- 1Division of Thoracic Tumor Multidisciplinary Treatment, Cancer Center and State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, China
- 2Physics Center, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
Purpose: To compare the differences between involved-field irradiation (IFI) and elective nodal irradiation (ENI) in selecting the optimal target area for neoadjuvant chemoradiotherapy (nCRT) in patients with locally advanced esophageal squamous cell carcinoma (LA-ESCC).
Materials and methods: We retrospectively analyzed 267 patients with LA-ESCC, of whom 165 underwent ENI and 102 underwent IFI. Dosimetry, treatment-related complications, pathological responses, recurrence/metastasis patterns, and survival were compared between the two groups.
Results: The median follow-up duration was 27.9 months. The R0 resection rates in the IFI and ENI groups were 95.1% and 92.7%, respectively (p=0.441), while the pathological complete response (pCR) rates were 42.2% and 34.5%, respectively (p=0.12). The ENI group received higher radiation doses to the heart (HV30:23.9% vs. 18%, p=0.033) and lungs (LV30:7.7% vs. 4.9%, p<0.001) than the IFI group. Consequently, the ENI group showed a higher incidence of grade 2 or higher radiation pneumonitis (30.3% vs. 17.6%, p=0.004) and pericardial effusion (26.7% vs. 11.8%, p=0.021) than the IFI group. Post-operation fistulas were observed in 3 (2.9%) and 17 cases (10.3%) in the IFI and ENI groups, respectively (p=0.026). In the multivariate analysis, smoking, positive lymph node involvement (pN+), and anastomotic fistula were independent predictors of overall survival (OS). The pN+ patients exhibited a greater propensity for recurrence compared to pN- patients, especially in the first year of follow-up (6.67% vs. 0.56%, p=0.003).
Conclusion: The ENI group had a higher incidence of radiation-induced adverse events compared to the IFI group, likely due to the higher radiation doses to normal tissues. Considering the similar disease-free survival (DFS) and OS rates in the two groups, IFI may be suitable for nCRT in patients with LA-ESCC, although further prospective studies are warranted.
Introduction
Esophageal cancer is the seventh most prevalent malignancy and the sixth leading cause of cancer-related fatalities (1). Despite advances in treatment, the disease is characterized by a high incidence of local and distant recurrence following surgical resection, leading to a dismal 5-year OS rate that rarely exceeds 30% (2). The long-term findings of the landmark CROSS trial established the survival benefits of neoadjuvant radiotherapy combined with surgery for resectable esophageal cancer, setting the standard of care for locally advanced cases (3, 4). However, the optimal radiation field for neoadjuvant radiotherapy remains controversial. Although the CROSS trial assessed the efficacy and recurrence patterns in the involved-field irradiation (IFI) group, it did not compare these parameters with those in the elective nodal irradiation (ENI) group (3). A retrospective study including 118 patients with esophageal squamous cell carcinoma (ESCC) receiving neoadjuvant chemoradiotherapy (nCRT) compared the efficacy and failure patterns between the ENI and non-ENI groups, as both were applicable to most of the population because of the similar prognoses of the two groups. However, considering the higher risk of complications in older patients (>70 years old), the authors recommended IFI only for this subgroup (5).
This study compared the application of ENI and IFI to evaluate the optimal radiation fields in terms of the dosimetric parameters, chemoradiotherapy-related and operational complications, impact on lymphocytes, pathological response, disease-progression patterns, and clinical outcomes.
Materials and methods
Patients
Patients with locally advanced esophageal cancer (AJCC Ver. 8, stages II–IVA) were reviewed at the West China Hospital between March 2017 and October 2022. All patients had pathologically confirmed squamous cell carcinoma and underwent nCRT followed by radical esophagectomy. Patients with performance status (PS) ≥ 2, distant metastases, prior chest radiation therapy, or coexisting other malignant tumors were excluded. The clinical stage was assessed by the following examinations, including esophagography, endoscopy, and computed tomography (CT), with some patients undergoing positron emission tomography.
Neoadjuvant chemo-radiotherapy
All patients received nCRT, with radiotherapy being delivered through intensity-modulated radiotherapy (IMRT). For IFI, the gross tumor volume (GTV) was the sum of the primary lesion (GTVp) and metastatic lymph node volume (GTVn). The lymph node clinical target volume (CTVn) included the GTVn with additional 5-mm expansion (6, 7). The CTV was defined as the sum of CTVn and GTVp plus a longitudinal 3-cm margin along the esophagus and a 5-mm radial margin. The planning target volume (PTV) was defined as the CTV with 5-10 mm expansion (8). Differently from IFI, the CTVn for ENI included both the clinically affected and uninvolved lymph node areas or stations (1/2/4/5/7, 2/4/5/7/8/9, and 4/5/7/8/9/16/17, respectively) for upper, middle, and lower thoracic ESCC (9). For normal tissues, the dose-volume constraints were as follows: to the spinal cord, ≤ 36 Gy; to the heart, V30 (volume receiving 30 Gy) ≤ 30%, mean heart dose (MHD) < 30 Gy; and to the whole lung, V20 ≤ 20%, V30 ≤ 15%, and mean lung dose (MLD) < 20 Gy (10). All patients were treated with paclitaxel in combination with carboplatin. Paclitaxel dose was calculated at 50 mg/m2 and carboplatin at an area under the curve of 2, weekly for 5 weeks (10).
Surgery
Surgery was scheduled 4 to 8 weeks after completion of nCRT. Most of the patients underwent McKeown esophagectomy (240/267, 89.9%). R0 resection was defined as complete resection of the tumor, with no tumor visible under the naked eye or microscope. A microscopic residual tumor (R1) was defined as a tumor located < 1 mm from the proximal, distal, or circumferential resection margins.
Pathological analysis
Pathological complete response (pCR) was defined as the absence of cancer cells in the primary lesion and regional lymph nodes after neoadjuvant therapy. Based on the degree of response of the primary tumor to treatment, the evaluation protocol for esophageal cancer (modified Ryan scheme for tumor regression score) from the College of American Pathologists classified the tumor regression grade (TRG) into four stages as follows: grade 0, no surviving cancer cells; grade 1, single cells or rare small groups of cancer cells; grade 2, residual cancer with evident tumor regression but more than single cells or rare small groups of cancer cells; and grade 3, extensive residual cancer with no evident tumor regression (11, 12).
Treatment-related complications
Complications of nCRT included hematological toxicity (anemia, thrombocytopenia, leukopenia, neutropenia, and lymphopenia), radiation pneumonia, radiation esophagitis, and radiation heart disease. Postoperative complications included fistula, esophageal stenosis, pleural effusion, pneumothorax, pulmonary atelectasis, acute respiratory distress syndrome (ARDS), mortality, and readmission within 30 days. We scored the severity of treatment-related complications using the Common Terminology Criteria for Adverse Events (CTCAE) 5.0.
Definition of endpoint and patterns of failure
The main endpoints were OS, disease-free survival (DFS), complications, pathological response, and failure modes. In-field failure (IFF) was defined as the presence of recurrence or metastasis within the irradiated field area. Out-of-field failure (OFF) was defined as the presence of recurrence or metastasis outside the irradiated field area. Patients in both groups had local recurrences.
Statistical analysis
The x2 test or Fisher’s exact test were used to compare the differences in the patient and tumor characteristics, toxicity, and first failure between the ENI and IFI groups. Spearman’s correlation analysis was performed to analyze the correlation between two continuous variables. The time between the start of nCRT or surgery and date of death, recurrence, or last follow-up was used to compute the survival outcomes. OS and DFS were calculated using the Kaplan-Meier method and compared using the log-rank test. Cox proportional hazard models were used to conduct both single-factor and multi-factor analyses as well as to compute the hazard ratios (HR) and 95% confidence intervals (CI). Variables with p <0.1 in the univariate analysis were entered into the multivariate model. SPSS (version 26.0), R Studio (version 4.2.3), and GraphPad Prism 9 software were used for all analyses. Statistical significance was set at p < 0.05.
Results
Patient characteristics
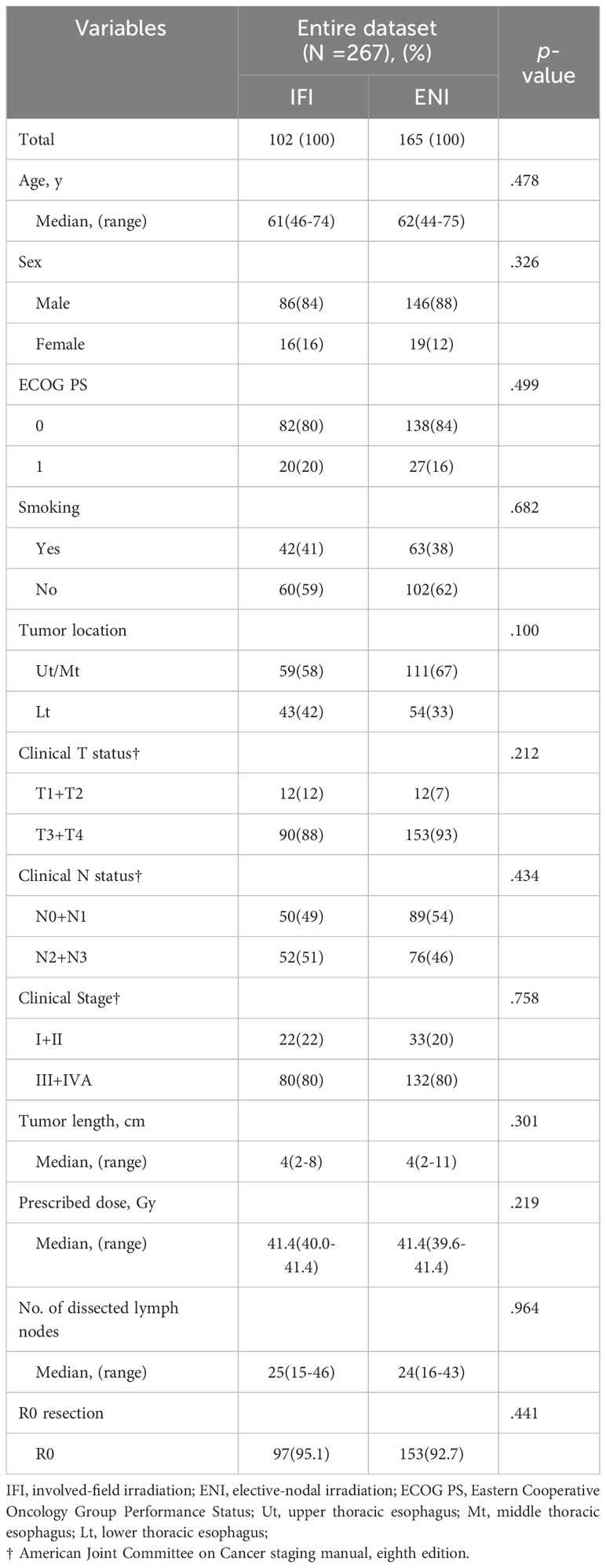
A total of 267 patients with esophageal cancer who completed preoperative nCRT followed by radical surgery were enrolled in the study. Among these, 102 (38%) were in the IFI group and 165 (62%) were in the ENI group. Table 1 shows the characteristics of the 267 patients. The median numbers of lymph node dissections were 25 (range:15–46) and 24 (range:16-43) in the IFI and ENI groups, respectively. The R0 resection rates in the IFI and ENI groups were 95.1% and 92.7%, respectively. Age sex, PS, smoking, tumor location, length, and stage, radiation dose, and R0 resection were not significantly different between the IFI and ENI groups (all p > 0.05).
Dosimetric parameters
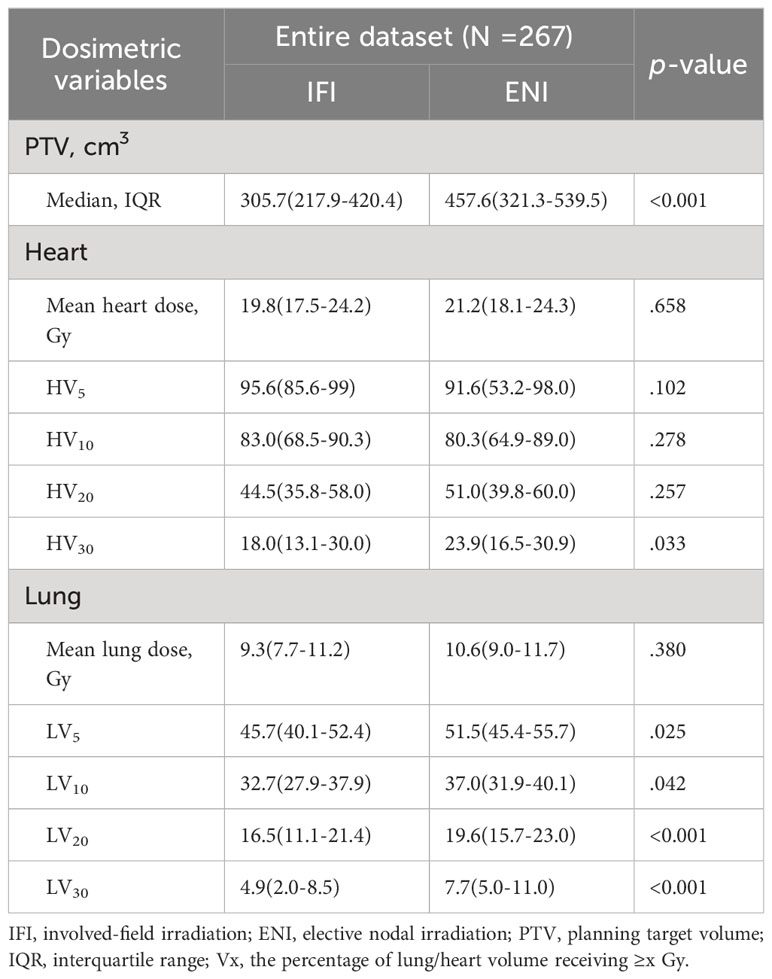
The radiation doses in the IFI and ENI groups were 40.0–41.4 Gy and 39.6–41.4 Gy in 1.8–2.0 Gy fractions, respectively. Table 2 presents the cardiac and pulmonary dosimetric parameters of the IFI and ENI groups. Patients who underwent ENI had significantly higher heart V30 and lung V5, V10, V20, and V30 values than those in the IFI group (p < 0.05).
Treatment-related complications
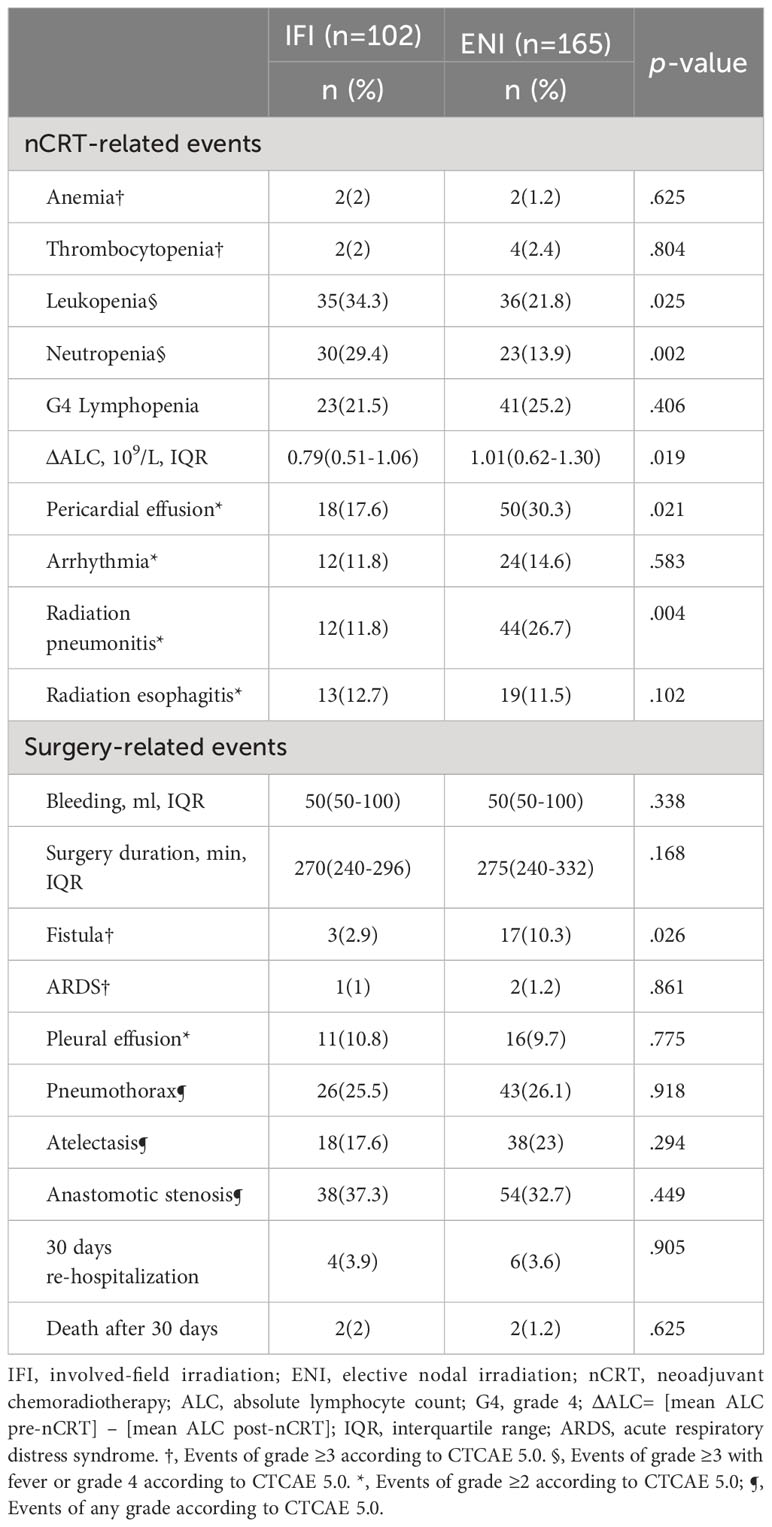
All serious adverse events that occurred during treatment are summarized in Table 3. Hematological toxicity has emerged as the most prevalent complication of radiation therapy. In the IFI and ENI groups, grade 3 or higher leukopenia was observed in 35 (34.3%) and 36 (21.8%) patients, respectively, and neutropenia was observed in 30 (29.4%) and 23(13.9%) (p < 0.05) patients, respectively. Grade 2 pericardial effusion (p=0.021) and radiation pneumonitis (p=0.004) occurred at considerably higher rates in the ENI group than in the IFI group (30.3% vs. 17.6% and 26.7% vs. 11.8%, respectively). No significant differences in arrhythmia or radiation esophagitis were observed between the two groups. There were 20 postoperative fistulas: three (2.9%) in the IFI group and 17 (10.3%) in the ENI group (p=0.026). Within 30 days of surgery, four patients in the IFI group and six in the ENI group were readmitted for anastomotic fistula (seven cases), respiratory failure (two cases), and wound infection (one case). There were two deaths in each group within 30 days after surgery: two from hemorrhage and two from severe pneumonia. Intraoperative bleeding, surgery duration, ARDS, pleural effusion, pneumothorax, atelectasis, and esophageal stenosis were not significantly different between the two groups (all p > 0.05).
Impact on lymphocytes
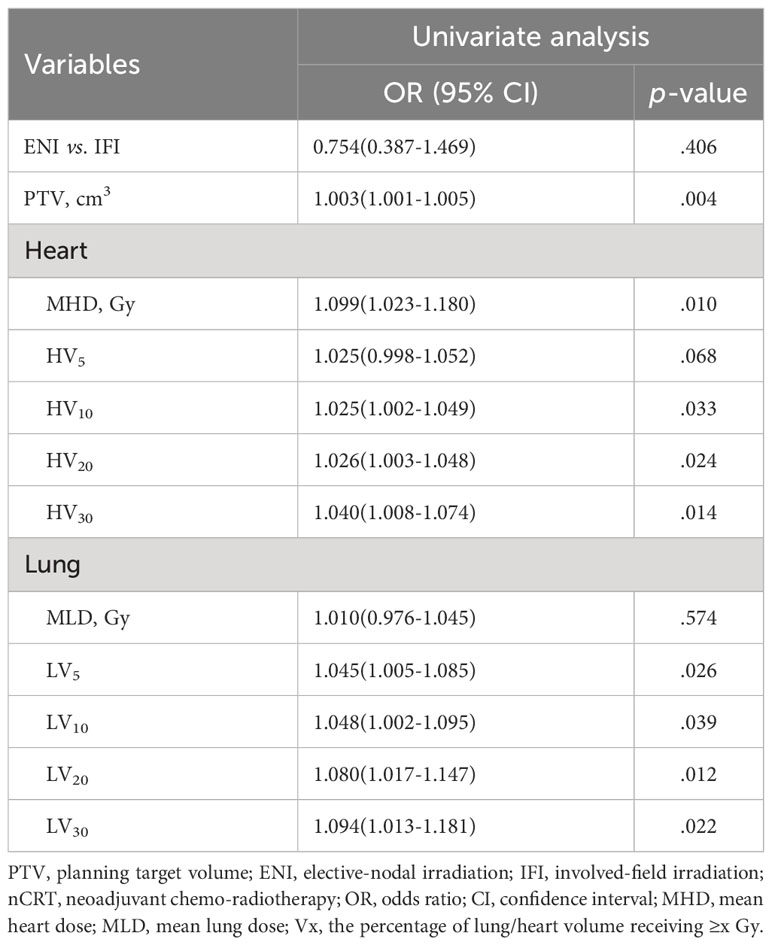
Table 4 shows that higher cardiopulmonary radiation doses (MHD, HV10-30, and LV5-30) were strongly associated with grade 4 (G4) lymphopenia (p<0.05). Table 3 demonstrates that the Δabsolute lymphocyte count (ΔALC= [mean ALC pre-nCRT] – [mean ALC post-nCRT]) was significantly lower in the IFI group than in the ENI group (0.79 vs. 1.01, p=0.019). Spearman’s correlation graph was plotted (including the leucocyte, neutrophil granulocyte, and lymphocyte) with the cardiopulmonary dosimetric parameters (Figure 1). A larger PTV and higher cardio-pulmonary doses (MHD, HV20, HV30, LV20, LV30) were strongly associated with ΔALC, while all cardio-pulmonary doses were highly correlated with the ALC nadir and neutrophil-lymphocyte ratio (NLR) (each p < 0.05). Only HV5 shows a significant correlation with leucocyte/neutrophil granulocyte (p<0.05), while other cardio-pulmonary parameters had not shown the significant correlation.
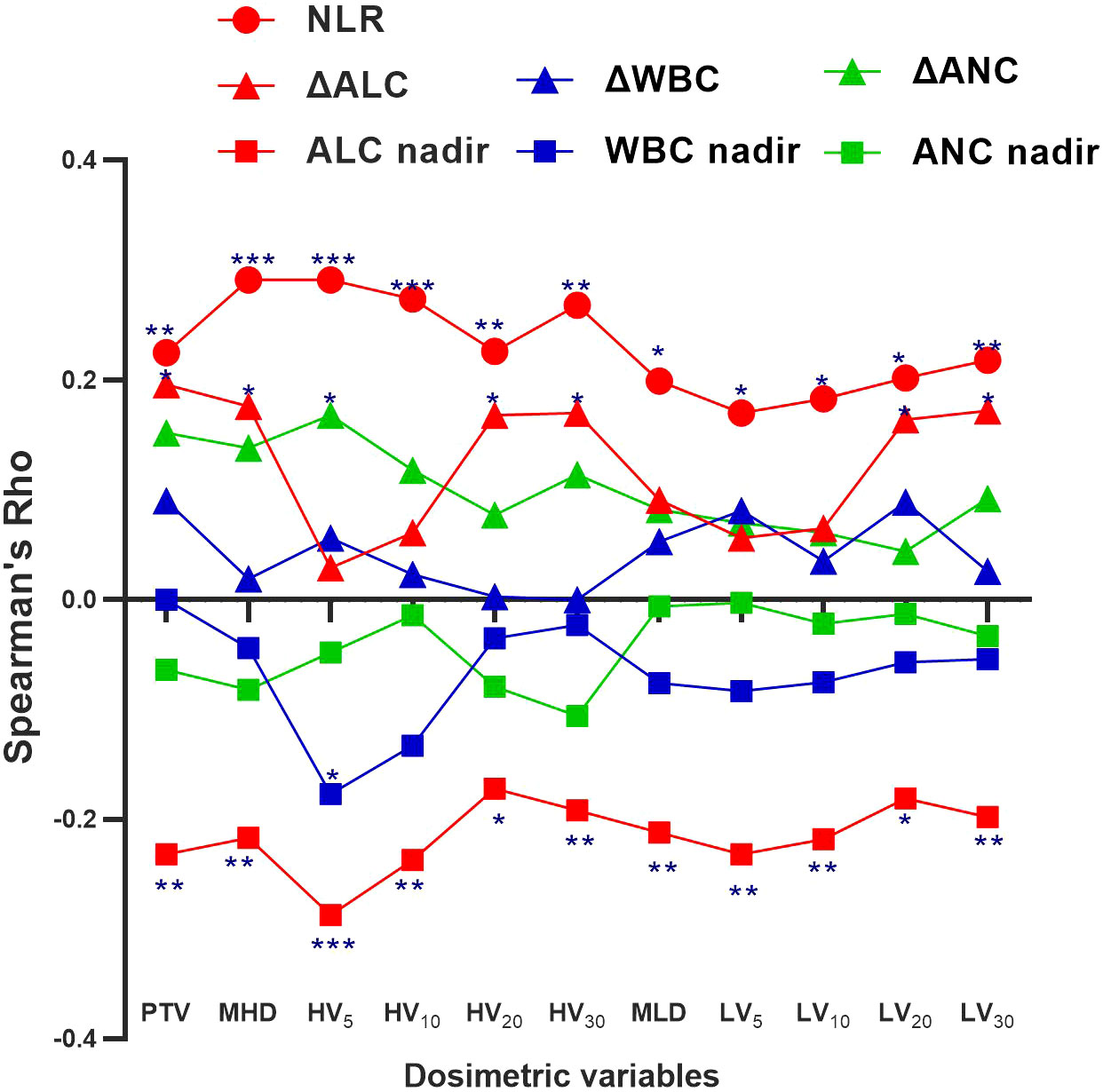
Figure 1 Spearman correlation coefficients (Rho) between the cardio-pulmonary dosimetric parameters and absolute lymphocyte count (ALC) nadir, neutrophil-lymphocyte ratio (NLR), and ΔALC, white blood cells (WBC) nadir, ΔWBC, absolute neutrophil count (ANC) nadir, and ΔANC. Significance indicated at ***p <.001, **p <.01, and *p <.05.
Pathological response
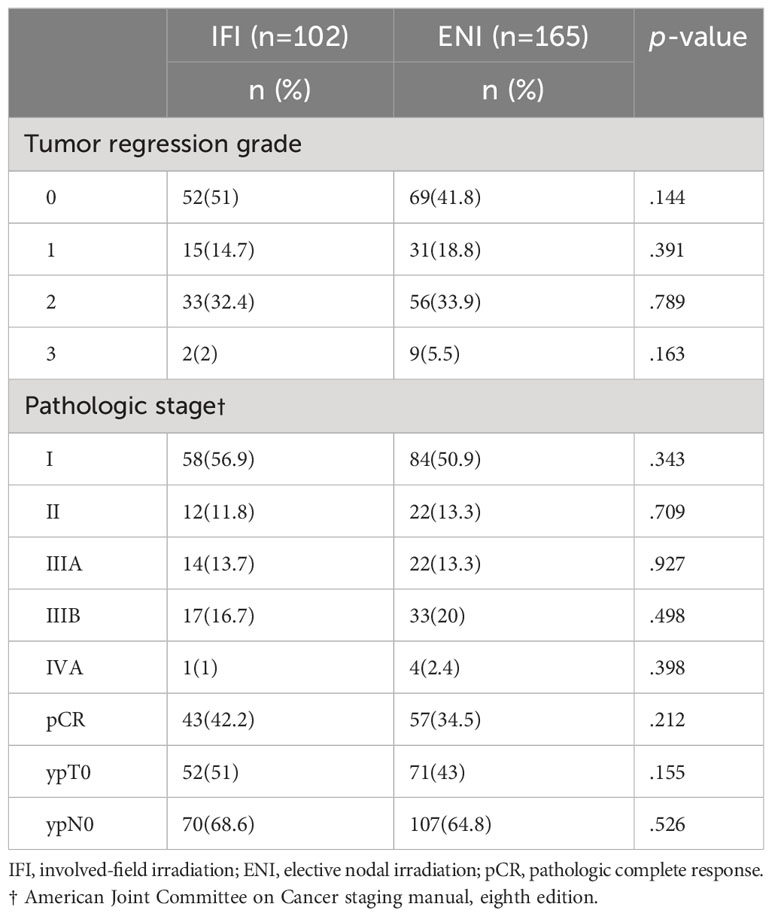
Table 5 summarizes the postoperative pathological responses and disease stages. The tumor regression grades in the IFI and ENI group were TRG0:52 (51%) vs. 69 (41.8%), TRG1:15 (14.7%) vs. 31 (18.8%), TRG2: 33 (32.4%) vs. 56 (33.95%), and TRG3:2 (2%) vs. 9 (5.5%), respectively. The rates of pCR, ypT0, and ypN0 in the IFI and ENI groups were 43 (42.2%) vs. 57 (34.5%), 52 (51%) vs. 71 (43%), and 70 (68.6%) vs. 107 (64.8%), respectively; however, the differences between the two groups were not statistically significant (p>0.05).
Patterns of failure
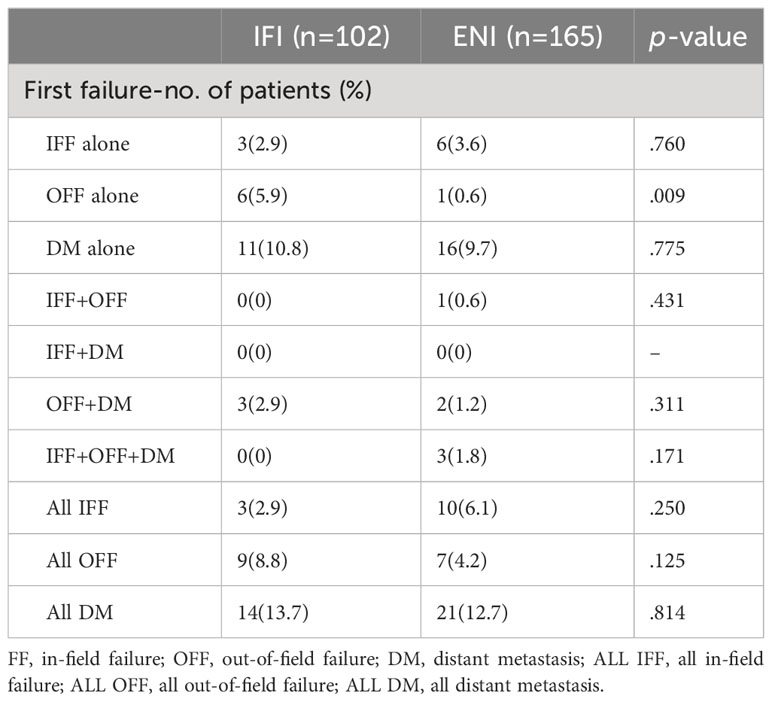
Table 6 summarizes the sites of the first treatment failure. Distant metastases (DM) were most common in 35 cases (13.1%) at the following sites: lung (21, 7.8%), bone (five, 1.9%), adrenal gland (four, 1.5%), liver (three, 1.1%), and brain (two, 0.7%). All in-field failure (ALLIFF) and all out-of-field failure (ALLOFF) were observed in 13 (4.9%) and 16 patients (6%), respectively. The probability of metastasis was significantly lower in the ENI group (one [0.6%]) than in the IFI group (six [5.9%]) in cases of isolated OFF (p=0.009). Although there was no significant difference between the two groups in the ALLOFF situation, the ENI group showed a lower ALLOFF rate than the IFI group (seven [4.2%] vs. nine [8.8%]).
Overall survival and prognostic factors
The median follow-up time for all patients was 27.9 months (range: 7.9–63.3). The 3-year OS and 3-year DFS were (78.2% vs. 74.0%) and (62.0% vs. 61.5%) for the IFI and ENI groups (Figures 2A, B), respectively, but there was no statistical difference (p>0.05).
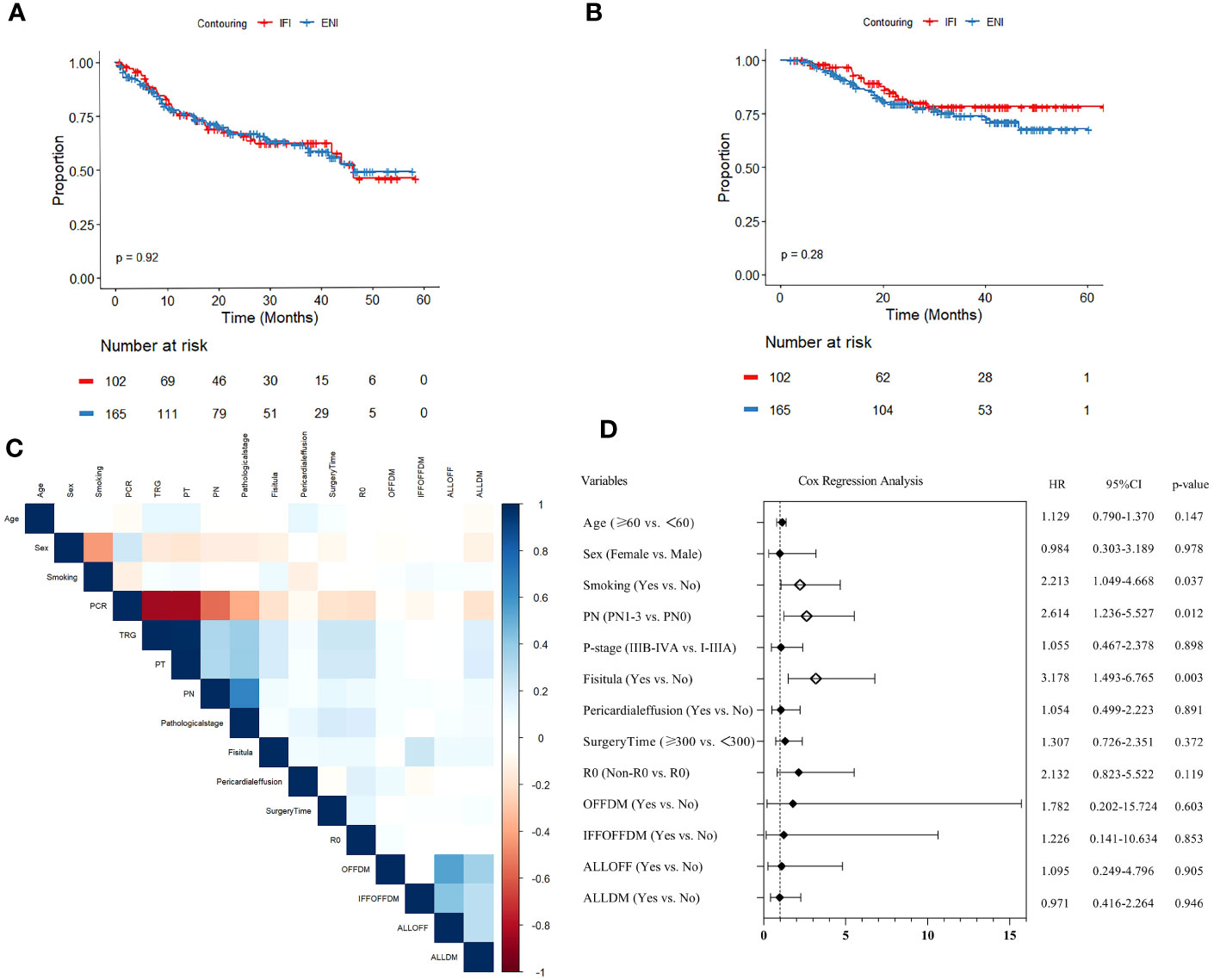
Figure 2 (A) Disease-free survival (DFS) analysis and (B) overall survival (OS) analysis of elective-nodal irradiation (ENI) and involved-field irradiation (IFI). (C) Heatmap of covariance test for variables with p<0.1 in univariate analysis. (D) Forest plot of Cox multivariable regression analysis for OS.
In univariate Cox regression analysis, age, sex, smoking, pCR, TRG, pathological T status (PT), pathological N status (PN), pathological stage, R0, duration of surgery, fistula, pericardial effusion, OFFDM, IFFOFFDM, ALLOFF, and ALLDM were associated with OS (all p<0.1). Subsequently, the variables for covariance mentioned above were analyzed, and pCR, TRG, and PT showed strong correlation (r>0.8); therefore, they were excluded from the final multivariate model (Figure 2C).
The results of the multivariate Cox regression analysis are presented as forest plots (Figure 2D). Ultimately, smoking (HR=2.213, 95% CI:1.049–4.668, p=0.037), pN+ (HR=2.614, 95% CI: 1.236–5.527, p=0.012), and fistula (HR=3.178, 95% CI: 1.493–6.765, p=0.003) were found to be significantly associated with OS.
Similar prognoses were recorded for TRG0 vs. TRG1 (p=0.127) and TRG2 vs. TRG3 (p=0.064), while TRG2/3 had a considerably worse prognosis than TRG0/1 (p=0.001). Further analysis was performed based on the combination of TRG and pN0/pN+ status (Figure 3A). The results showed that TRG0-1/pN+ patients had a significantly worse prognosis than TRG0-1/pN0 patients (p<0.001), while similar survival rates were observed in the other groups (TRG0-1/pN+ vs. TRG2-3/pN+, p=0.694; TRG0-1/pN+ vs. TRG2-3/pN0, p=0.234; TRG2-3/pN+ vs. TRG2-3/pN0, p=0.098).
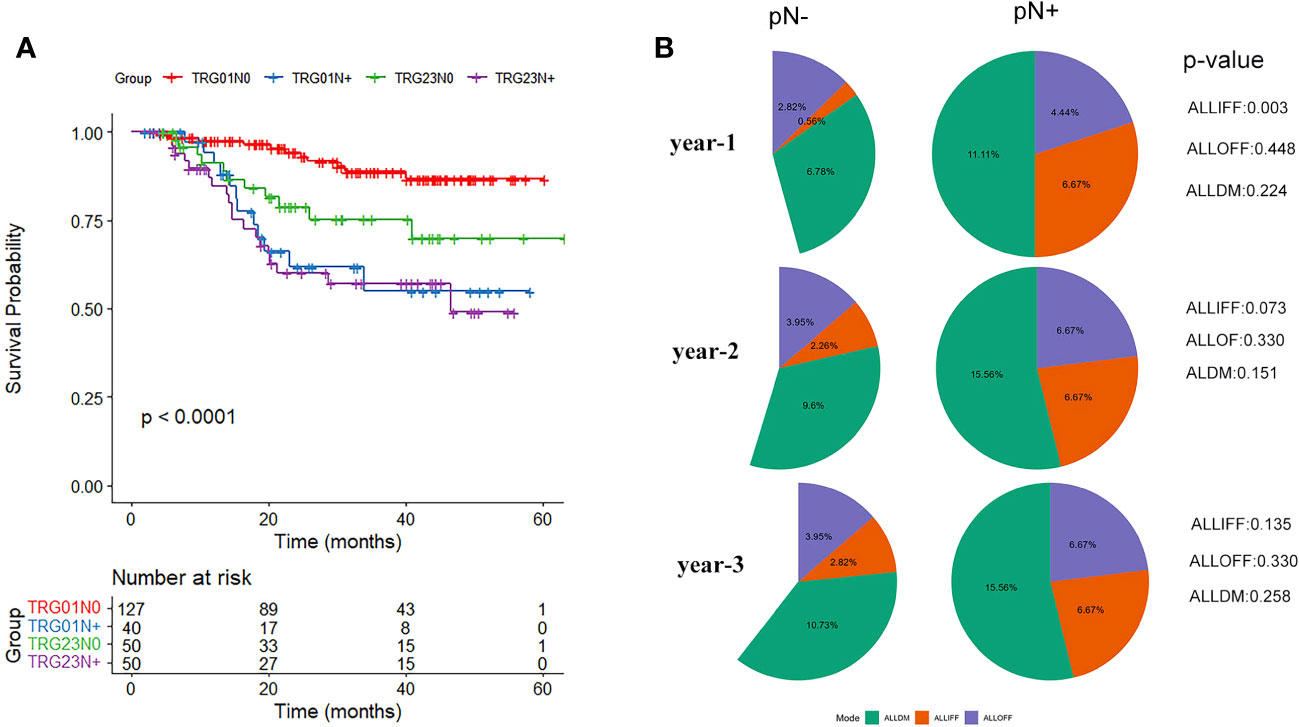
Figure 3 (A) Survival analysis curve of tumor regression grade (TRG) combined with pathological lymph node status (N0/N+). (B) Differences in pathological lymph node status (pN-/pN+) regarding recurrence and metastasis patterns of all in-field failure (ALLIFF), all out-of-field failure (ALLOFF), and all distant metastases (ALLDM).
The differences in the three disease progression patterns of ALLIFF, ALLOFF, and ALLDM between the pN-/pN+ groups are summarized in the follow-up years (Figure 3B). The risk of ALLIFF was greater in the pN+ group than in the pN- group (but only in the first year, p=0.003). This difference gradually diminished over the next two/three years. Although there was no significant difference in the overall pattern of recurrence between the pN-/pN+ groups, the pN+ group showed a higher tendency of recurrence (25.6% vs. 16.4%, p=0.074).
Discussion
ESCC is one of the most prevalent cancers in Asian countries and is typically locally advanced or advanced when first diagnosed and has a high fatality rate (13). In patients with LA-ESCC, the combined use of nCRT and surgery has a considerable survival advantage over surgery alone. The CROSS trial and NEOCRTEC 5010 study laid the foundation for nCRT plus surgery as the standard of care for this patient population (3, 14–16). A large sample study based on the National Cancer Database showed that in neoadjuvant radiotherapy, the pCR and OS did not differ between the three higher radiation doses [39.6-44.9 vs. 45-49.9 Gy vs. 50 Gy; pCR (p = 0.1) vs. OS (p = 0.097)] (17). While higher radiation doses could increase toxicity, all patients in this study had radiation doses of 39.6-41.4 Gy. However, the current guidelines do not provide explicit recommendations on the scope of target outlining for neoadjuvant RT. Based on the similar values of OS and DFS obtained in our study, the IFI technique might effectively decrease the radiation dose to normal tissues and consequently reduce the treatment-related adverse effects compared to ENI.
ESCC is more likely to metastasize through the esophageal axial lymphatics to multilevel lymph nodes or lymph nodes far from the primary site because of extensive longitudinal lymphatic connections within the esophageal wall (18, 19). The theoretical rationale for ENI is its ability to control lymph node micrometastases and potentially enhance the treatment efficacy by irradiating larger anatomical areas (19). However, the comparative efficacies of IFI and ENI have been found to be inconsistent in many studies on definitive chemoradiotherapy for LA-ESCC. A study conducted at the University of Tokyo Hospital involving 239 cases of esophageal cancer revealed that IFI did not increase the risk of lymph node failure in clinically unaffected nodal stations and demonstrated superior progression-free survival (PFS) and OS compared to the ENI group (20). Similarly, in a study of definitive radiotherapy for locally advanced non-small cell lung cancer (LA-NSCLC), IFI did not increase the incidence of lymph node failure in uninvolved nodal sites but significantly reduced esophageal toxicity (21). Conversely, a retrospective analysis of a larger sample of patients with ESCC favored ENI over IFI in terms of improved OS, with comparable toxicity profiles between the two groups (19). Furthermore, a meta-analysis indicated comparable rates of local control and OS in the ENI and IFI groups; however, the latter exhibited significantly lower incidences of esophageal and pulmonary toxicity (22). Several studies have shown that ENI can reduce local/regional failure; however, its survival benefits remain uncertain (23, 24). One possible explanation could be that the long-term toxicity associated with ENI diminishes the survival advantage (25). However, the comparatively high incidence of distant metastases exerts a more significant negative impact on survival; therefore, the relatively high localized control achieved through ENI may not be converted into a benefit for OS (26).
Few studies have investigated the differences in the efficacies of ENI and IFI in nCRT for LA-ESCC. Feng et al. examined the efficacy and failure patterns of ENI and non-ENI in patients with ESCC treated with preoperative irradiation; however, their analysis was not exhaustive (5). While they found that both ENI and IFI were suitable for LA-ESCC owing to similar efficacy outcomes, they recommended IFI for patients over 70 years of age without providing further analysis or explanation for this recommendation. In addition to the aforementioned findings, the present study aimed to address these shortcomings and provide additional insights by conducting new explorations. Our data indicated that patients in the ENI group might have received significantly higher radiation doses to the heart (HV30:23.9% vs. 18%, p=0.033) and lungs (LV5-LV30, each p<0.05) than those in the IFI group. Consequently, the ENI group had notably higher incidence rates of radiation pneumonia (26.7% vs. 11.8%, p=0.004) and pericardial effusion (30.3% vs. 17.6%, p=0.021).
Recently, immunotherapy has emerged as a critical therapeutic approach for advanced esophageal cancer, as recommended by the NCCN guidelines (10) In locally advanced solid tumor studies, radiotherapy combined with immunotherapy also appears to overturn the treatment guidelines, such as the KEYNOTE-799 study for LA-NSCLC, which reported a median DFS that far exceeds the current standard PACIFIC treatment paradigm (27, 28). In the field of esophageal cancer, the PALACE-1 study used pembrolizumab in combination with chemoradiotherapy for resectable LA-ESCC and showed a surgery rate of 90% and pCR rate of 55.6% in 20 patients, exceeding the results of the classic CROSS (49.0%) and NEOCRTEC5010 studies (43.2%) (29). Similarly, the NICE study enrolled patients with multisite lymph node metastatic LA-ESCC who were treated with neoadjuvant chemotherapy in combination with camrelizumab immunotherapy and reported a pCR rate of 45.4% (30). Exploratory analyses have highlighted the detrimental effects of excessive irradiation on the heart and large blood vessels, leading to damage to the lymphatic system, which ultimately affects patient survival (31–33). For the first time, our study recorded a significant association between higher cardio-pulmonary irradiation and G4 lymphopenia in the nCRT setting, and the ENI technique was more likely to lead to lymphopenia (p=0.019). A recently published study by Wang et al. on early-stage NSCLC radiotherapy showed that the estimated dose of radiation to immune cells is critical for treatment outcomes (34). The correlation between lymphocyte count and immunotherapy efficacy has been widely demonstrated in the treatment of a range of solid tumors (35, 36). In fact, the use of IFI to outline the target area provides better protection to the cardiopulmonary system area than ENI, consequently decreasing the incidence of lymphopenia. Thus, the IFI technique theoretically protects the patient’s immune function from irradiation, making it more appropriate to combine PD-1/PD-L1 drugs in the nCRT phase. Several prospective Phase III studies on this treatment are currently ongoing, including NCT05357846, NCT04807673, and NCT05244798. We eagerly await the reports of these studies regarding the clinical benefits, treatment-related toxicity, and other translational findings.
In addition, the present study demonstrated that the pN+ status was strongly associated with a poor prognosis. To further explore this relationship, we compared the recurrence patterns between the pN+/pN- groups, revealing a higher propensity for recurrence in the pN+ group. These findings highlight the importance of performing comprehensive lymph node dissection during surgery to accurately determine the lymph node status. A study by Samson et al. showed that compared to patients with esophageal cancer who did not receive adjuvant chemotherapy, those who received adjuvant chemotherapy benefited in terms of OS in all stages of lymph node positivity (37). Therefore, patients with pN+ ESCC may benefit from adjuvant chemotherapy. Recently, the results of the CheckMate-577 study confirmed that adjuvant immunotherapy may improve tumor-free survival in patients with high-risk esophageal cancer who did not achieve pCR after nCRT and R0 surgery, and that adjuvant nivolumab reduced the risk of distant metastases after surgery compared to placebo treatment (29% vs. 39%), with a median distant metastasis-free survival of 28.3 months and 17.6 months, respectively (38). Thus, patients with pN+ may benefit from postoperative adjuvant immunotherapy.
Interestingly, our data indicated an increased risk of anastomotic leakage in the ENI group compared to the IFI group according to the surgical procedure, whereas no significant differences were observed in the occurrence of pleural effusion, pneumothorax, esophageal stricture, and other postoperative complications. The larger radiotherapy target area of ENI may cover the anastomotic site in the currently used thoracoscopic approach for esophageal cancer surgery. Radiation exerts negative effects on wound repair through various mechanisms, including vascular system alterations, inflammatory response changes, and cellular function disruption (39, 40). Collagen, a vital matrix protein responsible for the strength and integrity of intestinal wall anastomosis, can be significantly hindered by high radiation doses, thereby affecting anastomotic healing (41, 42). Consequently, considering the range of target areas outlined for nCRT, the IFI may be a more suitable approach in the nCRT settings.
This study has some limitations. First, this study has the inherent limitations associated with both retrospective and observational studies. Future prospective studies with randomized controlled designs will provide stronger evidence in this field. Second, our study focused exclusively on patients with ESCC, which limits the generalizability of our findings to other histological types of esophageal cancer, although almost 95% of them are squamous cell carcinomas in Asian countries. Furthermore, as this was a single-center study, the external validity of our results may be limited. Findings from a single institution may not fully represent diverse patient populations encountered in broader clinical settings. Therefore, we anticipate the emergence of large-scale multicenter prospective studies in the future.
Conclusion
In conclusion, our study demonstrates that IFI is not inferior to ENI in terms of the pathological response and survival outcomes. The smaller target area of IFI has the potential to reduce cardiopulmonary irradiation, leading to a decrease in treatment-related adverse effects, which theoretically supports the utilization of IFI in nCRT for esophageal cancer. Patients with pN+ disease after nCRT are more likely to experience recurrence and metastasis, which are associated with a poorer prognosis, thus requiring more comprehensive treatment options.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of West China Hospital (approval number: 2021-1235). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XC: Data curation, Formal Analysis, Software, Writing – original draft, Writing – review & editing. YZ: Formal Analysis, Methodology, Software, Writing – review & editing. XZ: Data curation, Formal Analysis, Writing – review & editing. MW: Data curation, Formal Analysis, Writing – review & editing. FN: Investigation, Writing – review & editing. LZ: Investigation, Writing – review & editing. YX: Investigation, Writing – review & editing. BZ: Methodology, Writing – review & editing. JX: Methodology, Writing – review & editing. YL: Methodology, Writing – review & editing. YG: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by a grant from “1•3•5 project for discipline of excellence, West China Hospital, Sichuan University” (ZYJC21075 to YG).
Acknowledgments
Thanks to all the contributors and for the support of our clinical colleagues and mentors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med (1998) 339(27):1979–84. doi: 10.1056/NEJM199812313392704
3. Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the cross trials. J Clin Oncol (2014) 32(5):385–91. doi: 10.1200/JCO.2013.51.2186
4. Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (cross): long-term results of a randomised controlled trial. Lancet Oncol (2015) 16(9):1090–8. doi: 10.1016/S1470-2045(15)00040-6
5. Hsu FM, Lee JM, Huang PM, Lin CC, Hsu CH, Tsai YC, et al. Retrospective analysis of outcome differences in preoperative concurrent chemoradiation with or without elective nodal irradiation for esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys (2011) 81(4):e593–9. doi: 10.1016/j.ijrobp.2011.04.032
6. Jo YY, Yu J, Song KJ, Jang JY, Yoo YJ, Kim SB, et al. Definitive chemoradiotherapy versus esophagectomy in patients with clinical t1bn0m0 esophageal squamous cell carcinoma: a retrospective study. Radiother Oncol (2021) 162:112–8. doi: 10.1016/j.radonc.2021.07.006
7. Liu M, Zhao K, Chen Y, Jiang GL. Evaluation of the value of ENI in radiotherapy for cervical and upper thoracic esophageal cancer: a retrospective analysis. Radiat Oncol (2014) 9:232. doi: 10.1186/s13014-014-0232-4
8. Zhao KL, Ma JB, Liu G, Wu KL, Shi XH, Jiang GL. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys (2010) 76(2):446–51. doi: 10.1016/j.ijrobp.2009.02.078
9. Lyu J, Yisikandaer A, Li T, Zhang X, Wang X, Tian Z, et al. Comparison between the effects of elective nodal irradiation and involved-field irradiation on long-term survival in thoracic esophageal squamous cell carcinoma patients: A prospective, multicenter, randomized, controlled study in China. Cancer Med (2020) 9(20):7460–8. doi: 10.1002/cam4.3409
10. National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers NCCN guidelines Version 2 (2023). Available at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (Accessed 10 March 2023).
11. Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology (2005) 47(2):141–6. doi: 10.1111/j.1365-2559.2005.02176.x
12. Shi C. Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus. Available at: https://documents.cap.org/protocols/cp-giupper-esophagus-20-4100.pdf.
13. Chen SB, Weng HR, Wang G, Yang JS, Yang WP, Liu DT, et al. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: evaluation of the seventh edition of the American Joint Committee on Cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol (2013) 8(4):495–501. doi: 10.1097/JTO.0b013e3182829e2c
14. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
15. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (neocrtec5010): a phase iii multicenter, randomized, open-label clinical trial. J Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/JCO.2018.79.1483
16. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma. JAMA Surg (2021) 156(8):721–9. doi: 10.1001/jamasurg.2021.2373
17. Worrell SG, Towe CW A, Dorth J, Machtay M, Perry Y, Linden PA. Higher doses of neoadjuvant radiation for esophageal cancer do not affect the pathologic complete response rate or survival: A propensity-matched analysis. Ann Surg Oncol (2020) 27(2):500–8. doi: 10.1245/s10434-019-07849-z
18. Van De Voorde L, Larue RT, Pijls M, Buijsen J, Troost EG, Berbée M, et al. A qualitative synthesis of the evidence behind elective lymph node irradiation in oesophageal cancer. Radiother Oncol (2014) 113(2):166–74. doi: 10.1016/j.radonc.2014.11.010
19. Li Q, Zhu S, Li S, Deng W. Elective nodal irradiation provides a superior therapeutic modality for lymph node positivity esophageal squamous cell carcinoma patients receiving definitive radiotherapy versus involved-field irradiation. Med (Baltimore) (2019) 98(3):e14080. doi: 10.1097/MD.0000000000014080
20. Yamashita H, Takenaka R, Omori M, Imae T, Okuma K, Ohtomo K, et al. Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: a single institutional retrospective study. Radiat Oncol (2015) 10:171. doi: 10.1186/s13014-015-0482-9
21. Fernandes AT, Shen J, Finlay J, Mitra N, Evans T, Stevenson J, et al. Elective nodal irradiation (eni) vs. involved field radiotherapy (ifrt) for locally advanced non-small cell lung cancer (nsclc): a comparative analysis of toxicities and clinical outcomes. Radiother Oncol (2010) 95(2):178–84. doi: 10.1016/j.radonc.2010.02.007
22. Cheng YJ, Jing SW, Zhu LL, Wang J, Wang L, Liu Q, et al. Comparison of elective nodal irradiation and involved-field irradiation in esophageal squamous cell carcinoma: a meta-analysis. J Radiat Res (2018) 59(5):604–15. doi: 10.1093/jrr/rry055
23. Yamashita H, Okuma K, Wakui R, Kobayashi-Shibata S, Ohtomo K, Nakagawa K. Details of recurrence sites after elective nodal irradiation (eni) using 3d-conformal radiotherapy (3d-crt) combined with chemotherapy for thoracic esophageal squamous cell carcinoma – a retrospective analysis. Radiother Oncol (2011) 98(2):255–60. doi: 10.1016/j.radonc.2010.10.021
24. Onozawa M, Nihei K, Ishikura S, Minashi K, Yano T, Muto M, et al. Elective nodal irradiation (eni) in definitive chemoradiotherapy (crt) for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol (2009) 92(2):266–9. doi: 10.1016/j.radonc.2008.09.025
25. Ishikura S, Nihei K, Ohtsu A, Boku N, Hironaka S, Mera K, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol (2003) 21(14):2697–702. doi: 10.1200/JCO.2003.03.055
26. Li M, Zhang X, Zhao F, Luo Y, Kong L, Yu J. Involved-field radiotherapy for esophageal squamous cell carcinoma: theory and practice. Radiat Oncol (2016) 11:18. doi: 10.1186/s13014-016-0589-7
27. Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol (2021) 7(9):1–9. doi: 10.1001/jamaoncol.2021.2301
28. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med (2017) 377(20):1919–29. doi: 10.1056/NEJMoa1709937
29. Zheng Y, Li C, Yu B, Zhao S, Li J, Chen X, et al. Preoperative pembrolizumab combined with chemoradiotherapy for esophageal squamous cell carcinoma: Trial design. JTCVS Open (2021) 9:293–9. doi: 10.1016/j.xjon.2021.11.003
30. Yusupbekov AA. 125P analysis of esophageal cancer incidence for last 20 years in Uzbekistan. Ann Oncol (2020) 31:S1291–2. doi: 10.1016/j.annonc.2020.10.146
31. Xu C, Jin JY, Zhang M, Liu A, Wang J, Mohan R, et al. The impact of the effective dose to immune cells on lymphopenia and survival of esophageal cancer after chemoradiotherapy. Radiother Oncol (2020) 146:180–6. doi: 10.1016/j.radonc.2020.02.015
32. Contreras JA, Lin AJ, Weiner A, Speirs C, Samson P, Mullen D, et al. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol (2018) 128(3):498–504. doi: 10.1016/j.radonc.2018.05.017
33. Li Y, Fan X, Yu Q, Zhai H, Mo M, Sun J, et al. Proton and carbon ion radiation therapy decreased severe lymphopenia by reducing thoracic vertebra and aortic doses in non-small cell lung cancer versus intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys (2023) 116(3):579–89. doi: 10.1016/j.ijrobp.2022.12.030
34. Wang X, Bai H, Gao M, Guan Y, Yu L, Li J, et al. Impact of radiation dose to the immune system on disease progression and survival for early-stage non-small cell lung cancer treated with stereotactic body radiation therapy. Radiother Oncol (2023) 186:109804. doi: 10.1016/j.radonc.2023.109804
35. Dai D, Tian Q, Yu G, Shui Y, Jiang H, Wei Q. Severe radiation-induced lymphopenia affects the outcomes of esophageal cancer: A comprehensive systematic review and meta-analysis. Cancers (Basel) (2022) 14(12):3024. doi: 10.3390/cancers14123024
36. Damen PJJ, Kroese TE, van Hillegersberg R, Schuit E, Peters M, Verhoeff JJC, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys (2021) 111(4):936–48. doi: 10.1016/j.ijrobp.2021.07.1695
37. Samson P, Puri V, Lockhart AC, Robinson C, Broderick S, Patterson GA, et al. Adjuvant chemotherapy for patients with pathologic node-positive esophageal cancer after induction chemotherapy is associated with improved survival. J Thorac Cardiovasc Surg (2018) 156(4):1725–35. doi: 10.1016/j.jtcvs.2018.05.100
38. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
39. Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol (1997) 42(2):99–106. doi: 10.1016/s0167-8140(96)01880-4
40. Buell MG, Harding RK. Proinflammatory effects of local abdominal irradiation on rat gastrointestinal tract. Dig Dis Sci (1989) 34(3):390–9. doi: 10.1007/BF01536261
41. Hendriks T, Mastboom WJ. Healing of experimental intestinal anastomoses. Parameters repair. Dis Colon Rectum. (1990) 33(10):891–901. doi: 10.1007/BF02051930
Keywords: esophageal squamous cell carcinoma, neoadjuvant chemo-radiotherapy, involved-field irradiation, elective nodal irradiation, lymphopenia
Citation: Chen X, Zhang Y, Zhou X, Wang M, Na F, Zhou L, Xu Y, Zou B, Xue J, Liu Y and Gong Y (2023) Involved-field irradiation or elective-nodal irradiation in neoadjuvant chemo-radiotherapy for locally-advanced esophageal cancer: comprehensive analysis for dosimetry, treatment-related complications, impact on lymphocyte, patterns of failure and survival. Front. Oncol. 13:1274924. doi: 10.3389/fonc.2023.1274924
Received: 09 August 2023; Accepted: 25 September 2023;
Published: 10 October 2023.
Edited by:
Arunkumar Krishnan, Levine Cancer Institute, United StatesReviewed by:
Haihua Yang, Wenzhou Medical University, ChinaAtsuto Katano, The University of Tokyo Hospital, Japan
Copyright © 2023 Chen, Zhang, Zhou, Wang, Na, Zhou, Xu, Zou, Xue, Liu and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youling Gong, ZHJnb25neW91bGluZ0Bob3RtYWlsLmNvbQ==
 Xianyan Chen
Xianyan Chen Yingjie Zhang2
Yingjie Zhang2 Xiaojuan Zhou
Xiaojuan Zhou Feifei Na
Feifei Na Lin Zhou
Lin Zhou Bingwen Zou
Bingwen Zou Jianxin Xue
Jianxin Xue Youling Gong
Youling Gong