg+ group. Some of these genes are potential targets for activation-induced cytidine deaminase (AID), the driver of somatic hypermutation (SHM) in the immunoglobulin (Ig) genes during B-cell maturation and selection in the germinal center. The study also showed that the frequency of chromosomal translocations involving BCL6 was significantly increased in HBsAg+ DLBCL genomes (57% vs 28%; p = .0472), suggesting that BCL6 dysregulation plays a role in HBV-associated DLBCL. Finally, antigen processing and p53 signaling pathway-associated genes were significantly upregulated in HBsAg+ DLBCLs (Figure 3). These mutations or deletions carry a particularly poor prognosis for DLBCL. Interestingly, most of the genes that were mutated in HBV-associated DLBCLs were not mutated in HBV-associated HCC or HBV-positive lung adenocarcinoma, suggesting that genetic alterations found in HBV-associated DLBCL may be B-cell specific. The finding of APOBEC signatures and the lack of sequence homology of the CD3 region on characterization of the V(D)J region of immunoglobulin heavy chain (IgH) suggest that the lymphoma was due to direct carcinogenic effects of the virus rather than due to chronic antigen stimulation. Based on these observations, the authors proposed that HBsAg+ DLBCL should be considered and classified as a distinct subtype of DLBCL (Table 4).
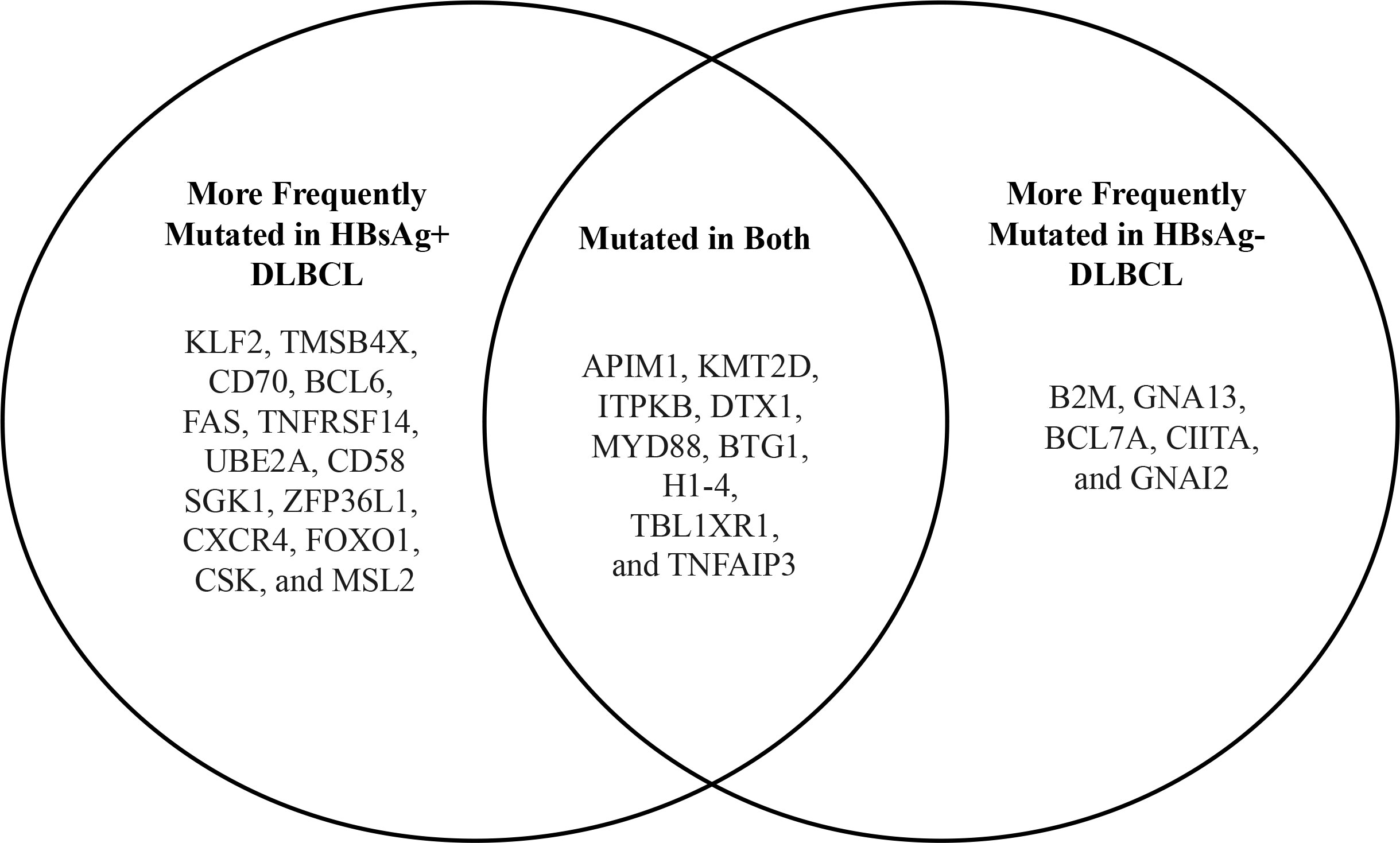
Figure 3 Comparison of mutated genes from HBsAg+ and HBsAg- DLBCL (data from Ren W. et al. (39)).
Most of the sero-epidemiological studies that have assessed the link between B-cell NHL and HBV, and in particular the impact of chronic HBV infection on clinical outcomes, have been performed in East Asian countries, where HBV is endemic. Several environmental and host- or tumor-specific factors, however, may confound the strength of the association between HBV infection and clinical outcome in B-cell NHL, including DLBCL. In addition, while two positive meta-analyses have been published, the relatively limited sample size of each individual study (the largest study by Ren et al. had 275 patients) and the bias inevitably present in retrospective datasets make any final conclusion about the impact of HBV on survival and response to treatment in DLBCL premature. It is therefore of significant interest to determine if such findings can be confirmed in non-endemic countries, in populations of different racial and ethnic backgrounds, and ideally in larger studies. In terms of ascertaining relative risk, considering that the prevalence of HBV infection is not as high in the U.S., it will be more challenging to validate the higher prevalence of B-NHL among patients with HBV infection, compared to endemic countries. However, focusing on subsets of U.S. patients with a higher prevalence of HBV infection but not from endemic areas may mitigate this challenge. A large enough multi-center retrospective study in North America or Europe in an unselected DLBCL population could provide a dataset addressing whether the higher prevalence and inferior survival outcomes of patients with concomitant HBV and DLBCL observed in Asia can be confirmed. If a study in non-endemic areas were to strongly suggest or confirm the inferior clinical outcome of DLBCL patients with concomitant HBV, it would also be important to determine if these disparities affect specific subsets of DLBCL patients, based on patient characteristics (ethnicity, race, age, comorbidities) or subtype of DLBCL (cell-of-origin, double hit, or double expressor status) defined by the standard of care methods (IHC, FISH). These questions can be addressed by a retrospective study, since all patients diagnosed with B-NHL in the U.S., including those with DLBCL, are screened for prior HBV infection prior to initiating therapy, with serologies for HBsAg, HBsAb, and HBcAB.
Of even greater interest is whether the mutational spectrum and the prevalence and specificity of the genomic signatures described by Ren and colleagues in HBV-associated DLBCL can be confirmed, although such studies will require more resources and coordination among centers. Given the increase in mutagenesis observed in HBsAg+ DLBCL (39), it would be important to determine if there is an association between HBV and one or more of the genetic subtypes of DLBCL defined by Schmitz et al. (34) and Chapuy et al. (32) The off-target AID-associated mutagenesis observed in lymphoma cases with HBV integration and the BCL6 chromosomal translocations in HBV associated DLBCL (39) could potentially lead to a higher prevalence of DH/TH lymphomas among HBV associated DLBCL. By specifying the DLBCL subtypes most impacted by HBV, it might also be possible to propose a mechanism by which HBV infection contributes to the development of DLBCL, and more broadly, B-cell NHL. This could also elucidate new prognostic factors and aid in earlier detection, treatment, or prevention of NHL in chronic HBV patients. Finally, it remains unclear, even from the retrospective studies conducted in Asia, whether past HBV exposure (HBsAg-, HBcAb+) confers an increased risk of B-NHL and worse clinical outcomes for B-NHL, or whether the presence of chronic HBV infection (HBsAg+, HBcAb+) is what ultimately confers these risks (discussed below).
Because HBV virus antigens can be detected in DLBCL cells via IHC in HBsAg+ patients using formalin-fixed paraffin-embedded (FFPE) tissue (16), the clinical relevance of such findings can be assessed in retrospective studies, to determine whether the presence of HBV antigens in the lymphoma cells of HBV associated DLBCL leads to distinct clinical characteristics or outcomes. For example: assuming that a linkage between chronic (or past) HBV infection is confirmed, is the presence of HBV antigens in the lymphoma cells necessary to confer worse clinical outcomes? This will be essential in the design of prognostic tools based on HBV status and for developing personalized therapies for these patients.
In need of further investigation is also the issue of occult HBV infection (OBI), defined as a condition where replication-competent HBV DNA is present in the liver or other tissues, with or without detectable HBV DNA in blood or plasma, in HBsAg-negative patients. HBV genome sequences were recently detected by NGS in plasma, normal B-cells, and tumor tissues from 40 HBsAg-negative DLBCL patients, 27 of which (68%) had OBI (72). Sequencing of these gene segments revealed a high frequency of viral DNA variants, including a T1762A/A1764G missense mutation in the basal core promoter and an HBsAg missense mutation that could account for HBsAg negativity and therefore OBI status. The accumulation of viral variants could provide HBV with a survival advantage and drive lymphomagenesis in DLBCL in the absence of clinically overt chronic HBV infection. Additional support for the clinical impact of OBI in patients with lymphoma comes from studies showing that HBV reactivation in HBsAg-negative lymphoma patients receiving chemo-immunotherapy can occur (73–75), suggesting the need for antiviral prophylaxis in this group, and from a case-control study from Japan showing that patients with OBI had a higher prevalence of DLBCL than other groups (76).
Lastly, the association of HBV with indolent B-cell NHL might also be of interest, considering that HBV-associated follicular lymphoma (FL) was recently reported to also have distinct clinical and genetic features and worse outcomes (77–79).
The available evidence shows that chronic HBV infection is associated with a higher risk of developing B-cell NHL, particularly DLBCL, with odds ratios consistently ranging between 2.0 and 4.0. The relative risk of lymphoma associated with HBV infection is significantly lower compared to that of HCC, but, considering the global prevalence of HBV, it amounts to a very high burden of aggregate lymphoma risk. There is also evidence that DLBCL patients with chronic HBV infection have more aggressive disease, greater frequency of high-risk IPI, and inferior outcomes with R-CHOP, compared to patients without chronic HBV infection. Differential risk, clinical presentation, and outcome studies come almost exclusively from endemic areas, such as China, Taiwan, and Japan, and need to be confirmed in non-endemic settings. HBV-positive DLBCL showed a distinct gene expression profile, spectrum of somatic mutations, and genetic signatures enriched in pathways associated with high mutagenesis, suggesting that HBV-associated DLBCL are a distinct subtype. Finally, the risk of developing B-NHL in patients with OBI needs to be better studied considering the magnitude of the population at risk.
MR: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. MP: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. CT: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. PS: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. AK: Conceptualization, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. PP: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to acknowledge Dr. Chari Cohen, President of the Hepatitis B Foundation in Philadelphia, and Dr. Amy Leader, Associate Professor in the Division of Population Science in the Department of Medical Oncology at Thomas Jefferson University, for their guidance in writing this report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med (2021) 384(9):842–58. doi: 10.1056/NEJMra2027612
2. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health (2016) 4(9):e609–16. doi: 10.1016/S2214-109X(16)30143-7
3. NIH launches clinical trial of Epstein-Barr virus vaccine . National Institutes of Health (NIH. Available at: https://www.nih.gov/news-events/news-releases/nih-launches-clinical-trial-epstein-barr-virus-vaccine (Accessed December 6, 2022).
4. Prockop S, Doubrovina E, Suser S, Heller G, Barker J, Dahi P, et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J Clin Invest (2020) 130(2):733–47. doi: 10.1172/JCI121127
5. Messick TE, Smith GR, Soldan SS, McDonnell ME, Deakyne JS, Malecka KA, et al. Structure-based design of small-molecule inhibitors of EBNA1 DNA binding blocks Epstein-Barr virus latent infection and tumor growth. Sci Transl Med (2019) 11(482). doi: 10.1126/scitranslmed.aau5612
6. Search orphan drug designations and approvals. Available at: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=847721 (Accessed December 7, 2022).
7. Haverkos BM, Alpdogan O, Baiocchi R, Brammer JE, Feldman TA, Capra M, et al. Nanatinostat (Nstat) and valganciclovir (VGCV) in relapsed/refractory (R/R) epstein-barr virus-positive (EBV +) lymphomas: final results from the phase 1b/2 VT3996-201 study. Blood (2021) 138(Supplement 1):623–3. doi: 10.1182/blood-2021-152603
8. Alter MJ. Epidemiology and prevention of hepatitis B. Semin Liver Dis (2003) 23(1):39–46. doi: 10.1055/s-2003-37583
9. Kruszon-Moran D, Paulose-Ram R, Martin CB, Barker LK, McQuillan G. Prevalence and trends in hepatitis B virus infection in the United States, 2015-2018. NCHS Data Brief (2020) 361):1–8.
10. Global progress report on HIV, viral hepatitis and sexually transmitted infections (2021). Available at: https://www.who.int/publications/i/item/9789240027077 (Accessed December 6, 2022).
11. Kim WR. Epidemiology of hepatitis B in the United States. Hepatology (2009) 49(5 Suppl):S28–34. doi: 10.1002/hep.22975
12. Nguyen MH, Burak Ozbay A, Liou I, Meyer N, Gordon SC, Dusheiko G, et al. Healthcare resource utilization and costs by disease severity in an insured national sample of US patients with chronic hepatitis B. J Hepatol (2019) 70(1):24–32. doi: 10.1016/j.jhep.2018.09.021
13. Kavosi Z, Zare F, Jafari A, Fattahi MR. Economic burden of hepatitis B virus infection in different stages of disease; a report from southern Iran. Middle East J Dig Dis (2014) 6(3):156–61.
14. Liang TJ. Hepatitis B: the virus and disease. Hepatology (2009) 49(5 Suppl):S13–21. doi: 10.1002/hep.22881
15. Chang M-H. Hepatitis B virus infection. Semin Fetal Neonatal Med (2007) 12(3):160–7. doi: 10.1016/j.siny.2007.01.013
16. Huang X, Young KH, Guo W, Wang Y, Wang X, Xi Y, et al. Identification of hepatitis B virus aetiologic antigens, HBx and Pre-S2, in diffuse large B-cell lymphoma. J Viral Hepat (2020) 27(9):948–50. doi: 10.1111/jvh.13301
17. Croagh CMN, Lubel JS. Natural history of chronic hepatitis B: phases in a complex relationship. World J Gastroenterol (2014) 20(30):10395–404. doi: 10.3748/wjg.v20.i30.10395
18. de Almeida Pondé RA. Detection of the serological markers hepatitis B virus surface antigen (HBsAg) and hepatitis B core IgM antibody (anti-HBcIgM) in the diagnosis of acute hepatitis B virus infection after recent exposure. Microbiol Immunol (2022) 66(1):1–9. doi: 10.1111/1348-0421.12943
19. Zhou K, Dodge JL, Grab J, Poltavskiy E, Terrault NA. Mortality in adults with chronic hepatitis B infection in the United States: a population-based study. Aliment Pharmacol Ther (2020) 52(2):382–9. doi: 10.1111/apt.15803
20. Ganem D, Prince AM. Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med (2004) 350(11):1118–29. doi: 10.1056/NEJMra031087
21. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology (2021) 73 Suppl 1(Suppl 1):4–13. doi: 10.1002/hep.31288
22. Nguyen MH, Wong G, Gane E, Kao J-H, Dusheiko G. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev (2020) 33(2). doi: 10.1128/CMR.00046-19
23. Siegel RL, Miller KD, Fuchs HE, Jemal A. CA: a cancer journal for clinicians. CA: A Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
24. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood (2016) 127(20):2375–90. doi: 10.1182/blood-2016-01-643569
25. Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology (2018) 50(1):74–87. doi: 10.1016/j.pathol.2017.09.006
26. Shenoy PJ, Malik N, Nooka A, Sinha R, Ward KC, Brawley OW, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer (2011) 117(11):2530–40. doi: 10.1002/cncr.25765
27. Li Y, Wang Y, Wang Z, Yi D, Ma S. Racial differences in three major NHL subtypes: descriptive epidemiology. Cancer Epidemiol (2015) 39(1):8–13. doi: 10.1016/j.canep.2014.12.001
28. Ayers AA, Lyu L, Dance K, Ward KC, Flowers CR, Koff JL, et al. Characterizing lymphoma incidence and disparities for a cancer center catchment region. Clin Lymphoma Myeloma Leuk (2019) 19(11):699–708.e5. doi: 10.1016/j.clml.2019.06.009
29. Nowakowski GS, Feldman T, Rimsza LM, Westin JR, Witzig TE, Zinzani PL. Integrating precision medicine through evaluation of cell of origin in treatment planning for diffuse large B-cell lymphoma. Blood Cancer J (2019) 9(6):48. doi: 10.1038/s41408-019-0208-6
30. Chiappella A, Diop F, Agostinelli C, Novo M, Nassi L, Evangelista A, et al. Prognostic impact of TP53 mutation in newly diagnosed diffuse large B-cell lymphoma patients treated in the FIL-DLCL04 trial. Br J Haematol (2022) 196(5):1184–93. doi: 10.1111/bjh.17971
31. Dodero A, Guidetti A, Marino F, Tucci A, Barretta F, Re A, et al. Dose-adjusted EPOCH and rituximab for the treatment of double expressor and double-hit diffuse large B-cell lymphoma: impact of TP53 mutations on clinical outcome. Haematologica (2022) 107(5):1153–62. doi: 10.3324/haematol.2021.278638
32. Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med (2018) 24(5):679–90. doi: 10.1038/s41591-018-0016-8
33. Wright GW, Huang DW, Phelan JD, Coulibaly ZA, Roulland S, Young RM, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell (2020) 37(4):551–568.e14. doi: 10.1016/j.ccell.2020.03.015
34. Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med (2018) 378(15):1396–407. doi: 10.1056/NEJMoa1801445
35. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med (1993) 329(14):987–94. doi: 10.1056/NEJM199309303291402
36. Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood (2007) 109(5):1857–61. doi: 10.1182/blood-2006-08-038257
37. Bourbon E, Maucort-Boulch D, Fontaine J, Mauduit C, Sesques P, Safar V, et al. Clinicopathological features and survival in EBV-positive diffuse large B-cell lymphoma not otherwise specified. Blood Adv (2021) 5(16):3227–39. doi: 10.1182/bloodadvances.2021004515
38. Hosry J, Miranda RN, Samaniego F, Economides MP, Torres HA. Clinicopathologic characteristics and outcomes of transformed diffuse large B-cell lymphoma in hepatitis C virus-infected patients. Int J Cancer (2018) 142(5):940–8. doi: 10.1002/ijc.31110
39. Ren W, Ye X, Su H, Li W, Liu D, Pirmoradian M, et al. Genetic landscape of hepatitis B virus-associated diffuse large B-cell lymphoma. Blood (2018) 131(24):2670–81. doi: 10.1182/blood-2017-11-817601
40. Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol (2016) 17(3):389–400. doi: 10.1016/S1470-2045(15)00533-1
41. Landsburg DJ, Petrich AM, Abramson JS, Sohani AR, Press O, Cassaday R, et al. Impact of oncogene rearrangement patterns on outcomes in patients with double-hit non-Hodgkin lymphoma. Cancer (2016) 122(4):559–64. doi: 10.1002/cncr.29781
42. Song JY, Perry AM, Herrera AF, Chen L, Skrabek P, Nasr MR, et al. Double-hit signature with TP53 abnormalities predicts poor survival in patients with germinal center type diffuse large B-cell lymphoma treated with R-CHOP. Clin Cancer Res (2021) 27(6):1671–80. doi: 10.1158/1078-0432.CCR-20-2378
43. Dunleavy K. Double-hit lymphoma: optimizing therapy. Hematol Am Soc Hematol Educ Program (2021) 2021(1):157–63. doi: 10.1182/hematology.2021000247
44. Ulcickas Yood M, Quesenberry CP, Guo D, Caldwell C, Wells K, Shan J, et al. Incidence of non-Hodgkin’s lymphoma among individuals with chronic hepatitis B virus infection. Hepatology (2007) 46(1):107–12. doi: 10.1002/hep.21642
45. Li M, Gan Y, Fan C, Yuan H, Zhang X, Shen Y, et al. Hepatitis B virus and risk of non-Hodgkin lymphoma: An updated meta-analysis of 58 studies. J Viral Hepat (2018) 25(8):894–903. doi: 10.1111/jvh.12892
46. Mahale P, Engels EA, Koshiol J. Hepatitis B virus infection and the risk of cancer in the elderly US population. Int J Cancer (2019) 144(3):431–9. doi: 10.1002/ijc.31643
47. Spradling PR, Xing J, Zhong Y, Rupp LB, Moorman AC, Lu M, et al. Incidence of Malignancies among patients with chronic hepatitis B in US health care organizations, 2006-2018. J Infect Dis (2022) 226(5):896–900. doi: 10.1093/infdis/jiac011
48. An J, Kim JW, Shim JH, Han S, Yu CS, Choe J, et al. Chronic hepatitis B infection and non-hepatocellular cancers: A hospital registry-based, case-control study. PloS One (2018) 13(3):e0193232. doi: 10.1371/journal.pone.0193232
49. Cheng C-L, Huang S-C, Chen J-H, Wei C-H, Fang W-Q, Su T-H, et al. Hepatitis B surface antigen positivity is an independent unfavorable prognostic factor in diffuse large B-cell lymphoma in the rituximab era. Oncologist (2020) 25(9):793–802. doi: 10.1634/theoncologist.2019-0756
50. Dalia S, Chavez J, Castillo JJ, Sokol L. Hepatitis B infection increases the risk of non-Hodgkin lymphoma: a meta-analysis of observational studies. Leuk Res (2013) 37(9):1107–15. doi: 10.1016/j.leukres.2013.06.007
51. Kim M, Lee YK, Park B, Oh DJ, Choi HG. Hepatitis virus B and C infections are associated with an increased risk of non-Hodgkin lymphoma: A nested case-control study using a national sample cohort. J Med Virol (2020) 92(8):1214–20. doi: 10.1002/jmv.25653
52. Lai Y-R, Chang Y-L, Lee C-H, Tsai T-H, Huang K-H, Lee C-Y. Risk of non-hodgkin lymphoma among patients with hepatitis B virus and hepatitis C virus in Taiwan: A nationwide cohort study. Cancers (Basel) (2022) 14(3):583. doi: 10.3390/cancers14030583
53. Li M, Shen Y, Chen Y, Gao H, Zhou J, Wang Q, et al. Characterization of hepatitis B virus infection and viral DNA integration in non-Hodgkin lymphoma. Int J Cancer (2020) 147(8):2199–209. doi: 10.1002/ijc.33027
54. Nath A, Agarwal R, Malhotra P, Varma S. Prevalence of hepatitis B virus infection in non-Hodgkin lymphoma: a systematic review and meta-analysis. Intern Med J (2010) 40(9):633–41. doi: 10.1111/j.1445-5994.2009.02060.x
55. Yi H, Chen J, Cen H, Yan W, Tan X. Association between infection of hepatitis B virus and onset risk of B-cell non-Hodgkin’s lymphoma: a systematic review and a meta-analysis. Med Oncol (2014) 31(8):84. doi: 10.1007/s12032-014-0084-7
56. Ma R, Xing Q, Shao L, Wang D, Hao Q, Li X, et al. Hepatitis B virus infection and replication in human bone marrow mesenchymal stem cells. Virol J (2011) 8:486. doi: 10.1186/1743-422X-8-486
57. Lau KC, Joshi SS, Gao S, Giles E, Swidinsky K, van Marle G, et al. Oncogenic HBV variants and integration are present in hepatic and lymphoid cells derived from chronic HBV patients. Cancer Lett (2020) 480:39–47. doi: 10.1016/j.canlet.2020.03.022
58. Coffin CS, Mulrooney-Cousins PM, Osiowy C, Michalak TI. Virological characteristics of occult hepatitis B virus in a North American cohort of human immunodeficiency virus type 1-positive patients on dual active anti-HBV/HIV therapy. J Clin Virol (2014) 60(4):347–53. doi: 10.1016/j.jcv.2014.04.021
59. Coffin CS, Mulrooney-Cousins PM, Michalak TI. Hepadnaviral lymphotropism and its relevance to HBV persistence and pathogenesis. Front Microbiol (2021) 12:695384. doi: 10.3389/fmicb.2021.695384
60. Zhao K, Liu A, Xia Y. Insights into Hepatitis B Virus DNA Integration-55 Years after Virus Discovery. Innovation (Camb) (2020) 1(2):100034. doi: 10.1016/j.xinn.2020.100034
61. Paterlini-Bréchot P, Saigo K, Murakami Y, Chami M, Gozuacik D, Mugnier C, et al. Hepatitis B virus-related insertional mutagenesis occurs frequently in human liver cancers and recurrently targets human telomerase gene. Oncogene (2003) 22(25):3911–6. doi: 10.1038/sj.onc.1206492
62. Saigo K, Yoshida K, Ikeda R, Sakamoto Y, Murakami Y, Urashima T, et al. Integration of hepatitis B virus DNA into the myeloid/lymphoid or mixed-lineage leukemia (MLL4) gene and rearrangements of MLL4 in human hepatocellular carcinoma. Hum Mutat (2008) 29(5):703–8. doi: 10.1002/humu.20701
63. Li W, Zeng X, Lee NP, Liu X, Chen S, Guo B, et al. HIVID: an efficient method to detect HBV integration using low coverage sequencing. Genomics (2013) 102(4):338–44. doi: 10.1016/j.ygeno.2013.07.002
64. Li X, Zhang J, Yang Z, Kang J, Jiang S, Zhang T, et al. The function of targeted host genes determines the oncogenicity of HBV integration in hepatocellular carcinoma. J Hepatol (2014) 60(5):975–84. doi: 10.1016/j.jhep.2013.12.014
65. Svicher V, Salpini R, D’Anna S, Piermatteo L, Iannetta M, Malagnino V, et al. New insights into hepatitis B virus lymphotropism: Implications for HBV-related lymphomagenesis. Front Oncol (2023) 13:1143258. doi: 10.3389/fonc.2023.1143258
66. Zeldis JB, Mugishima H, Steinberg HN, Nir E, Gale RP. In vitro hepatitis B virus infection of human bone marrow cells. J Clin Invest (1986) 78(2):411–7. doi: 10.1172/JCI112591
67. Stoll-Becker S, Repp R, Glebe D, Schaefer S, Kreuder J, Kann M, et al. Transcription of hepatitis B virus in peripheral blood mononuclear cells from persistently infected patients. J Virol (1997) 71(7):5399–407. doi: 10.1128/JVI.71.7.5399-5407.1997
68. Wieland SF. The chimpanzee model for hepatitis B virus infection. Cold Spring Harb Perspect Med (2015) 5(6). doi: 10.1101/cshperspect.a021469
69. Jaquet A, Boni SP, Boidy K, Tine J, Tchounga B, Touré SA, et al. Chronic viral hepatitis, HIV infection and Non-Hodgkin lymphomas in West Africa, a case-control study. Int J Cancer (2021) 149(8):1536–43. doi: 10.1002/ijc.33709
70. Rong X, Wang H, Ma J, Pan S, Wang H, Jing S, et al. Chronic hepatitis B virus infection is associated with a poorer prognosis in diffuse large B-cell lymphoma: a meta-analysis and systemic review. J Cancer (2019) 10(15):3450–8. doi: 10.7150/jca.31033
71. Vile RG, Melcher A, Pandha H, Harrington KJ, Pulido JS. APOBEC and cancer viroimmunotherapy: thinking the unthinkable. Clin Cancer Res (2021) 27(12):3280–90. doi: 10.1158/1078-0432.CCR-20-1888
72. Sinha M, Sundar K, Premalata CS, Asati V, Murali A, Bajpai AK, et al. Pro-oncogenic, intra host viral quasispecies in Diffuse large B cell lymphoma patients with occult Hepatitis B Virus infection. Sci Rep (2019) 9(1):14516. doi: 10.1038/s41598-019-51157-1
73. Yeo W, Chan TC, Leung NWY, Lam WY, Mo FKF, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol (2009) 27(4):605–11. doi: 10.1200/JCO.2008.18.0182
74. Pei S-N, Chen C-H, Lee C-M, Wang M-C, Ma M-C, Hu T-H, et al. Reactivation of hepatitis B virus following rituximab-based regimens: a serious complication in both HBsAg-positive and HBsAg-negative patients. Ann Hematol (2010) 89(3):255–62. doi: 10.1007/s00277-009-0806-7
75. Hsu C, Tsou H-H, Lin S-J, Wang M-C, Yao M, Hwang W-L, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology (2014) 59(6):2092–100. doi: 10.1002/hep.26718
76. Tajima K, Takahashi N, Ishizawa K, Murai K, Akagi T, Noji H, et al. High prevalence of diffuse large B-cell lymphoma in occult hepatitis B virus-infected patients in the Tohoku district in Eastern Japan. J Med Virol (2016) 88(12):2206–10. doi: 10.1002/jmv.24584
77. Ren W, Wang X, Yang M, Wan H, Li X, Ye X, et al. Distinct clinical and genetic features of hepatitis B virus-associated follicular lymphoma in Chinese patients. Blood Adv (2022) 6(9):2731–44. doi: 10.1182/bloodadvances.2021006410
78. Fernández-Rodríguez C, Rodríguez-Sevilla JJ, Fernández-Ibarrondo L, Sánchez-González B, Gibert J, Bento L, et al. Worse outcome and distinct mutational pattern in follicular lymphoma with anti-HBc positivity. Blood Adv (2022) 6(1):82–6. doi: 10.1182/bloodadvances.2021005316
Keywords: hepatitis B, B-cell, lymphoma, DLBCL, double hit, viral oncogenesis
Citation: Rosenberg M, Poluch M, Thomas C, Sindaco P, Khoo A and Porcu P (2023) Hepatitis B Virus and B-cell lymphoma: evidence, unmet need, clinical impact, and opportunities. Front. Oncol. 13:1275800. doi: 10.3389/fonc.2023.1275800
Received: 10 August 2023; Accepted: 09 October 2023;
Published: 20 October 2023.
Edited by:
Felix Seyfried, Ulm University Medical Center, GermanyReviewed by:
Walter Hanel, The Ohio State University, United StatesCopyright © 2023 Rosenberg, Poluch, Thomas, Sindaco, Khoo and Porcu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pierluigi Porcu, cGllcmx1aWdpLnBvcmN1QGplZmZlcnNvbi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.