
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 04 October 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1265304
This article is part of the Research TopicSuccessful Breast Cancer Survivorship Among Racial/Ethnic MinoritiesView all 9 articles
 Yujie Xie1†
Yujie Xie1† Yongqing Deng2†
Yongqing Deng2† Suosu Wei3†
Suosu Wei3† Zhen Huang1
Zhen Huang1 Lihui Li1
Lihui Li1 Kai Huang1
Kai Huang1 Chunyu Wei1
Chunyu Wei1 Jinan Xu1
Jinan Xu1 Lingguang Dong1
Lingguang Dong1 Qiuhuan Zhang4
Qiuhuan Zhang4 Jiehua Zhao1
Jiehua Zhao1 Quanqing Zou1*
Quanqing Zou1* Jianrong Yang1*
Jianrong Yang1*Background and Objectives: Age is a significant determinant of susceptibility to breast cancer. Currently, the available evidence regarding the non-linear correlation between the age of diagnosis and the prognosis of breast cancer patients is contradictory. Insufficient data currently exist regarding the influence of age at diagnosis on the prognosis of breast cancer. The objective of our investigation was to examine the relationship between age at diagnosis and overall survival (OS), breast cancer-specific survival (BCSS), and disease-free survival (DFS).
Methods: This retrospective cohort study included 1054 patients diagnosed with breast cancer between March 7, 2013 and December 31, 2019. The hazard ratios (HRs) and 95% confidence interval (CI) for OS, BCSS, DFS were assessed using Cox proportional hazard ratio models and restricted cubic splines (RCS).
Results: The study included 1054 breast cancer patients who met the criteria. With a median follow-up of 4.86 years, 71 patients (6.74%) died and 144 patients (13.66%) relapsed. After multivariable adjustment, age showed a U-shaped association with OS, BCSS, and DFS, with significantly higher risk at two ends, with age inflection points of 44, 44, and 41 years for OS, BCSS, and DFS, respectively. For OS, Quartile 1 (HR, 2.09; 95% CI: 0.90-4.84), Quartile 3 (HR, 2.44; 95% CI: 1.05-5.65) and Quartile 4 (HR, 3.38; 95% CI: 1.51-7.54) had poorer OS compared with Quartile 2. Similar results were found for BCSS and DFS.
Conclusions: This study confirmed a U-shaped association between age at diagnosis and breast cancer outcome.
Breast cancer is currently the most prevalent form of cancer among women, with an estimated 2.26 million diagnoses and 685,000 deaths in 2020 (1). The projected global cancer burden is anticipated to increase by 50% in 2040 as compared to 2020 (2).
Breast cancer is relatively common in middle-aged and elderly women. In China, about 30% of breast cancers are diagnosed in people over the age of 60, a proportion that exceeds 50% abroad (3, 4). Women aged 50 and above constitute roughly 82% of new breast cancer diagnoses (4). Conversely, breast cancer incidence in patients under the age of 40 and 30 accounts for only 6.5% and 0.6%, respectively (5). The above research shows that there may be a certain relationship between age and the occurrence of breast cancer.
The influence of age on the prognosis of breast cancer has been examined, yet there exists contradictory evidence regarding this matter (6–8). One study showed that older patients had a poorer prognosis than younger patients (9). In postmenopausal elderly breast cancer patients, Research has demonstrated that an increased age at diagnosis is associated with a unfavorable prognosis (6, 10). Nevertheless, surgical intervention has been found to enhance both overall survival (OS) and breast cancer-specific survival (BCSS) in elderly patients, thereby establishing it as an efficacious and secure treatment approach (11). In contrast to the prevailing notion that older patients experience a more adverse prognosis compared to their younger counterparts, certain studies have revealed that younger patients exhibit inferior survival outcomes in comparison to older patients (12–15). The 75-month OS incidence (78%) and DFS (62%) were significantly worse (p < 0.05) in women aged 35 years and younger compared to OS (89%) and disease-free survival (DFS) (78%) in women aged 36-45 years (16). It is worth noting that younger patients exhibited larger tumors and a higher likelihood of developing axillary lymph node metastases in comparison to older patients (17, 18). This discrepancy may contribute to the comparatively poorer survival outcomes observed in younger patients. Consequently, it is imperative to explore the correlation between age at diagnosis and survival among individuals with cancer.
The current historical cohort study included all inpatients diagnosed with breast cancer at the People’s Hospital of Guangxi Zhuang Autonomous Region, China, from March 7, 2013 to December 31, 2019 (registration site http://www.chictr.org.cn/index.aspx; registration number ChiCTR2200058542). In the present study 1579 patients were selected. Of these, only 1054 breast cancers were included. Patients based on the following criteria were excluded: breast cancer patients who had neoadjuvant chemotherapy, preoperative stage IV, bilateral breast cancer, male breast cancer and lost to follow-up. The inclusion and exclusion criteria for the enrolled patients were shown in Figure 1. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki (6th revision, 2008) and was approved by the Ethics Committees of the People’s Hospital of Guangxi Zhuang Autonomous Region, China. Individual informed consent was not obtained in this study because we analyzed anonymized electronic medical records data as aggregates, with no individual health data available.
This study focused on the age at diagnosis of breast cancer as the primary independent variable, stratified as Quartile 1 (Q1: age 22–43 years), Quartile 2 (Q2: 44 to 50 years), Quartile 3 (Q3: 51-58 years) and Quartile 4 (Q4: 59-90 years) by quartiles. That is, all breast cancer patients are listed in order of age, and we divided the total data into four equal parts, with each quartile containing 25% of breast cancer patients. Clinical characteristics included age at diagnosis, T category, N category, stage of TNM, tumor size, histopathological grade, lymph nodes in the axilla status (LN), molecular subtype, nuclear-associated antigen ki-67 (Ki-67) level, Status of both ER and PR hormone receptors, HER2 status, operation type, adjuvant therapy, hormonal therapy and follow-up time. Of which, TNM stage is divided into 0 (TisN0M0), 1 (T1N0M0), 2 (T1N1M0, T2N0-1M0 and T3N0M0), 3 (T1-2N2M0, T3N1-2M0, T4Nany M0 and Tany N3M0) and Unknown (unable to determine or sample size is missing). Operation type is divided into BCS (breast-conserving surgery) and M (mastectomy). With reference to the Chinese Anti-Cancer Society Breast Cancer Diagnosis and Treatment Guidelines and Specifications (2020) (19) and the Guidelines for HER-2 Detection in Breast Cancer (2019) (20), in our study the tumors were classified into Luminal A, Luminal B (HER-2 -), HER-2 + (HR* +), HER-2 + (HR* -) and triple negative subtypes.
The follow-up period was determined by the initial diagnosis date of breast cancer, and patients were monitored until June 30, 2022 or until their demise. Treatment specifics and pertinent data were obtained from electronic medical records or telephone follow-up. The study endpoints encompassed OS, BCSS and DFS. OS referred to the time elapsed between diagnosis of breast cancer and death caused by any cause. BCSS measured the total length of time between diagnosis and death from breast cancer-related causes. DFS was defined as the first breast cancer recurrence at any site after the surgery date. Patients without events were censored at the time of their last follow-up.
Categorical or continuous variables are presented as median and interquartile range (IQR) or frequency (percentage) base on the data of baseline characteristics, respectively. In order to compare the baseline characteristics of the groups, chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables were used. Multivariable Cox regression was used to assess the effect of age on OS, BCSS and DFS to adjust for the effects of independent variables (tumor size, molecular subtype, lymph nodes in the axilla, operation type, adjuvant therapy, hormonal therapy). Covariate selection and other potential confounders were identified using a backward selection procedure, clinical significance and literature data, respectively. The crude and adjusted (HR) and 95% (CI) were calculated for the risk for OS, BCSS and DFS according to age group. By modeling age group with restricted cubic splines (RCS) and adjusting for potential confounders, a possible nonlinear association was identified. Smooth curve fitting was used first to determine if the independent variable is interval-based. The intervals were fitted with segmented regression using separate line segments (also called piece-wise regression). The log-likelihood ratio test for nonlinearity of smooth curve fitting was conducted by comparing the non-segmented model with a segmented regression model to calculate the P value. The threshold level of age was determined when a maximum likelihood model was found at the inflection point. We used R 3.4.3 (http://www.R-project.org, The R Foundation) for all analyses. P values less than 0.05 were considered statistically significant.
The current historical cohort study included all inpatients diagnosed with breast cancer at the People’s Hospital of Guangxi Zhuang Autonomous Region, China, from March 7, 2013 to December 31, 2019. In the present study 1579 patients were selected. Of these, the study included 1054 breast cancer patients who met the criteria. There was a median age of 51.00 years (interquartile range, 44.00-59.00) at diagnosis. The baseline clinicopathologic characteristics of those selected according to their age quartile are shown in Table 1. The definition of “higher age quartile” is Q3(51-58y)-Q4(59-90y). Significant differences were observed in Ki-67 level, PR status, operation type, and adjuvant therapy by age quartile. For the higher age quartile, patients had a significantly higher PR-negative tumors and mastectomy rate. Q3 and Q4 had higher PR-negative tumors, Q3 had the highest (42.8%) vs Q4 (40.34%). The proportion of mastectomy was higher in Q3 and Q4, with the highest number of mastectomy in Q4 (90.34%) vs Q3 (83.76%). In contrast, Ki-67 levels, breast conserving surgery, and adjuvant chemotherapy were significantly lower in the higher age quartile (all P < 0.05). The proportion of tumors with high Ki-67 expression (Ki-67 > 30%) was lower in Q3 and Q4, with Q4 having the lowest rate of tumors with high Ki-67 expression (21.72%) compared to Ki-67 in Q3 (30.63%). The percentage of breast-conserving surgeries was lower in Q3 and Q4, where it was lowest in Q4 (9.66%) compared to Q3 (16.24%). The proportion of patients treated with adjuvant chemotherapy was lower in Q3 and Q4, with Q4 having the lowest proportion of adjuvant chemotherapy (35.86%) compared to Q3 (47.23%). Unlike the previous indicators, radiation therapy is U-shaped in all age groups, with the lowest in Q3 (2.58%) and the highest in Q4 (4.14%). Compared with Q2 (2.86%), Q1 (3.23%) has a higher radiation therapy rate.
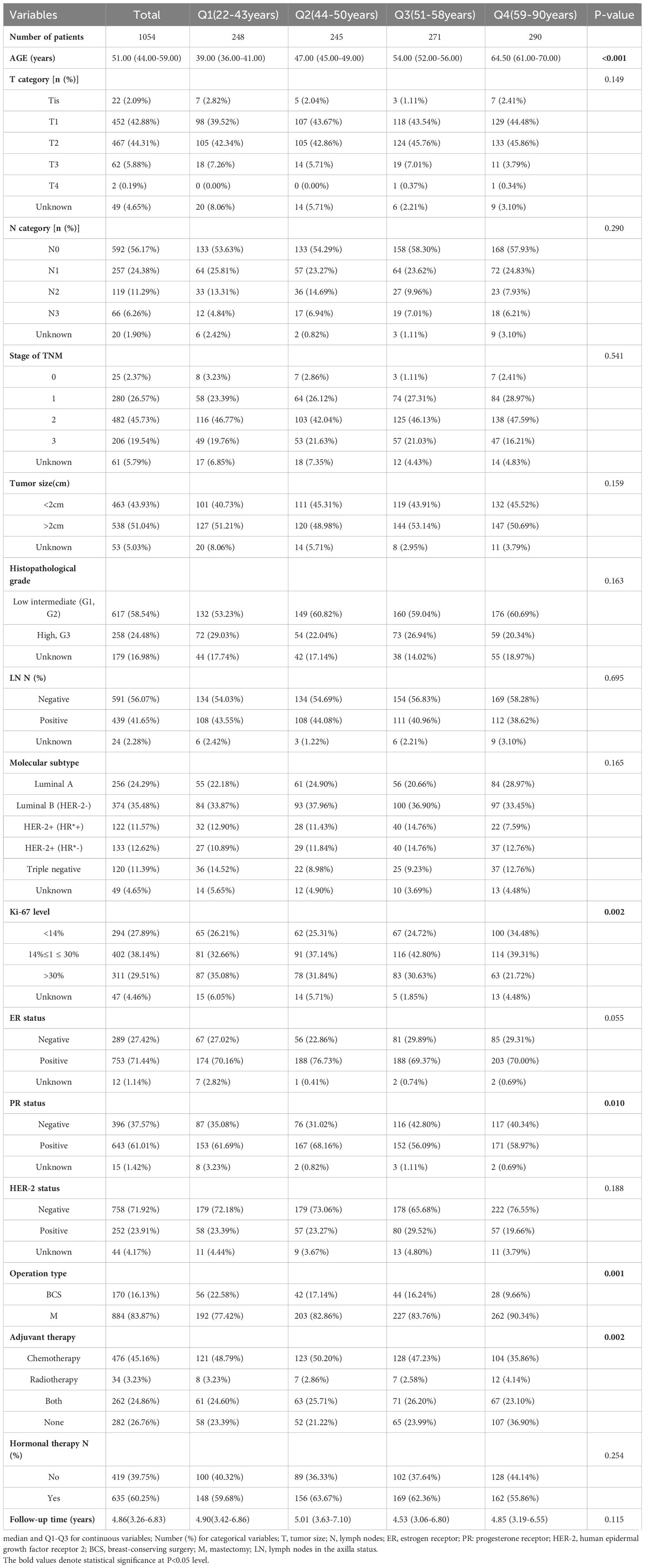
Table 1 Comparison of treatments and clinical and pathological characteristics of tumors according to age at breast cancer diagnosis.
Adjusted smooth curve fitting revealed a U-shaped relationship between age group and OS, BCSS, and DFS (Figure 2). As a result of threshold effect analysis (Table 2), age was associated with OS, BCSS, and DFS in a significant U-shaped relationship (P for log likelihood ratio test <0.05). OS, BCSS and DFS identified age inflection points of 44, 44 and 41 years, respectively. On the left of inflection point, the risk for OS (fully adjusted (HR), 0.94; 95% (CI), 0.88, 1.0), BCSS (fully adjusted (HR), 0.77; 95% (CI), 0.66, 0.89) and DFS (fully adjusted (HR), 0.92; 95% (CI), 0.86, 0.98) were negatively associated with age. Conversely, on the right of inflection point, the risk for OS (fully adjusted [HR], 1.05; 95% [CI], 1.02, 1.08), BCSS (fully adjusted [HR], 1.04; 95% [CI], 1.01, 1.07) and DFS (fully adjusted [HR], 1.02; 95% [CI], 1.01, 1.04) was positively associated with age.
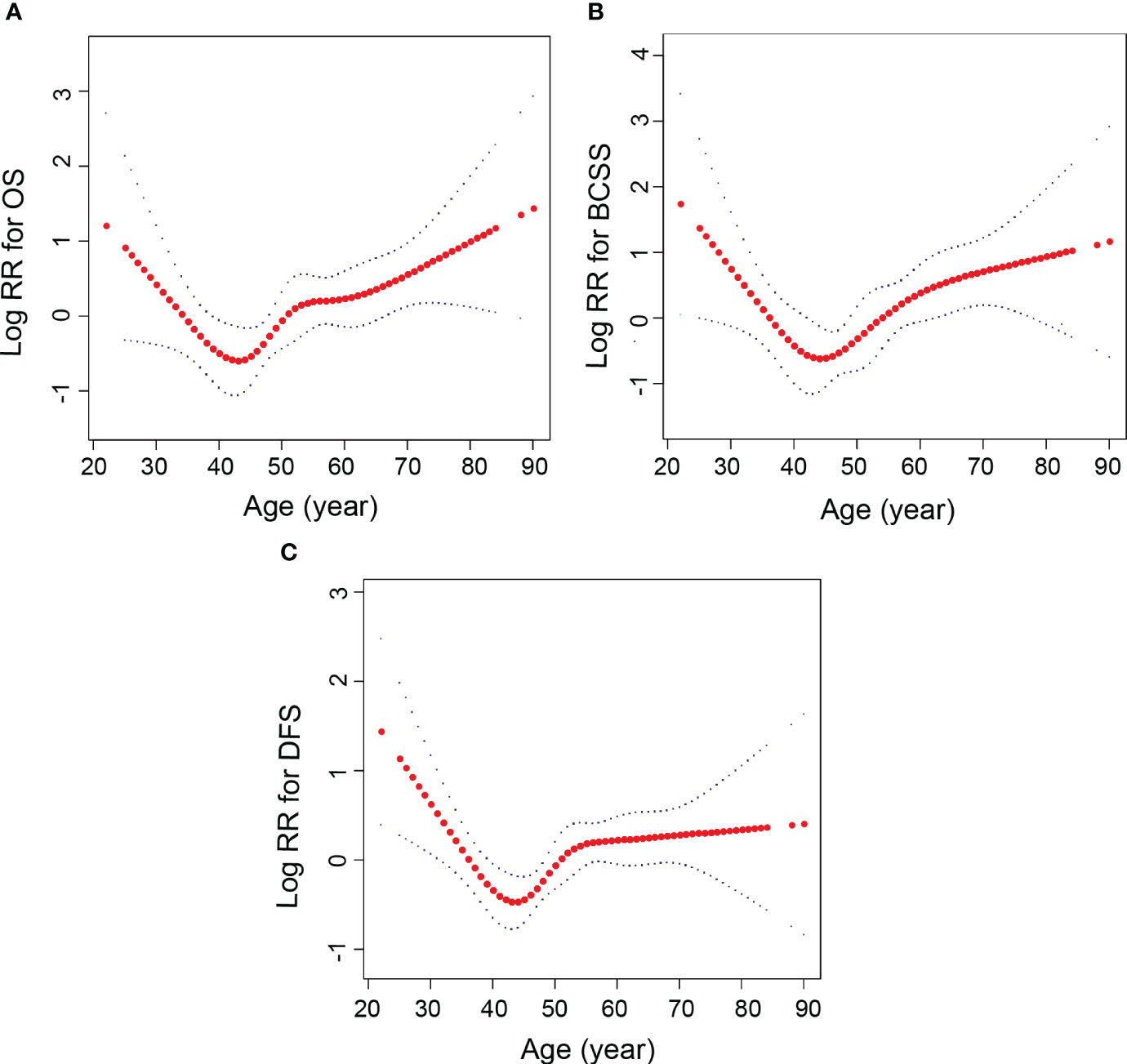
Figure 2 Relationships between age and the probability of OS, BCSS and DFS. (A) OS, (B) BCSS, (C) DFS. Non-linear associations between age and OS, BCSS and DFS were found (P < 0.05). The y-axis of Log RR is defined as the logarithm of the relative risk. Each red dot represents an age. The red line represents the age and the blue line represents its corresponding 95% confidence interval. Adjusted for tumor size, molecular subtype, lymph nodes in the axilla status, operation type, adjuvant therapy, hormonal therapy.
A median follow-up of 4.86 years after breast cancer diagnosis was observed, with an interquartile range of 3.26-6.83 years. Out of the total sample size, 71 patients (6.74%) experienced mortality, with 50 of those cases (70.42%) being attributed to breast cancer. Additionally, 144 patients (13.66%) encountered a recurrence of the condition. Kaplan–Meier outcomes showed that breast cancer patients age group in Q2 had a higher survival than in Q1, Q3 and Q4 patients (P<0.05) (Figure 3). The OS, BCSS, and DFS risks were higher in patients with Q1, Q3, and Q4 ages compared to Q2 (P<0.05). However, OS risk was non-significantly higher in Q1 compared with Q2 (P>0.05), and BCSS risk was non-significantly higher in Q3 compared with Q2 (P>0.05) (Table 3).
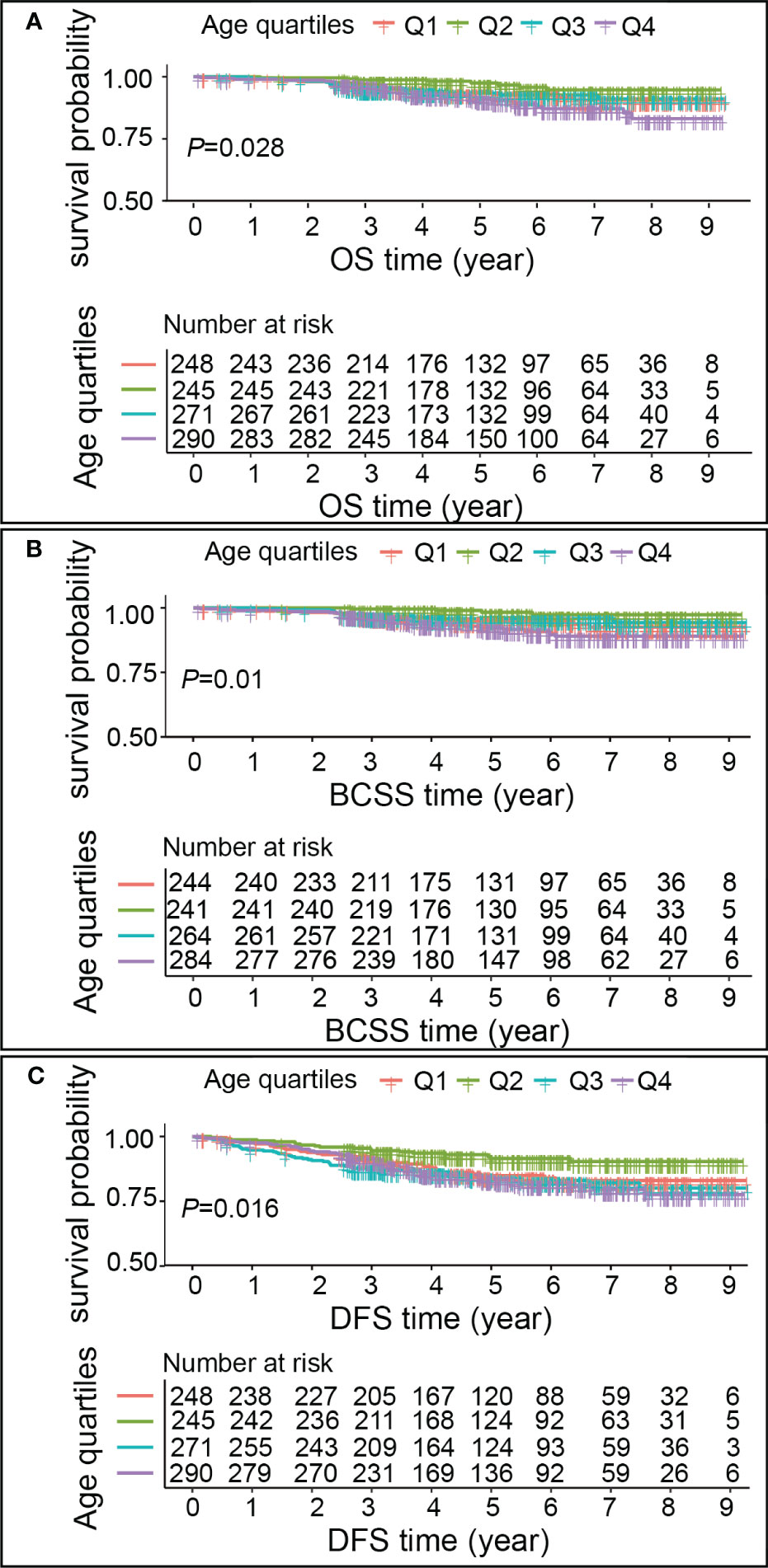
Figure 3 Kaplan–Meier estimates of OS, BCSS, DFS in different age groups. (A) OS, (B) BCSS, (C) DFS. Each graph comprises two components. The upper segment depicts the survival rates of breast cancer patients across various age groups, corresponding to the follow-up time. The horizontal axis represents the follow-up time, while the vertical axis denotes the survival probability. The lower segment of the graph illustrates the number of breast cancer patients in each age group who experienced a risk of survival at a specific follow-up time.
Extensive research has been conducted to investigate the correlation between age at diagnosis and survival rates in breast cancer (6, 10, 11, 16). Findings indicate that younger breast cancer patients are at a higher risk of developing malignancy and experiencing a less favorable prognosis (21, 22). Conversely, older women tend to exhibit indolent tumor characteristics (23), however, their prognosis is compromised by inadequate treatment or excessive intervention (24). Consequently, the prognostic implications of breast cancer remain a subject of controversy contingent upon the age at diagnosis.
In our study, we explored the relationship between diagnosis age and OS、BCSS and DFS. The findings revealed that breast cancer patients age group in Q2 had a higher survival than in Q1, Q3 and Q4 patients in our study.
Regarding OS, a recent study has revealed a significant improvement in the 10-year overall survival (OS) rates among breast cancer patients aged 40 years or older, as compared to those under the age of 40 (25). However, another study did not reveal a statistically significant difference in 5-year OS between the two age groups (26), which may be attributed to the shorter duration of follow-up. Wong et al. reported a U-shaped relationship between age at diagnosis and OS, with a nadir at 45 years (27). Our investigation also revealed a U-shaped association between diagnosis age and OS, with the lowest point at 44 years, consistent with Wong et al. findings. The study demonstrated a decline and subsequent rise in OS among breast cancer patients with advancing age. Additionally, there was a heightened susceptibility to competing background mortality following the lowest age, which exerted a greater impact on overall survival than breast cancer mortality alone. Notably, patients falling within the Q2 category exhibited superior OS compared to those in Q1, Q3, and Q4, with an age range of 44-50 years.
This finding aligns with the results conducted by Chen et al. which revealed that patients aged 40-49 years experienced significantly better OS than other patient groups (28). However, OS were non-significantly higher in Q1compared with Q2, which may be due to the short follow-up period in our study, resulting in a failure of statistical difference between the two.
For BCSS, several studies have suggested that younger age is associated with poorer BCSS in breast cancer patients (29–31). Wong et al. observed a non-linear relationship between age and BCSS, with the highest risk of breast cancer mortality observed in the youngest patients, followed by a linear decrease in hazard at a rate of 5% per year with increasing age (95% CI = 2%-8%; P = 0.001) (27). The risk of breast cancer mortality appears to remain low and relatively constant until approximately 50 years of age. Our investigation revealed a U-shaped relationship between age and BCSS. The study displayed that BCSS decreases with age until the age of diagnosis is 44 years and then shows an increase in breast cancer patients. The different findings of the two studies may be due to the fact that most patients with mastectomy in our study compared to Wong et al. study. Another study showed that patients aged 60-69 years had best BCSS among patients in other groups (28). However, our study findings indicate that individuals aged 44-50 years exhibited superior BCSS, while the BCSS in Q3 was insignificantly higher than that in Q2. It is worth considering that the limited sample size of our study, being conducted solely at a single center, may have influenced these results.
For DFS, the available literature indicates that younger breast cancer patients exhibit inferior DFS outcomes compared to their older counterparts (32, 33). Specifically, studies have demonstrated a statistically significant reduction in DFS among breast cancer patients under the age of 40 in comparison to those aged 40 years and above (25, 34). Furthermore, one study has identified a correlation between age and the formation of an L-shaped DFS curve (27). Notably, the evidence suggests that advancing age at diagnosis is associated with a decreased risk of breast cancer recurrence or mortality. The initial risk decreases rapidly with increasing age at presentation, suggesting that beyond approximately 40 years of age, the effect of age on breast cancer recurrence or death diminishes. In our study, however, age at diagnosis and DFS formed a U-shaped relationship. The initial risk decreases rapidly with increasing age at onset, with the curve showing the lowest value of this risk at approximately 41 years of age. this risk gradually increases with increasing age at onset after 41 years of age. And DFS rates was 13.66% (144 patients). Our result differed from Wong et al, possibly due to the inclusion of patients who were all breast-conserving patients in their study. Breast-conserving patients are generally younger, so an L-shape of age at diagnosis and DFS was observed. It May be due to different characteristics of the population of onset, too.
Our study showed that patients in the intermediate age (Q2) group had better survival compared to patients in the higher (Q3、Q4) and lower (Q1) age groups. Therefore, it suggests that we should not neglect the treatment of the last two. For lower age patients, the prognosis is poorer due to the more aggressive tumors such as TNBC diagnosed at a younger age of the tumor at the diagnosis time. And in the higher age patients, who often refuse standard treatment due to their poor health status, lower treatment acceptance or fear of poor tolerance to toxicity, such patients often do not receive standardized treatment leading to poor prognosis as well. For the higher age quartile in our study, patients had a significantly higher PR-negative tumors and mastectomy rate. In contrast, KI67 levels and adjuvant chemotherapy were significantly lower among the higher age quartile than among the lower quartile. One study showed that OS rates were not lower for young patients, despite their bigger tumors and poorer prognosis, results that patients may have benefitted from more aggressive treatment (35, 36). Additionally, another study has indicated that radiotherapy can improve OS and BCSS in women over 65 years of age with T3N0M0 (37). However, survival times did not differ between patients aged 18-45 years with and without radiotherapy. Therefore, for both groups of patients, clinical treatment plans should be individualized to improve their late survival.
Our study is based on data information from 1054 and is part of a large sample research center. However, it has several limitations. First, the median follow-up time in the study was only 4.86 years, which is not long enough. Second, the study used a retrospective data, which may have introduced sampling bias and incomplete information in case collection. for example, there was no geriatric assessment in our paper; missing histopathological characterization, TNM staging and molecular classification; the type of adjuvant treatment did not include the target treatment, no clear quantification of patients undergoing radiotherapy and chemotherapy; or no clarification of the percentage of patients with luminal tumors who received endocrine therap. Finally, it belongs to a monocentric study. These above may have unknown potential impact on our findings.
Upon examination of the sample size contained within our research center’s database, it has been determined that the prognostic significance of age in relation to OS, BCSS, and DFS is subject to variation based on age. Our analysis has revealed a U-shaped correlation between age and breast cancer outcomes. These findings hold potential for serving as a foundation for future research endeavors focused on formulating personalized treatment strategies for patients across different age groups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to Jianrong Yang, Z2FuZGFuc3VyZ2VyeTIwMTRAMTYzLmNvbQ==.
The studies involving humans were approved by The Ethics Committee of the Guangxi Zhuang Autonomous Region People’s Hospital (Ethics-KY-QT-202205). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YX: Writing – review & editing, Writing – original draft. YD: Writing – original draft, Data curation, Formal Analysis. SW: Data curation, Writing – review & editing. ZH: Writing – review & editing. LL: Supervision, Writing – review & editing. KH: Writing – review & editing. CW: Writing – review & editing. JX: Writing – review & editing. LD: Writing – review & editing. QHZ: Writing – review & editing. JZ: Writing – review & editing. QQZ: Methodology, Supervision, Writing – review & editing. JY: Data curation, Conceptualization, Methodology, Project administration, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Major Project of Science and Technology of Guangxi Zhuang Autonomous Region (grant number: Guike-AA22096018) and the Specific Research Project of Guangxi for Research Bases and Talents (grant number: Guike-AD21220042).
The authors thank all staff of the Guangxi Zhuang Autonomous Region People’s Hospital Information Network Center and Medical Record Information Quality Control Department for assisting with the retrieval of medical records.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
OS, overall survival; BCSS, breast cancer-specific survival; DFS, disease-free survival; RCS, restricted cubic splines; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor 2; IQR, interquartile range; BCS, breast-conserving surgery; M, mastectomy; LN, lymph nodes in the axilla.
1. Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Extermann M, Brain E, Canin B, Cherian M, Cheung K, de Glas N, et al. Priorities for the global advancement of care for older adults with cancer: an update of the International Society of Geriatric Oncology Priorities Initiative. Lancet Oncol (2021) 22(1):e29–36. doi: 10.1016/S1470-2045(20)30473-3
3. Chen W, Zheng R, Baade P, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
4. DeSantis C, Ma J, Gaudet M, Newman L, Miller K, Goding Sauer A, et al. Breast cancer statistics, 2019. CA: Cancer J Clin (2019) 69(6):438–51. doi: 10.3322/caac.21583
5. Hankey B, Miller B, Curtis R, Kosary C. Trends in breast cancer in younger women in contrast to older women. J Natl Cancer Institute Monogr (1994) 16):7–14.
6. Xie Y, Gou Q, Zhang Y, Xie K, Zheng D, Luo C, et al. Association between age at initial diagnosis and post-metastasis mortality among women with recurrent metastatic breast cancer in China. BMC Cancer (2022) 22(1):385. doi: 10.1186/s12885-022-09454-y
7. Ibrahim A, Salem M, Hassan R. Outcome of young age at diagnosis of breast cancer in South Egypt. Gulf J Oncol (2014) 1(15):76–83.
8. Alieldin N, Abo-Elazm O, Bilal D, Salem S, Gouda E, Elmongy M, et al. Age at diagnosis in women with non-metastatic breast cancer: Is it related to prognosis? J Egyptian Natl Cancer Institute (2014) 26(1):23–30. doi: 10.1016/j.jnci.2013.08.005
9. Grybach S, Polishchuk L, Chekhun V. Analysis of the survival of patients with breast cancer depending on age, molecular subtype of tumor and metabolic syndrome. Exp Oncol (2018) 40(3):243–8. doi: 10.31768/2312-8852.2018.40(3):243-248
10. van de Water W, Markopoulos C, van de Velde C, Seynaeve C, Hasenburg A, Rea D, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA (2012) 307(6):590–7. doi: 10.1001/jama.2012.84
11. Ojala K, Meretoja T, Mattson J, Leidenius M. Surgical treatment and prognosis of breast cancer in elderly - A population-based study. Eur J Surg Oncol (2019) 45(6):956–62. doi: 10.1016/j.ejso.2019.01.019
12. Billena C, Wilgucki M, Flynn J, Modlin L, Tadros A, Razavi P, et al. 10-year breast cancer outcomes in women ≤35 years of age. Int J Radiat oncology biology Phys (2021) 109(4):1007–18. doi: 10.1016/j.ijrobp.2020.10.022
13. Wei X, Li X, Xin X, Tong Z, Zhang S. Clinical features and survival analysis of very young (age<35) breast cancer patients. Asian Pacific J Cancer Prev APJCP (2013) 14(10):5949–52. doi: 10.7314/APJCP.2013.14.10.5949
14. Han W, Kang S. Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat (2010) 119(1):193–200. doi: 10.1007/s10549-009-0388-z
15. Zhong W, Tan L, Jiang W, Chen K, You N, Sanders A, et al. Effect of younger age on survival outcomes in T1N0M0 breast cancer: A propensity score matching analysis. J Surg Oncol (2019) 119(8):1039–46. doi: 10.1002/jso.25457
16. Szollár A, Újhelyi M, Polgár C, Oláh E, Pukancsik D, Rubovszky G, et al. A long-term retrospective comparative study of the oncological outcomes of 598 very young (≤35 years) and young (36-45 years) breast cancer patients. Eur J Surg Oncol (2019) 45(11):2009–15. doi: 10.1016/j.ejso.2019.06.007
17. Feng F, Wei Y, Zheng K, Li Y, Zhang L, Wang T, et al. Comparison of epidemiological features, clinicopathological features, and treatments between premenopausal and postmenopausal female breast cancer patients in western China: a retrospective multicenter study of 15,389 female patients. Cancer Med (2018) 7(6):2753–63. doi: 10.1002/cam4.1503
18. Wang K, Ren Y, Li H, Zheng K, Jiang J, Zou T, et al. Comparison of clinicopathological features and treatments between young (≤40 years) and older (>40 years) female breast cancer patients in west China: A retrospective, epidemiological, multicenter, case only study. PloS One (2016) 11(3):e0152312. doi: 10.1371/journal.pone.0152312
19. Jiang Z, Song E, Wang X, Wang H, Wang X, Wu J, et al. Guidelines of chinese society of clinical oncology (CSCO) on diagnosis and treatment of breast cancer (2020 version). Trans Breast Cancer Res (2020) 1(October):1–25. doi: 10.21037/TBCR-2020-2
20. Guideline for HER2 detection in breast cancer, the 2019 version. Zhonghua bing li xue za zhi = Chin J Pathol (2019) 48(3):169–75. doi: 10.3760/cma.j.issn.0529-5807.2019.03.001
21. Derks M, Bastiaannet E, van de Water W, de Glas N, Seynaeve C, Putter H, et al. Impact of age on breast cancer mortality and competing causes of death at 10 years follow-up in the adjuvant TEAM trial. Eur J Cancer (Oxford Engl 1990) (2018) 99:1–8. doi: 10.1016/j.ejca.2018.04.009
22. Bouferraa Y, Haibe Y, Chedid A, Jabra E, Charafeddine M, Temraz S, et al. The impact of young age (< 40 years) on the outcome of a cohort of patients with primary non-metastatic breast cancer: analysis of 10-year survival of a prospective study. BMC Cancer (2022) 22(1):27. doi: 10.1186/s12885-021-09100-z
23. Johnson R, Anders C, Litton J, Ruddy K, Bleyer A. Breast cancer in adolescents and young adults. Pediatr Blood Cancer (2018) 65(12):e27397. doi: 10.1002/pbc.27397
24. Di Lascio S, Tognazzo E, Bigiotti S, Bonollo M, Costa A, Pagani O, et al. Breast cancer in the oldest old (≥ 89 years): Tumor characteristics, treatment choices, clinical outcomes and literature review. Eur J Surg Oncol (2021) 47(4):796–803. doi: 10.1016/j.ejso.2020.10.008
25. Kim H, Kim S, Freedman R, Partridge A. The impact of young age at diagnosis (age <40 years) on prognosis varies by breast cancer subtype: A U. S. SEER Database analysis. Breast (Edinburgh Scotland) (2022) 61:77–83. doi: 10.1016/j.breast.2021.12.006
26. Walsh S, Zabor E, Flynn J, Stempel M, Morrow M, Gemignani M. Breast cancer in young black women. Br J Surg (2020) 107(6):677–86. doi: 10.1002/bjs.11401
27. Wong F, Tham W, Nei W, Lim C, Miao H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun (London England) (2018) 38(1):39. doi: 10.1186/s40880-018-0310-3
28. Chen H, Zhou M, Tian W, Meng K, He H. Effect of age on breast cancer patient prognoses: A population-based study using the SEER 18 database. PloS One (2016) 11(10):e0165409. doi: 10.1371/journal.pone.0165409
29. Fredholm H, Magnusson K, Lindström L, Garmo H, Fält S, Lindman H, et al. Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat (2016) 160(1):131–43. doi: 10.1007/s10549-016-3983-9
30. Dai D, Zhong Y, Wang Z, Yousafzai N, Jin H, Wang X. The prognostic impact of age in different molecular subtypes of breast cancer: a population-based study. PeerJ (2019) 7:e7252. doi: 10.7717/peerj.7252
31. Sun H, Huang W, Ji F, Pan Y, Yang L. Comparisons of metastatic patterns, survival outcomes and tumor immune microenvironment between young and non-young breast cancer patients. Front Cell Dev Biol (2022) 10:923371. doi: 10.3389/fcell.2022.923371
32. Lian W, Fu F, Lin Y, Lu M, Chen B, Yang P, et al. The impact of young age for prognosis by subtype in women with early breast cancer. Sci Rep (2017) 7(1):11625. doi: 10.1038/s41598-017-10414-x
33. Cvetanovic A, Popovic L, Filipovic S, Trifunovic J, Zivkovic N, Matovina-Brko G, et al. Young age and pathological features predict breast cancer outcome - report from a dual Institution experience in Serbia. J BUON (2015) 20(6):1407–13.
34. El Chediak A, Alameddine R, Hakim A, Hilal L, Abdel Massih S, Hamieh L, et al. Younger age is an independent predictor of worse prognosis among Lebanese nonmetastatic breast cancer patients: analysis of a prospective cohort. Breast Cancer (Dove Med Press) (2017) 9:407–14. doi: 10.2147/BCTT.S130273
35. Foo C, Su D, Chong C, Chng H, Tay K, Low S, et al. Breast cancer in young Asian women: study on survival. ANZ J Surg (2005) 75(7):566–72. doi: 10.1111/j.1445-2197.2005.03431.x
36. Abdel-Razeq H, Iweir S, Abdel-Razeq R, Rahman F, Almasri H, Bater R, et al. Differences in clinicopathological characteristics, treatment, and survival outcomes between older and younger breast cancer patients. Sci Rep (2021) 11(1):14340. doi: 10.1038/s41598-021-93676-w
Keywords: breast cancer, overall survival, breast cancer-specific survival, disease-free survival, prognosis, age
Citation: Xie Y, Deng Y, Wei S, Huang Z, Li L, Huang K, Wei C, Xu J, Dong L, Zhang Q, Zhao J, Zou Q and Yang J (2023) Age has a U-shaped relationship with breast cancer outcomes in women: a cohort study. Front. Oncol. 13:1265304. doi: 10.3389/fonc.2023.1265304
Received: 22 July 2023; Accepted: 19 September 2023;
Published: 04 October 2023.
Edited by:
Kate E. Dibble, Dana–Farber Cancer Institute, United StatesReviewed by:
Minghao Wang, Army Medical University, ChinaCopyright © 2023 Xie, Deng, Wei, Huang, Li, Huang, Wei, Xu, Dong, Zhang, Zhao, Zou and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianrong Yang, Z2FuZGFuc3VyZ2VyeTIwMTRAMTYzLmNvbQ==; Quanqing Zou, em91cXVhbnFpbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.