- 1Department of Radiology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Pathology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
Background: Anastomotic hemangioma is a rare subtype of capillary hemangioma primarily found in the genitourinary tract. We present a case of a patient with an anastomotic hemangioma located in the retroperitoneal space; then, we explore and summarize the imaging features from previously reported cases for accurate diagnosis.
Case presentation: A 57-year-old woman complained of left lower back pain. Contrast-enhanced ultrasound revealed a hypoechoic mass with “slow-in and slow-out” enhancement. Abdominal CT scan displayed a well-defined, round soft tissue mass in the right retroperitoneal region with obvious enhancement. MRI indicated low signal on T1-weighted imaging, high signal on T2-weighted imaging and diffusion-weighted imaging, and progressive enhancement after enhancement. Surgical removal of the tumor was performed. Histopathological examination exhibited a distinct tumor border with interconnected blood vessels and a cavity lined by a single layer of cubic endothelial cells. Immunohistochemistry confirmed the presence of CD31[+] and CD34[+]. The final pathological diagnosis was anastomotic hemangioma. No recurrence was observed during a 40-month follow-up.
Conclusion: Retroperitoneal anastomotic hemangioma is a rare and benign neoplasm that may be misdiagnosed as ectopic pheochromocytoma or angiosarcoma. This case report presents and analyzes the imaging characteristics of a series of retroperitoneal anastomotic hemangiomas, which can be valuable for future diagnoses and help prevent unnecessary surgeries.
Introduction
Anastomosing hemangioma (AH) is a rare but benign vascular tumor first documented by Montgomery and Epstein in 2009 (1). AH primarily appears in the urogenital tract, particularly in the kidney (2), and exhibits histopathological similarities to highly differentiated angiosarcoma. AH has been observed in various organs, including the renal and adrenal glands, liver, spleen, ovary, testicle, bladder, breast, gastrointestinal tract, retroperitoneum, mesentery/peritoneum, nasal cavity, larynx, left atrium, soft tissue, and bone (3–14). The 2020 WHO classification of Soft Tissue Tumors recognizes AH as a distinct benign vascular neoplasm (15). Retroperitoneal AH is rare, and limited research had been reported, particularly focusing on imaging features. In this study, we present the case of a 53-year-old woman with retroperitoneal AH, which was initially misdiagnosed as ectopic pheochromocytoma, and include an analysis and summary of clinical and imaging features from previously reported cases, which aimed to accurately diagnosing retroperitoneal AH preoperatively and avoiding unnecessary surgical interventions.
Case presentation
A 57-year-old woman with left lower back pain for 1 day was admitted to our hospital. The patient had a history of hypertension and hepatitis B infection. Physical examination and laboratory tests, including routine blood and tumor markers, yielded normal results. The outpatient doctor considered that the lower back pain was caused by the kidney. A renal CT scan was performed, revealing a 4.1×3.3×4.8 cm soft tissue density mass in the inferior cavity space of the right kidney. The CT values in the plain scan, arterial phase, and venous phase ranged from approximately 13 to 25 HU, 26 to 174 HU, and 29 to 138 HU, respectively. Notably, the arterial phase demonstrated significant enhancement of the mass, which extended to the center during the venous phase (Figures 1A–C). Subsequently, contrast-enhanced ultrasound (CEUS) was performed to further determine whether the lesion is benign or malignant. CEUS revealed a hypoechoic mass in the inferior anteromedial region of the right kidney. The mass displayed a clear boundary and a homogeneous echo signal, and exhibited gradual enhancement during “slow-in and slow-out” phases (Figures 1D–F). To further aid diagnosis, a contrast-enhanced magnetic resonance imaging (MRI) scan was conducted. The MRI indicated that the lesion was situated in the right retroperitoneal space, displaying a low signal on T1-weighted imaging (T1WI) and a high signal on T2-weighted imaging (T2WI), with a clear boundary and a slightly increased signal on the diffusion-weighted image (DWI) (Figures 2A–C). The enhancement of the mass showed progressive centripetal filling (Figures 2D, E). No evidence of adjacent organ invasion or retroperitoneal lymphadenopathy was noted. The preoperative diagnosis was initially considered ectopic pheochromocytoma or angiosarcoma.
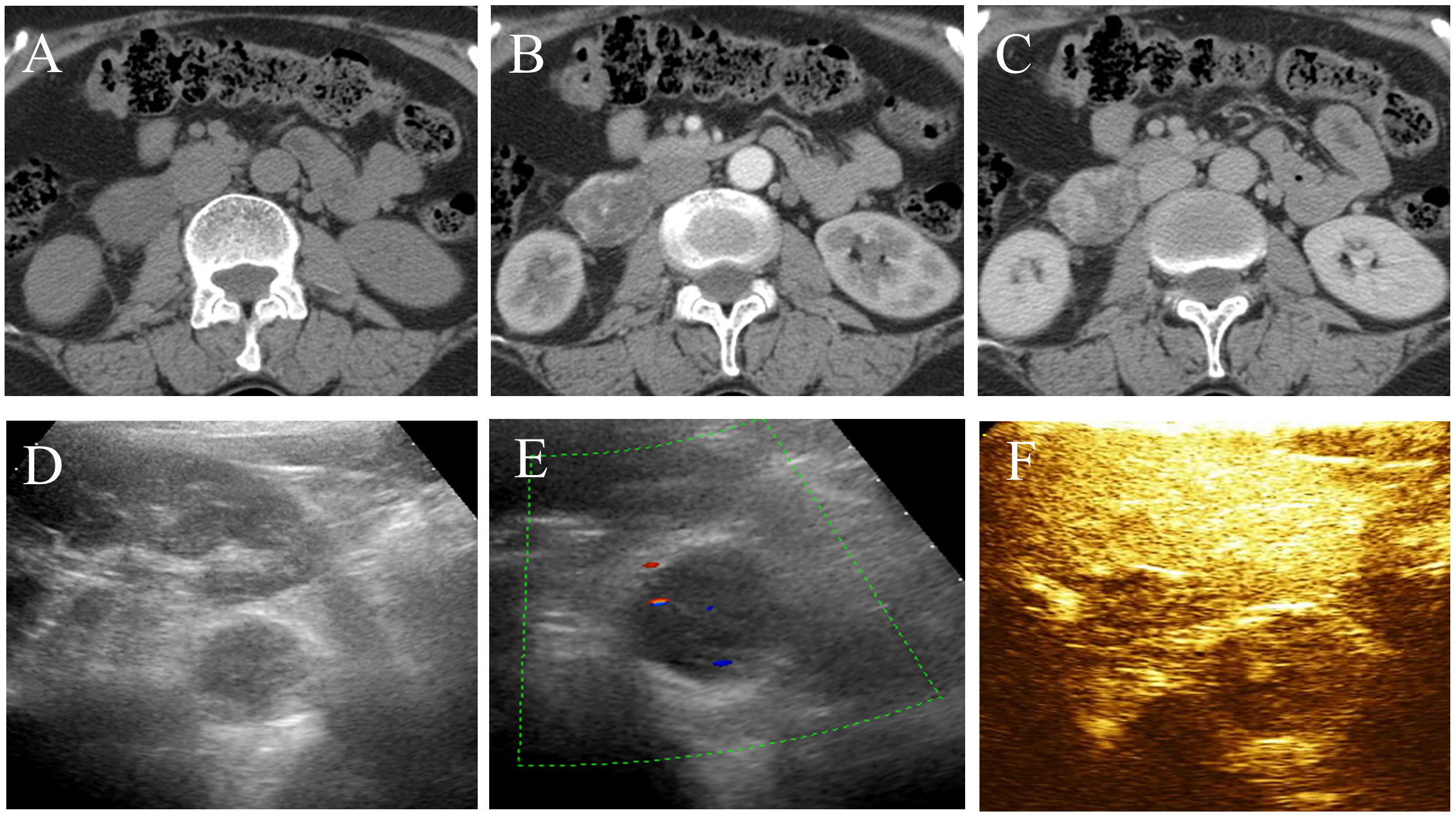
Figure 1 CT and ultrasound features of retroperitoneal anastomosing hemangioma. (A) Plain scan. (B) Arterial phase. (C) Venous phase. (D, E) The lesion exhibits a hypoechoic mass with slight blood flow signals in the inferior anteromedial region of the right kidney. (F) Contrast-enhanced ultrasound demonstrates homogeneous and “slow-in and slow-out” low enhancement.
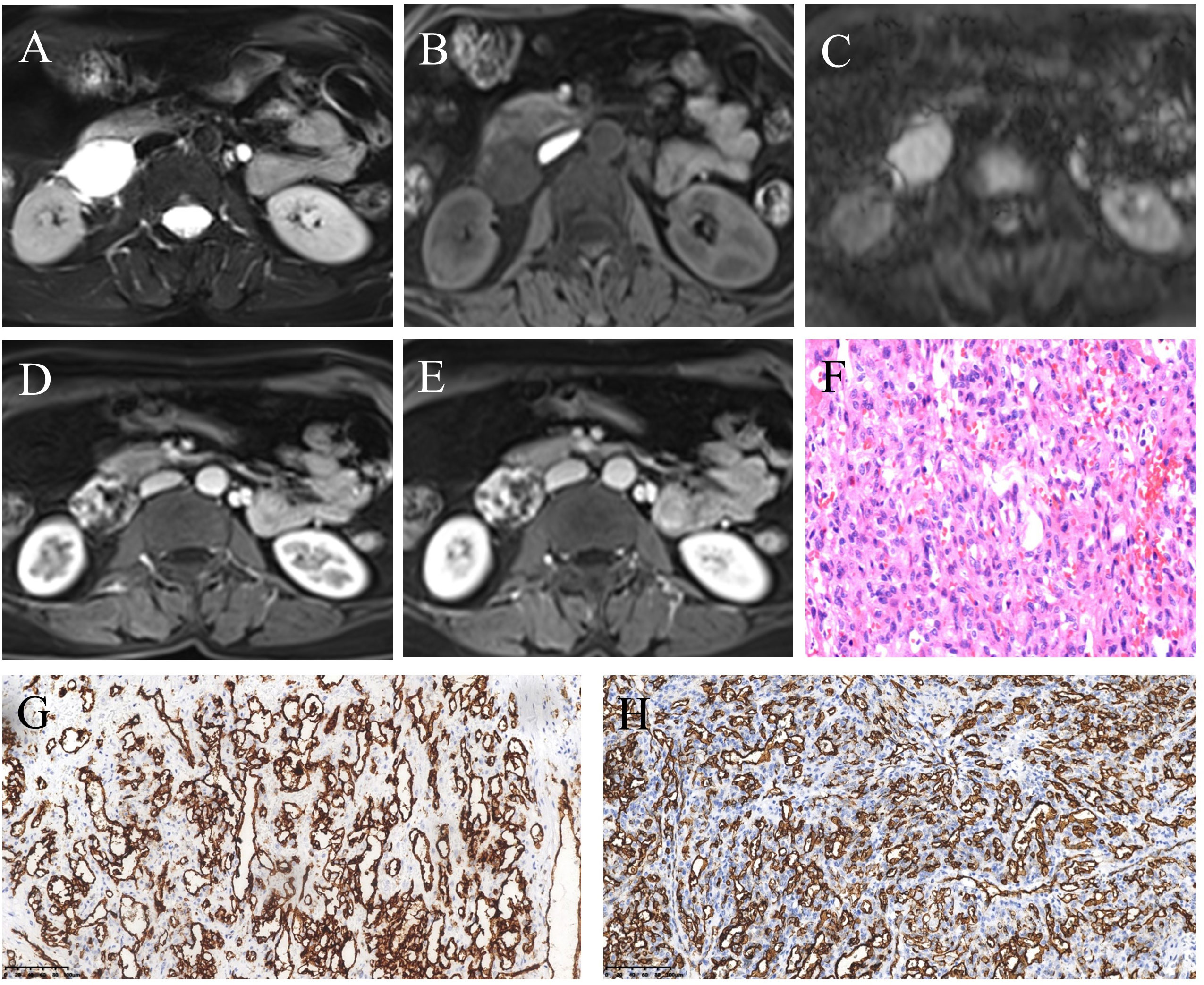
Figure 2 MRI and pathological features of retroperitoneal anastomosing hemangioma. (A) T2-weighted image. (B) T1-weighted image. (C) Diffusion-weighted image. (D) Arterial phase post-contrast T1-weighted image. (E) Venous phase post-contrast T1-weighted image. (F) Hematoxylin and eosin (HE) staining (200×). (G, H) Immunohistochemistry (200×) showed vascular endothelial cell markers CD31 (G) and CD34 (H) were positive.
During the surgical procedure, the mass was located behind the descending segment of the duodenum, displaying close association with the duodenum and inferior vena cava, along with severe adhesions. Pathological examination revealed a well-defined mass with nodular growth, composed of interconnected and anastomosing blood vessels (Figure 2F). Immunohistochemical analysis demonstrated positive staining for CD31 (Figure 2G), CD34 (Figure 2H), SMA, and focal Ki-67, while Calponin, S-100, Des CK, D2-40, CK5/6, HMB45, Melan-A, NSE, and Melan-A showed negative results. Based on the morphological and immunohistochemical findings, the diagnosis was confirmed as anastomosing hemangioma. The patient has been under follow-up for 40 months, with no recurrence or tumor metastasis observed.
Discussion
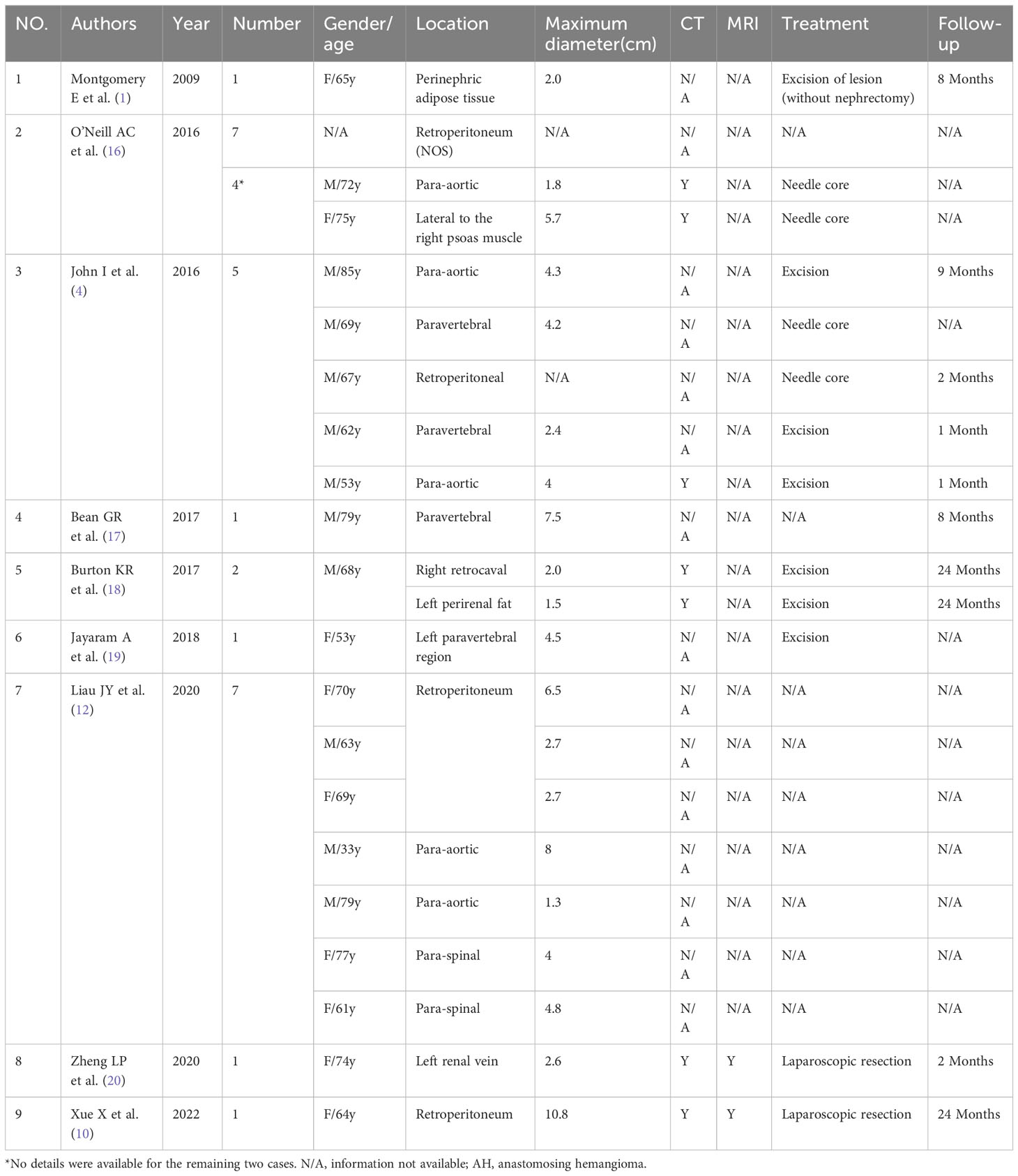
Retroperitoneal AH is an uncommon benign vascular tumor, with only approximately 30 cases reported in the literature (Table 1). These cases have been found in the retroperitoneum, para-aortic, paravertebral, and perinephric adipose tissue (1, 4, 10, 12, 16–20). While most of the reported cases focused on pathology or gene mutations, only a few examined the imaging findings.
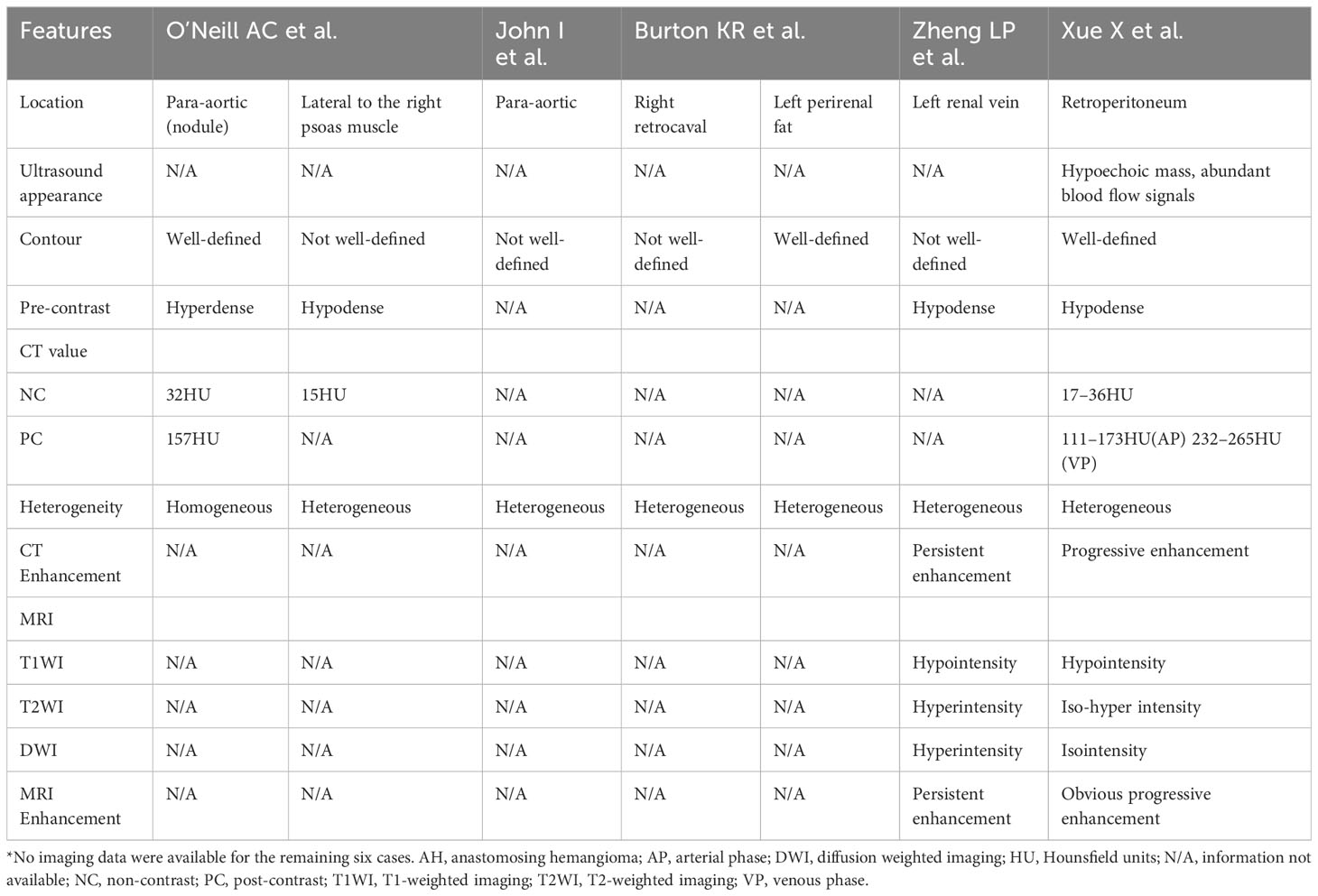
Previous literature indicates that AH predominantly affects men and typically presents asymptomatically, with a few cases showing low back pain, intermittent hematuria, and lower urinary tract symptoms. However, retroperitoneal AH often lacks specific clinical symptoms and signs, and it is frequently discovered incidentally during physical examinations (5). This is likely due to its deep retroperitoneal location, which hinders the appearance of symptoms. Among the 30 retroperitoneal cases collected in Table 1, there is a male-to-female ratio of approximately 1.2:1, with a slightly higher occurrence in men. The case that we report involves a middle-aged woman who presented with left low back pain, even though the lesion was located in the right retroperitoneal space. With a detailed history, we think that the lower back pain may be associated with muscle injury after physical labor, not the lesion. The age of AH patients ranges from 1 to 85 years old (14), with the median age for extrarenal AH being 65 years old. For retroperitoneal AH, the summarized age range is from 33 to 85 years old (average 66.9 ± 11.5 years) (Table 1), and the age in this case was 57 years old. Therefore, retroperitoneal AH appears to be more prevalent among the elderly. Existing literature reports a wide range of AH sizes, varying from 0.1 cm to 14 cm (14). According to the limited available data, the size of retroperitoneal AH ranges from 1.3 to 10.8 cm (average 4.2 cm). The maximum diameter in our case was 4.8 cm, consistent with previous reports. The imaging findings of reported AH cases have been limited, particularly in the retroperitoneal space, where only six patients with seven cases have been reported (Table 2). The ultrasound features of AH have not been extensively described in the literature. AH appears as lesions with a clear boundary and mixed echogenicity, and color Doppler flow imaging (CDFI) may show intense internal vascularity (5). The ultrasound characteristics of retroperitoneal AH were previously reported only by Xue et al. (10), who described it as a hypoechoic mass with abundant blood flow signals in CDFI. In our case, we observed a hypoechoic mass with some heterogeneity and a slight presence of blood flow, which differed from the previous study. Contrast-enhanced ultrasound revealed “slow-in and slow-out” with slight heterogeneous enhancement. Most AH cases typically appeared as low or equal density on CT scans, with a plain scan CT value of approximately 27–35 HU (11, 21). In contrast, the reported plain scan CT value for retroperitoneal AH ranged from approximately 15 HU to 37 HU, with our case measuring approximately 13–25 HU. Enhanced CT scans showed evident heterogeneous enhancement in the arterial phase for most lesions, followed by progressive enhancement in the venous phase, reaching values of 157–265 HU, similar to the aorta. In our case, the highest enhanced CT value was 174 HU, with visible nourishing vessels. Although the degree of enhancement decreased in the venous phase, it displayed centripetal filling enhancement. Variations in CT values may be associated with the number of vascular components within the lesion. Some AHs may exhibit unenhanced components, possibly due to surrounding fat wrapping or unenhanced areas resulting from degeneration or intravascular thrombosis. Xue et al. (10) reported the presence of a capsule in their case, but no other retroperitoneal AHs have been reported with this feature, suggesting the need for further verification. On MRI, typical AH appeared as a low signal on T1WI and a significantly high signal on T2WI. Additionally, there was evident enhancement in the arterial phase, followed by persistent centripetal filling in the venous phase. This enhancement pattern resembled that of cavernous hemangioma. DWI showed isointensity to high intensity in other locations (3, 20). In our study, DWI exhibited slightly high intensity, possibly due to the small number of cells and their loose arrangement. Merritt et al. (3) described the MRI imaging findings of intrahepatic AH, which showed progressive enhancement. This suggests that the enhancement features of AH in different locations may be similar. This case report contained multimodal imaging (ultrasound, CT, and MRI); enhancements of these examinations suggested that the mass may be a vascular neoplasm.
Retroperitoneal AH needs to be differentiated from the following diseases. First is ectopic pheochromocytoma, where most patients have rapid changes in blood pressure and a history of hypertension; it is prone to necrosis and cystic degeneration, with an obvious enhancement and slow washout. Second, retroperitoneal angiosarcoma is generally large and infiltrates the surrounding structures; it demonstrates a relatively high degree of malignancy and displays rapid short-term growth.
AH generally carries a favorable prognosis and is considered a benign lesion. No reports of metastasis or recurrence have been documented after resection. The longest follow-up for retroperitoneal AH has been 24 months without recurrence. In our case, the follow-up period was 40 months with no recurrence observed. However, it is important to note that the report of Burton et al. (18) indicated that multifocality or recurrence may still be possible.
Conclusion
Accurately diagnosing retroperitoneal AH preoperatively can be challenging due to its low incidence. Based on a summary of available imaging cases, AH should be considered in elderly patients exhibiting strong arterial phase enhancement with subsequent centripetal filling. AH is typically benign and slow growing, and early identification of this tumor may allow for appropriate follow-up, potentially avoiding unnecessary surgical interventions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LZ: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. JW: Data curation, Resources, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Medical Science Research Program of Zhejiang Province (No.2020KY692,No.2021KY861) and the Chinese Medical Science Research Program of Zhejiang Province (No. 2021ZQ072).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: A lesion mimicking angiosarcoma. Am J Surg Pathol (2009) 33:1364–9. doi: 10.1097/PAS.0b013e3181ad30a7
2. Cheon PM, Rebello R, Naqvi A, Popovic S, Bonert M, Kapoor A. Anastomosing hemangioma of the kidney: radiologic and pathologic distinctions of a kidney cancer mimic. Curr Oncol (2018) 25:220–3. doi: 10.3747/co.25.3927
3. Lunn B, Yasir S, Lam-Himlin D, Menias CO, Torbenson MS, Venkatesh SK. Anastomosing hemangioma of the liver: a case series. Abdom Radiol (2019) 44:2781–7. doi: 10.1007/s00261-019-02043-x
4. John I, Folpe AL. Anastomosing hemangiomas arising in unusual locations: A clinicopathologic study of 17 soft tissue cases showing a predilection for the paraspinal region. Am J Surg Pathol (2016) 40:1084–9. doi: 10.1097/PAS.0000000000000627
5. Shanbhogue K, Khandelwal A, Hajdu C, Cao W, Surabhi VR, Prasad SR. Anastomosing hemangioma: a current update on clinical, pathological and imaging features. Abdom Radiol (2022) 47:2335–46. doi: 10.1007/s00261-022-03559-5
6. Kryvenko ON, Gupta NS, Meier FA, Lee MW, Epstein JI. Anastomosing hemangioma of the genitourinary system. Am J Clin Pathol (2011) 136:450–7. doi: 10.1309/AJCPJPW34QCQYTMT
7. Rathore K, Yussouf R, Teh M, Jindal S, Wong D, Newman M. Left atrial anastomosing hemangioma causing recurrent pericardial effusion. Ann Thorac Surg (2020) 109:e157–9. doi: 10.1016/j.athoracsur.2019.06.082
8. Dutta R, Kakkar A, Sakthivel P, Kumar R. Anastomosing hemangioma of the larynx: A unicorn among head and neck tumors. Ann Otol Rhinol Laryngol (2021) 130:298–303. doi: 10.1177/0003489420943640
9. Huang Z, Chen C, Thingujam B. Anastomosing hemangioma of the nasal cavity. Laryngoscope (2020) 130:354–7. doi: 10.1002/lary.27998
10. Xue X, Song M, Xiao W, Chen F, Huang Q. Imaging findings of retroperitoneal anastomosing hemangioma: a case report and literature review. BMC Urol (2022) 22:77. doi: 10.1186/s12894-022-01022-7
11. Jin LU, Liu J, Li Y, Sun S, Mao X, Yang S, et al. Anastomosing hemangioma: The first case report in the bladder. Mol Clin Oncol (2016) 4:310–2. doi: 10.3892/mco.2015.699
12. Liau J-Y, Tsai J-H, Lan J, Chen C-C, Wang Y-H, Lee J-C, et al. GNA11 joins GNAQ and GNA14 as a recurrently mutated gene in anastomosing hemangioma. Virchows Arch (2020) 476:475–81. doi: 10.1007/s00428-019-02673-y
13. Bodman A, Goodman A, Olson JJ. Intracranial thrombosed anastomosing hemangioma: Case report. Neuropathology (2020) 40:206–10. doi: 10.1111/neup.12624
14. Zhang Z-Y, Hong P, Deng S-H, Tang S-Y, Liu Z, He H-Y, et al. Spermatic cord anastomosing hemangioma mimicking a Malignant inguinal tumor: A case report and literature review. Front Surg (2022) 9:930160. doi: 10.3389/fsurg.2022.930160
15. Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica (2020) 113:70–84. doi: 10.32074/1591-951X-213
16. O’Neill AC, Craig JW, Silverman SG, Alencar RO. Anastomosing hemangiomas: locations of occurrence, imaging features, and diagnosis with percutaneous biopsy. Abdom Radiol (2016) 41:1325–32. doi: 10.1007/s00261-016-0690-2
17. Bean GR, Joseph NM, Gill RM, Folpe AL, Horvai AE, Umetsu SE. Recurrent GNAQ mutations in anastomosing hemangiomas. Modern Pathol (2017) 30:722–7. doi: 10.1038/modpathol.2016.234
18. Burton KR, Jakate K, Pace KT, Vlachou PA. A case of recurrent, multifocal anastomosing haemangiomas. BMJ Case Rep (2017), bcr–2017-220076. doi: 10.1136/bcr-2017-220076
19. Jayaram A, Manipadam M, Jacob P. Anastomosing hemangioma with extensive fatty stroma in the retroperitoneum. Indian J Pathol Microbiol (2018) 61:120. doi: 10.4103/IJPM.IJPM_259_16
20. Zheng L-P, Shen W-A, Wang C-H, Hu C-D, Chen X-J, Shen Y-Y, et al. Anastomosing hemangioma arising from the left renal vein: A case report. WJCC (2020) 8:4986–92. doi: 10.12998/wjcc.v8.i20.4986
Keywords: retroperitoneal, anastomosing hemangioma, vascular tumors, magnetic resonance imaging, computed tomography
Citation: Zhang L and Wu J (2023) Multimodal imaging features of retroperitoneal anastomosing hemangioma: a case report and literature review. Front. Oncol. 13:1269631. doi: 10.3389/fonc.2023.1269631
Received: 30 July 2023; Accepted: 02 October 2023;
Published: 25 October 2023.
Edited by:
Wanhe Wang, Northwestern Polytechnical University, ChinaReviewed by:
Emina Talakic, Medical University of Graz, AustriaSung Jig Lim, Kyung Hee University, Republic of Korea
Carlos Bilreiro, Champalimaud Foundation, Portugal
Copyright © 2023 Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqing Zhang, emxxMTk5MHpscUAxNjMuY29t
 Liqing Zhang
Liqing Zhang Jian Wu2
Jian Wu2