
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 01 November 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1273719
Primary cutaneous follicle center lymphoma (PCFCL) differs from follicular lymphoma in biological behavior and molecular profile and is treated as a distinct entity, according to the 5th edition of the World Health Organization classification of hematolymphoid tumors. It is an uncommon cutaneous B-cell lymphoma that is considerably rare in children and adolescents. To date, only 13 cases of individuals younger than 20 years of age have been reported in the literature. The lack of relevant clinical epidemiological data in this population has hampered the investigation of its clinical and diagnostic aspects. Here we report the case of a 17-year-old male with PCFCL, who may be the first PCFCL patient under 20 years of age reported in China. He was admitted to the hospital with a solitary nodule on his face. After complete surgical excision, the patient’s facial mass was histologically identified as PCFCL. The patient’s prognosis was favorable, with no recurrence at 17 months of follow-up after the surgical resection. We present a case of an adolescent PCFCL patient and systematically review the literature with a view to increase the awareness of the disease and inform the diagnosis and treatment of this age group.
Primary cutaneous follicle center lymphoma (PCFCL) is a rare indolent cutaneous B-cell lymphoma (PCBCL) derived from follicle center cells and accounts for less than 1% of B-cell lymphoma cases. It is confined to the skin and rarely involves the lymph nodes or other extra nodal organs (1). PCFCL is much rarer in adolescents, with only 13 cases of PCFCL in children and adolescents reported to date (Table 1) (2–10). In this article, we report the case of a 17-year-old boy with PCFCL who was treated in our hospital and systematically review the literature to better understand the clinical and pathological characteristics of PCFCL in teenagers.
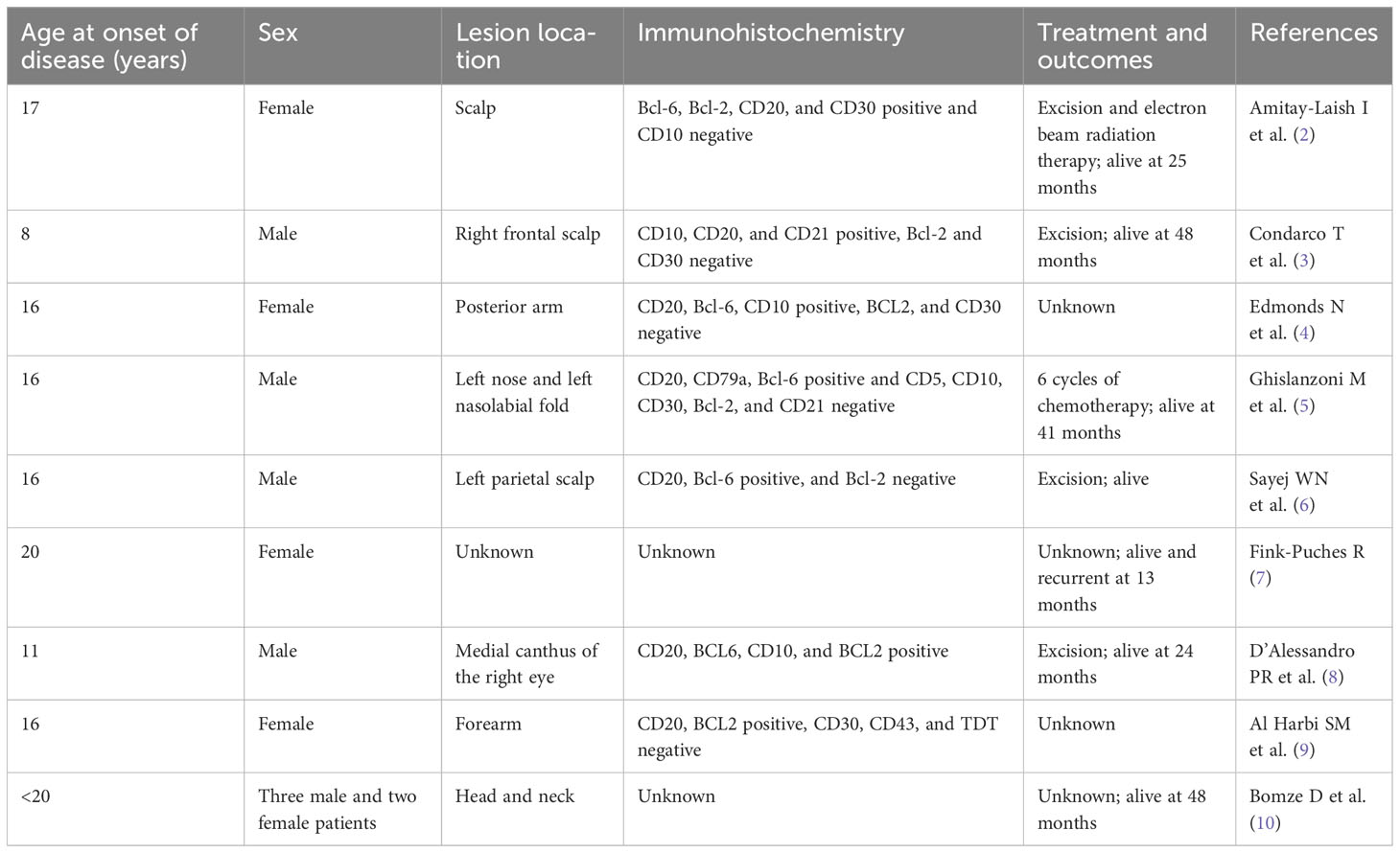
Table 1 Patients with primary cutaneous follicle center lymphoma under 20 years of age as reported in the literature.
An Asian male aged 17 was admitted to our hospital in March 2022 due to “a facial nodule found for about 6 months.” A right temporal mass, initially the size of a peanut, was accidentally discovered in September 2021. The patient was previously healthy; the lump seemed to increase in size over time, and he visited our dermatology center in January 2022. An examination revealed a right temporal mass of approximately 2 cm in diameter; it was tough, with good mobility, clear borders, and a smooth surface. When the mass was imaged using ultrasound, a 2.2 cm ×1.9 cm ×1.1 cm very hypoechoic nodule with a regular shape, distinct borders, and uneven internal echogenicity was detected in the fatty layer of the right temporal region (Figure 1). An orbital MRI, performed in February 2022, showed an oval T1 and T2 compression lipid high-signal shadow with clear borders in the right temporal subcutis that was approximately 15 mm × 27 mm (Figure 2).
The patient was hospitalized in our department for oral and maxillofacial surgery on February 9, 2022. The ECOG score was 0. No significant abnormalities were observed on routine blood tests. The lactate dehydrogenase was 188.7 U/L (reference range, 120–250 U/L). It might be difficult to distinguish between benign and malignant skin cancers without a biopsy. On February 11, 2022, the patient underwent a right temporal mass resection and adjacent flap transfer repair under general anesthesia, during which the mass was completely removed, and the postoperative specimen was sent for pathological examination. The pathology showed multiple pieces of grayish-white disorganized tissue, totaling 2.3 cm ×1.8 cm ×0.7 cm. Hematoxylin and eosin (H&E) staining revealed lymphocyte infiltration with a follicular growth pattern. The follicular structure was atypical and lacked distinct mantle zones. Infiltrating cells comprised mainly follicular centrocytes and centroblasts, along with several histiocytes (Figure 3). The immunohistochemical staining showed a strong diffuse CD20 positivity for germinal center B-cells with PAX5 and PD1 positivity (Figure 4A). The follicular dendritic cell network was compressed with CD21-positive cells (Figure 4B). There was variability in Bcl-2 expression in the follicle center and interstitial areas (Figure 4C). In interstitial areas, there was positive Bcl-6 and Bcl-10 expression, and the ki67 proliferation index was 70%. The fluorescence in situ hybridization results suggested rearrangements of immunoglobulin heavy chains. Combined with the patient’s clinical condition (an isolated lesion with distinct borders) and the results of immunohistochemistry and Ig gene rearrangement tests, we considered a diagnosis of PCFCL.
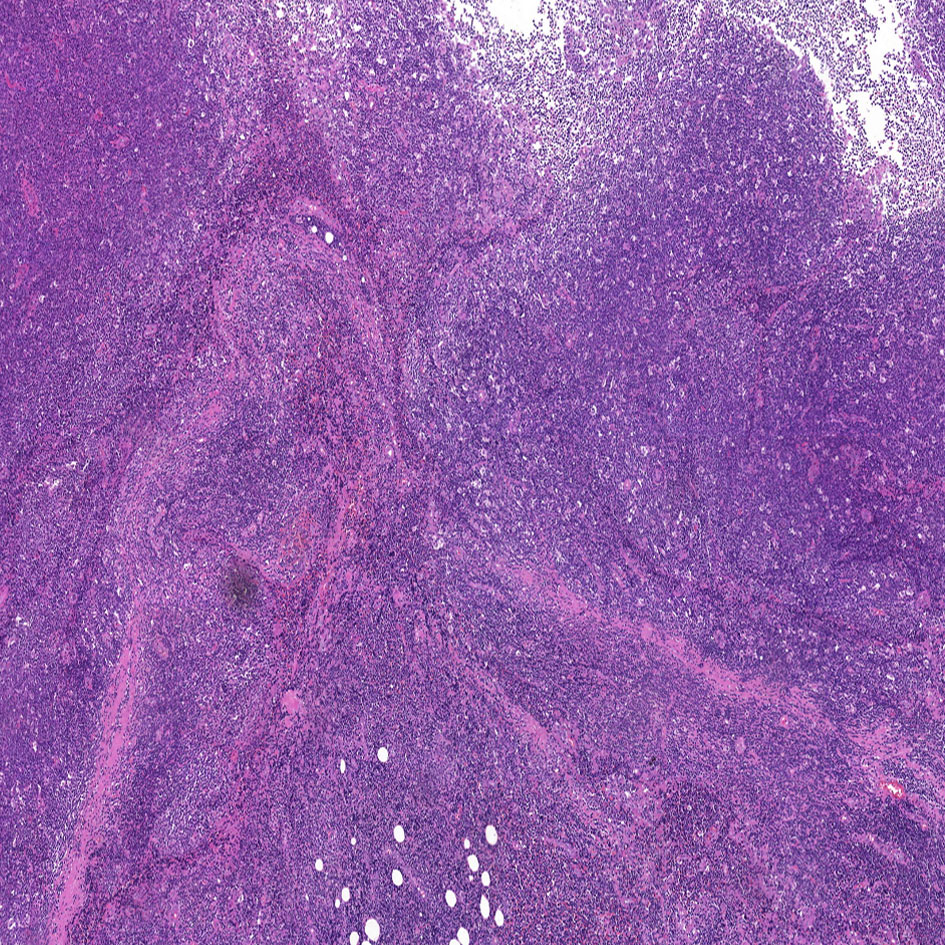
Figure 3 Hematoxylin and eosin staining of the tumor tissue (H&E, ×5). Neoplastic cells are small to moderately sized, with little cellular heterogeneity, and some infiltration of small lymphocytes and histiocytes can be observed in the background.

Figure 4 (A) Immunohistochemistry showing neoplastic cells diffusely positive for CD20 (×10). (B) Follicular dendritic cell networks strongly expressing CD21 (×10). (C) BCL-2 is variably expressed at different sites, and in this figure BCL-2 is negative (×10).
The patient underwent enhanced CT of the neck, chest, and abdomen on March 28, 2022, which revealed no enlarged lymph nodes. The patient underwent bone marrow aspiration on March 28, 2022, and the bone marrow smear, flow cytology, and biopsy revealed no obvious abnormalities. The patient was diagnosed with PCFCL, and the TNM stage was T1N0M0. In view of the complete resection of the patient’s lesion, we advised that the patient be followed up and reviewed regularly. The patient cooperated well with regular telephone follow-ups. The patient underwent a cranial MR enhancement scan 45 days after surgery, which showed no significant abnormalities in brain MR enhancement and a postoperative change in the right temporal subcutaneous area. The patient was satisfied with the outcome of the procedure. At the time of submission, the patient had not relapsed during the telephone follow-up.
In the 2018 World Health Organization for Research and Treatment of Cancer updated classification of cutaneous lymphomas, PCBCL has been divided into five categories: cutaneous marginal zone B-cell lymphoma (PCMZL), PCFCL, primary cutaneous diffuse large B-cell lymphoma, leg type (PCDLBCL-LT), intravascular large B-cell lymphoma, and EBV-positive mucocutaneous ulcer-provisional (11).
Clinically, PCFCL is more common in middle-aged men (>50 years old), with a preference for the head, neck, and back, and it typically presents as painless or pruritic. They can manifest morphologically as isolated or numerous plaques, nodules, papules, or masses (12). However, the incidence of PCFCL in adolescents differs significantly from that in adults. According to a retrospective study in the United States, which included 5,176 cases of cutaneous lymphoma, patients younger than 20 years of age accounted for only 10.4% of PCFCL cases, with an annual incidence rate of 0.12 per one million people. This represents an incidence rate of only one in 40 compared to the adult population (10).
According to histology, centroblast and follicle center cells make up the majority of tumor cells in PCFCL. Tumor cells show three growth patterns: follicular, diffuse, and follicular diffuse. Tumor cells were immunohistochemically tested positive for B-cell markers, such as CD20, CD79a, PAX5, and Bcl-6, but negative for CD5 and CD43 and mostly negative for MUM1. The expression of CD10 and bcl-2 varied, and their co-expression indicated a higher chance of skin recurrence (13). The strong expression of CD10 and bcl-2 suggests the possibility of systemic lymph node disease involving the skin, necessitating an appropriate differential diagnosis (14). Therefore, it is necessary to establish an appropriate differential diagnosis. PCMZL expresses CD20, CD79a, and bcl-2 but does not express CD10 and bcl-6 (15). The aggressive disease PCDLBCL-LT usually shows a strong expression of bcl-2 and MUM1. Unlike adult follicular lymphoma, most children and adolescents with PCFCL do not have the t (14, 16) chromosomal translocation involving BCL-2 protein expression.
Prior to staging assessment, it is essential to conduct a thorough medical history review, a comprehensive physical examination, blood routine tests, biochemistry tests (including lactate dehydrogenase), and either a CT or PET-CT scan. The necessity of bone marrow aspiration and biopsy remains a subject of debate. The TNMB staging system, originally designed for mycosis fungoides (MF) and Se’zary syndrome (SS), is not applicable to other primary cutaneous lymphomas. Consequently, the International Society for Cutaneous Lymphomas (ISCL) has recommended using an alternative TNMB staging system. A new TNM staging system was proposed by ISCL and EORTC for primary cutaneous lymphomas other than MF/SS (17, 18). The International Extranodal Lymphoma Study Group found that elevated lactate dehydrogenase levels, more than two skin lesions, and nodular lesions were independent risk factors affecting the prognosis of PCFCL; therefore, the international prognostic index (CIPI) for cutaneous lymphoma was based on these three risk factors to determine the prognosis of indolent cutaneous lymphoma. The CIPI for cutaneous lymphoma was developed based on these three risk factors. Patients with low-, intermediate-, and high-risk CIPI scores had 5-year progression-free survival rates of 91%, 64%, and 48%, respectively (P = 0.001) (16). In addition, differences in growth patterns, centroblast density, chromosomal abnormalities, and BCL-2 protein expression have no impact on the prognosis of PCFCL (12).
The preferred treatments are complete surgical resection or field radiation therapy, both of which have a complete remission rate of nearly 100% (19). In this case, there was no sign of recurrence in the short term after complete surgical resection. Rituximab alone or in combination with chemotherapy can be used to treat adult patients with extensive skin lesions (20). Radiotherapy alone has an increased rate of local recurrence compared with surgical resection (21). Delaying radiotherapy after recurrence does not affect prognosis (22). It is uncertain whether PCFCL in children and adolescents requires a different therapeutic approach than that in adults because of the rarity of this condition. In adult patients, PCFCL has a fair prognosis and an indolent course, with a 5-year survival rate of >95%. PCFCL is currently considered to have similar prognoses in pediatric and adolescent patients.
In conclusion, PCFCL is relatively rare in the pediatric and adolescent populations. The diagnosis of the disease is challenging due to the paucity of relevant studies and the lack of awareness of the disease, which may lead to underdiagnosis or misdiagnosis of the disease. Here we report a rare case of PCFCL in an adolescent in China and its multidisciplinary collaborative diagnostic and treatment process, which we hope will further raise awareness of the disease. In the future, as more prospective studies are conducted, the clinical and biological characteristics of PCFCL in pediatric and adolescent populations will be further elucidated.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the ethics committee review board of The Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
W-YN: Data curation, Writing – original draft, Writing – review & editing. JD: Supervision, Writing – review & editing. X-SY: Writing – original draft. HQ: Writing – original draft. Y-JS: Writing – original draft. H-YG: Resources, Visualization. G-LL: Supervision, Methodology. Z-GC: Supervision, Methodology.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank the patients and their families. We would like to thank Editage for the English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kim MJ, Hong ME, Maeng CH, Jung HA, Hong JY, Choi MK, et al. Clinical and treatment outcomes of primary cutaneous B-cell lymphoma: A single-center analysis in South Korea. Int J Hematol (2015) 101:273–8. doi: 10.1007/s12185-014-1728-2
2. Amitay-Laish I, Feinmesser M, Ben-Amitai D, David M, Manor Y, Kidron D, et al. Juvenile onset of primary low-grade cutaneous B-cell lymphoma. Br J Dermatol (2009) 161:140–7. doi: 10.1111/j.1365-2133.2009.09121.x
3. Condarco T, Sagatys E, Prakash AV, Rezania D, Cualing H. Primary cutaneous B-cell lymphoma in a child. Fetal Pediatr Pathol (2008) 27:206–14. doi: 10.1080/15513810802319442
4. Edmonds N, Hernández-Pérez M, Holsinger M, Gru AA. Primary cutaneous follicle center lymphoma in a 16-year-old girl. J Cutan Pathol (2021) 48:663–8. doi: 10.1111/cup.13939
5. Ghislanzoni M, Gambini D, Perrone T, Alessi E, Berti E. Primary cutaneous follicular center cell lymphoma of the nose with maxillary sinus involvement in a pediatric patient. J Am Acad Dermatol (2005) 52(Suppl 1):S73–5. doi: 10.1016/j.jaad.2004.05.025
6. Sayej WN, Isakoff MS, DiGiuseppe JA, Moote D, Balarezo F, Finck C, et al. Primary cutaneous Follicle Center Lymphoma in a 16-year-old boy with Crohn disease Exposed to infliximab and methotrexate. J Pediatr Gastroenterol Nutr (2020) 70:e49–50. doi: 10.1097/MPG.0000000000002578
7. Fink-Puches R. The spectrum of cutaneous lymphomas in patients less than 20 years of age. Pediatr Dermatol (2010) 21(5):525–33. doi: 10.1111/j.0736-8046.2004.21500.x
8. D’Alessandro PR, Lo AC, Spencer MH, Farinha P, Armstrong L, Dolman PJ, et al. Primary cutaneous follicle center lymphoma of the medial canthus of the eye in an 11-year old. Pediatr Blood Cancer (2022) 69:e29630. doi: 10.1002/pbc.29630
9. Al Harbi SM, Al Natour S, Al Saif NM, Al Saif N, Al Bayat MI. Primary cutaneous follicle center lymphoma presenting as a solitary nodule on the forearm of an adolescent girl: A case report and literature review. Clin Cosmet Investig Dermatol (2023) 16:167–72. doi: 10.2147/CCID.S396326
10. Bomze D, Sprecher E, Goldberg I, Samuelov L, Geller S. Primary cutaneous B-cell lymphomas in children and adolescents: A SEER population-based study. Clin Lymphoma Myeloma Leuk (2021) 21:e1000–5. doi: 10.1016/j.clml.2021.07.021
11. Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood (2019) 133:1703–14. doi: 10.1182/blood-2018-11-881268
12. Fava P, Roccuzzo G, Alberti-Violetti S, Grandi V, Pileri A, Pimpinelli N, et al. Cutaneous B-cell lymphomas: Update on diagnosis, risk-stratification, and management. Presse Med (2022) 51:104109. doi: 10.1016/j.lpm.2022.104109
13. Pham-Ledard A, Cowppli-Bony A, Doussau A, Prochazkova-Carlotti M, Laharanne E, Jouary T, et al. Diagnostic and prognostic value of BCL2 rearrangement in 53 patients with follicular lymphoma presenting as primary skin lesions. Am J Clin Pathol (2015) 143:362–73. doi: 10.1309/AJCP4SUBR4NPSPTN
14. Pileri A, Agostinelli C, Bertuzzi C, Grandi V, Maio V, Lastrucci I, et al. BCL-2 expression in primary cutaneous follicle center B-cell lymphoma and its prognostic role. Front Oncol (2020) 10:662. doi: 10.3389/fonc.2020.00662
15. Ceppi F, Pope E, Ngan B, Abla O. Primary cutaneous lymphomas in children and adolescents. Pediatr Blood Cancer (2016) 63:1886–94. doi: 10.1002/pbc.26076
16. Mian M, Marcheselli L, Luminari S, Federico M, Cantonetti M, Sarris AH, et al. CLIPI: A new prognostic index for indolent cutaneous B cell lymphoma proposed by the International Extranodal Lymphoma Study Group (IELSG 11). Ann Hematol (2011) 90:401–8. doi: 10.1007/s00277-010-1083-1
17. Kim YH, Willemze R, Pimpinelli N, Whittaker S, Olsen EA, Ranki A, et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood (2007) 110:479–84. doi: 10.1182/blood-2006-10-054601
18. Olsen EA. Evaluation, diagnosis, and staging of cutaneous lymphoma. Dermatol Clin (2015) 33:643–54. doi: 10.1016/j.det.2015.06.001
19. Wilcox RA. Cutaneous B-cell lymphomas: 2015 update on diagnosis, risk-stratification, and management. Am J Hematol (2015) 90:73–6. doi: 10.1002/ajh.23863
20. Porkert S, Mai P, Jonak C, Weihsengruber F, Rappersberger K, Bauer W, et al. Long-term therapeutic success of intravenous rituximab in 26 patients with indolent primary cutaneous B-cell lymphoma. Acta Derm Venereol (2021) 101:adv00383. doi: 10.2340/00015555-3746
21. Hamilton SN, Wai ES, Tan K, Alexander C, Gascoyne RD, Connors JM. Treatment and outcomes in patients with primary cutaneous B-cell lymphoma: The BC Cancer Agency experience. Int J Radiat Oncol Biol Phys (2013) 87:719–25. doi: 10.1016/j.ijrobp.2013.07.019
Keywords: follicle center lymphoma, cutaneous lymphoma, B-cell lymphoma, adolescent, case report
Citation: Niu W-Y, Yan X-S, Qiao H, Sun Y-J, Gu H-Y, Li G-L, Cui Z-G and Du J (2023) An adolescent with primary cutaneous follicle center lymphoma: a case report and literature review. Front. Oncol. 13:1273719. doi: 10.3389/fonc.2023.1273719
Received: 07 August 2023; Accepted: 16 October 2023;
Published: 01 November 2023.
Edited by:
Francesco Onida, IRCCS Ca’ Granda Foundation Maggiore Policlinico Hospital, ItalyCopyright © 2023 Niu, Yan, Qiao, Sun, Gu, Li, Cui and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Yan Niu, d2VueWFubml1QHFkdS5lZHUuY24=; Juan Du, ZHVqdWFuQHFkdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.