
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Oncol. , 25 October 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1271647
This article is part of the Research Topic Testicular Cancer Awareness Month 2023: Exploring New Treatments for Improving Testicular Cancer Management and Patient Outcome View all 5 articles
 Georgina E. Wood1†
Georgina E. Wood1† Christopher P. Bunting2†
Christopher P. Bunting2† Mesel Veli1
Mesel Veli1 Rupali Arora3
Rupali Arora3 Daniel M. Berney4
Daniel M. Berney4 Constantine Alifrangis1
Constantine Alifrangis1 Nicola D. MacDonald5
Nicola D. MacDonald5 Rowan E. Miller1,6
Rowan E. Miller1,6 Jonathan Shamash6
Jonathan Shamash6 Sara Stoneham7
Sara Stoneham7 Michelle Lockley1,8*
Michelle Lockley1,8*Malignant germ cell tumours are a group of rare cancers whose incidence peaks in late adolescence and early adulthood. Dysgerminomas of the ovary and seminomas of the testis are analogous diseases, but seminomas have a 10-fold higher incidence. The two tumours are morphologically identical and are only differentiated by surrounding organ-specific tissue or testicular germ cell neoplasia in situ. They share genetic features including KIT and RAS mutations, amplification of chromosome 12p, and expression of pluripotency markers (NANOG (Nanog homeobox), OCT3/4 (Octamer-binding transcription factor 3/4), and SAL4 (Spalt-like trascription factor 4)). Both histologies are exquisitely sensitive to platinum chemotherapy, and the combination of bleomycin, etoposide, and cisplatin (BEP) yields survival rates greater than 90%. However, BEP causes significant, lifelong toxicity (cardiovascular, renal, respiratory, and neurological) in these young patients with an expectation of cure. Here, we comprehensively review the biological features of dysgerminoma and seminoma to demonstrate that they are biologically analogous diseases. We present available clinical trial data supporting de-escalation of chemotherapy treatment. Finally, we propose that future trials should enrol men, women, and children to benefit all patients regardless of age or sex.
Approximately 90% of malignant tumours of the testis have a germ cell histology Testicular Germ Cell Tumour (TGCT). Seminomas are the most common pathological subtype accounting for 50% of testicular cancers worldwide (1). In contrast, malignant ovarian germ cell tumours (mOGCTs) are rare, accounting for less than 5% of ovarian malignancies (2, 3), but, of these, dysgerminomas represent 30%–40% (4). Dysgerminomas have a 10-fold lower incidence than seminomas and an earlier age of onset, peaking at 1.2 per 100,000 in women aged 15–19 (5), whereas seminomas have an incidence of 10.1 per 100,000 men aged 15–40 (6) and a mean age of 35 years at diagnosis (7). Both tumours are characterised by exquisite sensitivity to chemotherapy and an excellent prognosis, even when patients present with metastatic disease. Current treatment guidelines recommend widespread use of BEP (bleomycin, etoposide, and cisplatin) or EP (etoposide and cisplatin) chemotherapy with significant associated toxicity.
Here, we present evidence outlining the biological and clinical similarities between seminoma and dysgerminoma. We discuss recent clinical trials and our regional clinical experience, both of which strongly support a move towards de-escalation of systemic chemotherapy in adults in line with paediatric practice. We propose that future clinical trials should enrol patients with dysgerminoma and seminoma of all ages to increase statistical power, promote scientific discovery, and improve outcomes for all patients.
TGCTs and mOGCTs have common developmental origins. Seminomas develop from premalignant germ cell neoplasia in situ (GCNIS) within seminiferous tubules of the testes (8), whereas dysgerminomas arise from primordial germ cells (PGCs) (9). Embryonic transcription factors such as NANOG and OCT4 promote proliferation and accumulation of mutations in PGCs (10). Immunohistochemical analysis has quantified an increase in NANOG and OCT4 expression of at least 1.5- fold compared with healthy testis in 33% and 56% of seminomas, respectively (11), whereas, in dysgerminomas, 67% exhibit positive staining for NANOG and 80% for OCT3/4 (12). In contrast, expression of these embryonic stem cell markers is minimal in other GCT histologies.
Seminomas and dysgerminomas also share genetic and epigenetic aberrations that are not found in other GCTs. Recurrent DNA copy number alterations have been identified in dysgerminoma, specifically gain of chromosomes 7, 8, 12, and 21 and loss of chromosome 13 (13). Although chromosome 12p accumulation is a common feature in many GCTs, it is considered a hallmark of seminoma and is also observed in up to 77% of mOGCTs (13, 14). Mutations in KIT and RAS proto-oncogenes are identified in approximately 30% of dysgerminoma and seminoma (13–15) with targeted sequencing revealing five mutations located in domains that are common to both seminoma and dysgerminoma (14). In seminoma, KIT mutation likely occurs pre-migration of PGC to the gonadal ridges, whereas these mutations arise post-migration in dysgerminoma. This difference is likely explained by sex-specific PGC differentiation into either oocytes or spermatozoa (13).
Seminoma and dysgerminoma are morphologically indistinguishable (16, 17) and can only be differentiated by surrounding organ-specific tissue or testicular GCNIS (Figure 1). Both tumours are composed of uniform round primitive germ cells with clear cytoplasm and macronucleoli arranged in nests or cords and separated by thin fibrovascular septae containing lymphocytes (16, 17). Vascular invasion is less common than other GCTs but is estimated to be present in 34% of seminomas (17) and may have prognostic significance (18). Although data are more limited in dysgerminoma, vascular invasion is also uncommon and has been described in approximately 20% of cases (19). Immunohistochemical staining reflects the shared genetic basis of the two cancers, and positive staining for OCT3/4 and SALL4 as well as strong membrane positivity for c-KIT (CD117) and D2-40 are used clinically to define both seminoma and dysgerminoma (20, 21).
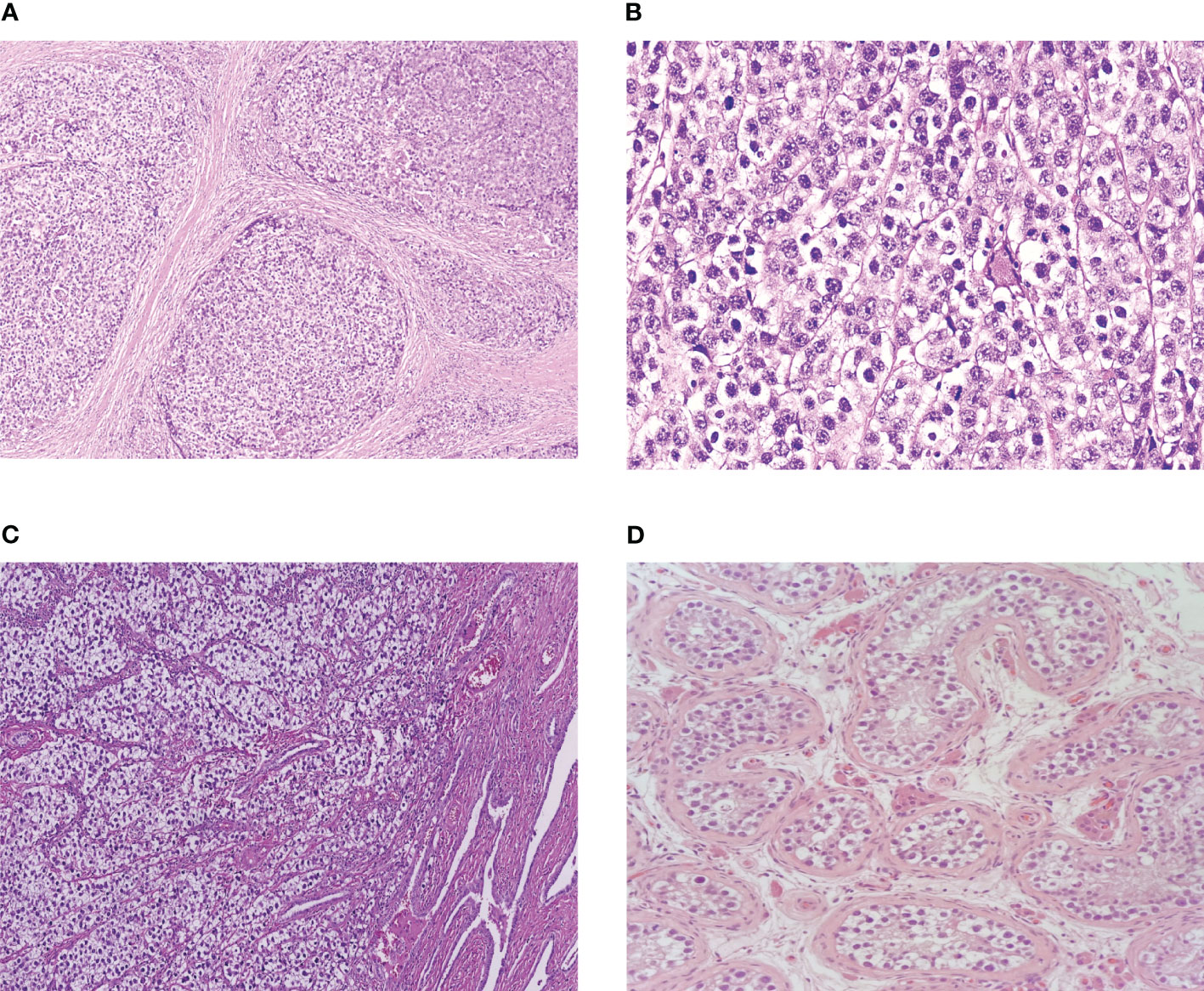
Figure 1 (A) Ovarian dysgerminoma at low power. (B) Ovarian dysgerminoma at high power. (C) Testicular seminoma invading rete testis. (D) Germ cell carcinoma neoplasia in situ.
In the UK, all patients with cancer are managed by diverse professional groups within multi-disciplinary teams (MDTs) based at high- volume, specialist oncology centres. For rare tumours like dysgerminomas and seminomas, “supra-regional” MDTs cover several hospitals and large geographical areas and include urological, gynaecological, and paediatric oncologists and surgeons. This specialised, collaborative approach has been shown to improve patient outcomes (22). Although surgery and radiotherapy for testicular, ovarian, and extra-gonadal mGCTs are necessarily different, owing to their different anatomical sites, there is a great deal of overlap in systemic chemotherapy.
GCTs at all anatomical sites show excellent sensitivity to platinum. This has been attributed to the preference of germ cells to respond to DNA damage by promoting apoptosis, as opposed to DNA repair pathways, perhaps to reduce the likelihood of altering the germline (23). Even among GCTs, seminoma and dysgerminoma are both particularly sensitive to platinum chemotherapy, potentially implying that common mechanisms of drug response are shared between these two tumours. This is reflected in an overall survival (OS) of 96% and 89% for good and intermediate prognosis metastatic seminoma, respectively (24). In dysgerminoma, first- line treatment of FIGO (International Federation of Gynaecology and Obstetrics) stage IV disease results in a 5-year OS greater than 90% (25, 26) compared with 72% for stage IV mOGCTs overall. Interestingly, a correlation has been shown between DNA methylation and response of TGCTs (excluding teratomas) to cisplatin chemotherapy, and, because cytogenetic studies have identified global hypomethylation in pure seminoma and dysgerminoma relative to other GCTs (27, 28), this could contribute to their extreme platinum sensitivity.
Current international guidelines for the treatment of mOGCTs in adults (National Comprehensive Cancer Network (29) and European Society of Medical Oncology) (3), children, and adolescents and young adults (AYA) (ESGO/SIOPE) (30) recommend that ovary confined, FIGO stage I pure dysgerminomas, which have been “properly surgically staged,” can be safely treated with surgery alone. Likewise, stage I seminomas can be treated with radical orchidectomy followed by surveillance without the need for adjuvant treatment (31) although discussion with patients regarding the marginal benefit of adjuvant carboplatin or radiotherapy to a para-aortic field is advised (32). In higher-stage dysgerminoma (FIGO stages II–IV), the guidelines recommend fertility sparing surgery followed by four cycles of BEP chemotherapy (omitting bleomycin from the fourth cycle). A similar treatment approach is recommended for adult men with metastatic seminoma, although patients presenting with IGCCCG (International Germ Cell Cancer Collaborative Group) good-prognosis metastatic disease receive three rather than four cycles of BEP or four cycles of EP (24, 33).
In the small number of patients who relapse, treatment is generally with combination chemotherapy, but there is no consensus on the optimal regimen. High-dose chemotherapy followed by autologous bone marrow transplant may also be used. Multiple novel targeted agents have been tested in GCTs. Although some activity has been observed with the cyclin dependent kinase 4/6 inhibitor palbociclib in patients with teratoma (34, 35), results from early phase trials testing other targeted agents such as angiogenesis inhibitors (36, 37), mammalian Target Of Rapamycin (mTOR) inhibitors (38, 39), and Programmed Death Ligand 1 (PDL1) inhibitors (40) have generally been poor. Even agents with a relevant target, for example, the c-KIT inhibitor imatinib in patients with c-KIT–expressing GCT (41), have not demonstrated efficacy.
Although chemotherapy is highly effective, it is associated with profound adverse short- and long-term effects. For example, BEP can cause ototoxicity (20%–50% patients), pulmonary toxicity (1%–3% fatality), renal impairment (mean decrease in glomerular filtration rate 25.9% with ≥3 cycles), neurotoxicity (20%–40% patients), cardiovascular morbidity (7% angina and 25%–61% Raynaud’s phenomenon), and secondary malignancies (e.g., approximately two-fold increased risk of leukaemia in patients treated with combined BEP and radiotherapy) (42–45). Because the vast majority of patients with seminoma and dysgerminoma are young and will ultimately be cured of their disease, a focus on survivorship and quality of life following curative treatment is of utmost importance.
One successful way to achieve this in seminoma has been the use of radiotherapy in stage I–IIb disease, which has been shown to prevent disease recurrence following orchidectomy in 15%–20% of these patients (32). However, this approach is associated with increased risk of secondary malignancies (42, 45). Dysgerminomas are also radiosensitive, but anatomical considerations including the frequently large size of the primary tumour, together with the risk of secondary cancers and premature ovarian failure, preclude radiotherapy in women with this disease (46).
In the absence of effective therapeutic alternatives to platinum, one approach to reducing chemotherapy toxicity, whilst maintaining anti-cancer efficacy has been to use carboplatin instead of cisplatin. Trials have focused on paediatric and AYA patients, perhaps because hearing loss in particular has significant educational, social, and vocational consequences in young patients (47). In pre-pubertal men and women up to age 18 with stage II–IV extracranial mGCTs, adjuvant chemotherapy with four cycles of JEB (bleomycin of 15 iu/m2 on day 1, etoposide of 120 mg/m2 on days 1–3, and carboplatin of 600 mg/m2 on day 1) has been used safely for over 20 years (48). Reported OS rates are more than 90% (49), which compares favourably with cisplatin-based regimens (50, 51), but treatment-related deafness and pulmonary and renal toxicity are extremely rare (49, 52).
In dysgerminoma, the most compelling evidence that carboplatin-based chemotherapy has equivalent efficacy to cisplatin in children and AYAs derives from a recent meta-analysis of paediatric and AYA patients treated in six international GCT trials including 126 patients with dysgerminoma (mean age, 20 years). There was no difference in Event-Free Survival (EFS) or OS between patients who were treated with cisplatin (n = 70) (5-year EFS rate, 93%; OS rate, 96%) and those who received carboplatin (n = 56) (5-year EFS rate, 96%; OS rate, 96%) (25). Another recent single-arm observational study of patients younger than 18 years, including 12 patients with dysgerminoma, demonstrated the safe use of JEB with an OS for the whole group of 97%, 5-year EFS of 92%, and no significant hearing or renal side effects (52).
Evidence for carboplatin-based chemotherapy in the treatment of adult patients is much more sparse. In the 1990s, two clinical trials compared single-agent carboplatin to cisplatin-based chemotherapy in adults with good prognosis metastatic seminoma. The first trial randomised between four and six cycles of carboplatin (400 mg/m2) and four cycles of VIP (etoposide of 100 mg/m2, ifosfamide of 1,200 mg/m2, and cisplatin of 20 mg/m2 on days 1–5), with both being administered every 28 days. The second trial compared four cycles of carboplatin (400 mg/m2) to EP chemotherapy (etoposide of 120 mg/m2 on days 1–3 and cisplatin 20 mg/m2 days 1–5) administered every 21 days. Both trials demonstrated higher rates of treatment failure with single-agent carboplatin compared with the combination regimens (53). This could be due to the additional drugs used in the combination regimens, and the trials have since been criticised for using an inadequate dose and frequency of carboplatin.
In dysgerminoma, a small single-arm study of 39 paediatric and adult patients investigated adjuvant carboplatin and etoposide without bleomycin following complete surgical resection (54) (the paediatric and AYA cases from this study were included in the meta-analysis presented in Section 3.6.1 (25). Notably, this trial included 21 patients with stage II/III disease and only 11 patients older than 30 years. Despite using carboplatin (400 mg/m2) every 28 days, survival rate was still 100% after a median follow up of 7.8 years with acceptable toxicity (54). This trial was unfortunately terminated early because of non-favourable results in parallel studies in non-seminomatous GCTs (55, 56). Because no other clinical trials have been conducted, the role of carboplatin in adult dysgerminoma remains unresolved.
To address the limitations of previous trials, we recently investigated the use of high-dose, single-agent carboplatin [area under the curve × 10 (AUC10) in accordance with the Calvert formula, every 3 weeks for four cycles] in 216 men with good-prognosis metastatic seminoma in a multi-centre phase II, non-randomised study. Efficacy was similar to BEP with a 5-year disease-specific OS of 98.3% (57). Importantly, we observed favourable toxicity in terms of febrile neutropenia and nephrotoxicity. We anticipate that long-term effects such as ototoxicity will be reduced although this remains to be determined.
These results lead us to adopt single-agent AUC10 as our preferred adjuvant treatment for good risk metastatic seminoma in our Supra-network Germ Cell Tumour MDT. On the basis of the similar embryological origins, morphologic, genetic, and clinical features of seminoma and dysgerminoma, we have extrapolated our use of carboplatin AUC10 to include ovarian dysgerminoma. This approach is subject to ongoing evaluation as a multi-centre, prospective, longitudinal study in the nine hospitals that make up our MDT. To promote further clinical investigation of this regimen, we now report the dysgerminoma cases that we have treated to date and have at least 3 years follow-up after completion of chemotherapy. The first patient who we treated with single-agent carboplatin was presented to our MDT in March 2017, and the last patient with sufficient follow-up was presented in July 2019. Between these two dates a total of eight patients with newly diagnosed dysgerminoma were discussed, and, because our MDT has a referral population of 7.5 million, this low case number highlights the very low incidence of this disease.
Two of the eight patients with dysgerminoma were FIGO stage I and were managed with post-operative observation. The other six all received single-agent carboplatin (Table 1). These six women have now been followed up for a median of 59.5 months (range, 36–73), and none have relapsed. Because most GCT recurrence occurs within 2 years of treatment (58), this implies that carboplatin AUC10 is sufficiently efficacious for dysgerminoma although we acknowledge that late relapse can occur and will only be detected by longer observation.
Carboplatin AUC10 was extremely well tolerated. Low-grade nausea and diarrhoea were common, occurring in four of the six patients. Bone marrow toxicity, particularly thrombocytopenia, was the most frequent grade 3/4 toxicity, occurring in four patients. All cases of haematological toxicity were readily managed with treatment delays and dose reductions and none developed neutropenic sepsis. We made a similar observation in men where 37% and 27% suffered grade 3/4 neutropenia and thrombocytopenia, respectively (57). Four of the six women were in the AYA age group, and none of these patients experienced long-term toxicity. This compares to our prior published data showing rates of chronic toxicity related to BEP in 20% of mOGCTs treated in our region (59).
It is of particular interest that efficacy was also seen in a 58-year-old patient with dysgerminoma, in whom BEP would have been contraindicated due to the high risk of pulmonary toxicity in patients older than 40 years (60, 61). This patient had poor risk disease due to the high stage (FIGO IVB) and the fact that age greater than 45 years is a known adverse risk factor in dysgerminoma (24, 62). Therefore, her relapse-free survival at 70 months follow-up is particularly encouraging. It is, however, important to note that this patient continues to have peripheral neuropathy and hearing impairment and so even carboplatin AUC10 should be used with caution in this patient age group, where GCTs are exceptionally rare.
Importantly, all six women remain in remission at a median of 5 years of follow-up. Our data, therefore, provides preliminary evidence of efficacy and tolerability of single-agent carboplatin AUC10 in metastatic dysgerminoma in line with our much larger metastatic seminoma cohort (n = 216) (57). In light of these new data, we now recommend investigation of this regimen in a larger, randomised clinical trial enrolling seminoma and dysgerminoma with the aim of reducing treatment-related toxicities for all patients.
The existing literature presented here demonstrates that dysgerminomas biologically resemble seminomas more closely than any other GCTs. In the 2022 World Health Organisation classification of genitourinary tumours, seminoma is now placed in the “germinoma family” of tumours (63), emphasising that dysgerminoma, seminoma, and germinoma are the same tumour arising at different sites. This similarity is borne out in clinical practice by comparable efficacy of systemic treatments.
We have now implemented carboplatin AUC10 as standard treatment for good prognosis metastatic seminoma in our region and single-agent carboplatin is already being used more widely across the UK in good prognosis disease (64). The new data that we present here show, for the first time, that women with dysgerminoma can also be successfully treated with carboplatin AUC10 with minimal associated toxicity. We are now prospectively collecting data for all patients with dysgerminoma and seminoma receiving this regimen as an ongoing multi-centre cohort study.
The rarity of dysgerminoma has resulted in a paucity of suitable clinical studies; thus, treatment advances for patients with dysgerminoma have fallen behind those for men with seminoma. This situation is highlighted by the fact that, at the time of writing, there are only seven clinical trials for patients with dysgerminoma listed on clinicaltrials.gov, of which only one is recruiting patients. This compares to 59 clinical trials for seminoma. In view of the notable similarities between dysgerminoma and seminoma, we propose that they should be investigated together in the same clinical trials. Such a commitment requires international collaboration, particularly in rare cancers. The ongoing AGCT1531 trial (65) for non-seminoma/dysgerminoma GCTs that will enrol patients regardless of patient age or sex demonstrates the feasibility of conducting clinical trials that transcend established professional boundaries. Future investigators should build on this approach by developing similarly inclusive trials to facilitate equitable improvements in outcome for all patients with these rare cancers.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
GW: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. CB: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. MV: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. RA: Data curation, Formal Analysis, Visualization, Writing – review & editing. DB: Data curation, Formal Analysis, Visualization, Writing – review & editing. CA: Conceptualization, Data curation, Writing – review & editing. NM: Conceptualization, Data curation, Writing – review & editing. RM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – review & editing. JS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – review & editing. SS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – review & editing. ML: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research Support: ML was supported by a Cancer Research UK Advanced Clinician Scientist Fellowship (C41405/A19694).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Oldenburg J, Fossa SD, Nuver J, Heidenreich A, Schmoll HJ, Bokemeyer C, et al. Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2013) 24 Suppl 6:vi125–32. doi: 10.1093/annonc/mdt304
2. Matz M, Coleman MP, Sant M, Chirlaque MD, Visser O, Gore M, et al. The histology of ovarian cancer: worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol Oncol (2017) 144:405–13. doi: 10.1016/j.ygyno.2016.10.019
3. Ray-Coquard I, Morice P, Lorusso D, Prat J, Oaknin A, Pautier P, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29 Suppl 4:iv1–iv18. doi: 10.1093/annonc/mdy001
4. Pectasides D, Pectasides E, Kassanos D. Germ cell tumors of the ovary. Cancer Treat Rev (2008) 34:427–41. doi: 10.1016/j.ctrv.2008.02.002
5. Quirk JT, Natarajan N, Mettlin CJ. Age-specific ovarian cancer incidence rate patterns in the United States. Gynecol Oncol (2005) 99:248–50. doi: 10.1016/j.ygyno.2005.06.052
6. Rajpert-De Meyts E, McGlynn KA, Okamoto K, Jewett MA, Bokemeyer C. Testicular germ cell tumours. Lancet (2016) 387:1762–74. doi: 10.1016/S0140-6736(15)00991-5
7. Vasdev N, Moon A, Thorpe AC. Classification, epidemiology and therapies for testicular germ cell tumours. Int J Dev Biol (2013) 57:133–9. doi: 10.1387/ijdb.130031nv
8. Nettersheim D, Schorle H. The plasticity of germ cell cancers and its dependence on the cellular microenvironment. J Cell Mol Med (2017) 21:1463–7. doi: 10.1111/jcmm.13082
9. Cheng L, Thomas A, Roth LM, Zheng W, Michael H, Karim FW. OCT4: a novel biomarker for dysgerminoma of the ovary. Am J Surg Pathol (2004) 28:1341–6. doi: 10.1097/01.pas.0000135528.03942.1f
10. Kushwaha R, Jagadish N, Kustagi M, Mendiratta G, Seandel M, Soni R, et al. Mechanism and role of SOX2 repression in seminoma: relevance to human germline specification. Stem Cell Rep (2016) 6:772–83. doi: 10.1016/j.stemcr.2016.04.002
11. Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer (2005) 104:2255–65. doi: 10.1002/cncr.21432
12. Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, Lothe RA. Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol Cancer (2007) 6:12. doi: 10.1186/1476-4598-6-12
13. Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, Skotheim RI, Abeler VM, Rajpert-De Meyts E, et al. Molecular characteristics of Malignant ovarian germ cell tumors and comparison with testicular counterparts: implications for pathogenesis. Endocr Rev (2013) 34:339–76. doi: 10.1210/er.2012-1045
14. Van Nieuwenhuysen E, Busschaert P, Neven P, Han SN, Moerman P, Liontos M, et al. The genetic landscape of 87 ovarian germ cell tumors. Gynecol Oncol (2018) 151:61–8. doi: 10.1016/j.ygyno.2018.08.013
15. Hersmus R, Stoop H, van de Geijn GJ, Eini R, Biermann K, Oosterhuis JW, et al. Prevalence of c-KIT mutations in gonadoblastoma and dysgerminomas of patients with disorders of sex development (DSD) and ovarian dysgerminomas. PloS One (2012) 7:e43952. doi: 10.1371/journal.pone.0043952
16. Cheng L, Roth LM, Zhang S, Wang M, Morton MJ, Zheng W, et al. KIT gene mutation and amplification in dysgerminoma of the ovary. Cancer (2011) 117:2096–103. doi: 10.1002/cncr.25794
17. Tickoo SK, Hutchinson B, Bacik J, Mazumdar M, Motzer RJ, Bajorin DF, et al. Testicular seminoma: a clinicopathologic and immunohistochemical study of 105 cases with special reference to seminomas with atypical features. Int J Surg Pathol (2002) 10:23–32. doi: 10.1177/106689690201000105
18. Mortensen MS, Lauritsen J, Gundgaard MG, Agerbaek M, Holm NV, Christensen IJ, et al. A nationwide cohort study of stage I seminoma patients followed on a surveillance program. Eur Urol (2014) 66:1172–8. doi: 10.1016/j.eururo.2014.07.001
19. Jha R, Jha S. Study of Malignant germ cell tumors with special reference to Malignant ovarian germ cell tumors: 5 years experience. J Pathol Nepal (2012) 2:289–92. doi: 10.3126/jpn.v2i4.6880
20. Sever M, Jones TD, Roth LM, Karim FW, Zheng W, Michael H, et al. Expression of CD117 (c-kit) receptor in dysgerminoma of the ovary: diagnostic and therapeutic implications. Mod Pathol (2005) 18:1411–6. doi: 10.1038/modpathol.3800463
21. Tian Q, Frierson HF Jr., Krystal GW, Moskaluk CA. Activating c-kit gene mutations in human germ cell tumors. Am J Pathol (1999) 154:1643–7. doi: 10.1016/S0002-9440(10)65419-3
22. Shamash J, Ansell W, Alifrangis C, Thomas B, Wilson P, Stoneham S, et al. The impact of a supranetwork multidisciplinary team (SMDT) on decision-making in testicular cancers: a 10-year overview of the Anglian Germ Cell Cancer Collaborative Group (AGCCCG). Br J Cancer (2021) 124:368–74. doi: 10.1038/s41416-020-01075-1
23. Bloom JC, Loehr AR, Schimenti JC, Weiss RS. Germline genome protection: implications for gamete quality and germ cell tumorigenesis. Andrology (2019) 7:516–26. doi: 10.1111/andr.12651
24. Gillessen S, Sauve N, Collette L, Daugaard G, de Wit R, Albany C, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG update consortium. J Clin Oncol (2021) 39:1563–74. doi: 10.1200/JCO.20.03296
25. Shah R, Xia C, Krailo M, Amatruda JF, Arul SG, Billmire DF, et al. Is carboplatin-based chemotherapy as effective as cisplatin-based chemotherapy in the treatment of advanced-stage dysgerminoma in children, adolescents and young adults? Gynecol Oncol (2018) 150:253–60. doi: 10.1016/j.ygyno.2018.05.025
26. Jorge S, Jones NL, Chen L, Hou JY, Tergas AI, Burke WM, et al. Characteristics, treatment and outcomes of women with immature ovarian teratoma, 1998-2012. Gynecol Oncol (2016) 142:261–6. doi: 10.1016/j.ygyno.2016.05.024
27. Rijlaarsdam MA, Tax DM, Gillis AJ, Dorssers LC, Koestler DC, de Ridder J, et al. Genome wide DNA methylation profiles provide clues to the origin and pathogenesis of germ cell tumors. PloS One (2015) 10:e0122146. doi: 10.1371/journal.pone.0122146
28. Singh R, Fazal Z, Freemantle SJ, Spinella MJ. Mechanisms of cisplatin sensitivity and resistance in testicular germ cell tumors. Cancer Drug Resist (2019) 2:580–94. doi: 10.20517/cdr.2019.19
29. Armstrong DK AR, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM, et al. NCCN clinical practice guidelines in oncology., Version 4.2017.
30. Sessa C, Schneider DT, Planchamp F, Behbakht K, Berchuck A, Chen LM, et al. ESGO-SIOPE guidelines for the management of adolescents and young adults with non-epithelial ovarian cancers. Lancet Oncol (2020) 21:e360–8. doi: 10.1016/S1470-2045(20)30091-7
31. Nichols CR, Roth B, Albers P, Einhorn LH, Foster R, Daneshmand S, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol (2013) 31:3490–3. doi: 10.1200/JCO.2012.47.6010
32. Oliver RT, Mason MD, Mead GM, von der Maase H, Rustin GJ, Joffe JK, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet (2005) 366:293–300. doi: 10.1016/S0140-6736(05)66984-X
33. Honecker F, Aparicio J, Berney D, Beyer J, Bokemeyer C, Cathomas R, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol (2018) 29:1658–86. doi: 10.1093/annonc/mdy217
34. Vaughn DJ, Hwang WT, Lal P, Rosen MA, Gallagher M, O'Dwyer PJ, et al. Phase 2 trial of the cyclin-dependent kinase 4/6 inhibitor palbociclib in patients with retinoblastoma protein-expressing germ cell tumors. Cancer (2015) 121:1463–8. doi: 10.1002/cncr.29213
35. Narayan V, Hwang WT, Lal P, Rosen MA, Gallagher M, O'Dwyer PJ, et al. Cyclin-dependent kinase 4/6 inhibition for the treatment of unresectable mature teratoma: long-term follow-up of a phase II study. Clin Genitourin Cancer (2016) 14:504–10. doi: 10.1016/j.clgc.2016.03.010
36. Oechsle K, Honecker F, Cheng T, Mayer F, Czaykowski P, Winquist E, et al. Preclinical and clinical activity of sunitinib in patients with cisplatin-refractory or multiply relapsed germ cell tumors: a Canadian Urologic Oncology Group/German Testicular Cancer Study Group cooperative study. Ann Oncol (2011) 22:2654–60. doi: 10.1093/annonc/mdr026
37. Necchi A, Lo Vullo S, Giannatempo P, Raggi D, Calareso G, Togliardi E, et al. Pazopanib in advanced germ cell tumors after chemotherapy failure: results of the open-label, single-arm, phase 2 Pazotest trial. Ann Oncol (2017) 28:1346–51. doi: 10.1093/annonc/mdx124
38. Mego M, Svetlovska D, Miskovska V, Obertova J, Palacka P, Rajec J, et al. Phase II study of everolimus in refractory testicular germ cell tumors. Urol Oncol (2016) 34:122 e17–22. doi: 10.1016/j.urolonc.2015.10.010
39. Fenner M, Oing C, Dieing A, Gauler T, Oechsle K, Lorch A, et al. Everolimus in patients with multiply relapsed or cisplatin refractory germ cell tumors: results of a phase II, single-arm, open-label multicenter trial (RADIT) of the German Testicular Cancer Study Group. J Cancer Res Clin Oncol (2019) 145:717–23. doi: 10.1007/s00432-018-2752-z
40. Adra N, Einhorn LH, Althouse SK, Ammakkanavar NR, Musapatika D, Albany C, et al. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: a Hoosier Cancer Research Network Study GU14-206. Ann Oncol (2018) 29:209–14. doi: 10.1093/annonc/mdx680
41. Einhorn LH, Brames MJ, Heinrich MC, Corless CL, Madani A.. Phase II study of imatinib mesylate in chemotherapy refractory germ cell tumors expressing KIT. Am J Clin Oncol (2006) 29:12–3. doi: 10.1097/01.coc.0000195086.47548.ef
42. Fung C, Dinh P Jr., Ardeshir-Rouhani-Fard S, Fossa SD, Travis LB. Toxicities associated with cisplatin-based chemotherapy and radiotherapy in long-term testicular cancer survivors. Adv Urol (2018) 2018:8671832. doi: 10.1155/2018/8671832
43. Lauritsen J, Mortensen MS, Kier MGG, Christensen IJ, Agerbaek M, Gupta R, et al. Renal impairment and late toxicity in germ-cell cancer survivors. Ann Oncol (2015) 26:173–8. doi: 10.1093/annonc/mdu506
44. Kerns SL, Fung C, Monahan PO, Ardeshir-Rouhani-Fard S, Abu Zaid MI, Williams AM, et al. Cumulative burden of morbidity among testicular cancer survivors after standard cisplatin-based chemotherapy: A multi-institutional study. J Clin Oncol (2018) 36:1505–12. doi: 10.1200/JCO.2017.77.0735
45. Kier MG, Hansen MK, Lauritsen J, Mortensen MS, Bandak M, Agerbaek M, et al. Second Malignant neoplasms and cause of death in patients with germ cell cancer: A Danish nationwide cohort study. JAMA Oncol (2016) 2:1624–7. doi: 10.1001/jamaoncol.2016.3651
46. Duhil de Benaze G, Pacquement H, Faure-Conter C, Patte C, Orbach D, Corradini N, et al. Paediatric dysgerminoma: Results of three consecutive French germ cell tumours clinical studies (TGM-85/90/95) with late effects study. Eur J Cancer (2018) 91:30–7. doi: 10.1016/j.ejca.2017.11.030
47. Cejas I, Coto J, Sanchez C, Lorenzo NE. Prevalence of depression and anxiety in adolescents with hearing loss. Otol Neurotol (2021) 42:e470–5. doi: 10.1097/MAO.0000000000003006
48. Nicholson J, Stoneham S, Murray M, Ajithkumar T. Prevalence of depression and anxiety in adolescents with hearing loss. Otol Neurotol (2021) 42:e470–5.
49. Mann JR, Raafat F, Robinson K, Imeson J, Gornall P, Sokal M, et al. The United Kingdom Children’s Cancer Study Group’s second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with Malignant extracranial germ cell tumors, with acceptable toxicity. J Clin Oncol (2000) 18:3809–18. doi: 10.1200/JCO.2000.18.22.3809
50. Cushing B, Giller R, Cullen JW, Marina NM, Lauer SJ, Olson TA, et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk Malignant germ cell tumors: a pediatric intergroup study–Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol (2004) 22:2691–700. doi: 10.1200/JCO.2004.08.015
51. Rogers PC, Olson TA, Cullen JW, Billmire DF, Marina N, Rescorla F, et al. Treatment of children and adolescents with stage II testicular and stages I and II ovarian Malignant germ cell tumors: A Pediatric Intergroup Study–Pediatric Oncology Group 9048 and Children’s Cancer Group 8891. J Clin Oncol (2004) 22:3563–9. doi: 10.1200/JCO.2004.01.006
52. Depani S, Stoneham S, Krailo M, Xia C, Nicholson J. Results from the UK Children’s Cancer and Leukaemia Group study of extracranial germ cell tumours in children and adolescents (GCIII). Eur J Cancer (2019) 118:49–57. doi: 10.1016/j.ejca.2019.05.001
53. Bokemeyer C, Kollmannsberger C, Stenning S, Hartmann JT, Horwich A, Clemm C, et al. Metastatic seminoma treated with either single agent carboplatin or cisplatin-based combination chemotherapy: a pooled analysis of two randomised trials. Br J Cancer (2004) 91:683–7. doi: 10.1038/sj.bjc.6602020
54. Williams SD, Kauderer J, Burnett AF, Lentz SS, Aghajanian C, Armstrong DK. Adjuvant therapy of completely resected dysgerminoma with carboplatin and etoposide: a trial of the Gynecologic Oncology Group. Gynecol Oncol (2004) 95:496–9. doi: 10.1016/j.ygyno.2004.07.044
55. Bajorin DF, Sarosdy MF, Pfister DG, Mazumdar M, Motzer RJ, Scher HI, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol (1993) 11:598–606. doi: 10.1200/JCO.1993.11.4.598
56. Horwich A, Sleijfer DT, Fossa SD, Kaye SB, Oliver RT, Cullen MH, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol (1997) 15:1844–52. doi: 10.1200/JCO.1997.15.5.1844
57. Alifrangis C, Sharma A, Chowdhury S, Duncan S, Milic M, Gogbashian A, et al. Single-agent carboplatin AUC10 in metastatic seminoma: A multi-centre UK study of 216 patients. Eur J Cancer (2022) 164:105–13. doi: 10.1016/j.ejca.2020.08.031
58. Murugaesu N, Schmid P, Dancey G, Agarwal R, Holden L, McNeish I, et al. Malignant ovarian germ cell tumors: identification of novel prognostic markers and long-term outcome after multimodality treatment. J Clin Oncol (2006) 24:4862–6. doi: 10.1200/JCO.2006.06.2489
59. Newton C, Murali K, Ahmad A, Hockings H, Graham R, Liberale V, et al. A multicentre retrospective cohort study of ovarian germ cell tumours: Evidence for chemotherapy de-escalation and alignment of paediatric and adult practice. Eur J Cancer (2019) 113:19–27. doi: 10.1016/j.ejca.2019.03.001
60. Watson RA, de la Pena H, Tsakok MT, Joseph J, Stoneham S, Shamash J, et al. Development of a best-practice clinical guideline for the use of bleomycin in the treatment of germ cell tumours in the UK. Br J Cancer (2018) 119:1044–51. doi: 10.1038/s41416-018-0300-x
61. Shamash J, Sarker SJ, Huddart R, Harland S, Joffe JK, Mazhar D, et al. A randomized phase III study of 72 h infusional versus bolus bleomycin in BEP (bleomycin, etoposide and cisplatin) chemotherapy to treat IGCCCG good prognosis metastatic germ cell tumours (TE-3). Ann Oncol (2017) 28:1333–8. doi: 10.1093/annonc/mdx071
62. Mangili G, Sigismondi C, Gadducci A, Cormio G, Scollo P, Tateo S, et al. Outcome and risk factors for recurrence in Malignant ovarian germ cell tumors: a MITO-9 retrospective study. Int J Gynecol Cancer (2011) 21:1414–21. doi: 10.1097/IGC.0b013e3182236582
63. Moch H, Amin MB, Berney DM, Comperat EM, Gill AJ, Hartmann A, et al. The 2022 World Health Organization classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol (2022). doi: 10.1016/j.eururo.2022.06.016
64. Joffe JK, Cafferty F, Butcher E, Noor D, Rustin G, Kaplan RS, et al. 794P UK practices for treatment of relapse in seminoma testicular cancer. Ann Oncol (2020) 31(Supplement 4):S603. doi: 10.1016/j.annonc.2020.08.866
Keywords: systemic chemotherapy, carboplatin, germ cell tumours, seminoma, dysgerminoma, de-escalating chemotherapy
Citation: Wood GE, Bunting CP, Veli M, Arora R, Berney DM, Alifrangis C, MacDonald ND, Miller RE, Shamash J, Stoneham S and Lockley M (2023) Seminoma and dysgerminoma: evidence for alignment of clinical trials and de-escalation of systemic chemotherapy. Front. Oncol. 13:1271647. doi: 10.3389/fonc.2023.1271647
Received: 02 August 2023; Accepted: 18 September 2023;
Published: 25 October 2023.
Edited by:
Giovanni Rosti, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyReviewed by:
Giuseppe Schepisi, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyCopyright © 2023 Wood, Bunting, Veli, Arora, Berney, Alifrangis, MacDonald, Miller, Shamash, Stoneham and Lockley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michelle Lockley, bS5sb2NrbGV5QHFtdWwuYWMudWs=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
From Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.