- 1Department of Hepatobilology, General Hospital of Ningxia Medical University, Yinchuan, China
- 2School of Nursing, Shanxi University of Chinese Medicine, Xianyang, China
- 3Department of Neonatal Intensive Care Unit, General Hospital of Ningxia Medical University, Yinchuan, China
- 4School of Nursing, Ningxia Medical University, Yinchuan, China
Objective: This study aims to retrieve, evaluate, and summarize domestic and foreign evidence on the prevention and treatment of embolism syndrome following transcatheter arterial chemoembolization (TACE) for primary liver cancer and to provide an evidence-based foundation for clinical practice.
Methods: Utilizing the “6S” model, we conducted a systematic search of UpToDate, BMJ Best Practice, domestic and foreign guidelines, and related databases on the prevention and treatment of embolism syndrome following TACE for primary liver cancer. This search included clinical decision-making, guidelines, systematic reviews, evidence summaries, randomized controlled trials, and expert consensus. The search time frame extended from January 1, 2013 to May 1, 2023. Evidence was synthesized after an independent review of the included studies by two investigators.
Results: A total of 11 articles were included in the analysis, comprising one clinical decision-making article, three clinical guidelines, six expert consensus articles, and one randomized controlled trial. We summarized 31 pieces of evidence across three categories: preoperative preparation, intraoperative interventions, and postoperative symptom management.
Conclusion: This study presents a comprehensive summary of the best available evidence on the prevention and treatment of embolism syndrome following TACE for primary liver cancer. These findings can serve as a valuable reference for clinical practitioners, enabling nurses to deliver individualized care based on the symptoms and specific needs of liver cancer patients.
Systematic review registration: http://ebn.nursing.fudan.edu.cn, identifier ES20232398.
1 Introduction
Primary liver cancer (PLC) is a malignant condition that predominantly includes hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and combined hepatocellular-cholangiocarcinoma (cHCC-CCA) (1). These cancers are characterized by their insidious onset, rapid progression, and poor prognosis (2). According to the 2020 global cancer statistics report, there were approximately 906,000 new cases of liver cancer, resulting in 830,000 deaths. This malignancy has become the fourth most prevalent malignant tumor worldwide and the second leading cause of cancer-related deaths in China (3, 4). It seriously affects the health of Chinese residents, and the burden of prevention and treatment of liver cancer diseases is increasing.
Primary liver cancer often presents without typical or early-stage symptoms, resulting in missed opportunities for surgical intervention. Surgical resection is feasible for only 10% to 30% of cases, and postoperative recurrence rates are notably high. Despite significant advancements in liver surgery, the majority of newly diagnosed HCC cases are not amenable to surgical resection (5, 6).
With the advancement of interventional medicine, transcatheter arterial chemoembolization (TACE) has emerged as the primary non-surgical palliative treatment for liver cancer patients. TACE involves the injection of embolization agents into tumor blood vessels through the hepatic artery, leading to the blockage of the tumor’s blood supply and inducing ischemic necrosis. This approach serves as a preventive and consolidative measure against recurrence in patients with PLC and has been shown to significantly improve overall patient survival rates (7–9).
While TACE is generally considered a safe procedure, it can lead to various postoperative complications that require caution and consideration. The most common complication is postembolization syndrome (PES), characterized by symptoms such as fever, liver pain, nausea, and vomiting (10). The reported incidence of PES varies widely, ranging from 15% to 90%, which can be attributed to bias arising from differing definitions of embolic syndrome and inadequate follow-up (11). Although PES is typically self-limiting, it significantly affects patient comfort, prolongs hospital stays, and may impact compliance to subsequent treatments (12).
Numerous studies focus on preventing and treating embolism syndrome following TACE, predominantly relying on original research, literature reviews, and meta-analyses. Nonetheless, their conclusions often lack the requisite evidence-based foundation for clinical translation. To address this gap, our study systematically searched both domestic and foreign evidence pertaining to the prevention and treatment of embolism syndrome after TACE and subsequently extracted and summarized this evidence. This study has been registered with the Evidence-Based Nursing Center of Fudan University (ES20232398).
2 Materials and methods
2.1 Determining the problem and inclusion criteria
Herein the Population, Intervention, Professional, Outcome, Setting, Type of evidence (PIPOST) model was used to determine the basic research questions (13). The inclusion criteria for evidence were as follows: (1) P (population): The evidence’s target population is patients with primary liver cancer after TACE, (2) I (intervention): The measures are aimed at preventing post-TACE syndrome, (3) P (professional): The evidence is applicable to medical staff, patients undergoing TACE, and their family members, (4) O (outcome): The outcome is the incidence of embolism syndrome after TACE, (5) S (setting): The evidence is applicable in medical institutions at all levels, and (6) T (type of evidence): The type of evidence resource include clinical decision-making, best practice, clinical guidelines, systematic reviews, evidence summaries, expert consensus, and relevant original research.
The exclusion criteria were as follows: (1) documents with incomplete data and (2) repeatedly published or updated documents.
2.2 Literature search strategy
According to the “6S” model (14) and employing the top-down retrieval principle, we conducted computerized searches in the following evidence-based resource databases: BMJ Best Practice, UpToDate, JBI Library, Cochrane Library, CINAHL, Ontario Registered Nurses Association Guidelines Network, and the National Institute of Health and Clinical Excellence Evidence-based resource databases such as the Guideline Network, the Scottish Intercollegiate Guidelines Network, the New Zealand Guidelines Research Group, the International Guidelines Collaboration Network, the Canadian Medical Association Clinical Practice Guidelines Library, and Medline. A supplementary search of comprehensive databases PubMed, EMBASE, China Biomedical Literature Service System, CNKI, Wanfang database, and VIP database was also carried out. Furthermore, we explored guidelines from reputable organizations, including the European Society of Liver Diseases, the National Comprehensive Cancer Network, the Australian Society of Gastroenterology, the American Society of Liver Diseases and the American Society of Transplantation, the European Society of Nuclear Medicine, International Society for Multidisciplinary Interventional Oncology, Japanese Liver Cancer Research Group, and the European Society for Enhanced Recovery After Surgery. The search period was from January 1, 2013 to May 1, 2023.
We searched for clinical decisions, recommended practices, evidence summaries, clinical practice guidelines, and websites of professional societies. The Chinese search terms included “primary liver cancer/liver tumors/hepatocellular carcinoma/hepatic malignancies”, “percutaneous transarterial chemoembolization/interventional therapy for liver cancer”, “treatment/hepatic arterial embolization/hepatic arterial chemoembolization”, and “post-embolization syndrome/embolism syndrome/abdominal pain/fever/nausea/vomiting”. The English search terms included “primary liver cancer/liver neoplasms/hepatocellular carcinoma”, “transarterial chemoembolization/transfemoral artery intervention/hepatic chemoembolization/chemoembolization/hepatic artery * embolization/embolization”, and “postembolization/postembolization syndrome/pain/nausea/vomiting/fever”.
The Chinese search strategy using CNKI as an example was as follows: SU % = (‘primary liver cancer’ + ‘liver cancer’ + ‘primary liver tumor’ + ‘primary liver cancer’ + ‘hepatocellular carcinoma’ + ‘cholangiocarcinoma’ + ‘intrahepatic cholangiocarcinoma’ + ‘hepatic angiosarcoma’ + ‘hepatoblastoma’ + ‘TACE’ + ‘percutaneous hepatic arterial chemoembolization’ + ‘transarterial chemoembolization for hepatocellular carcinoma’ + ‘interventional therapy for liver cancer’ + ‘hepatic arterial embolization’ + ‘hepatic arterial chemoembolization’) * (‘post-embolism syndrome’ + ‘embolism syndrome’ + ‘PES’ + ‘abdominal pain’ + ‘nausea’ + ‘vomiting’ + ‘fever’ + ‘pain in the liver area’ + ‘abdominal distension’ + ‘anorexia’) * (‘treatment’ + ‘prevention’ + ‘management’ + ‘diagnosis and treatment’ + ‘nursing’) * (‘guidelines’ + ‘meta’ + ‘systematic review’ + ‘evidence summary’ + ‘practice guideline’ + ‘expert consensus’ + ‘randomized control’ + ‘RCT’).
The English search strategy using PubMed as an example was as follows:
1 (TACE [Title/Abstract] OR transcatheter arterial chemoembolization [Title/Abstract] OR transfemoral artery intervention [Title/Abstract] OR transcatheter hepatic arterial chemoembolization [Title/Abstract] OR transcatheter hepatic arterial chemoembolization [Title/Abstract] OR percutaneous hepatic artery chemoembolization [Title/Abstract] OR trans-arterial chemoembolization [Title/Abstract] OR percutaneous transcatheter arterial chemoembolization [Title/Abstract] OR transarterial chemo-embolization [Title/Abstract]), #2 (embolization syndrome [Title/Abstract] OR embolism syndrome [Title/Abstract] OR embolic syndrome [Title/Abstract] OR post-embolization syndrome [Title/Abstract] OR embolism syndrome after interventional therapy [Title/Abstract] OR PES [Title/Abstract]), #3 (systematic review [Title/Abstract] OR meta-analysis [Title/Abstract] OR guideline [Title/Abstract] OR evidence summary * [Title/Abstract] OR consensus [Title/Abstract] OR RCT * [Title/Abstract]), and #4 (#1 AND #2 AND #3 AND Filters: from 2013 to 2023).
2.3 Literature quality evaluation
For guideline evaluation, we employed the British “Clinical Guidelines Research and Evaluation System” (AGREE II) (15) for quality evaluation, including 23 items in six areas: scope and purpose, participants, rigor of formulation, clarity, applicability, and editorial independence. Each item was rated on a scale of 1 to 7, with 7 indicating full compliance and 1 indicating non-compliance. For expert consensus evaluation, we followed the JBI Evidence-Based Health Care Center Expert Consensus (2017) evaluation standard (16) for quality assessment. Randomized controlled trials (RCTs) were evaluated using the JBI Evidence-Based Health Care Center’s RCT paper quality evaluation tool (17).
2.4 Summary, classification, and recommendation level of evidence
The quality of evidence and the level of recommendation were assessed independently by two researchers. In cases of conflicting assessments, a third researcher participated in discussions to reach a consensus. When discrepancies arose among evidence from different sources, we applied principles prioritizing evidence-based, high-quality, and recently published evidence.
The clinical practice guidelines and clinical decisions included were graded using their original grading system, while evidence lacking a grading system was graded using the JBI Evidence Pre-grading System (2014 version) to grade the original study on a scale of 1–5, with level 1 being the highest and level 5 being the lowest.
The recommendation level of evidence adopts the JBI Evidence Pre-grading and Evidence Recommendation Level System (2014 version) and refers to the grading system provided by the evidence itself.
3 Results
3.1 Literature search results
We initially retrieved a total of 1,057 documents through a systematic search. After removing duplicates using NoteExpress and applying our inclusion criteria, we obtained the full-text copies of potentially relevant documents. Each full-text copy was carefully reviewed to confirm whether it met the inclusion criteria. Eventually, we included a total of 11 documents, comprising one clinical decision (18), three guidelines (7, 19, 20), six expert consensus (21–26), and one randomized controlled trial (27). The details of the included literature are summarized in Table 1.
3.2 Quality evaluation results of the included literature
3.2.1 Quality evaluation results of the guideline
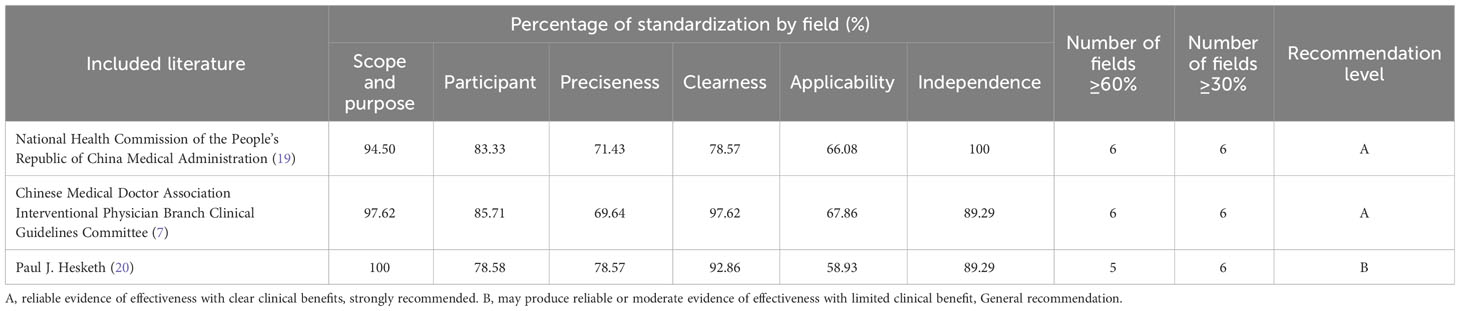
In this study, we included three guidelines (7, 19, 20), and the quality evaluation results are detailed in Table 2.
3.2.2 Quality evaluation results of the expert consensus
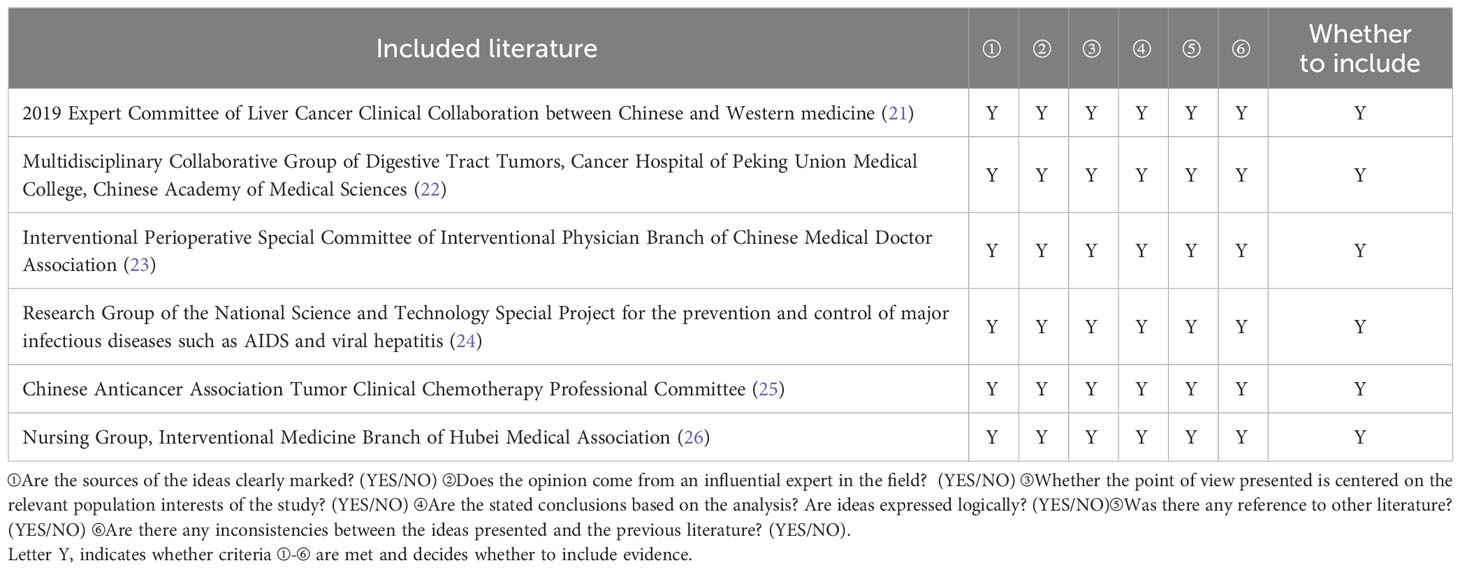
Six expert consensus articles were included in this study (21–26), and the quality evaluation results are shown in Table 3.
3.2.3 Quality evaluation results of clinical decision-making
One clinical decision (18) from UpToDate was directly included.
3.2.4 Quality evaluation results of randomized controlled trials
A randomized controlled trial (27) from the Cochrane Library was included, and its quality was evaluated using evaluation tools. The evaluation result of item 6, “Whether the results evaluator was blinded,” was “unclear”, and the evaluation result of the other item was “Yes”. The overall quality was high.
3.3 Evidence description and summary
According to the principle of evidence synthesis, the researchers summarized the evidence. A total of 31 pieces of evidence were summarized, divided into 3 evidence topics, as shown in Table 4 (preoperative preparation, intraoperative interventions, and postoperative symptom management). A flow chart of evidence transformation for the prevention and treatment of embolism syndrome after TACE is shown in Figure 1.
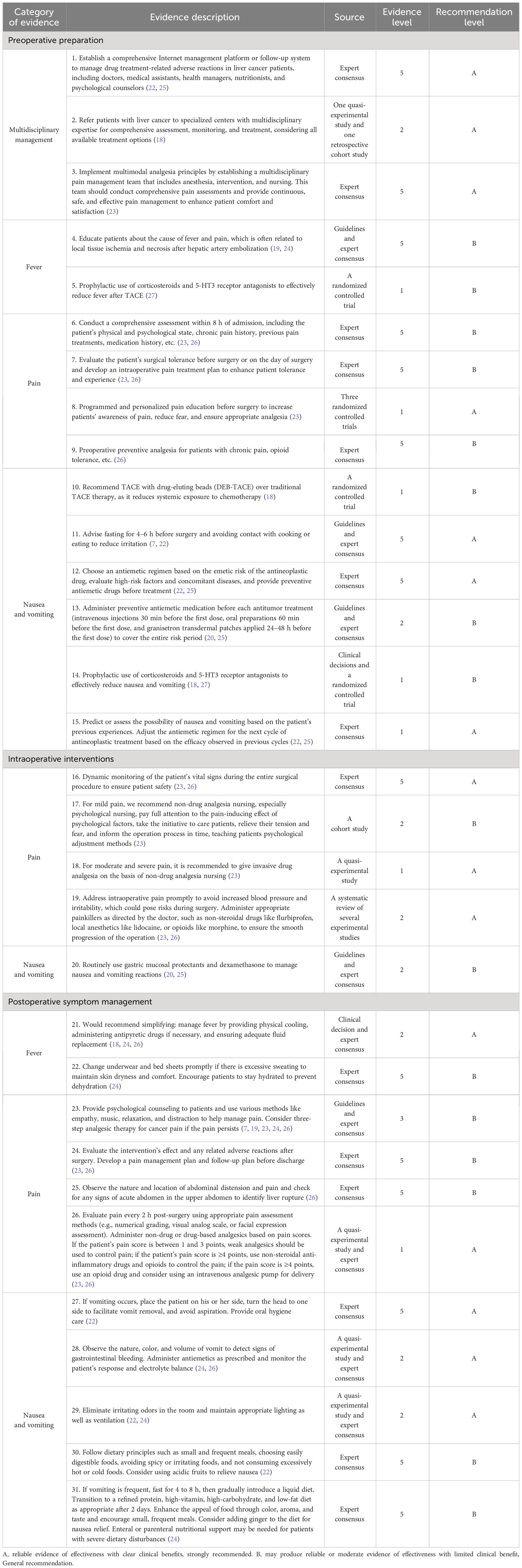
Table 4 Summary of the best evidence for the prevention and treatment of embolism post-TACE syndrome for primary liver cancer.
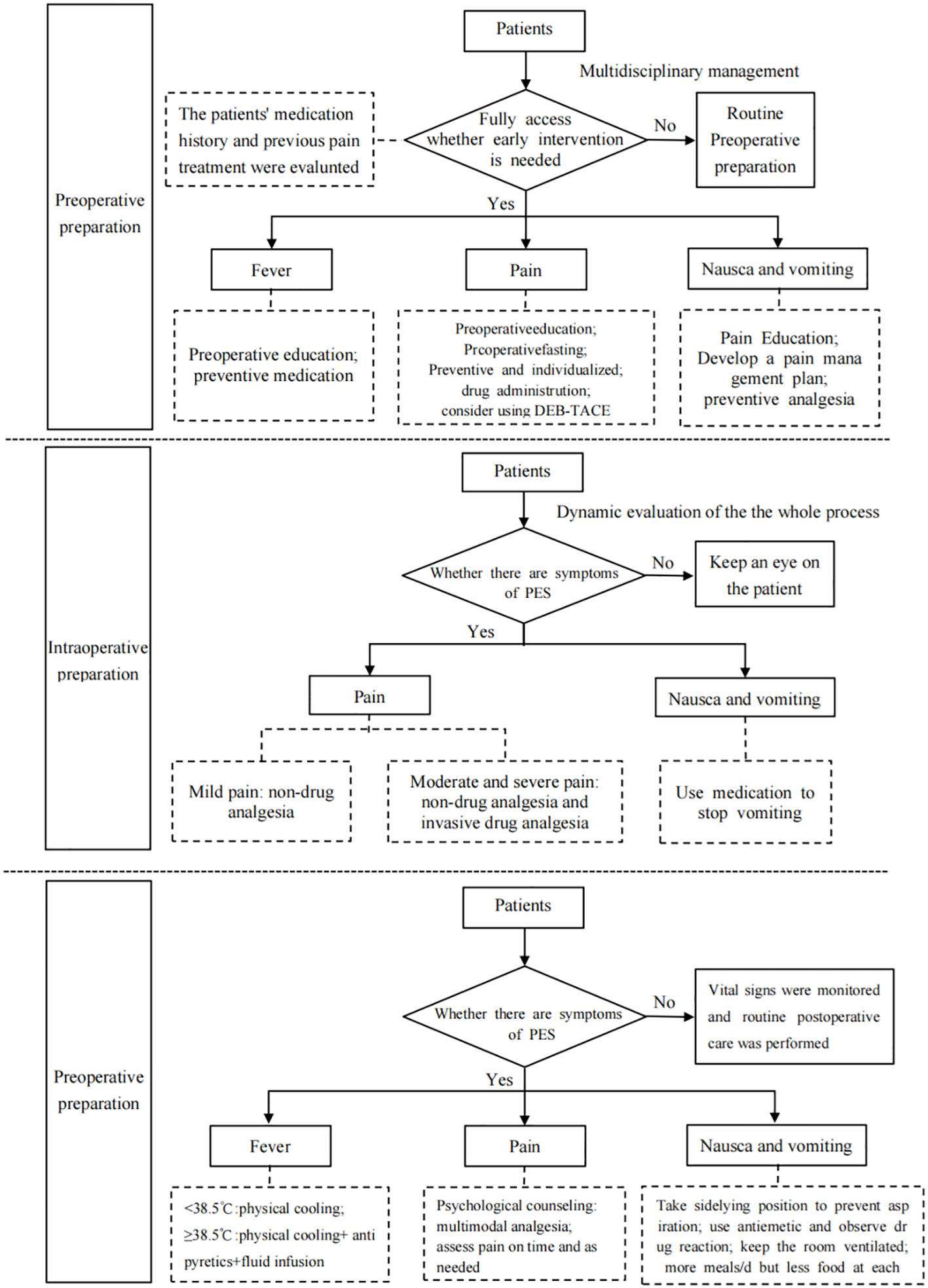
Figure 1 Flow chart of evidence transformation for prevention and treatment of embolic syndrome after transcatheter arterial chemoembolization.
4 Discussion
4.1 Clinical significance of the prevention and treatment of embolism syndrome after TACE based on evidence summary
TACE is an established treatment option for patients with unresectable liver disease, prolonging survival and improving the quality of life (28). Embolism syndrome, or at least some characteristic symptoms, is almost unavoidable following TACE (11). The predictive model constructed by Meredith C. Mason indicated that the bilobal and single-lobe distribution of tumors and the use of DEB-TACE were independent predictors of PES (11). The study of Mohamed H. Khalaf also pointed out that PES history, tumor burden, and DEB-TACE were important predictors of prolonged recovery (29). According to the study of Natascha Roehlen, preoperative tumor size, degree of cirrhosis, and DEB-TACE are predictors of PES (30). Embolism syndrome not only greatly impacts patient comfort post-treatment but also frequently leads to protracted hospital stays and treatment progression. Moreover, it emerges as a complicating factor in the diagnostic and therapeutic processes for healthcare practitioners, potentially giving rise to diagnostic complexities in cases characterized by fever and pain, thereby necessitating supplementary diagnostic tests (31). Effective management of embolic syndrome is important from a clinical practice point of view, but there are currently no established guidelines. Although UpToDate and several countries have published guidelines for the diagnosis and treatment of liver cancer or clinical decision-making information, the evidence is long and the evidence for the prevention and treatment of embolic syndrome after TACE is relatively scattered. Therefore, following a rigorous process of quality assessment and evidence grading, this study aims to consolidate the most robust evidence pertaining to the prevention and treatment of embolism syndrome in patients undergoing TACE.
This summary of evidence fully focuses on the evidence related to the prevention and treatment of embolic syndrome after TACE surgery, which will help nursing practitioners to acquire and understand the evidence efficiently, establish standardized prevention and management procedures for embolic syndrome after TACE, improve nursing practitioners’ sensitivity to PES, reduce the incidence of PES, and reduce patient hospitalization time and cost.
4.2 Forming a multidisciplinary team and an Internet platform to strengthen the health education and follow-up system
Embolism syndrome after TACE is an unavoidable clinical problem marked by its multifaceted symptoms. Formulating and implementing effective and precise prevention and treatment strategies necessitate the collaborative efforts of a multidisciplinary team. This team typically comprises oncologists, nurses specializing in oncology care, gastroenterologists, pain management specialists and nurses, psychological counselors, nutritionists, and other healthcare professionals. Within this framework, physicians shoulder the responsibilities of symptom assessment and the development of tailored management plans, while nurses oversee patients, ensuring regular monitoring and recording of patient data. Nutritionists and psychological counselors, on the other hand, are entrusted with personalized management strategies tailored to the unique needs of individual patients. Patients with liver cancer have recurrent symptoms, and continuous management outside the hospital is an important part of systemic treatment. Establishing an Internet-based management platform for follow-up care is invaluable for healthcare managers. This platform streamlines the identification of symptoms and the monitoring of critical health indicators, enabling timely treatment recommendations. Beyond its utility in facilitating multidisciplinary team collaboration, the Internet management platform also extends practical guidance to liver cancer patients as they navigate the complexities of systemic treatment outside the hospital setting to maximize the benefits of treatment for these patients (22).
4.3 Strengthening the evaluation of each node is an important measure to prevent and treat embolism syndrome
Effective symptom management in surgical care relies on timely and methodical symptom assessment. Prior to surgery, there is a need to promote robust public education by introducing patients to the surgery’s purpose, process, and benefits. This builds patient confidence and provides essential psychological support (32). During surgery, treatment plans to address adverse reactions are implemented, improving patient tolerance and experience. Continuously monitoring patient complaints, maintaining open communication, and promptly identifying and addressing issues also enhance surgical safety. The patients are monitored post-surgery for adverse reactions and discomfort, offering empathetic responses to their concerns. To boost patient tolerance and treatment compliance before discharge, thorough assessments are conducted, psychological support is provided, and follow-up plans are developed.
4.4 Limitations of this study
This study has certain limitations. Notably, some systematic reviews and meta-analyses were excluded during our systematic search as they focused on survival time or tumor response as outcome measures. Additionally, several studies comparing various TACE techniques (cTACE or DEB-TACE), combinations of TACE with other treatments, and different drug predictions were omitted due to incomplete study designs or low-quality assessments. At the same time, the literatures included in this study were mainly from Asia. The included people had different concepts and attitudes toward PES, and there were regional and cultural differences caused by different medical service systems and medical environments. Therefore, future research should aim to incorporate high-quality original studies to gain deeper insights into the effects of various TACE techniques and drug dosages on embolism syndrome.
5 Summary
This study summarized 31 pieces of evidence for preventing and treating embolism syndrome in patients after TACE, serving as a valuable resource for clinical medical practitioners. The results presented here are derived from existing research conclusions. Nevertheless, individual patient cases may vary, necessitating tailored treatment plans based on specific patient conditions. When incorporating this evidence into clinical practice, healthcare professionals should exercise their expertise and consider the unique clinical circumstances, using the best available evidence as a foundation for informed decision-making in clinical nursing practice.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed toeWFveWFucm9uZzExMEAxNjMuY29t.
Author contributions
YY: Funding acquisition, Writing – original draft, Writing – review & editing, Data curation, Methodology, Project administration. XH: Data curation, Formal analysis, Writing – original draft. CZ: Methodology, Writing – review & editing. XW: Formal analysis, Writing – review & editing. GM: Conceptualization, Resources, Writing – review & editing. JL: Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the 2021 Ningxia Nursing Society Research Project Youth Project (NXHL21-30), 2023 Autonomous Region Health System Research Project Youth Science and Technology Cultivation Project (2023-NWKYP-052), and 2023 Ningxia Natural Science Foundation General Project (2023AAC03622). This study was also support by Ningxia science and technology benefit people project (2021CMG03015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Health Commission of the People’s Republic of China Medical Administration Bureau. Guidelines for the diagnosis and treatment of primary liver cancer (2022 edition). Chin J Digestive Surg (2022) 21(2):143–68. doi: 10.3760/cma.j.cn115610-20220124-00053
2. Treska V, Bruha J, Liska V, Fichtl J, Prochazkova K, Petrakova T, et al. Pros and cons of portal vein embolization with hematopoietic stem cells application in colorectal liver metastases surgery. In Vivo (2020) 34(5):2919–25. doi: 10.21873/invivo.12121
3. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality,morbidity,and risk factors in China and its provinces1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. J Lancet (2019) 394(10204):1145–58. doi: 10.1016/S0140-6736(19)30427-1
4. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China,2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
5. Li Y, Zhou F, Liu F, Wang M, Xing W. Experimental study on evaluation of blood supply level and embolization ratio of liver cancer based on I-flow software. Technol Cancer Res Treat (2020) 19:1533033820970665–10. doi: 10.1177/1533033820970665
6. Mejia JC, Pasko J. Primary liver cancers: intrahepatic cholangiocarcinoma and hepatocellular carcinoma. Surg Clin North Am (2020) 100(3):535–49. doi: 10.1016/j.suc.2020.02.013
7. Clinical Diagnosis and Treatment Guidelines Special Committee of Interventional Physician Branch of Chinese Medical Doctor Association. Clinical practice guidelines for transarterial chemoembolization (TACE) treatment of hepatocellular carcinoma in China (2021 edition). Chin J Med (2021) 101(24):1848–62. doi: 10.3760/cma.j.cn112137-20210425-00991
8. Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev (2019) 72:28–36. doi: 10.1016/j.ctrv.2018.11.002
9. Li D, Pang Y, Xu L, Xu X. Efficacy and safety of sorafenib combined with TACE in the treatment of advanced hepatocellular carcinoma: A meta-analysis. J BUON (2021) 26(4):1355–64.
10. Blackburn H, West S. Management of postembolization syndrome following hepatic transarterial chemoembolization for primary or metastatic liver cancer. Cancer Nurs (2016) 39(5):E1–E18. doi: 10.1097/NCC.0000000000000302
11. Mason MC, Massarweh NN, Salami A, Sultenfuss MA, Anaya DA. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford) (2015) 17(12):1137–44. doi: 10.1111/hpb.12487
12. Arslan M, Degirmencioglu S. Risk factors for postembolization syndrome after transcatheter arterial chemoembolization. Curr Med Imaging Rev (2019) 15(4):380–5. doi: 10.2174/1573405615666181122145330
13. Zhu Zh, Hu Y, Xing WJ, Zhou YF, Gu Y. Composition of different types of evidence-based questions. J Nurses Training (2017) 32(21):1991–4.
14. Alper BS, Haynes RB. EBHC pyramid 5.0 for accessing preappraised evidence and guidance. Evid Based Med (2016) 21(4):123–125.
15. Wei D, Wang CY, Xiao XJ, Chen YL, Yao L. Interpretation of guidelines research and evaluation (AGREEII) tool examples. Chin J Evidence-Based Pediatr (2013) 8(4):316–9.
16. Joanna Briggs Institute. Joanna Briggs Institute reviewers’manual:2017 edition. Australia: The Joanna Briggs Institute (2017).
17. Zhou YF, Gu Y, Hu Y, Xing WJ. JBI evidence-based health care center’s quality evaluation tool for different types of research–quality evaluation of intervention research (1). J Nurses Training (2018) 33(01):24 –26.
18. Curley SA, Stuart KE, Schwartz JM, Carithers RL, Hunter KU. Localized hepatocellular carcinoma: Liver-directed therapies for nonsurgical candidates not eligible for local thermal ablation. UpToDate (2022). Available at: https://www.uptodate.cn/.
19. National Health Commission of the People’s Republic of China Medical Administration Bureau. Guidelines for the diagnosis and treatment of primary liver cancer (2022 edition). Chin J Hepat (2022) 30(4):367–88. doi: 10.3760/cma.j.cn501113-20220413-00193
20. Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: ASCO guideline update. J Clin Oncol (2020) 38(24):2782–97. doi: 10.1200/JCO.20.01296
21. 2019 Expert Committee on Clinical Collaboration of Traditional Chinese and Western Medicine for Liver Cancer, Zheng JS, Yang GW, Gong J, Gao C. Expert consensus on interventional diagnosis and treatment of primary liver cancer with integrated traditional Chinese and Western medicine (Trial version 1). J Interventional Radio (2021) 30(11):1079–90.
22. Feng Y, Huang ZH, Sun YK, Zhang YF. Expert guidance on internet management of adverse reactions related to drug treatment in patients with liver cancer. Liver Cancer Electronic J (2021) 8(02):16–22.
23. Wang XY, Jia ZZ, Xu XF, Du RJ, Xue YH, Xiao SP. Expert consensus on perioperative pain management in interventional therapy for Malignant liver tumors (2022). J Interventional Radio (2022) 31(10):943–8.
24. Research group of the National Science and Technology Special Project for the prevention and control of major infectious diseases such as AIDS and viral hepatitis, Ma SP, Chen XJ, Chen XQ. Transhepatic Arterial Chemoembolization for Primary Liver Cancer Expert consensus on postoperative rehabilitation of integrated traditional Chinese and Western medicine. Clin J Hepatobiliary Dis (2021) 37(07):1545–9.
25. Chinese Anti-Cancer Association Cancer Clinical Chemotherapy Professional Committee, Chinese Anti-Cancer Association Cancer Supportive Care Professional Committee. Chinese expert consensus on prevention and treatment of nausea and vomiting associated with cancer drug therapy (2022 edition). Chin J Med (2022) 102(39):3080 –3094. doi: 10.3760/cma.j.cn112137-20220810-01724
26. Xiao SP, Xiao F, Chen DP, Mao YJ, Rao M. Expert consensus on perioperative nursing strategies for transarterial chemoembolization for hepatocellular carcinoma. J Clin Radio (2022) 41(02):212–6.
27. Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, et al. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatol (2018) 67(2):575–85. doi: 10.1002/hep.29403
28. Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci (2020) 21(21):8165. doi: 10.3390/ijms21218165
29. Khalaf MH, Sundaram V, AbdelRazek Mohammed MA, Shah R, Khosla A, Jackson K, et al. A predictive model for postembolization syndrome after transarterial hepatic chemoembolization of hepatocellular carcinoma. Radiology, 180257. doi: 10.1148/radiol.2018180257
30. Roehlen N, Stoehr F, Müller L, Luxenburger H, Gairing SJ, Reincke M, et al. Prediction of postembolization syndrome after transarterial chemoembolization of hepatocellular carcinoma and its impact on prognosis. Hepatol Commun (2023) 7(10):e0252. doi: 10.1097/HC9.0000000000000252
31. Piscaglia F, Tovoli F, Pini P, Salvatore V. A new horizon in the prevention of the postembolization syndrome after transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatology (2018) 67(2):467–9. doi: 10.1002/hep.29517
Keywords: primary liver cancer, TACE, embolism syndrome, evidence-based primary liver cancer, evidence-based nursing, best evidence summary
Citation: Yao Y, Huang X, Zhao C, Wang X, Mi G and Liu J (2024) Summary of the evidence of best practices for the prevention and treatment of embolism syndrome after TACE in primary liver cancer. Front. Oncol. 13:1274235. doi: 10.3389/fonc.2023.1274235
Received: 06 October 2023; Accepted: 14 December 2023;
Published: 15 January 2024.
Edited by:
Jianxun J. Song, Texas A&M Health Science Center, United StatesReviewed by:
Liqing Wang, Texas A&M Health Science Center, United StatesAlfredo Caturano, University of Campania Luigi Vanvitelli, Italy
Anil Kumar, Texas A&M Health Science Center, United States
Copyright © 2024 Yao, Huang, Zhao, Wang, Mi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingli Liu, emNsNjY2QHllYWgubmV0
†These authors have contributed equally to this work
 Yanrong Yao
Yanrong Yao Xi Huang
Xi Huang Chengsi Zhao1
Chengsi Zhao1