- Department of Oncology, The Second Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
Human epidermal growth factor 2 (HER2) mutations are uncommon in non-small cell lung cancer (NSCLC), and the lack of established, effective, targeted drugs has resulted in a persistently poor prognosis. Herein, we report the case of a non-smoking, 58-year-old man diagnosed with lung adenocarcinoma (cT3N0M1c, stage IVB) harboring a HER2 mutation (Y772_A775dupYVMA) and PD-L1 (-). The patient’s Eastern Cooperative Oncology Group performance status (PS) score was assessed as 1. He commenced first-line treatment with chemotherapy, followed by immuno-chemotherapy, and with disease progression, he received HER2-targeted therapy and chemotherapy with an anti-angiogenic agent. However, HER2-targeted therapy, including pan-HER tyrosine kinase inhibitors (afatinib, pyrotinib, and pozitinib) and antibody–drug conjugate (T-DM1), produced only stable disease (SD) as the best response. After the previously described treatment, primary tumor recurrence and multiple brain metastases were observed. Despite the patient’s compromised overall physical condition with a PS score of 3-4, he was administered T-DXd in addition to whole-brain radiotherapy (WBRT). Remarkably, both intracranial metastases and primary lesions were significantly reduced, he achieved a partial response (PR), and his PS score increased from 3-4 to 1. He was then treated with T-DXd for almost 9 months until the disease again progressed, and he did not discontinue the drug despite the occurrence of myelosuppression during this period. This is a critical case as it exerted an effective response to T-DXd despite multiple lines therapy, including T-DM1. Simultaneously, despite the occurrence of myelosuppression in the patient during T-DXd, it was controlled after aggressive treatment.
Background
Lung cancer is the most common cause of cancer-related death worldwide (1). Non-small cell lung cancer (NSCLC) is the predominant type of lung cancer, accounting for 85% of all cases. However, human epidermal growth factor 2 (HER2, erbB-2/neu) mutations occur in only 2–4% of NSCLC patients, more commonly in women, never-smokers, and adenocarcinoma (2). HER2 is a member of the ErbB receptor tyrosine kinase family, and its mutations in NSCLC are predominantly an in-frame insertion of exon 20 into the tyrosine kinase structural domain, including Y772_A775dupYVMA, G778_P780dup, and G776delinsVC (G776delinsLC) (3, 4), which is associated with poor overall survival (OS) of only 18–21 months (5). Although HER2 can be regarded as a therapeutic target, the efficacy of targeted therapy in HER2-mutant NSCLC has been disputed. Therefore, the standard first-line treatment remains a reference for advanced “driverless” NSCLC. Antibody-drug conjugates (ADCs) are novel antitumor agents that combine the high specificity of antibody targeting with potent cytotoxic drugs (6). For cancer patients with HER2 mutation or amplification, particularly breast and gastric cancer, numerous clinical trials have demonstrated the promise of ADCs as an effective treatment strategy. Nevertheless, research into potentially effective treatments for advanced NSCLC with HER2 mutations is still ongoing.
Herein, we present a patient with an overall survival (OS) of 46.5 months who underwent chemotherapy, immune checkpoint inhibitors (ICIs), anti-angiogenesis agents (bevacizumab and anlotinib), pan-HER tyrosine kinase inhibitors (afatinib, pyrotinib, and poziotinib), and ADCs (ado-trastuzumab emtansine [T-DM1], trastuzumab deruxtecan [T-DXd]), and attempt to provide new evidence for effective treatment of patients with HER2-mutant cancer (Figure 1-I).
Case presentation
A 58-year-old male never-smoker was found to have an occupying lesion in the right hilar lung on chest computed tomography (CT) during a medical examination on 12.05.2019 (Figure 1-IIa). A biopsy was performed, and pathology revealed adenocarcinoma. The patient had diabetes, but it was under control. He then completed a systemic evaluation, which revealed pericardial and sacrococcygeal metastases. Next-generation sequencing identified a HER2 mutation (p.Y772_A775dupYVMA) and a BRCA2 germline mutation with PD-L1 (-) and microsatellite stability. He was ultimately diagnosed with advanced-stage lung adenocarcinoma (cT3N0M1c [pericardial and sacral metastases], stage IVB) with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 1. The pericardial effusion was drained, and the patient was administered one cycle of cisplatin. Subsequently, he underwent six cycles of first-line chemotherapy (pemetrexed plus cisplatin plus bevacizumab) and four cycles of maintenance therapy. A partial response (PR) was achieved at each efficacy evaluation, and progression-free survival (PFS) from first-line treatment was 11.3 months. In May 2020, with progressive disease (PD), the subject received a second-line immuno-chemotherapy regimen (paclitaxel albumin plus pembrolizumab plus bevacizumab) and was deemed to have achieved PR after two cycles. However, he discontinued paclitaxel albumin owing to unbearable bone pain. Strikingly, after two cycles, new frontal lobe and lung metastases were observed (Figure 1-IIb). Based on the HER2 mutation, he then received four anti-HER2 therapies, including ADCs (afatinib, T-DM1, pyrotinib, and poziotinib) for each relapse at recurrence (Figures 1-IIc, d). During this period, the patient underwent stereotactic radiotherapy (45 Gy/3 Gy/15 fractions) for significant enlargement of the intracranial metastasis (Figure 1-IId). Subsequently, a chest CT revealed an increase in bilateral pulmonary nodules. He was treated with anlotinib plus paclitaxel albumin for six cycles. After the second cycle, CT revealed a reduction in the size of the nodules in both lungs, with some lesions exhibiting cavity formation (Figure 1-IIe).
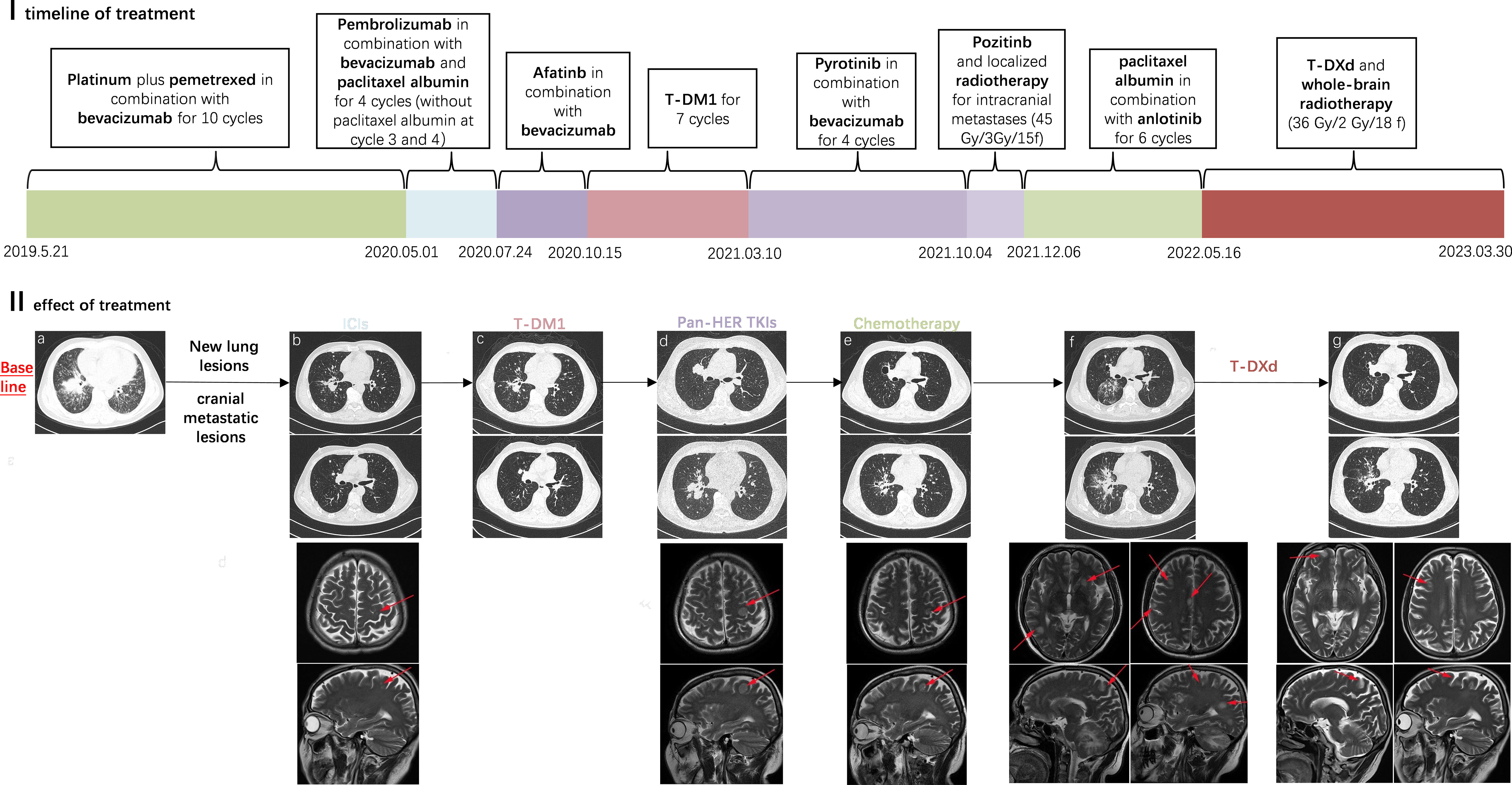
Figure 1 I (x-coordinate is the time, the unit is months): timeline diagram of the case receiving multiple lines of treatment; the PFS during this treatment period is expressed in length. II: Efficacy of treatment as demonstrated by chest CT and cranial MRI; (a) Primary lesions in the lungs (b) After treatment with ICIs, new lesions in the lungs and cranial metastatic lesions were observed (c) During treatment with T-DM1 (d) Disease progression after multiple lines pan-HER TKI therapy (e) Significant reduction in lung and brain lesions during treatment with re-challenge chemotherapy combination (f) Disease progression and multiple metastases in the brain (g) Radiotherapy was given for the cranial metastases, and subsequently in treatment with T-Dxd.
On 16/05/2022, significantly enlarged primary foci and multiple brain metastases were observed (Figure 1-IIf). The patient subsequently underwent whole-brain radiotherapy (36 Gy/2 Gy/18 f). Despite the previous treatment with T-DM1 and its poor efficacy, he chose to try T-DXd (400mg q3w) with a poor PS of 3-4. Interestingly, the patient responded to T-DXd even after heavy treatment and achieved PR (Figure 1-IIg), showing a significant improvement in PS (1–2). He was treated with T-DXd for almost 9 months before his disease progressed again, and he experienced neutropenia of grade 4 caused by T-DXd, which improved after supportive care. The patient died on 30.03.2023, having achieved an OS of 46.5 months.
Discussion and conclusion
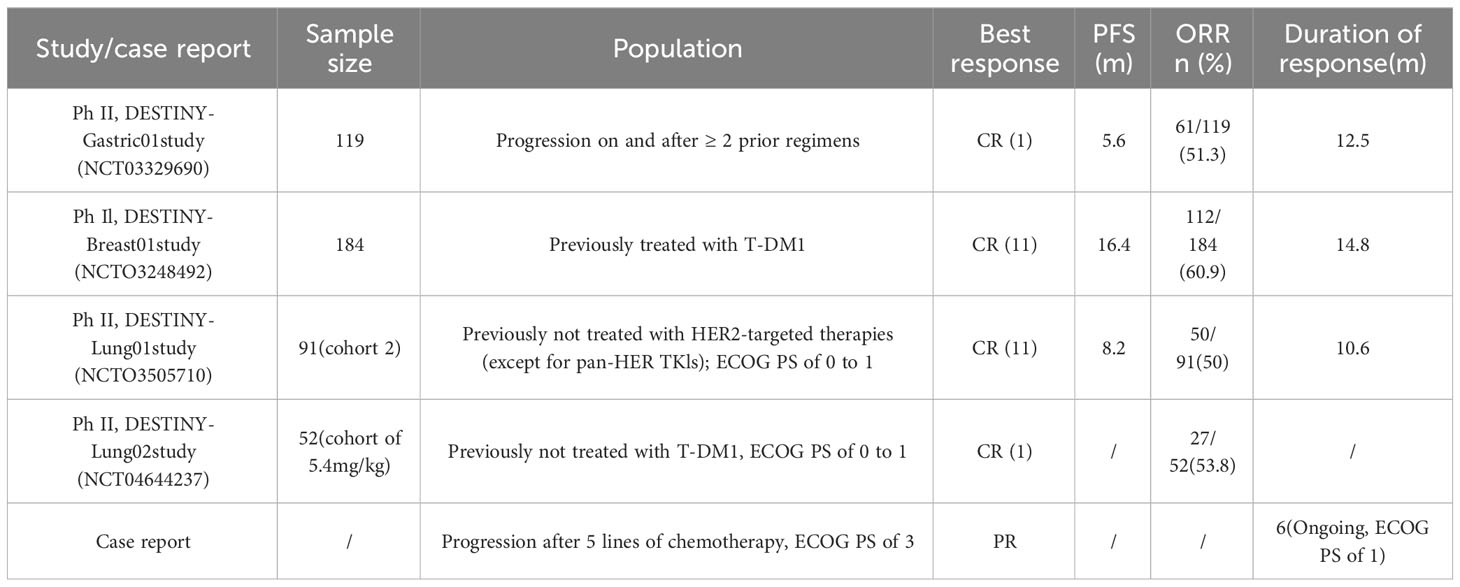
HER2 mutations are uncommon in advanced-stage NSCLC with poor prognosis, and the current standard treatment still refers to the National Comprehensive Cancer Network (NCCN) (7) recommended driverless NSCLC using platinum-pemetrexed plus bevacizumab or pembrolizumab. Also, a European EUHER2 cohort reported that in first-line treatment, PFS was 6 months and 4.8 months for chemotherapy and HER2-targeted therapy, respectively (8). In our case, we chose pemetrexed plus cisplatin plus bevacizumab as first-line treatment, and the patient was evaluated as PR during treatment with a PFS of 11.3 months. Additionally, the KEYNOTE-189 trial reported significant improvements in PFS and OS in chemotherapy-naive patients with non-squamous NSCLC, regardless of their PD-L1 expression status, using immuno-combination therapy (9). In this study, our patient received second-line albumin paclitaxel, bevacizumab, and pembrolizumab, and unfortunately, his PFS was only 2.8 months, similar to that reported in the IMMUNOTARGET retrospective study of HER2-mutant NSCLC receiving immunotherapy (10). Notably, although PR was achieved at the first evaluation, disease progression occurred after the last two cycles without albumin-paclitaxel due to bone pain. Whether disease progression is related to the discontinuation of chemotherapy or the ineffectiveness of immunotherapy remains inconclusive. However, in the first two lines of treatment, we noticed that this patient appeared to have a better response to chemotherapy. In addition, it was confirmed that afatinib and pirotinib had similar mPFS and produced responses in patients with HER2-mutant NSCLC with or without prior treatment (11–13). Also, the ZENITH20-2 trial demonstrated that poziotinib had antitumor activity in previously treated patients with HER2 exon 20 insertion NSCLC. This patient was treated with multiple pan-HER TKIs (afatinib, pyrotinib, and pozitinib) and ADC (T-DM1) based on the HER2 mutation, but neither reached an objective response. For T-DM1, the duration of response (DoR) in our case was similar to the lung cancer cohort in the phase 2 basket study (14). Our multiple treatment attempts have failed at this point. According to the ALTER0303 trial, the mPFS of the anlotinib group in advanced NSCLC beyond third-line treatment was 5.4 months; thus, we chose conventional treatment plus anlotinib (15). Every efficacy evaluation showed PR, with a PFS of 5.7 months. In this case, we chose multiple lines therapy including chemotherapy, immunotherapy, pan-HER TKIs, and ADC, and despite the chemotherapy used in front-line and back-line, which both achieved PR and contributed to delayed progression, the disease still inevitably progressed.
With the previous multiple lines treatment having failed, the DESTINY series of studies (Table 1), which reported the positive efficacy of third-generation ADC (T-DXd) in HER2-altered breast and gastric cancer, has given us a new direction for treatment options (16, 17). T-DXd is a novel HER2-targeted ADC that consists of trastuzumab and a topoisomerase I inhibitor. Subsequently, in Destiny-Lung01 by Li et al., previously treated HER2-mutant NSCLC patients receiving T-DXd (6.4 mg/kg) had an mPFS of 8.2 months and a median overall survival of 17.8 months, while the most common drug-related adverse event of grade 3 or higher was neutropenia, and drug-related interstitial lung disease occurred in 26% of patients (18). The pivotal findings from the Destiny-Lung02 study for T-DXd dose exploration significantly contributed to the subsequent FDA approval of T-DXd on 11/08/2022 for adults with HER2-mutant NSCLC who have experienced disease progression following systemic platinum-based therapy (19). Our patient received T-DXd in failure of T-DM1 treatment and a poor PS score (3-4) and maintained PFS for almost 9 months. The best response was assessed as PR, and the ECOG PS score improved to 1-2, which is similar to a case that reported an objective response and ECOG PS score improvement following treatment with T-DXd in poor PS (20). However, both Destiny-Lung01 and Destiny-Lung02 excluded patients with a PS score >2 or who had previously received T-DM1. Prior to this, only the ‘DESTINY-Breast’ clinical trial series reported a significant delay in disease progression with T-DXd in HER2-positive breast cancer patients who had previously received T-DM1 (17, 21). Our case suggests that the efficacy and safety of T-DXd were maintained in patients with advanced NSCLC who had previously received multiple lines therapy, despite the failure of T-DM1, and with poor PS scores. Although at that time we chose to administer T-DXd at a dose of 6.4 mg/kg according to Destiny-lung01, the recent Destiny-lung02 clinical trial demonstrated the efficacy of low-dose T-DXd in treating HER2-mutant NSCLC with a lower incidence of grade 3 or higher adverse events than the high-dose group (22). In addition, we caution clinicians about the four-degree myelosuppression caused by T-DXd. Based on the limitations of individual cases, the efficacy and safety of T-DXd in this group of patients still require further clinical case accumulation for validation.
This patient had both HER2 and BRCA2 germline mutations. Our therapeutic work has focused on standard treatment for driverless advanced NSCLC in addition to treatment for the HER2 gene mutation. Notably, the patient achieved a positive response with T-DXd after T-DM1 resistance demonstrated promising survival benefits. The efficacy of T-DXd on T-DM1-resistant HER2-positive cancer cells has been suggested to be related to the elevated expression of ABCC2 (MPR2) and ABCG2 (BCRP) (23). Furthermore, for lung cancer patients with BRCA germline mutations, BRCA inhibitors in combination with chemotherapeutic agents are emerging as a viable option. Taofeek et al. reported the efficacy and safety of veliparib compared to conventional platinum-based chemotherapeutic agents for the treatment of SCLC (24, 25). Lynnette et al. revealed a significant anti-tumor effect when combining olaparib and icotinib (26). Based on the above positive results of combination therapy with BRCA inhibitors in the treatment of lung cancer, more extensive research is required to ascertain whether combining ADCs with BRCA inhibitors could offer a novel and potential treatment approach for patients with concurrent HER2 and BRCA germline mutations.
In conclusion, we have reported a case with the HER2 mutation in an NSCLC patient who tried various treatments after progressing on standard therapy and achieved an OS of 46.5 months. This case is a reminder to clinicians that such patients could still benefit from T-DXd despite having poor PS scores after multiple lines therapy, including T-DM1, which still needs to be confirmed by more data and prospective clinical trials.
Data availability statement
The data are not available for public access due to patient privacy concerns but are available from the corresponding author upon reasonable request.
Ethics statement
This study complied with the tenets of the Declaration of Helsinki. It was conducted following formal approval by the Ethics Committee of the Second Affiliated Hospital of Zunyi Medical University (YXLL(KY-R)-2021-012). Written informed consent was obtained from the patient for the publication of any identifiable images or data contained herein.
Author contributions
JX: Writing – original draft. BH: Writing – review & editing. YW: Writing – review & editing. MW: Writing – review & editing. YL: Writing – review & editing. ZS: Writing – review & editing. SL: Writing – review & editing. FY: Writing – review & editing. JZ: Funding acquisition, Writing – review & editing. WH: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Science and Technology Department of Guizhou Province, grant no.: ZK (2021)-452, China, the Zunyi Medical University School-level Education Reform, grant no.: XJJG2021-45, the National Natural Science Foundation of China (Grant No. 82060475), and the Natural Science Foundation of Guizhou Province (Grant No. ZK2022-YB632).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADCs, antibody-drug conjugates; CT, computed tomography; DoR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; I+C+A, immune checkpoint inhibitors + chemotherapy + angiogenesis inhibitors; ICI, immune checkpoint inhibitor; IMRT, intensity-modulated radiotherapy; MRI, magnetic resonance imaging; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; NCCN, National Comprehensive Cancer Network; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria In Solid Tumors; SD, stable disease; T-DM1, ado-trastuzumab emtansine; T-DXd, trastuzumab deruxtecan.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Takeda M, Sakai K, Hayashi H, Tanaka K, Tanizaki J, Takahama T, et al. Clinical characteristics of non-small cell lung cancer harboring mutations in exon 20 of EGFR or HER2. Oncotarget (2018) 9(30):21132–40. doi: 10.18632/oncotarget.24958
3. Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature (2018) 554(7691):189–94. doi: 10.1038/nature25475
4. Wei XW, Gao X, Zhang XC, Yang JJ, Chen ZH, Wu YL, et al. Mutational landscape and characteristics of ERBB2 in non-small cell lung cancer. Thorac Cancer (2020) 11(6):1512–21. doi: 10.1111/1759-7714.13419
5. Wang Y, Jiang T, Qin Z, Jiang J, Wang Q, Yang S, et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol (2019) 30(3):447–55. doi: 10.1093/annonc/mdy542
6. Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discovery (2020) 10(5):674–87. doi: 10.1158/2159-8290.CD-20-0215
7. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines® Insights: non-small cell lung cancer, version 2.2023. J Natl Compr Cancer Network JNCCN (2023) 21(4):340–50. doi: 10.6004/jnccn.2023.0020
8. Mazières J, Barlesi F, Filleron T, Besse B, Monnet I, Beau-Faller M, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol (2016) 27(2):281–6. doi: 10.1093/annonc/mdv573
9. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
10. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol (2019) 30(8):1321–8. doi: 10.1093/annonc/mdz167
11. Fan Y, Chen J, Zhou C, Wang H, Shu Y, Zhang J, et al. Afatinib in patients with advanced non-small cell lung cancer harboring HER2 mutations, previously treated with chemotherapy: A phase II trial. Lung Cancer (2020) 147:209–13. doi: 10.1016/j.lungcan.2020.07.017
12. Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: A multicenter, open-label, single-arm, phase II study. J Clin Oncol (2020) 38(24):2753–61. doi: 10.1200/JCO.20.00297
13. Yang G, Xu H, Hu J, Liu R, Hu P, Yang Y, et al. Specific HER2 exon 20 gly776 deletion-insertions in non-small cell lung cancer: structural analysis and sensitivity to HER2-targeted tyrosine kinase inhibitors. Front Pharmacol (2022) 13:806737. doi: 10.3389/fphar.2022.806737
14. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol (2018) 36(24):2532–7. doi: 10.1200/JCO.2018.77.9777
15. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol (2018) 4(11):1569–75. doi: 10.1001/jamaoncol.2018.3039
16. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med (2020) 382(25):2419–30. doi: 10.1056/NEJMoa2004413
17. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med (2020) 382(7):610–21. doi: 10.1056/NEJMoa1914510
18. Li B, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med (2021) 386:241–51. doi: 10.1056/NEJMoa2112431
19. FDA gives nod to T-DXd for HER2-mutant NSCLC. Cancer Discov (2022) 12(10):2224. doi: 10.1158/2159-8290.CD-NB2022-0053
20. Kato Y, Kato Y, Minegishi Y, Suzuki T, Nakamichi S, Matsumoto M, et al. Efficacy with trastuzumab deruxtecan for non-small-cell lung cancer harboring HER2 exon 20 insertion mutation in a patient with a poor performance status: A case report. Onco Targets Ther (2021) 14:5315–9. doi: 10.2147/OTT.S341290
21. André F, Hee Park Y, Kim SB, Takano T, Im SA, Borges G, et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet (London England) (2023) 401(10390):1773–85. doi: 10.1016/S0140-6736(23)00725-0
22. Goto K, Goto Y, Kubo T, Ninomiya K, Kim SW, Planchard D, et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-lung02 trial. J Clin Oncol: Official J Am Soc Clin Oncol (2023) 41(31):4852–63. doi: 10.1200/JCO.23.01361
23. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy, Nature reviews. Clin Oncol (2021) 18(6):327–44. doi: 10.1038/s41571-021-00470-8
24. Owonikoko TK, Dahlberg SE, Khan SA, Gerber DE, Dowell J, Moss RA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer (2015) 89(1):66–70. doi: 10.1016/j.lungcan.2015.04.015
25. Atrafi F, Groen HJM, Byers LA, Garralda E, Lolkema MP, Sangha RS, et al. A phase I dose-escalation study of veliparib combined with carboplatin and etoposide in patients with extensive-stage small cell lung cancer and other solid tumors. Clin Cancer Res (2019) 25(2):496–505. doi: 10.1158/1078-0432.CCR-18-2014
Keywords: HER2 mutation, non-small cell lung cancer, chemotherapy, target-therapy, T-DXd, ADCS
Citation: Xu J, He B, Wang Y, Wu M, Lu Y, Su Z, Liu S, Yin F, Zhou J-G and Hu W (2024) Positive response to trastuzumab deruxtecan in a patient with HER2-mutant NSCLC after multiple lines therapy, including T-DM1: a case report. Front. Oncol. 13:1268260. doi: 10.3389/fonc.2023.1268260
Received: 27 July 2023; Accepted: 22 December 2023;
Published: 18 January 2024.
Edited by:
Shanye Yin, Albert Einstein College of Medicine, United StatesReviewed by:
Wei Ke, Rutgers, The State University of New Jersey, United StatesWenjun Deng, Massachusetts General Hospital and Harvard Medical School, United States
Yunjia Song, Johns Hopkins University, United States, in collaboration with reviewer WD
Copyright © 2024 Xu, He, Wang, Wu, Lu, Su, Liu, Yin, Zhou and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Hu, aHV3ZWlrZXlhbkAxNjMuY29t; Jian-Guo Zhou, amlhbmd1by56aG91QHlhaG9vLmNvbQ==
 Junzhu Xu
Junzhu Xu Bo He
Bo He Jian-Guo Zhou
Jian-Guo Zhou Wei Hu
Wei Hu