
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 17 November 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1271722
This article is part of the Research TopicCytogenetics, Genomics and Epigenomics in Myelodysplastic Neoplasm and Acute Myeloid Leukemia: From Biology to TreatmentView all 6 articles
CPX-351, a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a 1:5 molar ratio, is approved for the treatment of newly diagnosed therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes. In a pivotal phase III trial, CPX-351 significantly improved overall survival compared with standard-of-care 7 + 3 chemotherapy (7 days cytarabine; 3 days daunorubicin) in adults aged 60–75 years with newly diagnosed high-risk or secondary AML (median = 9.56 months vs. 5.95 months; hazard ratio = 0.69; 95% confidence interval = 0.52–0.90; p = 0.003). Approximately 30% of patients with newly diagnosed AML have mutations in the FLT3 gene, which may be associated with poor outcomes. Here, we review the current in vitro, clinical, and real-world evidence on the use of CPX-351 in patients with AML and mutations in FLT3. Additionally, we review preliminary data from clinical trials and patient case reports that suggest the combination of CPX-351 with FLT3 inhibitors may represent another treatment option for patients with FLT3 mutation-positive AML.
CPX-351 is a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a synergistic 1:5 molar ratio (1). Phase III trial data have shown CPX-351 to be significantly more effective than standard-of-care 7 + 3 chemotherapy (7 days cytarabine; 3 days daunorubicin) in treating newly diagnosed adults aged 60–75 years with high-risk/secondary acute myeloid leukemia (AML). Among 309 randomized patients, overall survival (OS) was significantly improved with CPX-351 vs. 7 + 3 (median = 9.56 months vs. 5.95 months; hazard ratio (HR) = 0.69; 95% confidence interval (CI) = 0.52–0.90; one-sided p = 0.003) (2). At 5-year follow-up, the median OS was 9.33 months with CPX-351 and 5.95 months with 7 + 3 (HR = 0.70; 95% CI = 0.55–0.91), and the 5-year OS rate was 18% with CPX-351 and 8% with 7 + 3 (3). CPX-351 treatment also resulted in a significantly higher overall remission rate compared with 7 + 3 (47.7% vs. 33.3%; two-sided p = 0.016) (2). Among patients who subsequently underwent hematopoietic cell transplantation, 3-year OS landmarked from the date of hematopoietic cell transplantation was 56% with CPX-351 and 23% with 7 + 3 (3). Based on these data, CPX-351 was approved for the treatment of newly diagnosed, therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC) in adult and pediatric patients aged 1 year and older in the USA and in adults in the European Union (4, 5). It should be noted that in the 2022 update of the World Health Organization classification for hematolymphoid tumors, the classification AML-MRC was replaced with “AML, myelodysplasia-related” (AML-MR), which requires the presence of cytogenetic or molecular abnormalities and/or a history of myelodysplastic neoplasms (MDS) or MDS/myeloproliferative neoplasms for diagnosis (6).
FLT3 is expressed in most AML blasts and plays a key role in normal hematopoiesis and leukemogenesis (7, 8). Approximately 30% of patients with newly diagnosed AML have mutations in the FLT3 gene, most commonly an internal tandem duplication (ITD; ~25% of cases) or point mutations in the tyrosine kinase domain (TKD; 7%–10% of cases) (9). Among patients with t-AML and normal karyotype, the frequencies of FLT3-ITD and FLT3-TKD mutations are 23% and 9%, respectively (10). The FLT3-ITD mutation is associated with poor outcomes in patients with AML, whereas the prognostic impact of FLT3-TKD mutations is unclear (9). Here, we review the preclinical and clinical evidence on the use of CPX-351 in patients with AML and mutations in FLT3.
Following initial reports of CPX-351 activity compared with conventional drug combinations in phase II trials, the cytotoxic activity of CPX-351 was assessed across risk groups using an ex vivo assay with freshly harvested blasts from 53 patients with AML (11). Primary AML leukemia blasts showed high sensitivity to CPX-351, with 50% growth inhibition concentration (IC50) values ranging from 0.035:0.007 μM to 9.77:1.95 μM. Responses to CPX-351 were similar across conventional cytogenetic risk groups (per 2010 European LeukemiaNet criteria), including blasts with intermediate II or adverse cytogenetic and molecular abnormalities, and were not correlated with prior response to 7 + 3 treatment. In total, samples from 14 patients with AML were FLT3-ITD-positive, and these blasts were found to be nearly five times more sensitive to CPX-351-induced cytotoxicity than FLT3-ITD-negative AML blasts (mean IC50 values = 0.29:0.058 μM and 1.32:0.26 μM, respectively; p = 0.047 for difference). In contrast, mutations in NPM1 or CEBPA were found to have no significant impact on CPX-351 treatment responses. FLT3-ITD-positive AML blasts were also observed to have an increased uptake of CPX-351 as compared with FLT3-ITD-negative blasts (Figure 1). Overall, the sensitivity of AML blasts to CPX-351 in this ex vivo assay was consistent with the observed clinical activity in patient populations (11).
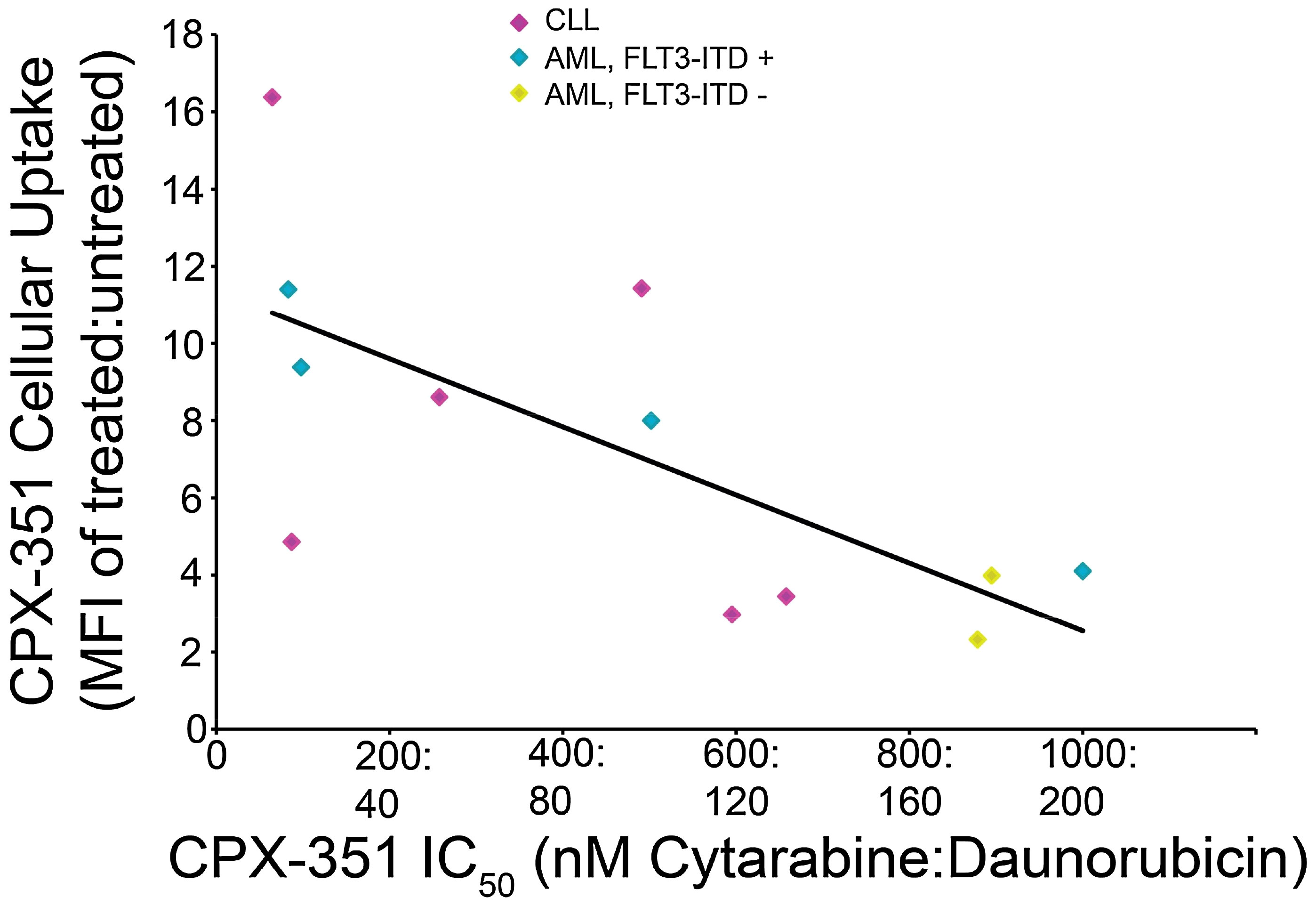
Figure 1 Correlation of CPX-351 cytotoxicity with cellular uptake in AML blasts. Patient blasts (n = 12) were exposed to graded concentrations of CPX-351 for 24 h, and the number of viable cells at 3 days was used to calculate IC50 values for each sample. CPX-351 uptake was assessed by analysis of daunorubicin fluorescence uptake using flow cytometry; the ratio of mean fluorescence intensity (MFI) in treated vs. untreated cells was calculated and plotted against CPX-351 IC50. The correlation coefficient between IC50 and MFI was 0.703. Figure reproduced with permission from Gordon et al. 2017 (11).
The above findings were corroborated by analysis of AML cell lines (MOLM-13, MOLM-14, and ME1), which also showed increased sensitivity to CPX-351 and increased drug uptake in the presence of FLT3-ITD or FLT3-activating mutations, compared with other genetic abnormalities (12). It has been hypothesized that dysregulation of FLT3 signaling may lead to the activation of liposome uptake pathways, resulting in increased sensitivity to CPX-351. Notably, pretreatment of AML cell lines with the FLT3 inhibitor, quizartinib, for 16 h resulted in ~50% of the total cell population exhibiting a decrease in daunorubicin fluorescence, an indicator of drug uptake, suggesting that prolonged FLT3 inhibition may decrease CPX-351 uptake. It follows that alterations in the timing of FLT3 inhibitor treatment may impact effectiveness. Consistent with this, it was found that combining CPX-351 with FLT3 inhibitors (quizartinib and midostaurin) had a synergistic effect when the drugs were used simultaneously or when CPX-351 was scheduled 24 h before the FLT3 inhibitor. However, when FLT3 inhibitors were administered 24 h prior to CPX-351, less synergy was seen, and at certain doses, the effects were antagonistic (12). The authors concluded that these results provide additional evidence that FLT3 activation leads to increased uptake of CPX-351.
In the pivotal phase III study, 22 (16%) patients in the CPX-351 arm and 21 (15%) patients in the 7 + 3 control arm had mutations in the FLT3 gene (2, 3). Response rates were higher with CPX-351 vs. 7 + 3 in patients with FLT3 mutations (complete remission (CR)/CR with incomplete neutrophil or platelet count recovery (CRi) rate = 68.2% vs. 23.8%; odds ratio = 6.86; 95% CI = 1.78–26.36; CR rate = 54.5% vs 19.0%; odds ratio = 5.10; 95% CI = 1.29–20.17) (2). Furthermore, post-hoc subgroup analysis showed a trend toward improved OS with CPX-351 vs. 7 + 3 in patients with FLT3 mutations (median OS = 10.25 months vs. 4.60 months; HR = 0.76; 95% CI = 0.34–1.66), although caution should be exerted when interpreting these data due to the small number of patients with baseline FLT3 mutations included in this analysis. In patients with wild-type FLT3, the median OS was 9.33 months with CPX-351 and 5.98 months with 7 + 3 (HR = 0.64; 95% CI = 0.47–0.87). These findings have been supported by data generated in a real-world setting, as outlined below and summarized in Table 1.
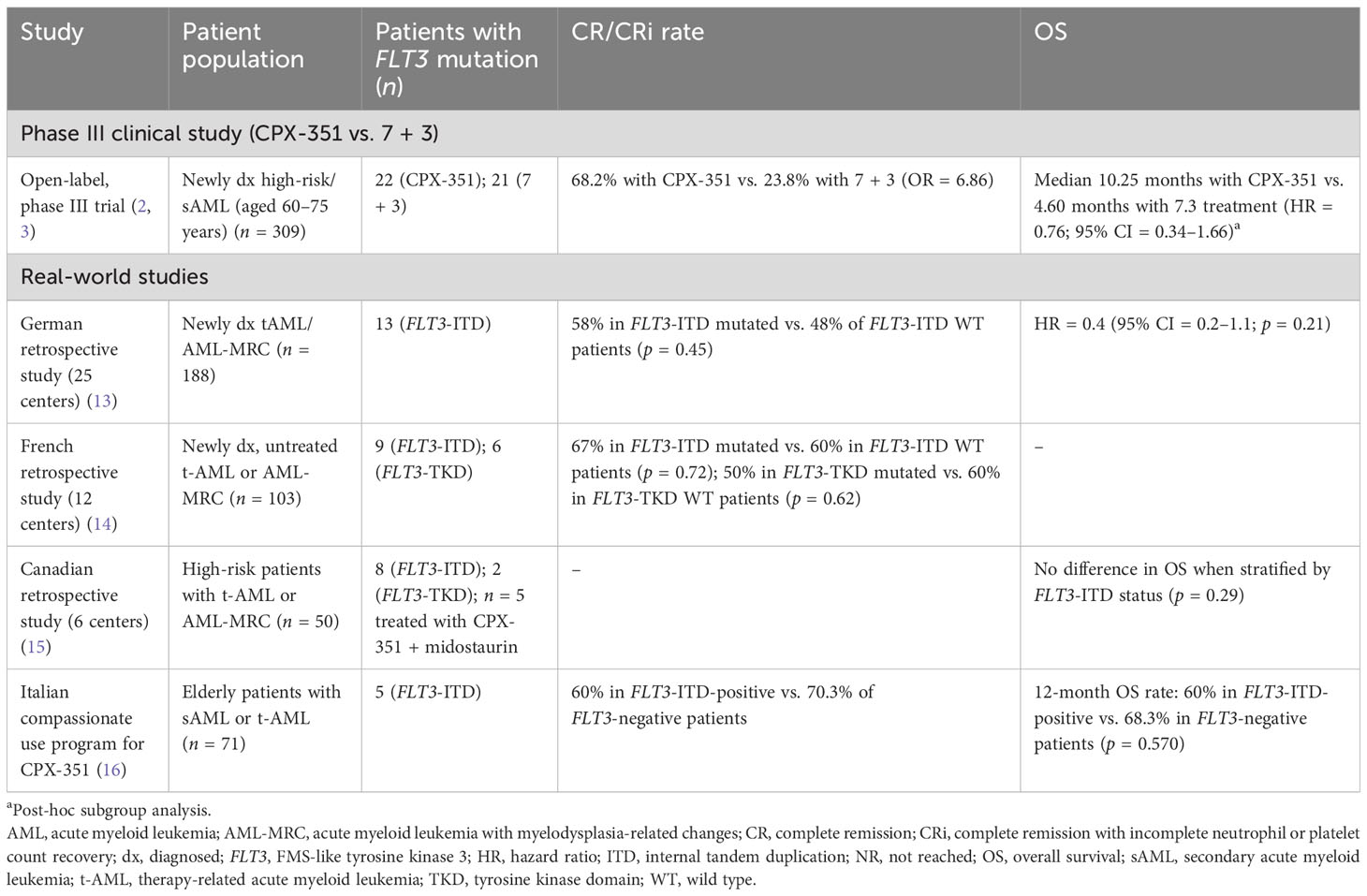
Table 1 Summary of published clinical study data and real-world evidence for use of CPX-351 monotherapy in patients with FLT3-ITD/TKD mutations.
A German real-world study of first-line CPX-351 efficacy and safety in adults with newly diagnosed AML-MRC or t-AML (n = 188) included 13 patients with FLT3-ITD mutations (13). The CR/CRi rate was 58% in patients with FLT3-ITD mutations and 48% in patients with wild-type FLT3-ITD (p = 0.45 for difference). The presence of a FLT3-ITD mutation was not found to be a factor impacting OS in univariate analysis (HR = 0.4; 95% CI = 0.2–1.1; p = 0.21). In a retrospective study that assessed CPX-351 efficacy and safety in adults with newly diagnosed, untreated t-AML or AML-MRC in France (n = 103), the CR/CRi rate for adults with FLT3-ITD mutations was 67% (n = 6). In patients without FLT3-ITD mutations, the CR/CRi rate was 60% (n = 53; there was no statistical difference between the groups; p = 0.72) (14). In patients with FLT3-TKD mutations, the CR/CRi rate was 50% (n = 3), and in patients with nonmutated FLT3-TKD, the CR/CRi rate was 60% (n = 56; p = 0.62 for difference) (14). Another real-world study that supports the data from the phase III study described above is a Canadian multicenter study of 50 patients who received CPX-351 for t-AML and AML-MRC, 10 of whom had mutations in FLT3 (eight ITD and two TKD). Analysis of OS by risk factors showed no differences, including when stratified by the presence of a FLT3-ITD mutation (p = 0.29) (15). Furthermore, in an Italian compassionate use program that recruited patients aged 52–79 years with secondary AML, three of five patients with a FLT3-ITD mutation achieved CR/CRi with CPX-351; the 12-month OS was 60% (16). It is important to note that all real-world studies described here had limited numbers of patients with FLT3 mutations; however, these findings support a role for CPX-351 as early-line treatment in patients with FLT3-mutated AML.
The phase Ib V-FAST master study (NCT04075747) was initiated to investigate the recommended phase II dose and the safety and tolerability of CPX-351 when administered in combination with targeted agents in patients with AML who are fit to receive intensive chemotherapy (17, 18). Patients with mutated FLT3 were assigned to receive CPX-351 in combination with the FLT3-inhibitor midostaurin.
Preliminary results from the ongoing V-FAST study suggest that the combination of CPX-351 + midostaurin is a feasible treatment strategy for adults with newly diagnosed AML who have an FLT3 mutation (17, 18). In total, 23 patients with FLT3-mutated AML received CPX-351 + midostaurin treatment. Of these, 18 (78%) had FLT3-ITD, and six (26%) had FLT3-TKD mutations (one patient had both mutations and was included in both subgroups). Most patients had de novo disease (78% of patients with FLT3-ITD and 100% of patients with FLT3-TKD mutations). The majority of patients achieved CR with CPX-351 + midostaurin, including 14/17 (82.4%) patients with FLT3-ITD mutations, and five of six (83.3%) patients with FLT3-TKD mutations. Among patients with a CR and known measurable residual disease status (as assessed by multicolor flow cytometry at local laboratories), rates of measurable residual disease negativity after induction were 50% for those with FLT3-ITD mutations and 33.3% for those with FLT3-TKD mutations. At the time of analysis, 10 of 18 patients (55.6%) with FLT3-ITD mutations and two of six patients (33.3%) with FLT3-TKD mutation had proceeded to hematopoietic cell transplantation following treatment with CPX-351 + midostaurin. Hematologic recovery times in the V-FAST study were consistent with CPX-351 monotherapy. The overall safety profile was manageable, with serious adverse events reported in 33.3% of patients and no deaths occurring prior to study day 60 (17, 18). These findings are supported by case reports from three patients who received CPX-351 + midostaurin for the treatment of FLT3 mutation-positive secondary AML. These were a 75-year-old man with AML-MRC and FLT3-ITD mutation, a 73-year-old woman with t-AML and FLT3-TKD mutation, and a 60-year-old woman with AML-MRC and FLT3-TKD mutation. In each case, combination treatment did not cause any unexpected adverse events and resulted in a CR. Overall, the median time to neutrophil recovery (≥ 500/µL) was 31 days, while the median time to platelet recovery (≥ 50,000/µL) was 32 days. OS in the three treated patients was 17, 4, and 12 months, respectively (19). One of the three patients went on to receive an allogeneic stem-cell transplant and remained in CR post-transplant.
This mini-review summarizes the growing evidence supporting a role for CPX-351 in the treatment of AML associated with mutations in FLT3. Furthermore, preliminary data from clinical trials and patient case reports suggest that the combination of CPX-351 + midostaurin may represent an additional treatment option for patients with FLT3 mutation-positive AML. Combination therapies using an induction chemotherapy backbone with targeted agents are increasingly being used in eligible patient populations for the treatment of AML (20). The benefit of combining standard chemotherapy with midostaurin in patients with AML and FLT3 mutations has been demonstrated previously. In the randomized phase III RATIFY study (NCT00651261), the addition of midostaurin to chemotherapy significantly improved OS and event-free survival in adults with newly diagnosed AML and an FLT3 mutation compared with placebo (21). The combination of CPX-351 + FLT3 inhibitors for AML will be further investigated in the phase I/II NCT04128748 study (CPX-351 + quizartinib), the phase I/II NCT04982354 study (CPX-351 + midostaurin), and the phase III NCT04293562 study (CPX-351 + gilteritinib), which are currently recruiting patients. Interestingly, preclinical data have suggested a synergistic effect with combination treatment when FLT3 inhibitors were administered after CPX-351 (12). While the use of CPX-351 as a combination therapy with FLT3 inhibitors continues to be evaluated in clinical trials, the pivotal CPX-351 clinical trial and real-world studies have demonstrated that CPX-351 monotherapy may be of benefit in AML patients with FLT3 mutations; no significant differences in CPX-351 treatment outcomes were observed between patients with versus without an FLT3 mutation.
A caveat to the findings described in this review is the relatively small number of patients included in each analysis; further validation of these data is warranted in larger studies. In addition, the data described in this article also do not consider the allelic ratio of FLT3-ITD mutations, which is known to have an impact on disease prognosis (7). It remains to be determined whether FLT3-ITDhigh or FLT3-ITDlow genotypes show differential responses to CPX-351 treatment. However, using allelic ratio testing in clinical practice presents challenges as it is often unavailable to treating physicians, and there is a lack of validated and standardized methods between laboratories. Furthermore, FLT3-ITD allelic ratios are no longer part of the European LeukemiaNet risk classification at initial diagnosis for AML (22).
Also worthy of consideration and future study are the mechanisms of resistance to CPX-351 treatment, such as TP53 mutation (23), and how these may specifically impact patients with FLT3 mutations.
In summary, the findings reviewed in this article highlight the potential for CPX-351 use in the treatment of patients with AML and mutations in FLT3. Combination approaches with CPX-351 and FLT3 inhibitors are supported by preclinical data and initial findings from clinical studies.
CA: Writing – original draft, Writing – review & editing. VP: Writing – original draft, Writing – review & editing. CR: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This article was supported by Jazz Pharmaceuticals.
Medical writing support, under the direction of the authors, was provided by Paul O’Neill, PhD, of CMC Affinity, a division of IPG Health Medical Communications, in accordance with Good Publication Practice (GPP 2022) guidelines. This assistance was funded by Jazz Pharmaceuticals.
CA has participated in advisory board meetings for Jazz Pharmaceuticals and AOP Orphan and has received an honorarium from Incyte. VP has received speaker and consultancy fees from Jazz Pharmaceuticals. CR has received grants from AbbVie, Amgen, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, IQVIA, and MaaT Pharma; has received personal fees from AbbVie, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, Novartis, Servier, and Takeda; and has received nonfinancial support from AbbVie, Astellas, Bristol Myers Squibb, Jazz Pharmaceuticals, Novartis, and Servier.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ALT, alanine aminotransferase; AML, acute myeloid leukemia; CI, confidence interval; CR, complete remission; CRi, complete remission with incomplete neutrophil or platelet count recovery; HR, hazard ratio; IC50, 50 percent growth inhibition concentration; ITD, internal tandem duplication; MRC, myelodysplasia-related changes; OS, overall survival; t-AML, therapy-related acute myeloid leukemia; TEAE, treatment-emergent adverse event; TKD, tyrosine kinase domain.
1. Tardi P, Johnstone S, Harasym N, Xie S, Harasym T, Zisman N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res (2009) 33(1):129–39. doi: 10.1016/j.leukres.2008.06.028
2. Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol (2018) 36(26):2684–92. doi: 10.1200/jco.2017.77.6112
3. Lancet JE, Uy GL, Newell LF, Lin TL, Ritchie EK, Stuart RK, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol (2021) 8(7):e481–e91. doi: 10.1016/s2352-3026(21)00134-4
4. Jazz Pharmaceuticals Inc. VYXEOS® (daunorubicin and cytarabine) Prescribing Information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/209401s011lbl.pdf (Accessed March 14).
5. European Medicines Agency. Vyxeos liposomal summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/vyxeos-liposomal-epar-product-information_en.pdf (Accessed March 14).
6. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia (2022) 36(7):1703–19. doi: 10.1038/s41375-022-01613-1
7. Kiyoi H, Kawashima N, Ishikawa Y. FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development. Cancer Sci (2020) 111(2):312–22. doi: 10.1111/cas.14274
8. Ambinder AJ, Levis M. Potential targeting of FLT3 acute myeloid leukemia. Haematologica (2021) 106(3):671–81. doi: 10.3324/haematol.2019.240754
9. Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia (2019) 33(2):299–312. doi: 10.1038/s41375-018-0357-9
10. Samra B, Richard-Carpentier G, Kadia TM, Ravandi F, Daver NG, Dinardo CD, et al. Characteristics and outcomes of patients with therapy-related acute myeloid leukemia with normal karyotype. Blood Cancer J (2020) 10(5):47. doi: 10.1038/s41408-020-0316-3
11. Gordon MJ, Tardi P, Loriaux MM, Spurgeon SE, Traer E, Kovacsovics T, et al. CPX-351 exhibits potent and direct ex vivo cytotoxicity against AML blasts with enhanced efficacy for cells harboring the FLT3-ITD mutation. Leuk Res (2017) 53:39–49. doi: 10.1016/j.leukres.2016.12.002
12. Edwards DK, Javidi-Sharifi N, Rofelty A, Gordon M, Roth-Carter R, Tardi P, et al. Abstract 1087: CPX-351 works synergistically in combination with FLT3 inhibitors against AML with FLT3-ITD. Cancer Res (2017) 77(13_Supplement):1087. doi: 10.1158/1538-7445.Am2017-1087
13. Rautenberg C, Stölzel F, Röllig C, Stelljes M, Gaidzik V, Lauseker M, et al. Real-world experience of CPX-351 as first-line treatment for patients with acute myeloid leukemia. Blood Cancer J (2021) 11(10):164. doi: 10.1038/s41408-021-00558-5
14. Chiche E, Rahmé R, Bertoli S, Dumas P-Y, Micol J-B, Hicheri Y, et al. Real-life experience with CPX-351 and impact on the outcome of high-risk AML patients: a multicentric French cohort. Blood Adv (2021) 5(1):176–84. doi: 10.1182/bloodadvances.2020003159
15. Andrews C, Young T, Atenafu EG, Assouline SE, Brandwein JM, Chan SM, et al. CPX351 has short remission duration but is an effective bridge to allogeneic transplant in high risk AML: results from Canadian real-world multi-centre study. Blood (2020) 136(Suppl 1):6–7. doi: 10.1182/blood-2020-142990
16. Guolo F, Fianchi L, Minetto P, Clavio M, Gottardi M, Galimberti S, et al. CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: results of the Italian compassionate use program. Blood Cancer J (2020) 10(10):96. doi: 10.1038/s41408-020-00361-8
17. McCloskey JK, Pullarkat VA, Mannis GN, Lin TL, Strickland SA, Fathi AT, et al. V-FAST master trial: Preliminary results of treatment with CPX-351 plus midostaurin in adults with newly diagnosed FLT3-mutated acute myeloid leukemia. J Clin Oncol (2022) 40(16_suppl):7043. doi: 10.1200/JCO.2022.40.16_suppl.7043
18. McCloskey J, Pullarkat VA, Mannis GN, Lin TL, Strickland SA, Fathi AT, et al. V-FAST master trial: subgroup analysis of outcomes with CPX-351 plus midostaurin in adults with newly diagnosed acute myeloid leukemia by FLT3 mutation type. Blood (2022) 140(Supplement 1):3312–4. doi: 10.1182/blood-2022-159680
19. Andrews C, Maze D, Murphy T, Sibai H. Combination treatment with CPX-351 and midostaurin in patients with secondary acute myeloid leukaemia that are FLT3 mutated: three cases and review of literature. Br J Haematol (2020) 190(3):467–70. doi: 10.1111/bjh.16800
20. Tang K, Schuh AC, Yee KW. 3+7 combined chemotherapy for acute myeloid leukemia: is it time to say goodbye? Curr Oncol Rep (2021) 23(10):120. doi: 10.1007/s11912-021-01108-9
21. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med (2017) 377(5):454–64. doi: 10.1056/NEJMoa1614359
22. Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood (2022) 140(12):1345–77. doi: 10.1182/blood.2022016867
Keywords: acute myeloid leukemia, chemotherapy, CPX-351, FLT3 inhibitors, FLT3 mutations
Citation: Andrews C, Pullarkat V and Recher C (2023) CPX-351 in FLT3-mutated acute myeloid leukemia. Front. Oncol. 13:1271722. doi: 10.3389/fonc.2023.1271722
Received: 02 August 2023; Accepted: 31 October 2023;
Published: 17 November 2023.
Edited by:
Joshua Zeidner, University of North Carolina at Chapel Hill, United StatesReviewed by:
Amanda Przespolewski, University at Buffalo, United StatesCopyright © 2023 Andrews, Pullarkat and Recher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claire Andrews, Y2xhaXJlYW5kcmV3c0BzdC12aW5jZW50cy5pZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.