- 1Department of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Institute of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Prostate cancer is viewed as the second most common cancer in men worldwide. In our study, we used bibliometric analysis to construct a visual map of the relationship between prostate cancer and exosomes with the intent of uncovering research trends and current hotspots in this field.
Method: We searched the Web of Science Core Collection for all publications in the prostate cancer associated with exosome field came out since 2010. With the assistance of bibliometric analysis software such as VOSviewer and CiteSpace, we conducted data extraction and analysis for countries/regions, institutions, authors, journals, references and keywords.
Results: A bibliometric analysis of 990 publications was performed. Since 2010, the published quantity and cited frequency of the prostate cancer-associated exosome field have revealed an increasing tendency. In this field, we visualized the research trends by the means of analyzing the references and keywords. We obtained the statistical data: the total citations of publications have increased to 55,462, the average citation per article has reached 55.3 times, and the H-index has amounted to 110. Our findings supported that USA, China and Italy rank the top countries with both the maximum publications and strongest cooperations. Harvard Medical School, Cedars Sinai Med Ctr, Johns Hopkins University, are top institutions in the center of research as they are held to be. Thery C, Skog J and Taylor DD are the leading and outstanding professors and researchers. And top journals like Prostate, Plos One and Journal of Extracellular Vesicle expressed keen interests in this field. Based on our analysis and research, we believe that this field is attracting more and more attention and will focus on tumor bone metastasis, drug delivery, and tumor suppressor.
Conclusion: In the past 12 years, researchers have dedicated their efforts to prostate cancer associated exosome. On the basis of previous studies, scientists are showing increasingly solicitude for the role of exosome in prostate cancer progression and potential therapy such as drug delivery.
1 Introduction
Prostate cancer (PCa), with the highest incidence of human disease, impacts patients’ health condition and quality of life and remains the second leading cause of male mortality with an estimated 34,130 deaths in 2021 in the US (1). This makes the public pay attention to screening for prostate cancer to alleviate their anxiety about risk of cancer. Therefore, in the past decade, the content, principles, methods, safety, reliability, and efficiency of prostate cancer examination have also become a hot research topic in this field. Besides, adopting the most advanced examination methods so as to find potential PCa at early period and give accurate diagnosis is believed to be a useful way to improve cure rate.
Compared with rectal proctoscopy, Computed Tomography (CT) and Magnetic Resonance Imaging (MRI), biomarkers for PCa are less traumatic, more convenient in operation, and more economical and practical. During the tumor progression, a variety of bioactive substance will be released to the serum, which will lead to changes in concentration of these special substances. Regular serum prostate-specific antigen (PSA) evaluation and digital rectal examination (DRE) are recognized as most commonplace methods for detecting PCa (2). The serum PSA level is among the best of the screening tools available in medicine today and is viewed as the best marker for early detection (3). While PSA examination has little effect in distinguishing whether tumors are benign or malignant, so researchers aspire for the role of other small molecule biomarkers. In the tumor microenvironment, exosomes are considered to be diverse and carry various functions (4–8). The research on exosomes related to prostate cancer has gradually entered people’s vision.
Extracellular vesicles (EVs) are lipid-enclosed vesicles released by cells into the extracellular environment. The three main subtypes of EVs are microvesicles (MVs), exosomes, and apoptotic bodies, which are differentiated based upon their biogenesis, release pathways, size, content, and function. Exosomes are derived and secreted by different cells, containing specific RNA and protein which maintain bioactive functions (9–11). These small molecules can be released and uptaken by various target cells. The composition and cargo of exosomes vary from others owing to their derived cells. For this reason, exosomes are thought to be potential approach for examine PCa at early period. However, the identity and mechanism of exosomes still retain obscure. On the other hand, prior studies demonstrate that exosomes have traits to be effective therapeutic tool against prostate cancer (11–13). Since they are not only able to deliver medicine to specific target cells but also useful for enhancing the cytotoxic effect of drug. As a result, exosome mediated drug delivery is promising research which will be important for promoting the accuracy of examination and treatment of PCa.
In the past decade, research in this field has shown an upward trend, and more and more literature has emerged. Although there are a few systematic reviews summarizing recent research on exosome and the relationship between exosome and cancer, among others. However, there is no relevant quantitative analysis on the role of exosomes in prostate cancer. Bibliometric analysis is the process of analyzing a certain amount of literature to identify topics and popular citations that have been well researched in this field (14–16). It analyzes the focus that has gradually gained widespread attention in the development process of this field and helps researchers discover hot topics that can be explored and have not yet been studied (14–16). This study presents a visual, bibliometric and scientometric analysis based on the application of various literature analysis software, providing emerging hot research directions.
2 Method
2.1 Database
As widely recognized as one of the most reputable and comprehensive databases in the field of scientific research, WOSCC encompasses a vast collection of interdisciplinary literature and research (17). Consequently, for bibliometric studies, WOSCC is often regarded as the most suitable database for acquiring data (18, 19). For the purposes of this study, we conducted a thorough literature search utilizing the Web of Science Core Collection (WosCC).
2.2 Search strategies
Our search strategy was as follows: ((TS= (“exosom*”) OR TS= (“exosc*”) NOT TS= (“exoscreen”) NOT TS= (“exoscop*”) NOT TS= (“exosca*”)) AND (TS=(“prostat*” NEAR/1 “cancer*” OR “prostat*” NEAR/1 “tumor*” OR “prostat*” NEAR/1 “tumour*” OR “prostat*” NEAR/1 “oncology” OR “prostat*” NEAR/1 “neoplasm*” OR “prostat*” NEAR/1 “carcinoma*” OR “prostat*” NEAR/1 “adenocarcinoma*” OR “prostat*” NEAR/1 “adenocarcinoma*”))) (20, 21). The language of the literature was restricted to English. And the type of 92 literature was limited to articles and reviews. Literature from 2010 to 2022 was selected. All 93 information of the retrieved literature was saved in plain text format for analysis.
2.3 Data extraction & analysis
To eliminate duplication, the plain text files extracted from the WOSCC were imported into CiteSpace V (6.1.R2 Basic version, Drexel University, United States). Subsequently, the non-duplicate files were gathered and imported into Microsoft Excel for analysis of publication authors, countries, journals, and institutions. Additionally, data on publication countries, journals, authors, institutions, citation frequencies, and H-index were obtained from WOS on 17th January 2023. VOSviewer was utilized to analyze and acquire the Total Link Strength (TLS), while CiteSpace was employed for centrality analysis.
2.4 Data visualization
In this study, we conducted bibliometric analysis and visualization using various tools including VOSviewer (version 1.6.18), CiteSpace V (version 6.1.R2 basic), Tableau, an online bibliometric analysis platform (bibliometric.com), and Microsoft Excel. VOSviewer is a widely utilized software for bibliometric analysis that facilitates visual representation of the relationships between different nodes and displays associated information through their characteristics (22). In our study, we employed VOSviewer to generate visualizations of co-authorship among countries, institutions, authors, journals, citation relationships of 109 references, and co-occurrence of keywords, resulting in concise and clear visual representations. CiteSpace, a Java-based bibliometric analysis software developed by Prof. Chaomei Chen (23), was chosen due to its outstanding performance in burst detection, centrality calculation, citation relationship visualization, and clustering analysis (24). Thus, we employed CiteSpace to visualize dual maps of journals, citation bursts of keywords or references, timelines, and authors’ co-citation relationships.
3 Results
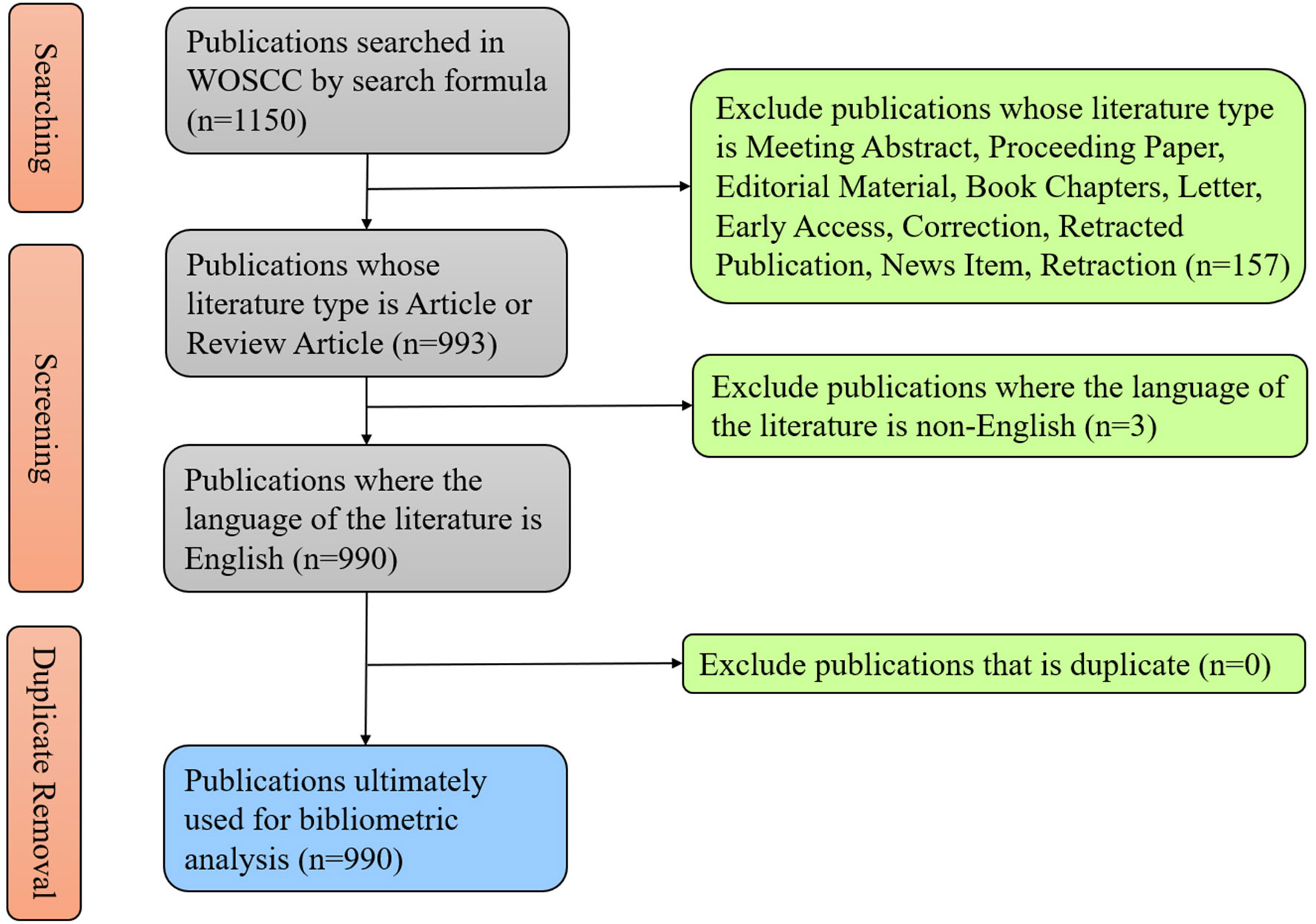
According to the previous literature, we used a search formula to conduct a research about the literature and data in the field of exosome in prostate cancer on WoSCC and finally collected 1150 literatures published from 2010 to 2022. After restricting some conditions, we sifted out 990 publications to carry out following analysis (Figure 1). As of the search date, the total citations of publications have reached up to 55,462, with 55.3 times for average per item, and the H-index have reached 110.
3.1 Global trends in publication volume and citation frequency
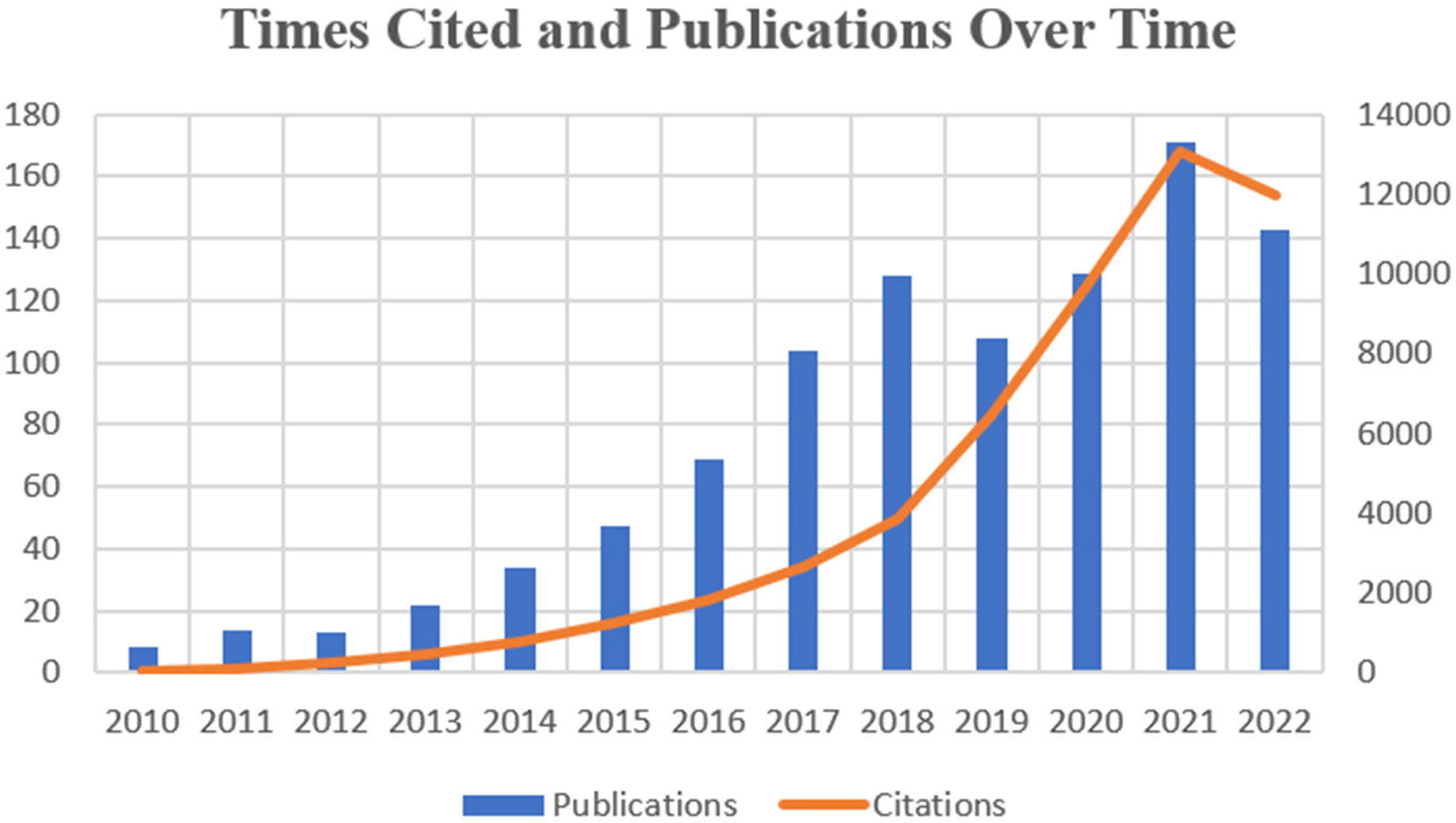
The number and its changes of research paper published in different periods can help reflect the prevalence and developmental trend of research in a specific field. We collected and sorted 990 publications which came out in the last 12 years focusing on exosome in the field of prostate cancer. Figure 2 shows the variation in global trends of publication outputs and citations. In 2010, there was merely 8 papers published in this field, while in 2017, the publication number firstly surpassed 100. During the last 6 years, the average annual publications remained above 100. And 2021 witnessed both the largest publication number of 172 and the highest citation frequency of 13146. On the whole, the global cited times is growing at a skyscraping rate, exceeding drastically from 31 to 13084 at the peak. Until the search date, the total literatures have attained an overall H-index of 110.
3.2 Country/region analysis
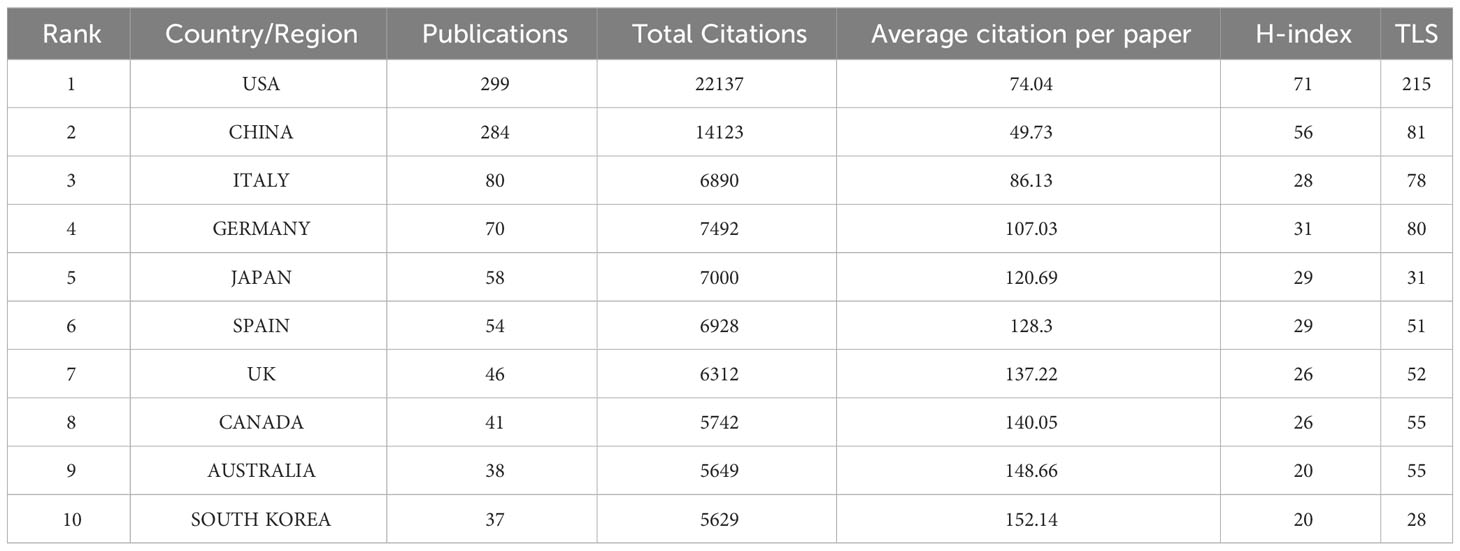
To present a more vivid analysis, we have conducted a geographical visualization of publications on prostate cancer-associated exosome. As shown in Figure 3B, the research in this area is mainly concentrated in North America, East Asia, West Europe and the Oceania. In Table 1, we have concluded the top 10 countries whose publication number rank the highest and attached their relevant information. USA (occupied the first place with total publication of 299), China (284) and Italy (80) have the maximum publications. In terms of Average citations per paper, South Korea, Australia and Canada rank the top three, albeit not so many publications. The H-index and TLS of USA are way ahead, 71 and 215 respectively, suggesting that its cooperation with countries is the broadest and most relevant.
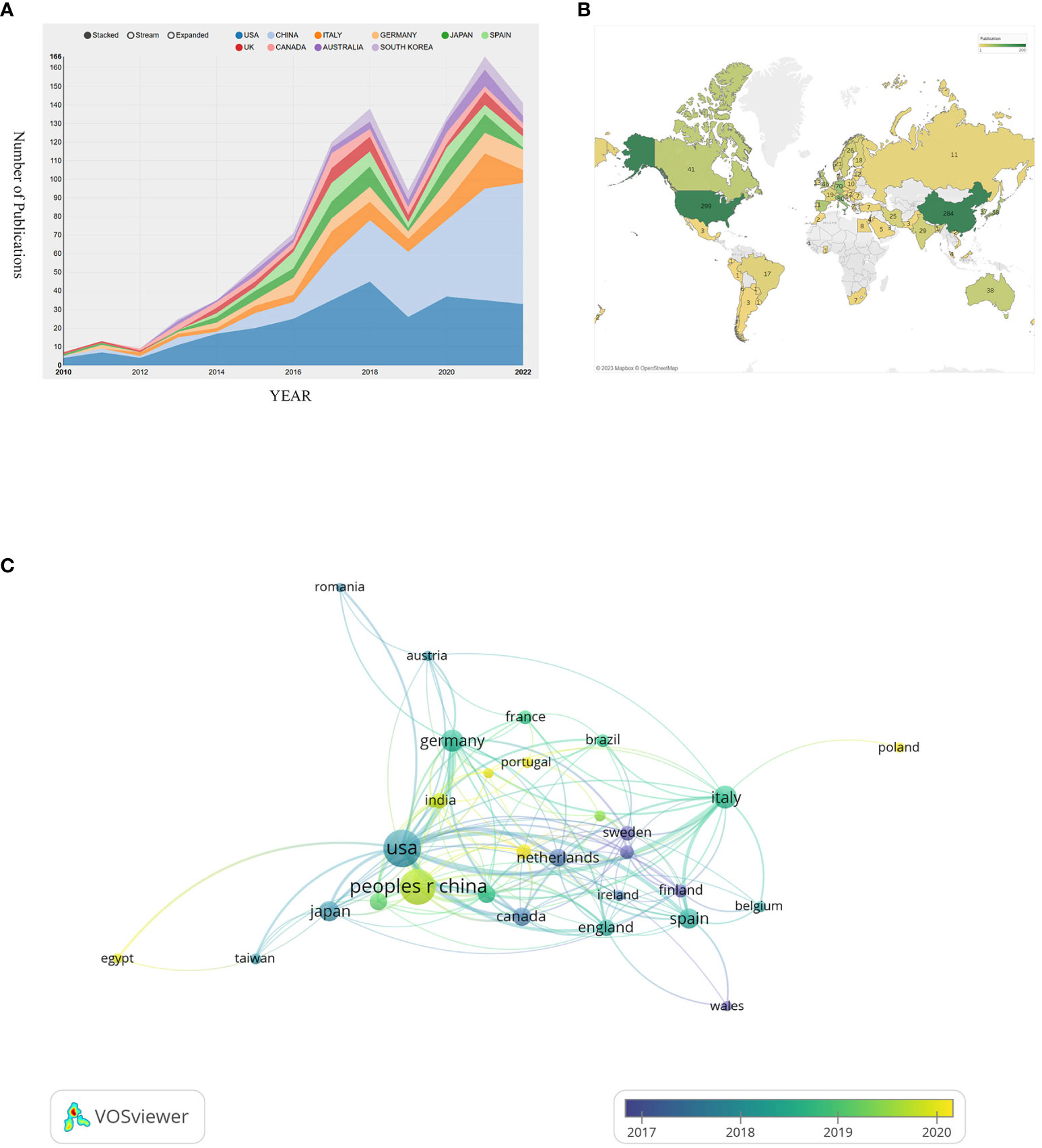
Figure 3 (A) Visualizes the annual change in the volume of publications. (B) Visualizes a geographical map of contributions of different countries/regions. (C) Visualizes the collaborative connection between countries/regions.
All the countries/regions in this field fully cooperate with each other and have complex connections. We use VOSviewer to analyze and visualize this connection network. As shown in Figure 3C, USA and China occupy the most center location and have the maximum cooperation number of 34 times, with the strongest connection between them. This indicates that they have such sufficient coordination in exploring this field. While the cooperation frequency between USA and German, USA and Italy are 23 and 19 respectively, ranking the following two. The node’s color can represent the time order sequence when they started the research. It’s not hard to find that European countries such as Norway, Finland, Netherlands and Sweden, whose relevant nodes are purple, searched this field at the earliest time. Compared with them, the yellow nodes are represented the latest years. In comparison to USA whose average research publication year is 2017.87, China (2019.6), India (2019.71), and Iran (2020.04) are up-and-comers. Though China started this area late, China’s total publication quantity exceeded USA and became the most published country in this area (Figure 3A).
3.3 Institutions and funding agencies analysis
As shown in Figure 4A, we analyzed the co-authorship of literatures. Three institutions that conducted research in this field earlier are Harvard Medical School, Cedars Sinai Med Ctr, Johns Hopkins University, are located at the core place of the co-authorship analysis. They have the highest TLS, which are 76, 65 and 62 respectively. The purple nodes mean that these institutions started this area research in earlier period, taking Cedars Sinai Med Ctr as an example. Comparing with these European or American institutions whose nodes are mostly purple, Chinese institutions are presented with much green or yellow nodes, which indicate that China’s late start.
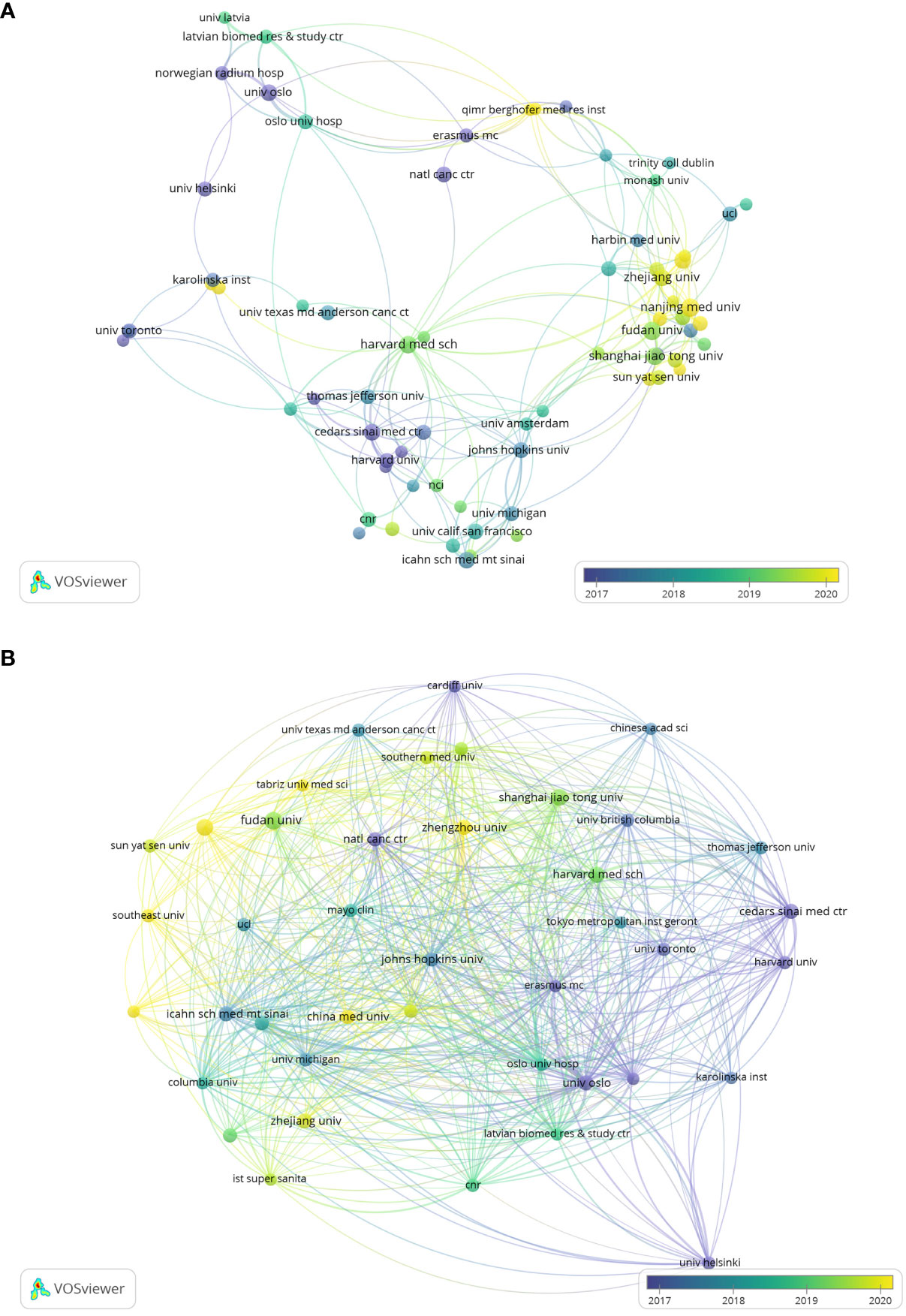
Figure 4 (A) Visualizes co-authorship between research institutions. (B) Visualizes the citation relationships between research institutions.
Figure 4B demonstrates the citation relationships between research institutions, we can find that the most strengthened connection occurred in University Oslo, Oslo University Hospital and Erasmus University Rotterdam, with the highest TLS, in proper order 1718, 1367, 1050.
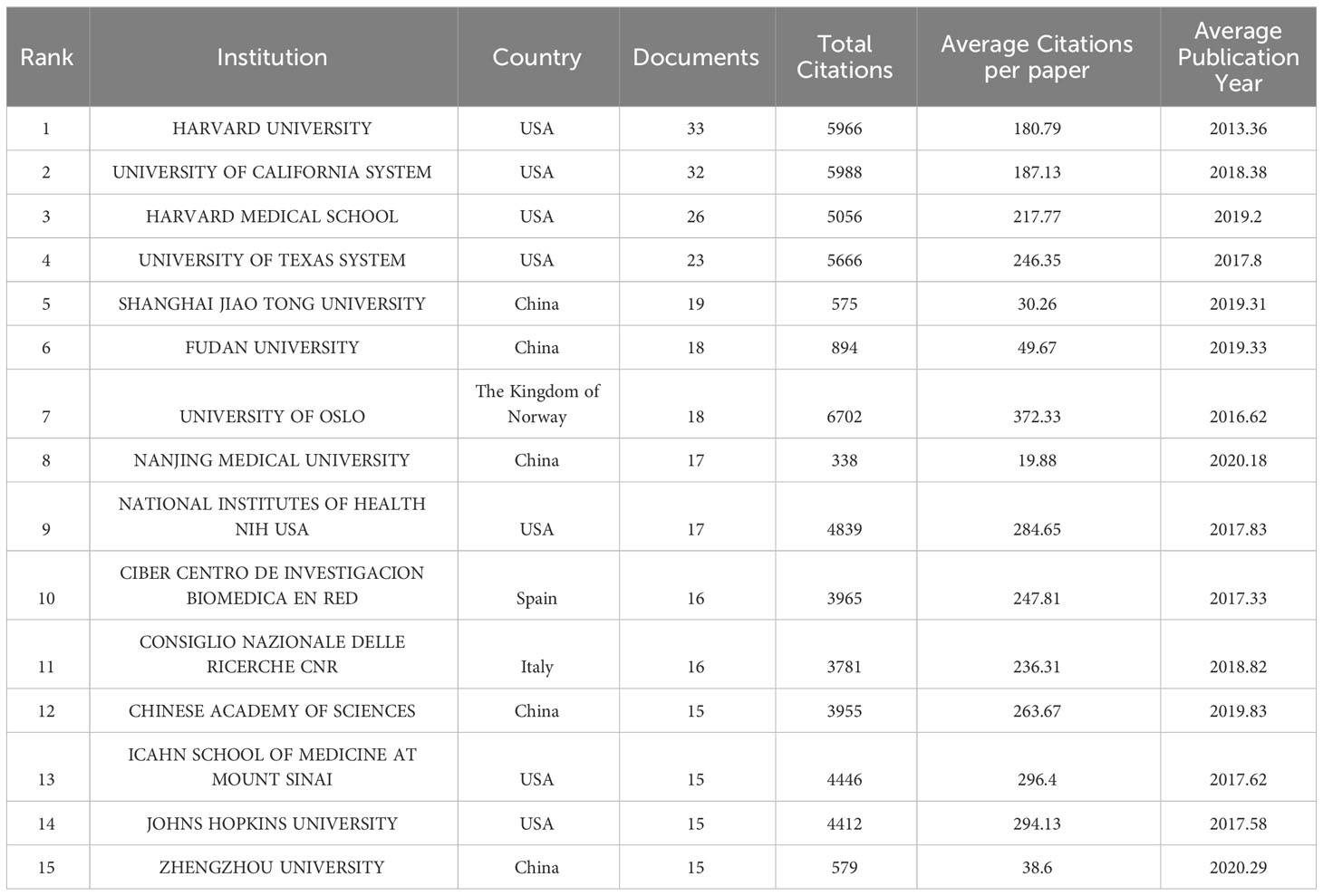
As shown in Table 2, among the top 15 publication research institutions all over the world in this field, USA owns 7 of them, while China has 5. Additionally, Norway, Spain and Italy each have one institution involved. Harvard University, University of California System, Harvard Medical School have the maximum number of publications, 33,32 and 26 respectively. Institution with the highest total citations (6702) and the highest average citations per paper (372.33) is University of Oslo, University of California System and Harvard University just fall behind with total citations of 5988 and 5966. In this field, Harvard University has the earliest average publication year which takes place in 2013.36, while the average publication year of most of Chinese institutions is around 2019, nearly 6 years later.
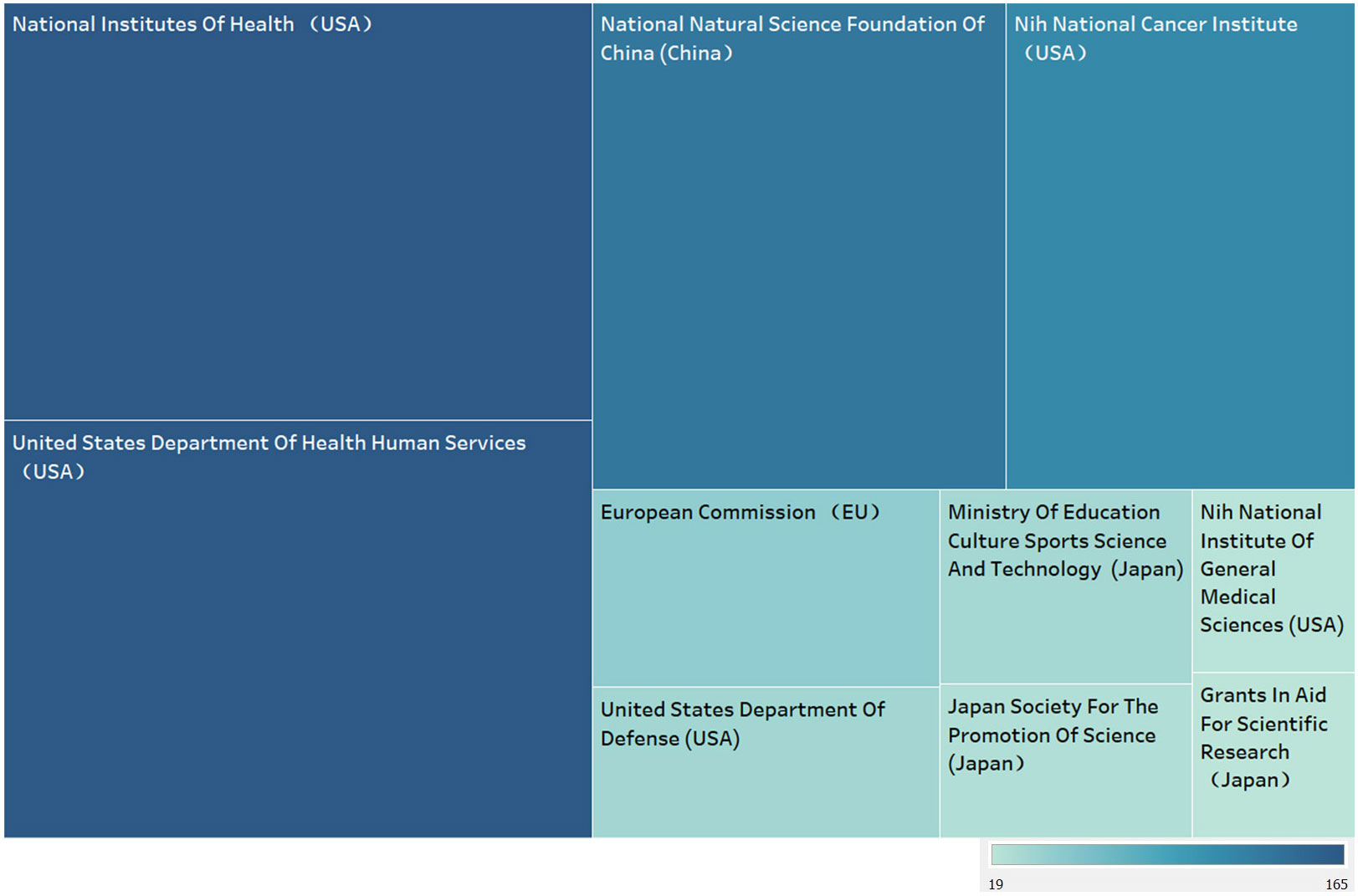
Figure 5 shows the top 10 funding agencies in terms of publication volume, including 7 American agencies and 3 Japanese ones. Amongst all these agencies, National Institutes of Health (USA), United States Department of Health Human Services (USA), National Natural Science Foundation Of China (China) relying on publication of 165, 165, 135 respectively head the table. USA, known as the biggest contributor, tops the chart, while China also takes great percent albeit one institution.
3.4 Journal analysis
Figure 6A shows the citation relationships, of all the 365 journals which have all published literatures of this field, Prostate, Plos One, Journal of Extracellular Vesicle are at the center. Since the color of the nodes can reveal the time when papers are published, we can calculate and thus find the average publication year of Prostate and Plos One are before 2017, and the yellow nodes which stand for the journals that show solicitude for the research of this field recently, involving Frontiers in Oncology, American Journal of Cancer Research, Frontiers in Cell and Development, Biomedicines, Biosensors and Bioelectronics, Biomedicine and Pharmacotherapy, whose average publication year are behind 2020.
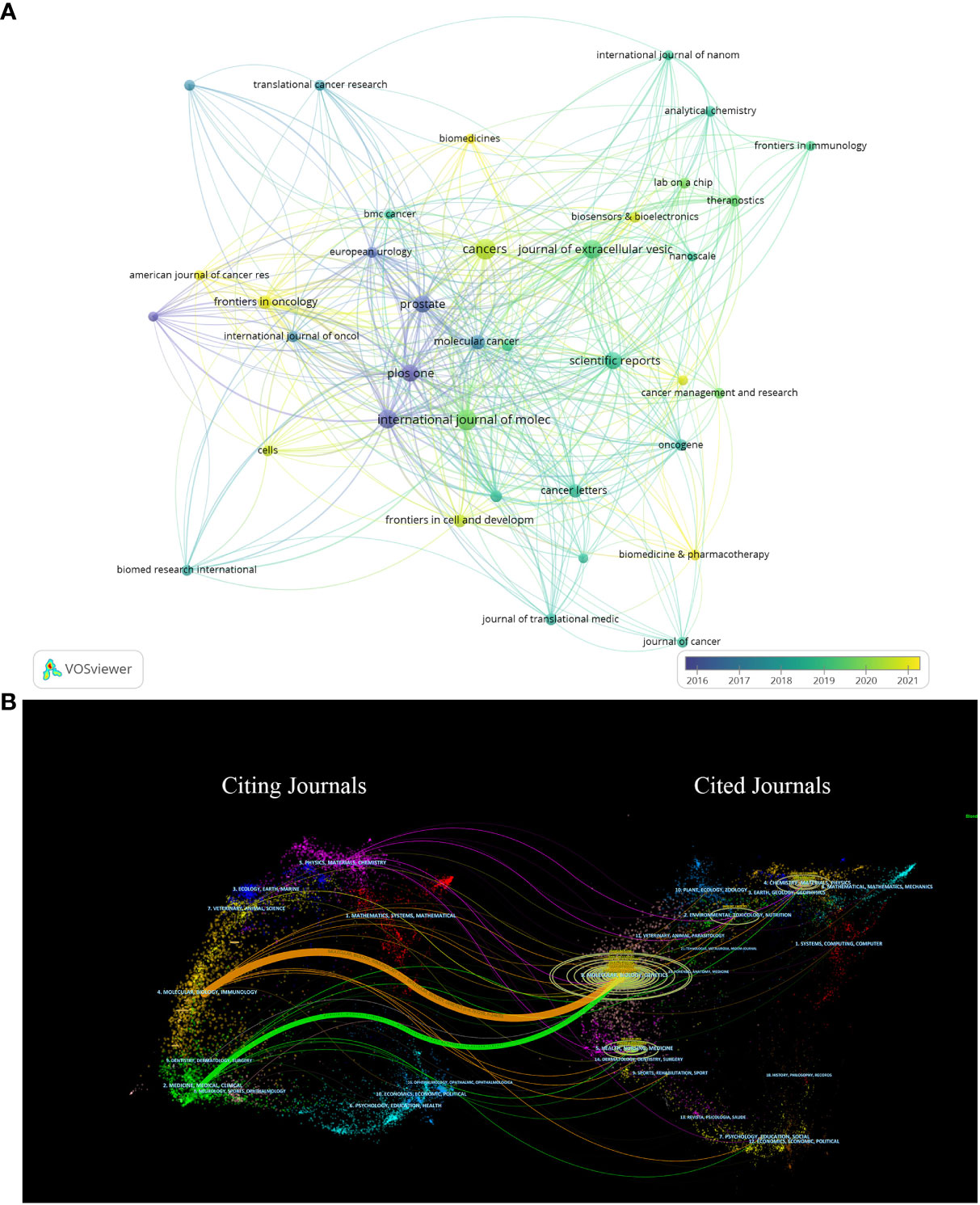
Figure 6 (A) Shows the citation relationships between journals. (B) Is the journal dual-map produced through CiteSpace.
Figure 6B vividly displays the citation relationships between journals by using a journal dual-map, with the thickest green and yellow lines representing the strongest citation relationships in this field. These thick lines suggest that literatures published on Molecular/Biology/Immunology and Medicine/Medical/Clinical are usually cited by Molecular/Biology/Genetics or Health/Nursing/Medicine.
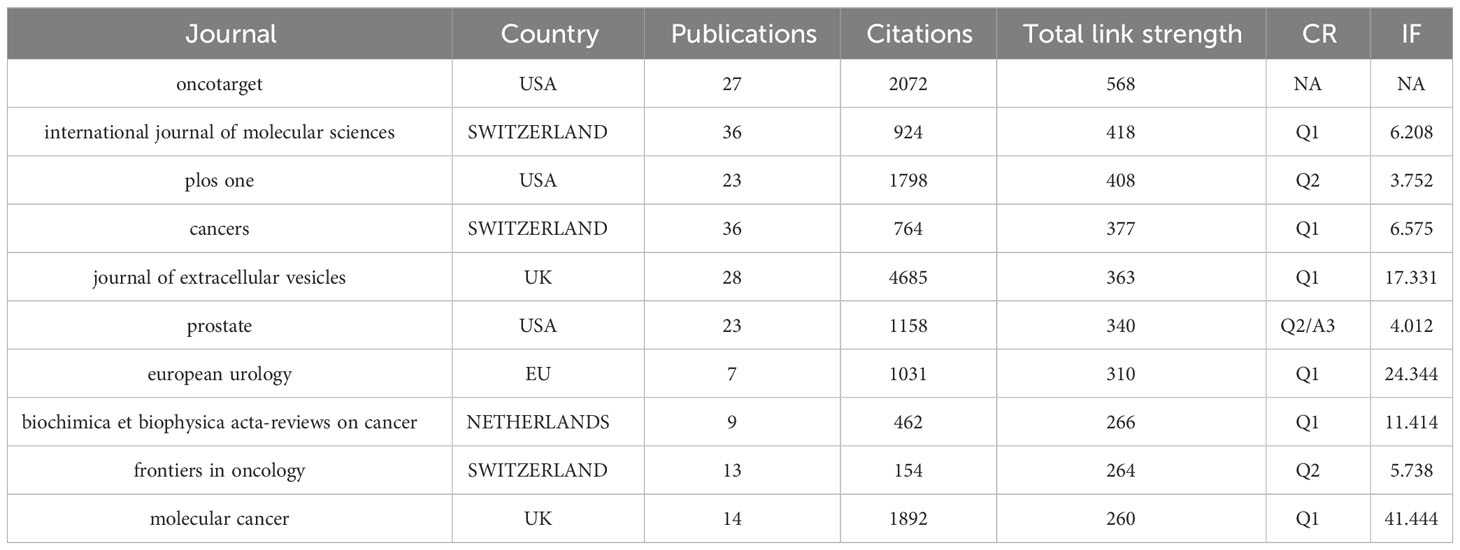
Table 3 summarizes the relevant information of these ten journals, among which USA and Switzerland each have three journals. International Journal of Molecular Sciences (25), Cancers (25), Journal of Extracellular Vesicles (26) have the most publications and Journal of Extracellular Vesicles takes the top spot in term of citations which reaches 4685 times. Furthermore, Molecular cancer, Journal of Extracellular Vesicles, European Urology with much high IF of 41.444, 17.331 and 11.414 carry a lot of clout in this field.
3.5 Author and co-cited author analysis
Different researchers pay close attention to quite different point in this field, as shown in Figure 7A, researchers are divided into four groups, separately led by Kosaka Nobuyoshi, Jenster Guido, Line Aija and Llorenta Alicia. It is Ochiya Takahiro and Soekmadji Carolina that make contribution to connect these four groups to a whole.
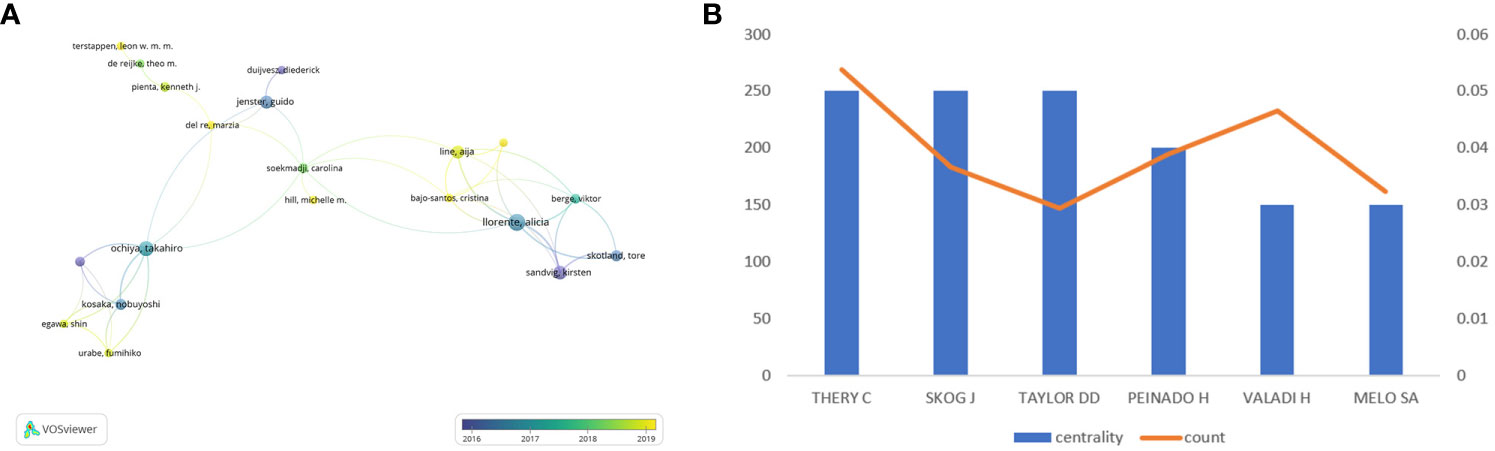
Figure 7 (A) Visualizes the collaborations of the leading authors in the field. (B) Shows the top 6 authors with the highest centrality in the co-citation analysis.
As shown in Figure 7B, we analyzed the information of six authors with the highest centrality. Among them, Thery C, Skog J and Taylor DD have the highest centrality in comparison with others.
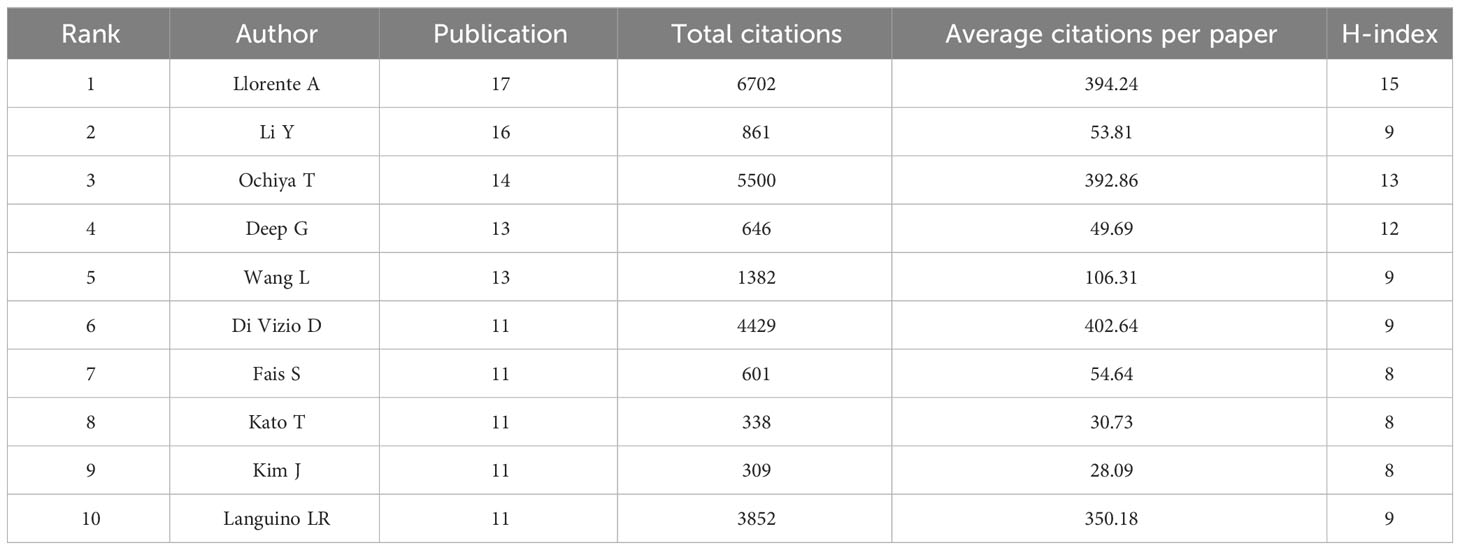
Table 4 summarizes the information of authors whose publications and co-citation rank the top ten. Liorenta A not only has the maximum publications and citations, but also has the highest H-index. Li Y (16) and Ochiya T (14) also have very high publications. Besides, Ochiya T has the citation of 5500 and average citations per paper of 392.86 high; Di Vizio D, who is just a bit behind, has 4429 publications with average citations per paper of 402.64; which indicates their far-reaching implications.
3.6 References and co-cited references analysis
We conducted a co-citation analysis on 990 publications included in this study using VosViewer and CiteSpace. As shown in Figure 8A, the clustering analysis of reference presents that the top 8 popular research directions combine and connect with each other tightly, with K=9, Modularity Q=0.5928, and Weighted Mean Silhouette S=0.8419, indicating excellent clustering effect and network homogeneity.
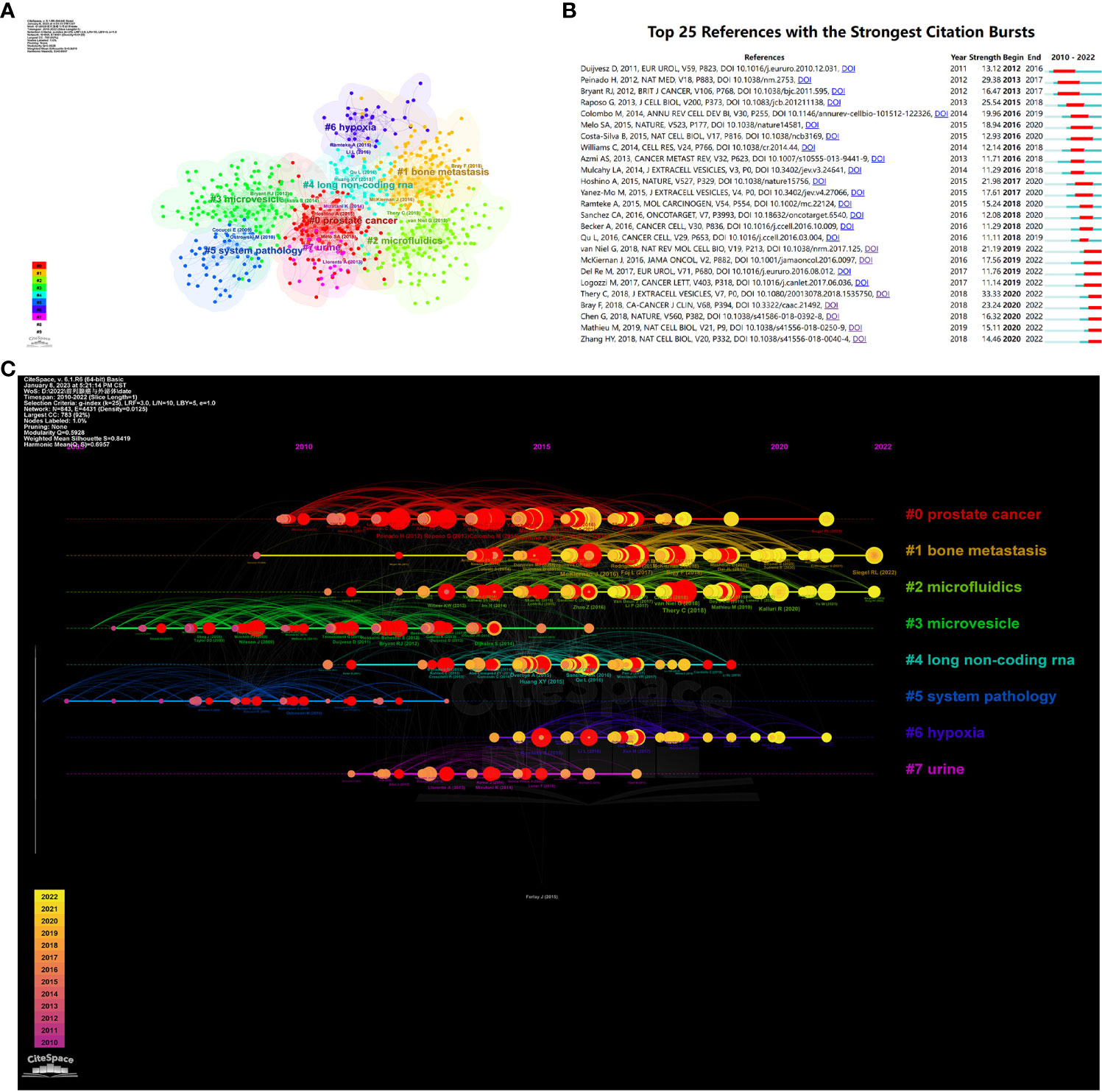
Figure 8 (A) Visualizes the cluster analysis for references. (B) Shows the top 25 references with the strongest citation bursts. (C) Shows the timeline of the references.
Moreover, we visualized the timeline analysis of reference on the basis of the clustering analysis. As shown in Figure 8B, widely concerning problems in previous time comprised microvesicle and long non-coding RNA. However, semen, hypoxia, bone metastasis and microfluidics came into the eyesight of researchers currently and became research hotspots of this field.
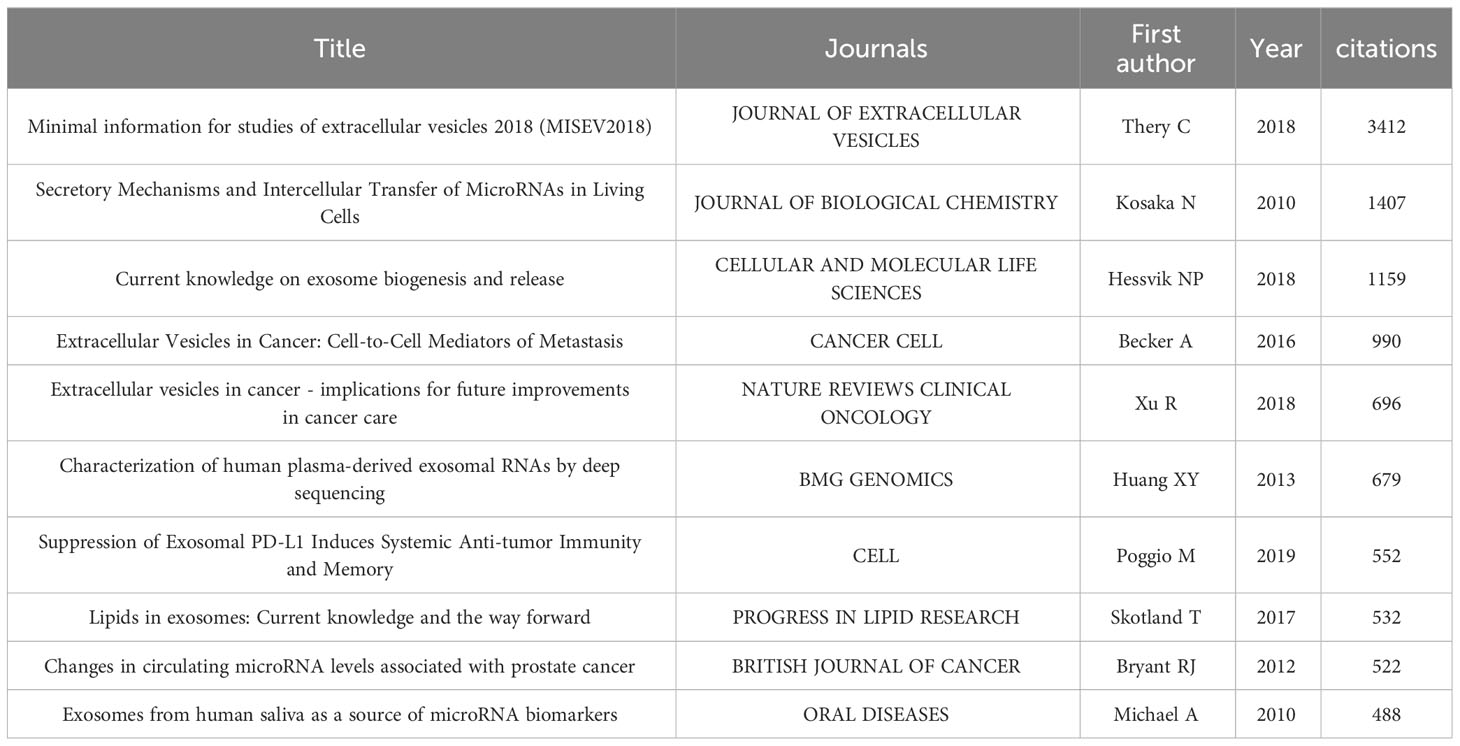
We sorted out the top 25 references with the strongest citation bursts, as shown in Figure 8B. Amongst them, the paper written by Duijvesz D and published in European Urology burst at the earliest time, in 2016. Thery C’s paper named “Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines” has the maximum burst strength of 33.33 and burst in 2022, obviously revealing the research trend of this area.
3.7 Keywords analysis
We conducted a co-occurrence analysis on author keywords, of the total 1919 keywords, Figure 9A displays 54 main keywords. The color of nodes can indicate the order of research time, which clearly reminds us of the tidal current and future orientation of research in this field. Prostate, plasma and prostasomes are the concerns of former research, which can be recognized as purple nodes. Then the study began to focus on biomarkers, exosome and prostate cancer, which are at the core place of the map of keywords. The slight yellow nodes indicate recent hot academic topics, demonstrating that scientific workers turned their eyes to diagnosis, radiotherapy, drug delivery, liquid biopsies, therapy, extracellular vesicles, cancer diagnosis, tumor-derived exosome, tumor environment, bone metastasis, chemoresistance.
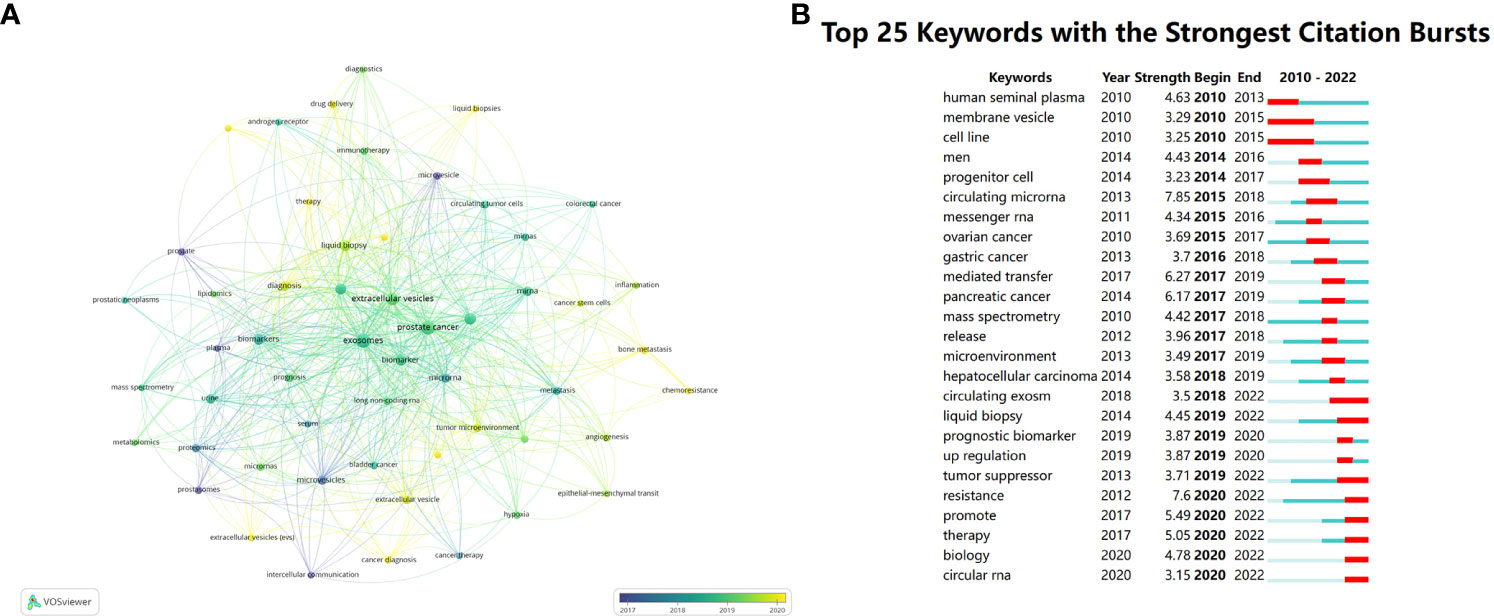
Figure 9 (A) Visualizes the co-occurrence analysis of author keywords. (B) Summarizes the top 25 keywords with the strongest citation bursts.
Using Burst Detection of CiteSpace, we analyzed 25 keywords with the strongest citation outbreaks. The burst keywords in earlier period consist of human seminal plasma, membrane vesicle and cell line, stronger burst happened to circulating microRNA and mediated transfer with strength of 7.85 and 6.27. However, current burst appears at resistance, promote, therapy and biology. (Figure 9B)
4 Discussion
In the current rapid development of science and technology, millions of researchers are faced with difficulty in selecting a new project among numerous choices. At the same time, they also created myriad achievements in latest researches, which filled in the gap of a specific subject, not only trying to answer the questions which were not focused before but equally pivotal, but also striving to construct connections between different projects. In face front of such countless literatures and researches, bibliometric analysis, an extensively used method which aims at analyzing total papers in one certain field, offers a very convenient and quick way for researchers to get basic understanding of a new field. It greatly helps researchers analyze the history, previous achievements and the most cutting-edged research interests that have not been concentrated on before. Generally, grasping the current research trend is a necessity for scientific research personnel. Thus, bibliometric analysis, the research we have done, exactly met the need to providing materials to lead the selection of future research and spark inspirations by using data analysis and creating visual images.
As shown in Figure 2, there’s an increasing tendency in the publication number and the total citation times in the field of exosome in PCa. Although scarcely and barely less than ten papers published in 2010, the publication number grew steadily from 2013 and achieved the peak in 2021.
This was probably because the Nobel Prize in Physiology or Medicine was awarded to scientists who have made outstanding contributions to the field of intercellular vesicular transport regulation mechanisms in 2013, pushing the research of exosome into heat wave. The decline in 2018 and 2019 was probably owing to the Nobel Prize in Physiology/Medicine in 2018 was awarded to American scientist James P. Allison and Japanese scientist Tasuku Honjo for their discovery and contribution to new methods of cancer treatment. As a result, this partially transferred public attention towards exosome which was believed to be a potential star in future medicine. In addition, the exponential growth rates of global citation also demonstrated the popularity and prevalence of this field in recent years.
As shown in Figure 3B, countries involved in this field research are majorly developed countries which are quite prosperous, affluent, and tech savvy, while the involved developing countries are still minority. This can partly convey that the research in this field may consume heavy cost and represent a major expenditure of time and effort. Developing countries may have difficulty in paying the cost of these relevant research proceed from their national actual conditions. Another thing deserved to be mentioned is that developed countries like USA, England, Sweden and Canada also have an earlier start in this field, which prevails their keen insight and sharp perception of the unprecedented scientific interests. Despite that China has a late start in this field (China’s average publication year is 2019.6), almost 2 years after USA whose average publication year is 2017.87, China’s contribution to this field is nonnegligible and remarkable. As shown in Figure 3A, China’s total publication quantity exceeded USA and became the most published country in this area. With the second highest total publications and citations shown in Table 1, as well as the H-index and TLS at the second place, China has grown up to be a powerful and influential country in this area research, revealing great potential to have long-lasting innovations and achievements in the following years. Other East Asia countries also present passion and strength in researching this area, with high average citation per paper in Japan (120.69) and South Korea (152.14) shown in Table 1. There will be a brighter future in seeing more findings coming from East Asia with their intimate cooperation.
We also conducted research focusing on contributions of top institutions and funding agencies. The top three institutions which have the earliest start to carry out researches in this field, Harvard Medical School, Cedars Sinai Medical Center (Cedars Sinai Med Ctr), Johns Hopkins University, are all in the lead in USA academic community. And as shown in Figure 4B, Johns Hopkins University took over a central position in the whole field, which mains a perfect choice for researchers to get previous achievements. Besides, USA has the most institutions with the maximum publications at the same time. It’ s quite evident that USA has significant influence and is worthy of scientists’ and institutions’ attention. These institutions should be given priority when considering cooperations. As mentioned above, China has commanded our attention in recent years for its rapid development and great progress. A large amount of Chinese institutions such as Fudan University and Zhejiang University have made cooperations with American top institutions now.
Notably, it’s equally pivotal for researchers to quickly find proper journals which contains cutting-edged publications in a certain field. By means of VOSviewer and CiteSpace, we analyzed the journals and citations in the field of exosome in prostate cancer. Molecular cancer, Journal of extracellular vesicles and European urology stand out accounting for their high publications and citations. These journals perfectly satisfy researchers’ need and sight. Additionally, they can know which journals have tendency to accept papers in this area and probably obtain guidance to publish their documentary with great influence.
Thery C and Skog J have the highest centrality (Figure 7B), which is sufficient to illustrate their leadership roles in the field of exosomes and prostate cancer research.
Thery C, as an early pioneer in exosomal research, has led a research team investigating exosomes and tumor growth. She was the first to describe the physical properties of Exosomes, defining them as specific populations of secreted vesicles, and summarized their biological effects, particularly their role in immune system responses (27, 28). Thery discussed the potential of secreted vesicles as intercellular messengers. Moreover, different cells mediate specific secretion of Exosomes and uptake of their cargo, indicating the presence of specificity (26, 29). Additionally, Thery C developed commonly used methods for the isolation and purification of Exosomes from various sources, and discussed approaches to evaluate the purity and homogeneity of purified EV preparations (30, 31). These methods have provided convenience for studying the properties and specific mechanisms of action of exosomes. Furthermore, in recent articles, Thery C explored the role of exosomes in normal physiological and pathological processes, particularly in communication between tumor cells and the establishment of the tumor microenvironment (28, 32).
Skog J is a renowned leader scientist in the field of exosomes and has been at the forefront of groundbreaking discoveries regarding the crucial role played by exosomes and other microvesicles as cellular messengers in disease dissemination. Dr. Skog has demonstrated that tumor-derived exsomes RNA mutations can be detected in serum and other bodily fluids, offering a heterogeneous biomarker for tumor diagnosis (33). His team has also made significant contributions to liquid biopsy techniques for prostate cancer, detecting valuable RNA, DNA, and protein biomarkers in active cell-secreted exosomes, enabling precise diagnostics and aiding clinicians in monitoring cancer progression and determining each patient’s unique genetic composition of the disease. These contribute to our understanding of the leadership roles of Thery C and Skog J in the research on exosomes.
We carried out co-citation analysis (Table 5), cluster analysis (Figure 8A), timeline analysis (Figure 8C), and burst detection on the references (Figure 8B). In recent years, the focus can be summarized as the bone metastasis of prostate cancer in relation to exosome, the role of exosome in drug delivery in prostate cancer, the relationship between exosome and tumor suppressors, as well as the association between exosome and drug resistance (Figures 8, 9).
Although prostate cancer is the second most common diagnosed cancer in males, localized or regional lesions do not directly lead to death. However, the prognosis for metastatic disease is usually not optimistic, which may be the main reason why prostate cancer ranks fifth in global cancer-related mortality. It can be inferred that the ability of prostate cancer to acquire metastasis is the most lethal part of its disease progression. The bone is a relatively common site of metastasis, with 70% of advanced prostate cancer patients found to have bone metastasis (34).
Normal prostate epithelial cells are tightly adhered to neighboring cells and the surrounding extracellular matrix (ECM). However, malignant prostate cells have reduced adhesion and detach from adjacent cells and ECM, facilitating migration and invasion. Therefore, tumor cells in advanced prostate cancer can detach, migrate into the bloodstream, and enter the bone marrow through the lymphatic or hematogenous system, leading to additional bone metastasis. The growth of prostate cancer after metastasis initially relies on the establishment of the premetastatic niche, which requires factors that can be produced endogenously by osteoclasts or generated, transmitted, and secreted by cancer cell-derived exosomes (35, 36). Factors such as interleukins, bone morphogenetic proteins, VEGF, and chemokines secreted by primary PCa cells have been shown to play a role in promoting tumor metastasis and progression (25, 37–40). Exosomes have been shown to carry tumor-related molecules that play a role in cancer and premetastatic niche (41, 42). Additionally, research has indicated the importance of the bone microenvironment in promoting tumor colonization and proliferation, similar to the tumor cells themselves (43).
It is noteworthy that, in line with the seed and soil hypothesis of tumor development, the tumor microenvironment constructed as a result complements and influences the occurrence and progression of prostate cancer. The primary PCa needs to undergo a lengthy process of bone remodeling to develop into bone metastasis, which involves metastatic cancer cells, as well as intrinsic bone cells such as osteoblasts, osteoclasts, and stromal cells (44). The metastatic cancer cells are partially in a dormant state, awaiting suitable conditions for activation, and their role is to promote new bone formation. Therefore, the activation of osteoclasts and osteoblasts during bone remodeling is key to promoting tumor growth. Research has shown that cancer cells secrete more than 10 times the amount of exosomes compared to normal cells. Moreover, exosomes derived from tumors promote intercellular communication through growth factors, chemokines, miRNA, and other small molecules (45, 46). Tumor-derived exosomes play an important role in mediating intercellular communication during the bone metastasis process of osteoblastic cells. Osteoblasts specifically take up the secreted exosomes from PCa cells, undergo reprogramming, and subsequently promote PCa cell homing and local proliferation (47). Furthermore, human bone marrow stromal cells can also take up exosomes derived from PCa cells, leading to changes in transcriptional level and signal transduction, promoting cancer cell metastasis (48). This highlights the importance of establishing a signaling network in the tumor microenvironment.
In bone metastasis, tumor-derived exosomes also have an osteogenic differentiation-inducing effect on the local mesenchymal cell pool. In addition to tumor cell-derived exosomes, exosomes derived from other cells also participate in this process. For example, osteoblast-derived exosomes can regulate the proliferation of cancer cells in the metastatic microenvironment (49, 50). However, the molecular mechanisms underlying PCa bone metastasis formation are not fully understood. Specifically, it is unclear how certain substances present in the microenvironment, such as cellular factors and immune molecules, are secreted into the ECM from tumor cells, osteoclasts, fibroblasts, etc., whether through exocytosis, exosomes, or other forms. Exosomes are typically regarded as carriers of proteins, lipids, and RNA, capable of targeted delivery to specific cells and acting on membrane receptors to initiate signal transduction.
However, the role of exosomes in this pathological process is not well elucidated. Therefore, we believe that exosomes may play a role in prostate cancer bone metastasis. Research on the specific contributions of exosomes from various sources to the tumor ECM could potentially improve the accuracy of metastatic prostate cancer diagnosis and address the current issue of overdiagnosis. The composition and characteristics of exosomes derived from different cell sources exhibit heterogeneity. Revealing the substances secreted by tumor cell-derived exosomes, compared to those released by bone-related cells and fibroblasts into the ECM, may contribute to targeted therapy for PCa.
Exosomes are small particles which can be seen in and secreted by all cells in the organism, thus are a heterogeneous population of lipid bilayer membrane-enclosed vesicles. The promising prospects of exosomes as drug delivery vehicles are attracting the attention of more researchers. The characteristics of exosomes themselves align with the requirements of targeted drugs for cancer treatment: high targeting specificity, ability to traverse cell membranes, immune tolerance, multifunctionality, and feasibility for large-scale production. Exosomes exhibit high targeting specificity, as they can achieve precise targeted delivery through interactions between the heterogeneous surface proteins of exosomes and the matching proteins on target cells (26). The molecular regulation of fusion between different cell-derived exosomes and target cell plasma membranes varies and is likely dependent on the organism, cell type, and exosome subtype. Currently, there are no specific targeted drugs that have been proven effective for the treatment of prostate cancer, which exhibits high heterogeneity. There may be differences in tumor characteristics and gene expression between different patients or within different lesions of the same patient. This can result in a lack of available options for some patients with advanced prostate cancer who may be resistant to mainstream treatment drugs or methods. Therefore, under the premise of understanding the mechanism of exosome action, it is possible to artificially design vesicles that assemble RNA with therapeutic effects, which can improve the limitations of existing personalized medicine. Additionally, the ability of exosomes to traverse biological membranes, including cell membranes and the blood-brain barrier (26), demonstrates their feasibility and effectiveness in delivering drugs to target cells or tissues. Exosomes are considered a natural drug delivery system with low immunogenicity and toxicity, and they may also participate in immune responses. Compared to other nanocarriers such as nanoparticles or liposomes, exosomes are more readily accepted by the body, reducing immune reactions and improving biocompatibility. In addition to serving as drug carriers, exosomes can also exert other functions during drug delivery. For example, they may influence cell signaling and gene expression. This multifunctionality enables exosomes not only to transport drugs but also to modulate disease environments. In recent years, with the advancement of technology, exosomes can be derived from various cell sources including cell lines cultured in vitro and endogenous sources in vivo. This provides diverse options for exosome production and holds the potential for achieving large-scale production to meet the demands of drug delivery. Despite the existing limitations (51), an increasing number of new technologies such as “exosomes for protein loading via optically reversible protein–protein interactions” (EXPLORs) (52) and cellular nanoporation (53) should be implemented to facilitate the loading of exogenous cargoes into exosomes. In conclusion, due to their low cytotoxicity, ability to maximize the bioavailability of drugs, and precise targeted homing specificity (26, 54), there has been an increasing number of clinical trials confirming the therapeutic potential of exosomes. They have been successfully applied in the treatment of chronic kidney disease (55), non-small cell lung cancer (56), colon cancer (57), breast cancer (58), and more. We believe that this will also provide help in the treatment prospects of prostate cancer.
Exosomes have a unique potential role in anti-tumor effects. Factors mediated by exosomes are involved in cell communication within the tumor microenvironment (TME), thus promoting tumor initiation and metastasis (59–61). Multiple studies have revealed that the TME affects tumor behavior by altering the malignant behavior of tumor cells (62). Exosomes, located within the TME, influence tumor progression through various tumor-promoting pathways such as angiogenesis, metastasis, and hypoxia induced by epithelial-mesenchymal transition (EMT) (62). As depicted in Figure 9, these have been research hotspots. Apart from the well-known role of gene mutations reaching a certain threshold in the initiation of tumors, the functional changes of exosomes in the TME also contribute to tumor initiation (62–64). In the reshaping of the TME, the promotion of cancer cell invasion may be attributed to the activation of exosomes leading to the release of tumor progression factors, including growth factors (65), RNA, and proteins (66). It may also be due to EMT, in which scientists have observed an increase in the release of mesenchymal markers associated with exosomes (67). In the field of prostate cancer, exosomes-mediated cooperation of miR-21-5p, miR-100-5p, and miR-139-5p has been shown to promote the progression and metastasis of PCa (68). Previous studies have indicated that the removal of exosomes from circulation can inhibit tumor progression (69). Tumor-derived exosomes can enhance cancer cell migration and invasion by downregulating tumor suppressor factors, as observed in breast cancer cells (70). Moreover, dendritic cells (DC cells), as the most effective antigen-presenting cells in the body, play a central role in initiating and regulating innate and adaptive immunity in the tumor microenvironment. They have the ability to present tumor-associated antigens on MHC molecules, provide co-stimulatory molecules or soluble factors to induce anti-tumor cellular responses (71). Immunogenic cell death can enhance tumor antigen exposure and promote tumor killing (72–74). Therefore, exosomes derived from dendritic cells (DCs) are potential candidates for specific cancer therapy, as they have been proven to induce immune system activation (75). Tumor-derived exosomes (TDEs) can theoretically also serve as therapeutic carriers for in situ DC activation through tumor microenvironment infiltration. Anti-tumor drugs based on this principle have been developed (76). This provides a new approach to inhibit the progression of prostate cancer. In addition, drug-loaded exosomes can improve the anti-tumor outcomes of chemotherapy drugs, such as paclitaxel-loaded exosomes for the treatment of various cancers including prostate cancer and lung cancer (77, 78).
Exosomes have been found to be closely related to tumor therapy resistance (59–61, 79). Exosomes may be hijacked in cancer chemotherapy. Its mechanism is related to the process of epithelial-mesenchymal transition (EMT) (80–82). Exosomes play a central role in multiple steps of EMT, ranging from the generation of invasive phenotypes to distant metastasis (83, 84). Resistant cancer cells can package chemotherapy drugs into exosomes and shuttle anticancer drugs out of tumor cells (85). Exosomes can carry and exchange genetic material with target cells, and resistant cancer cells utilize this to confer resistance on sensitive cells (86, 87). Additionally, TDE can induce resistance by promoting pathways of apoptosis inhibition (88–91). Cancer stem cells (CSC), which are also critical members in tumor development, maintain self-renewal and certain characteristics, as well as participate in cell migration, differentiation, and proliferation, through the transfer of their derived exosomes carrying cargo. Therefore, they enhance resistance to cancer treatment (92). With the advancement of cancer therapy, the emergence of resistance poses new challenges for researchers and medical teams. While exploring novel therapies for prostate cancer using exosomes, it is crucial for researchers to also be aware of the potential issue of resistance that may be encountered.
5 Conclusion
Through a comprehensive analysis of entire aspects of the PCa associated exosome field, we conducted a bibliometric and scientometric analysis of the research trends and hotspots in the field of exosome related to prostate cancer. USA and China have been in the center of this area with the high citations and publications, which makes us believe their potential in exploring the pathogenesis of exosomes in PCa. Over the past 12 years, the research focus has shifted from the generation and function of exosome to their role in tumor progression and drug therapy. Based on our findings, we predict that the research focus in this field will revolve around the role of exosomes in bone metastasis of prostate cancer, tumor treatment resistance, and the potential for future drug development. Researchers have started to pay attention to the unique properties of exosome in order to explore their potential effects in drug delivery. Accordingly we have sufficient reasons to believe that this field is about to witness more research interests and development prospects.
6 Limitations
This bibliometric analysis may have a few limitations. Firstly, we have relied solely on the WOSCC database for literature extraction. While WOSCC is widely regarded as a comprehensive database, it is still possible that some relevant publications may have been overlooked. Furthermore, our analysis may have excluded certain important publications due to restrictions on language and types of publications. Additionally, for the purpose of enhancing the clarity of our visual representations, we have selectively screened and included significant literature in the field for our analysis and mapping.
Explanation of nouns
1. Cluster Analysis is a data mining technique that uses statistical analysis to divide a data set into categories based on some similarity measure. The purpose of cluster analysis is to allocate data items with similar features to the same group, and data items with different features to different groups. The goal is to organize large amounts of data into meaningful clusters for better understanding and interpretation of the data.
2. Co-citation analysis refers to when two papers appear in the reference directory of a third paper, forming a co-citation relationship. The co-citation analysis method evaluates the similarity of papers by examining the number of times they are cited after publication. If two authors’ papers are cited by the same third author’s paper, then these two authors have a co-citation relationship, and the higher the frequency of their co-citation, the closer their academic relationship. The clustering function of CiteSpace was used for cluster analysis of literature co-citation, and the common themes of similar papers can be mined. The core papers of the co-citation cluster analysis may become research trends and hotspots.
3. Co-occurrence Analysis refers to the analysis of the frequency of occurrence of different entities within the same time or space, in order to evaluate their degree of association. Co-occurrence analysis is a quantitative study of co-occurrence phenomena, to reveal the content associations and knowledge implied by the features. This type of analysis is commonly used in fields such as data mining and social network analysis to discover relationships or patterns and to serve as support for decision-making.
4. Centrality is a metric that measures the proximity of a node in a network to the center of the entire network. By evaluating a node’s centrality, the importance of the node in the network can be determined. In graph theory and network analysis, centrality metrics can identify the most critical node in a graph.
5. Total Link Strength (TLS) refers to the sum of the strength of a node’s relationships with other nodes in a network. This strength can be represented by weights and can be evaluated based on the characteristics of the relationship between two nodes. TLS can be used to assess a node’s influence and centrality in a network.
6. A timeline is a graphical representation of a timeline that displays the sequence of events or historical events. It can help people clearly understand the order of events, the relationship between events and the relative length of each event. Its purpose is to visualize content and present it in a graphic and textual form to assist the reader in understanding.
7. Burst detection is a technique in data mining and information processing that detects sudden growth or events in data. It is commonly applied to time-series data to identify instances where the volume of data significantly increases in a short period and determine the cause of this growth.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
ZZ: Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft. YZ: Conceptualization, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. HL: Resources, Writing – original draft. WX: Resources, Writing – original draft. TW: Data curation, Writing – original draft. JL: Resources, Writing – original draft. HJ: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1270104/full#supplementary-material
Supplementary Material 1 | Shows the ten journals with the highest TLS in citation analysis.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Lee F, Littrup PJ, Torp-Pedersen ST, Mettlin C, McHugh TA, Gray JM, et al. Prostate cancer: comparison of transrectal US and digital rectal examination for screening. Radiology. (1988) 168(2):389–94. doi: 10.1148/radiology.168.2.3293108
3. Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen-based screening for prostate cancer: evidence report and systematic review for the US preventive services task force. JAMA. (2018) 319(18):1914–31. doi: 10.1001/jama.2018.3712
4. Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther (2020) 5(1):242. doi: 10.1038/s41392-020-00359-5
5. Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol (2022) 15(1):83. doi: 10.1186/s13045-022-01305-4
6. Yang C, Wu S, Mou Z, Zhou Q, Dai X, Ou Y, et al. Exosome-derived circTRPS1 promotes Malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments. Mol Ther (2022) 30(3):1054–70. doi: 10.1016/j.ymthe.2022.01.022
7. Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther (2020) 5(1):145. doi: 10.1038/s41392-020-00261-0
8. Zhang X, Xu Y, Ma L, Yu K, Niu Y, Xu X, et al. Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun (Lond). (2022) 42(4):287–313. doi: 10.1002/cac2.12275
9. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. (2019) 8(7):727. doi: 10.3390/cells8070727
10. Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. (2015) 65(8):783–97. doi: 10.1093/biosci/biv084
11. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (2020) 367(6478):eaau6977. doi: 10.1126/science.aau6977
12. Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther (2018) 188:1–11. doi: 10.1016/j.pharmthera.2018.02.013
13. Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A, et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv (2018) 36(1):328–34. doi: 10.1016/j.bioteChadv.2017.12.010
14. Ahmad P, Slots J. A bibliometric analysis of Periodontology. Periodontology (2000) 2020:85:237–40. doi: 10.1111/prd.12376
15. Ge Y, Chao T, Sun J, Liu W, Chen Y, Wang C. Frontiers and hotspots evolution in psycho-cardiology: A bibliometric analysis from 2004 to 2022. Curr Problems Cardiol (2022) 47:101361. doi: 10.1016/j.cpcardiol.2022.101361
16. Shen Z, Hu J, Wu H, Chen Z, Wu W, Lin J, et al. Global research trends and foci of artificial intelligence-based tumor pathology: A Scientometric study. J Trans Med (2022) 20:409. doi: 10.1186/s12967-022-03615-0
17. Shen Z, Wu H, Chen Z, Hu J, Pan J, Kong J, et al. The Global Research of Artificial Intelligence on Prostate Cancer: A 22-year bibliometric analysis. Front Oncol (2022) 12:843735. doi: 10.3389/fonc.2022.843735
18. Miao L, Zhang J, Zhang Z, Wang S, Tang F, Teng M, et al. A bibliometric and knowledge-map analysis of CAR-T cells from 2009 to 2021. Front Immunol (2022) 13:840956. doi: 10.3389/fimmu.2022.840956
19. Liu K, Zhao S, Li J, Zheng Y, Wu H, Kong J, et al. Knowledge mapping and research hotspots of immunotherapy in renal cell carcinoma: A text-mining study from 2002 to 2021. Front Immunol (2022) 13:969217. doi: 10.3389/fimmu.2022.969217
20. Zhong W, Shen Z, Wu Y, Mao X, Kong J, Wu W. Knowledge mapping and current trends of immunotherapy for prostate cancer: A bibliometric study. Front Immunol (2022) 13:1014981. doi: 10.3389/fimmu.2022.1014981
21. Ma D, Guan B, Song L, Liu Q, Fan Y, Zhao L, et al. A bibliometric analysis of exosomes in cardiovascular diseases from 2001 to 2021. Front Cardiovasc Med (2021) 8:734514. doi: 10.3389/fcvm.2021.734514
22. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for Bibliometric mapping. Scientometrics (2009) 84:523–38. doi: 10.1007/s11192-009-0146-3
23. Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol (2006) 57:359–77. doi: 10.1002/asi.20317
24. Chen C. Searching for intellectual turning points: Progressive Knowledge Domain Visualization. Proc Natl Acad Sci (2004) 101:5303–10. doi: 10.1073/pnas.0307513100
25. Kang J, La Manna F, Bonollo F, Sampson N, Alberts IL, Mingels C, et al. Tumor microenvironment mechanisms and bone metastatic disease progression of prostate cancer. Cancer Lett (2022) 530:156–69. doi: 10.1016/j.canlet.2022.01.015
26. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol (2019) 21(1):9–17. doi: 10.1038/s41556-018-0250-9
27. Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol (2002) 2(8):569–79. doi: 10.1038/nri855
28. Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. (2016) 164(6):1226–32. doi: 10.1016/j.cell.2016.01.043
29. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol (2009) 9(8):581–93. doi: 10.1038/nri2567
30. Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol (2006). doi: 10.1002/0471143030.cb0322s30
31. Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles (2012) 1(1). doi: 10.3402/jev.v1i0.18397
32. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol (2014) 30:255–89. doi: 10.1146/annurev-cellbio-101512-122326
33. Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol (2008) 10(12):1470–6. doi: 10.1038/ncb1800
34. Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, et al. Bone metastases: an overview. Oncol Rev (2017) 11(1):321. doi: 10.4081/oncol.2017.321
35. Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, et al. MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell (2005) 7(5):485–96. doi: 10.1016/j.ccr.2005.04.013
36. Kruger S, Abd Elmageed ZY, Hawke DH, Wörner PM, Jansen DA, Abdel-Mageed AB, et al. Molecular characterization of exosome-like vesicles from breast cancer cells. BMC Cancer. (2014) 14:44. doi: 10.1186/1471-2407-14-44
37. Hall CL, Kang S, MacDougald OA, Keller ET. Role of Wnts in prostate cancer bone metastases. J Cell Biochem (2006) 97(4):661–72. doi: 10.1002/jcb.20735
38. Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res (2005) 65(18):8274–85. doi: 10.1158/0008-5472.CAN-05-1891
39. Bellido T, Borba VZ, Roberson P, Manolagas SC. Activation of the Janus kinase/STAT (signal transducer and activator of transcription) signal transduction pathway by interleukin-6-type cytokines promotes osteoblast differentiation. Endocrinology. (1997) 138(9):3666–76. doi: 10.1210/endo.138.9.5364
40. Adekoya TO, Richardson RM. Cytokines and chemokines as mediators of prostate cancer metastasis. Int J Mol Sci (2020) 21(12):4449. doi: 10.3390/ijms21124449
41. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. (2015) 527(7578):329–35. doi: 10.1038/nature15756
42. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. (2017) 17(5):302–17. doi: 10.1038/nrc.2017.6
43. Loriot Y, Fizazi K, de Bono JS, Forer D, Hirmand M, Scher HI. Enzalutamide in castration-resistant prostate cancer patients with visceral disease in the liver and/or lung: Outcomes from the randomized controlled phase 3 AFFIRM trial. Cancer. (2017) 123(2):253–62. doi: 10.1002/cncr.30336
44. Fornetti J, Welm AL, Stewart SA. Understanding the bone in cancer metastasis. J Bone Miner Res (2018) 33(12):2099–113. doi: 10.1002/jbmr.3618
45. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. (2013) 113(1):1–11. doi: 10.1007/s11060-013-1084-8
46. Mao L, Li X, Gong S, Yuan H, Jiang Y, Huang W, et al. Serum exosomes contain ECRG4 mRNA that suppresses tumor growth via inhibition of genes involved in inflammation, cell proliferation, and angiogenesis. Cancer Gene Ther (2018) 25(9-10):248–59. doi: 10.1038/s41417-018-0032-3
47. Probert C, Dottorini T, Speakman A, Hunt S, Nafee T, Fazeli A, et al. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene. (2019) 38(10):1751–63. doi: 10.1038/s41388-018-0540-5
48. Liu CM, Hsieh CL, Shen CN, Lin CC, Shigemura K, Sung SY. Exosomes from the tumor microenvironment as reciprocal regulators that enhance prostate cancer progression. Int J Urol. (2016) 23(9):734–44. doi: 10.1111/iju.13145
49. Hashimoto K, Ochi H, Sunamura S, Kosaka N, Mabuchi Y, Fukuda T, et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc Natl Acad Sci U S A. (2018) 115(9):2204–9. doi: 10.1073/pnas.1717363115
50. Morhayim J, van de Peppel J, Demmers JA, Kocer G, Nigg AL, van Driel M, et al. Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth. FASEB J (2015) 29(1):274–85. doi: 10.1096/fj.14-261404
51. Familtseva A, Jeremic N, Tyagi SC. Exosomes: cell-created drug delivery systems. Mol Cell Biochem (2019) 459(1-2):1–6. doi: 10.1007/s11010-019-03545-4
52. Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun (2016) 7:12277. doi: 10.1038/ncomms12277
53. Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T, et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat BioMed Eng. (2020) 4(1):69–83. doi: 10.1038/s41551-019-0485-1
54. Choi JS, Cho WL, Choi YJ, Kim JD, Park HA, Kim SY, et al. Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J Extracell Vesicles. (2019) 8(1):1565885. doi: 10.1080/20013078.2019.1565885
55. Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res (2016) 20:21. doi: 10.1186/s40824-016-0068-0
56. Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. (2015) 5(4):e1071008. doi: 10.1080/2162402X.2015.1071008
57. Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther (2008) 16(4):782–90. doi: 10.1038/mt.2008.1
58. Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, Toffoli G, et al. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine (Lond). (2016) 11(18):2431–41. doi: 10.2217/nnm-2016-0154
59. Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol (2014) 184(1):28–41. doi: 10.1016/j.ajpath.2013.09.027
60. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med (2013) 19(11):1423–37. doi: 10.1038/nm.3394
61. Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev (2013) 32(3-4):623–42. doi: 10.1007/s10555-013-9441-9
62. Deep G, Panigrahi GK. Hypoxia-induced signaling promotes prostate cancer progression: exosomes role as messenger of hypoxic response in tumor microenvironment. Crit Rev Oncog (2015) 20(5-6):419–34. doi: 10.1615/CritRevOncog.v20.i5-6.130
63. DeCosse JJ, Gossens CL, Kuzma JF, Unsworth BR. Breast cancer: induction of differentiation by embryonic tissue. Science. (1973) 181(4104):1057–8. doi: 10.1126/science.181.4104.1057
64. Fujii H, Cunha GR, Norman JT. The induction of adenocarcinomatous differentiation in neoplastic bladder epithelium by an embryonic prostatic inductor. J Urol. (1982) 128(4):858–61. doi: 10.1016/s0022-5347(17)53221-8
65. Hendrix A, Maynard D, Pauwels P, Braems G, Denys H, Van den Broecke R, et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst (2010) 102(12):866–80. doi: 10.1093/jnci/djq153
66. He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, et al. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. (2015) 36(9):1008–18. doi: 10.1093/carcin/bgv081
67. Franzen CA, Blackwell RH, Todorovic V, Greco KA, Foreman KE, Flanigan RC, et al. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis. (2015) 4(8):e163. doi: 10.1038/oncsis.2015.21
68. Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. (2016) 7(4):3993–4008. doi: 10.18632/oncotarget.6540
69. Hannafon BN, Ding WQ. Cancer stem cells and exosome signaling. Stem Cell Investig (2015) 2:11. doi: 10.3978/j.issn.2306-9759.2015.05.02
70. Ding J, Xu Z, Zhang Y, Tan C, Hu W, Wang M, et al. Exosome-mediated miR-222 transferring: An insight into NF-κB-mediated breast cancer metastasis. Exp Cell Res (2018) 369(1):129–38. doi: 10.1016/j.yexcr.2018.05.014
71. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol (2020) 20(1):7–24. doi: 10.1038/s41577-019-0210-z
72. Wang Z, Chen J, Hu J, Zhang H, Xu F, He W, et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J Clin Invest. (2019) 129(11):4850–62. doi: 10.1172/JCI127471
73. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med (2007) 13(1):54–61. doi: 10.1038/nm1523
74. Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW, et al. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med (2016) 8(328):328ra27. doi: 10.1126/scitranslmed.aae0105
75. Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, et al. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. (2005) 114(4):613–22. doi: 10.1002/ijc.20757
76. Huang L, Rong Y, Tang X, Yi K, Qi P, Hou J, et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol Cancer (2022) 21(1):45. doi: 10.1186/s12943-022-01515-x
77. Saari H, Lázaro-Ibáñez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release (2015) 220(Pt B):727–37. doi: 10.1016/j.jconrel.2015.09.031
78. Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. (2018) 14(1):195–204. doi: 10.1016/j.nano.2017.09.011
79. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. (2019) 18(1):75. doi: 10.1186/s12943-019-0991-5
80. Lobb RJ, van Amerongen R, Wiegmans A, Ham S, Larsen JE, Möller A. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance. Int J Cancer. (2017) 141(3):614–20. doi: 10.1002/ijc.30752
81. Zeng AL, Yan W, Liu YW, Wang Z, Hu Q, Nie E, et al. Tumour exosomes from cells harbouring PTPRZ1-MET fusion contribute to a Malignant phenotype and temozolomide chemoresistance in glioblastoma. Oncogene. (2017) 36(38):5369–81. doi: 10.1038/onc.2017.134
82. Crow J, Atay S, Banskota S, Artale B, Schmitt S, Godwin AK. Exosomes as mediators of platinum resistance in ovarian cancer. Oncotarget. (2017) 8(7):11917–36. doi: 10.18632/oncotarget.14440
83. Syn N, Wang L, Sethi G, Thiery JP, Goh BC. Exosome-mediated metastasis: from epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol Sci (2016) 37(7):606–17. doi: 10.1016/j.tips.2016.04.006
84. Whiteside TL. The role of tumor-derived exosomes in epithelial mesenchymal transition (EMT). Transl Cancer Res (2017) 6(Suppl 1):S90–2. doi: 10.21037/tcr.2017.02.13
85. Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther (2005) 4(10):1595–604. doi: 10.1158/1535-7163.MCT-05-0102
86. Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res (2003) 63(15):4331–7.
87. Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci (2013) 14(3):5338–66. doi: 10.3390/ijms14035338
88. Dong H, Wang W, Chen R, Zhang Y, Zou K, Ye M, et al. Exosome-mediated transfer of lncRNA−SNHG14 promotes trastuzumab chemoresistance in breast cancer. Int J Oncol (2018) 53(3):1013–26. doi: 10.3892/ijo.2018.4467
89. Jing C, Cao H, Qin X, Yu S, Wu J, Wang Z, et al. Exosome-mediated gefitinib resistance in lung cancer HCC827 cells via delivery of miR-21. Oncol Lett (2018) 15(6):9811–7. doi: 10.3892/ol.2018.8604
90. Vella LJ, Behren A, Coleman B, Greening DW, Hill AF, Cebon J, et al. Intercellular resistance to BRAF inhibition can be mediated by extracellular vesicle-associated PDGFRβ. Neoplasia. (2017) 19(11):932–40. doi: 10.1016/j.neo.2017.07.002
91. Fornari F, Pollutri D, Patrizi C, La Bella T, Marinelli S, Casadei Gardini A, et al. In Hepatocellular Carcinoma miR-221 Modulates Sorafenib Resistance through Inhibition of Caspase-3-Mediated Apoptosis. Clin Cancer Res (2017) 23(14):3953–65. doi: 10.1158/1078-0432.CCR-16-1464
Keywords: exosome, prostate cancer, bibliometric analysis, bone metastasis, tumor suppressor, drug resistance
Citation: Zhu Z, Zhou Y, Li H, Xu W, Wang T, Liu J and Jiang H (2023) Research trends and hotspots in prostate cancer associated exosome: a bibliometric analysis. Front. Oncol. 13:1270104. doi: 10.3389/fonc.2023.1270104
Received: 01 August 2023; Accepted: 30 October 2023;
Published: 21 November 2023.
Edited by:
Yusuke Shiozawa, Wake Forest University, United StatesReviewed by:
Fumihiko Urabe, Jikei University School of Medicine, JapanHojat Dehghanbanadaki, Tehran University of Medical Sciences, Iran
Copyright © 2023 Zhu, Zhou, Li, Xu, Wang, Liu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyang Jiang, amlhbmcuaG9uZ3lhbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zhengjia Zhu
Zhengjia Zhu Yingjian Zhou
Yingjian Zhou Hao Li
Hao Li Wenchao Xu
Wenchao Xu Tao Wang
Tao Wang Jihong Liu
Jihong Liu Hongyang Jiang
Hongyang Jiang