- 1Department of Internal Medicine, King Hussein Cancer Center, Amman, Jordan
- 2School of Medicine, The University of Jordan, Amman, Jordan
Breast cancer continues to be the most common cancer diagnosed among women worldwide. Family history of breast cancer is frequently encountered, and 5-15% of patients may carry inherited pathogenic germline variants, identification of which can be helpful for both; patients themselves and their unaffected close relatives. The availability and affordability of molecular diagnostics, like next generation sequencing (NGS), had resulted in wider adoption of such technologies to detect pathogenic variants of cancer-predisposing genes. International guidelines had recently broadened the indications for germline genetic testing to include much more patients, and also expanded the testing to include multi-gene panels, while some professional societies are calling for universal testing of all newly diagnosed patients with breast cancer, regardless of their age, personal or family history. The risk of experiencing a contralateral breast cancer (CBC) or ipsilateral recurrence, is well known. Such risk is highest with variants like BRCA1 and BRCA2, but less well-studied with other less common variants. The optimal local therapy for women with BRCA-associated breast cancer remains controversial, but tends to be aggressive and may involve bilateral mastectomies, which may not have any survival advantage. Additionally, surgical management of unaffected women, known to carry a pathogenic cancer-predisposing gene, may vary from surveillance to bilateral mastectomies, too. The oncological safety, and the higher satisfaction of unaffected women and patients with new surgical techniques, like the skin-sparing (SSM) and nipple-sparing (NSM) mastectomies, eased up the process of counselling. In this review, we address the oncological safety of less aggressive surgical options for both; patients and unaffected carriers.
1 Introduction
Breast cancer is the most common cancer worldwide and is considered one of the leading causes of cancer-related mortality in both developed and developing countries. In 2020, about 2.3 million women were diagnosed with breast cancer worldwide and 685,000 died of their disease (1). In 2023, almost 300,000 women will be diagnosed with breast cancer in the U.S alone (2). Almost one in five patients with newly diagnosed breast cancer report a family history of breast cancer (3–5). However, smaller fraction may be attributed to an inherited cancer-predisposing gene, mostly in BRCA1 or BRCA2 (6). Based on one meta-analysis, the estimated mean cumulative risk for developing breast cancer by age 70 for carriers of the BRCA1 variant is 57%, whereas the risk for carriers of the BRCA2 variant is a little lower at 49% (7). However, other studies reported higher cumulative breast cancer risk (72%) to age 80 for BRCA1 and 69% for BRCA2 carriers (8). The extent to which other pathogenic variants, like CHEK2, PALB2, ATM, TP53, are associated with breast cancer susceptibility varies significantly (9, 10).
Molecular diagnostics, like next generation sequencing (NGS), is becoming affordable and is widely utilized to detect variants in cancer predisposing genes (11, 12). For patients without BRCA1/2 variants, breast-conserving surgery (BCS), with or without neoadjuvant chemotherapy, followed by radiation therapy, is the treatment of choice for most patients; it offers similar survival to that of mastectomy (13–16). More recent study claimed even better survival outcome with BCS followed by radiation therapy, compared to mastectomy (17–20). In a recent study that used the Surveillance, Epidemiology and End Results (SEER) database which identified 205,788 women with breast cancer diagnosed from 1988 to 2018, patients who underwent BCS and radiotherapy had higher competing risk of breast cancer recurrence (adjusted hazard ratio [HR]: 1.996, 95% CI: 1.925-2.069, p<0.001) and lower competing risk of breast cancer-specific death (BSD) when compared to mastectomy (adjusted HR: 0.584, 95% CI: 0.572-0.597, p<0.001) (21). Another study that also used the SEER database reached almost similar conclusions (22). Additionally, BCS provides better quality of life; a recent study concluded that patients treated with BCS were more satisfied with their cosmetic outcome compared to those who had mastectomy with or without reconstruction (23).
In this review, we discuss surgical treatment options for patients with breast cancer known to have a high-penetrant cancer-predisposing gene, like the BRCA1 and BRCA2, and address the oncological safety of less aggressive surgical options, for both patients and unaffected carriers.
2 The prevalence of germline mutations
Depending on population studied and method of testing, 5-15% of breast cancer patients are carriers of one of the increasingly recognized hereditary predisposition genes. Multiple studies have evaluated the prevalence of pathogenic (PV) or likely pathogenic variants (LPV) in breast cancer patients; majority of such studies were retrospective and from single institution. In a large industry sponsored study, over 35,000 women with breast cancer underwent germline genetic testing with a 25-gene panel. PV/LPVs were detected in 9.3% of women tested; 51.5% were in genes other than BRCA1 or BRCA2, including CHEK2, ATM and PALB2. Rates were significantly higher among younger women aged < 40 years (24). In another study, all women 20 years of age or older diagnosed with breast (or ovarian cancer) in the state of California and Georgia in 2013 and 2014, and reported to the SEER registries were reviewed. Over 77,000 patients with breast cancer were included; almost 25% of them had genetic test results. Pathogenic variants were mostly in BRCA1 (3.2%), BRCA2 (3.1%), CHEK2 (1.6%), PALB2 (1.0%) and ATM (0.7%) (25).
We recently reported our experience on 1,310 non-Western patients diagnosed with breast cancer. Patients were tested as per the National Comprehensive Cancer Network (NCCN) guidelines. Age ≤ 45 years was the most common indication for testing, while positive family history of breast, ovarian, pancreatic or prostate cancers, and triple-negative disease were among other frequent indications. Among the whole group, 184 (14.0%) patients had PV/LPVs; only 90 (48.9%) were in BRCA1 or BRCA2, while 94 (51.1%) others had pathogenic variants in other genes; mostly in APC, TP53, CHEK2 and PALB2. Mutation rates were higher among patients with positive family history (p=0.009); especially if they were 50 years or younger at the time of breast cancer diagnosis (p<0.001). Patients with triple-negative disease had relatively higher rate (17.5%) and mostly in BRCA1/2 genes (71.4%) (26).
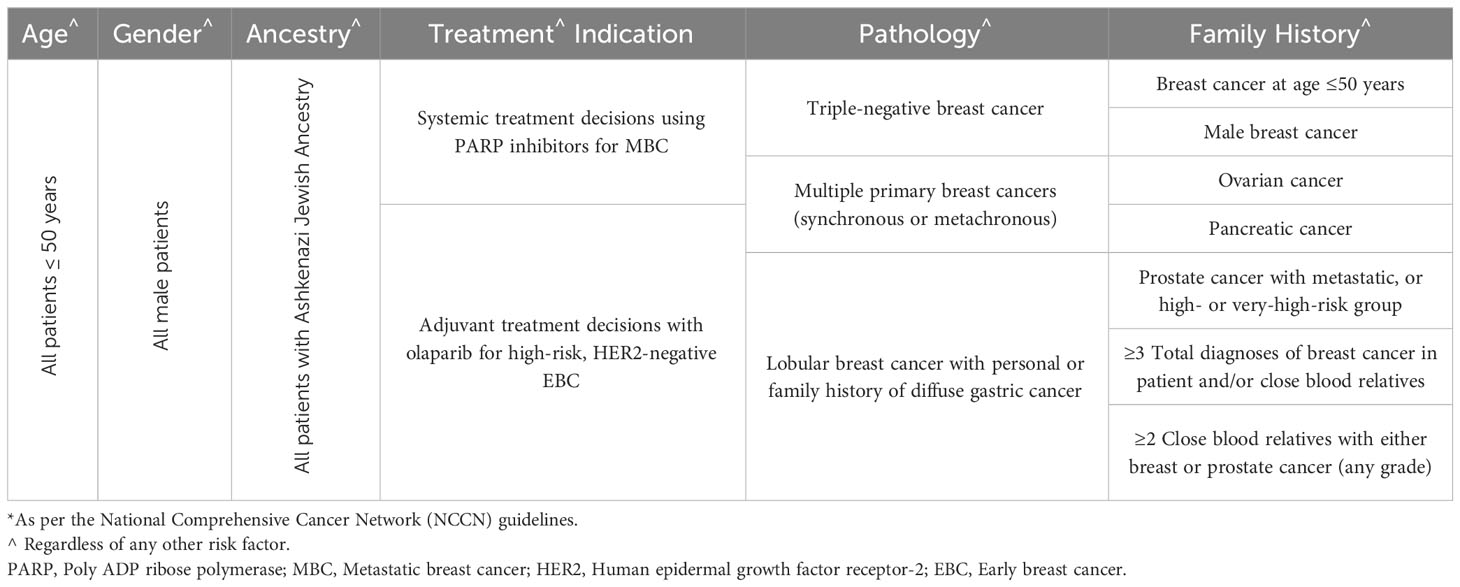
3 Patients at risk
Several international guidelines, including the American Society of Clinical Oncology (ASCO) (27), the NCCN (28), the American Society for Radiation Oncology (ASTRO) (29), and the European Society for Medical Oncology (ESMO) (30), attempted to select patients at higher risk for carrying PV/LPVs. Most of these guidelines were based on consensus, and not a result of randomized clinical trials. The NCCN guidelines are updated frequently and often such updates might not be closely followed by practicing community oncologists. The most recent criteria were expanded to include older patients (50 instead of 40 years), and all patients with triple negative disease regardless of their age (Table 1). However, the recent introduction of poly ADP ribose polymerase (PARP) inhibitors to treat patients with BRCA1/2 variants resulted in more expansion of the testing guidelines to include all patients who may potentially benefit from certain anti-cancer therapy used in the setting of BRCA1/2 variants. A randomized phase-3 trial (OlympiAD) showed that olaparib, a PARP inhibitor, when compared to palliative chemotherapy, in human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer patients, with pathogenic germline BRCA1/2 variants, was associated with better progression-free survival (PFS) (31). Similar results were reported using talazoparib, another PARP inhibitor (32). More recently, PARP inhibitors were also tried in the setting of high-risk early-stage breast cancer with germline pathogenic BRCA1/2 variants (Olympia trial). When compared to placebo, adjuvant olaparib for one year was associated with significant improvement in distant (dDFS) and invasive (iDFS), disease-free survivals, and possibly overall survival (OS), too (33).
Given this expansion in the indications for genetic testing, it’s estimated that almost two-thirds of breast cancer patients will have at least one indication for genetic testing. However, many studies had shown that the current testing guidelines are restrictive and only a fraction of eligible patients are tested (34, 35). Additionally, several other studies had shown that the prevalence of PV/LPVs in the other non-tested patients are high enough to justify testing all patients in a testing approach known as “universal testing” (36). This approach was adopted by the American Society of Breast Surgeons, which called for testing all breast cancer patients regardless of their age, personal or family history of cancer.37
4 Surgery for the diseased breast
Options for the diseased breast varies and can range from BCS (followed by radiation therapy) to many forms of mastectomies. Each option has its own advantages and obviously some potential setbacks (37).
4.1 BCS versus mastectomy
Tumor’s characteristics, including size and site, and patient’s characteristics, like breast size, may determine the extent of surgery; mastectomy versus BCS, regardless of the existence of BRCA1/2 variants. Patients with newly diagnosed early-stage breast cancer who carry a PV/LPV in BRCA1 or BRCA2 are often advised to undergo mastectomy, which can be skin-sparing or nipple-sparing. BCS was never compared, in a randomized study, to mastectomy in this setting. Much of our knowledge, however, is based on small retrospective studies and pooled analysis of such studies.
In one systematic review that included 3,807 patients in 23 observational studies, differences in outcomes between mastectomy and BCS among breast cancer patients with BRCA1/2 variants were analyzed. Patients were young with a median age at breast cancer diagnosis of 41 years; 2,200 (57.7%) had BRCA1 variants while 1,212 (31.8%) had BRCA2. BCS was performed on 2,157 (56.7%) while 1,408 (41.5%) patients had mastectomy. Risk of loco-regional relapse (LRR) was significantly higher in the BCS group (HR: 4.54, 95% CI: 2.77-7.42, p<0.001). However, disease-specific recurrence (HR: 1.58, 95% CI: 0.79-3.15, p=0.200), disease recurrence (HR: 1.16, 95% CI: 0.78-1.72, p=0.470), contralateral breast cancer (HR: 1.51, 95% CI: 0.44-5.11, p=0.510), and death (HR: 1.10, 95% CI: 0.72-1.69, p= 0.660) were not higher in the group who underwent BCS (38).
In another systematic review of 18 studies that compared BCS and mastectomy, OS at 5, 10, and 15 years were comparable (83%, 86.0%, and 83.2%) with mastectomy, and with BCS (88.7%, 89.0% and 83.6%), respectively. However, the ipsilateral breast cancer recurrence rates at 5, 10, and 15 years were significantly lower with mastectomy (3.4%, 4.9%, and 6.4%, respectively) than with BCS group (8.2%, 15.5%, and 23%, respectively). Researchers concluded that BCS can be offered for select patients with BRCA1/2 mutation after proper counseling and with intensive follow-up (39).
Patient’s satisfaction for cosmetic results should always be balanced against oncological safety. The need for adjuvant radiation therapy following BCS and the possible increase in the risk of complications that may lead to a possible subsequent mastectomy with immediate breast reconstruction should always be addressed with patients when considering BCS versus mastectomy.
4.2 BCS in BRCA1/2 vs sporadic breast cancer
Several other studies had attempted to answer the question of the oncological safety of BCS by comparing the outcomes of patients with BRCA1/2 mutation to a control group of patients with sporadic breast cancer. In one retrospective study that reviewed the clinical and pathological records of 501 patients who underwent BCS in China between 2005 and 2018, 63 patients had BRCA1 or BRCA2 variants. After a median follow-up of 61 months for carriers and 70 months for noncarriers, the DFS (p=0.424) and the OS (p=0.173) were not significantly different. Interestingly, there was no difference between the two groups in ipsilateral breast tumor recurrence (p=0.348). However, CBC was significantly worse in carriers; 9.5% versus 0.68%, p<0.001 (40). No significant difference in ipsilateral-breast tumor recurrence (IBTR) was also reported in another Chinese study (41).
In another meta-analysis that included 13 studies with 701 BRCA-mutation carriers and 4,788 controls, IBTR was significantly higher in BRCA-mutation carriers (RR: 1.589; 95% CI 1.247-2.024; p<0.001). As expected, risk of recurrence increased as the follow up increases; (RR: 1.601; 95% CI 1.201-2.132) with 10 or more years of follow up and (RR: 1.505; 95% CI 1.184-1.913) with median follow up of 7 or more years. However, overall survival in three included cohort studies found no evidence to suggest a deterioration in OS in patients with BCS (38). Multiple other studies had confirmed the high rate of IBTR in BRCA1/2 carriers treated with BCS compared to matched controls with sporadic breast cancer (42).
5 Risk-reducing mastectomy
Compared with non-carriers, patients with BRCA1/2 mutation have a higher risk for contralateral breast cancer with BRCA1-mutation is associated with higher risk compared to those with BRCA2. Several studies had compared outcomes of women who underwent risk-reducing mastectomies with those who opted to continue on surveillance (43). Surgical decision-making process is quite complex and should take into consideration several risk-modifying factors including age at first breast cancer diagnosis, the use of adjuvant endocrine therapy and planned, or already performed oophorectomy. Younger patients who have not received adjuvant endocrine therapy or undergone oophorectomy, might be at higher risk for ipsilateral breast cancer recurrence (IBCR) and CBC, and thus might benefit from a more aggressive surgical approach. Women with strong family history, like those with family member diagnosed or died, with breast cancer at younger age, tend to choose mastectomy, while younger patients aged 30 or less are more likely to choose surveillance. Anxiety and fear of getting a second breast cancer are significantly lower following RRM, which impacts positively on the quality of life of such patients (44). Several surgical options are available to manage the contralateral breast but mostly nipple-sparing, skin-sparing mastectomy, which is usually associated with excellent cosmetic and oncological results.
5.1 Skin-sparing and nipple-sparing mastectomies: how effective and how safe?
In skin-Sparing mastectomy (SSM), a radial, axillary or an inframammary incision is utilized, much of the breast skin is spared but carefully dissected off breast tissue with removal of the entire breast glands to create a pocket that facilitates immediate breast reconstruction with implant or autologous graft. Nipple-sparing mastectomy (NSM) is similar to SSM, but the nipple-areola complex (NAC) is preserved, as well (45, 46). Both techniques are increasingly utilized in clinical practice and are associated with superior cosmetic outcomes and better patients’ satisfaction compared to mastectomy (47–50). In addition to the usual complication encountered with other types of breast reconstructions, NAC necrosis is the main complication of NSM and tends to be higher among smokers, obese and those with large breasts, and following radiotherapy (51, 52).
However, one of the main concerns associated with both SSM and NSM is the risk of local breast cancer recurrence at the NAC secondary to occult nipple involvement or a second new primary cancer in the retained breast tissue (53–57). Such risk is obviously higher among patients who carry a pathogenic germline breast cancer predisposing genes. Breast cancer recurrence at the NAC, often referred to as “oncologic safety” can be a concern. Several studies, mostly retrospective ones, attempted to answer the question in two groups; the affected patients who underwent contralateral prophylactic surgery, and among unaffected carriers.
The oncologic safety of SSM and NSM was initially studied in the setting of sporadic breast cancer. In a 2010 meta-analysis of 9 studies that enrolled 3,739 patients, rates of local recurrence in SSM did not differ significantly from those who underwent non-SSM (53). Another meta-analysis of 20 studies involving 5,594 women with early-stage breast cancer did not detect any differences in local recurrence, DFS or OS between those receiving SSM compared to those receiving conventional mastectomy without reconstruction (54). Another large systematic review of 17 retrospective studies included 7,107 patients; majority (85.4%) of them had the procedure for invasive carcinoma. Following a median follow up of 48 months (range 25-94), the mean rates of local recurrence was 5.4% (0.9-11.9), and recurrence involving the NAC was 1.3% (0-4.9) (55). Another large retrospective study from Korea that involved 944 patients, reached similar conclusions. Multicentricity or multifocality, negative hormone receptor, or HER2-positive subtype, high histologic grade, and extensive intraductal component, were independently associated with cancer recurrence at the NAC after NSM (56).
Several other studies addressed issues related to oncologic safety among patients harboring a pathogenic cancer-predisposing gene. In one study, researchers examined tissues from 62 NACs from 33 women (25 BRCA1, 8 BRCA2) who underwent mastectomy between 1987 and 2009 at Mayo Clinic. Atypical hyperplasia, carcinoma in situ, or invasive carcinoma were not found in any of the 33 prophylactic mastectomy specimens performed. However, 2 (7%) of the 29 breasts with cancer, and available tissue, had malignant findings, and 1 (3%) had atypia in the NAC (57).
More recently, Rocco et al. reviewed 9 studies reported on the incidence of primary breast cancer following NSM in BRCA1/2 unaffected carriers who undergo prophylactic bilateral mastectomy. From an oncological point of view, NSM appears to be a safe option for BRCA mutation carriers, with low reported rates of new breast cancers. Additionally, the procedure was associated with low rates of postoperative complications, and high levels of satisfaction and postoperative quality of life (58). In another study, researchers reviewed 114 NSM performed from 2008 to 2019 on patients with breast cancer in 105 BRCA1/2 carriers (56 BRCA1, 47 BRCA2, and two women with both mutations). Five (4.4%) patients had positive nipple margins on final pathology and all underwent nipple excision. Systemic therapy was offered to 76% patients; 65 (62%) with chemotherapy and 48 (46%) received endocrine therapy. Patients were followed up for a median of 70 months (range 15-150), no patient had a recurrence in the retained NAC or at the site of a nipple excised for a positive margin. The rate of locoregional recurrence outside the nipple and distant recurrence were also low at 2.6% and 3.8%, respectively (59).
In another study from 9 major institutions in the US, researchers retrospectively reviewed their experience on 548 prophylactic NSM performed in a cohort of 346 patients with BRCA1 or BRCA2 variants. Unilateral risk-reducing NSM secondary to a concurrent, or prior cancer in the contralateral breast, were performed on 144 (41.6%) patients, while bilateral prophylactic NSM were performed on 202 (58.4%) patients. With median and mean follow-up of 34 and 56 months, respectively, no ipsilateral breast cancers were reported after prophylactic NSM. Similarly, breast cancer did not occur in any patients undergoing bilateral risk-reducing NSM (60).
6 Moderate penetrance genes
The recent advances in NGS technologies resulted in an increase use of multigene panel testing and enabled sequencing of BRCA1/2 concomitantly with many additional genes. Recent studies suggest that other cancer predisposing genes, including PALB2, ATM, CHEK2, TP53, RAD51C, RAD51D, and many others, confer variable risks of breast and other cancers (61–63). Rates of such variants are very variable, depending on population studied and testing method utilized. Figure 1 illustrates an example of such variation in a study that used a 25-multi gene panel, and enrolled over 35,000 patients; half of them were non-Western with different ethnic background (24), and a recently published study from our group that enrolled over 1,000 Arab breast cancer patients utilizing a multi-gene panel, too (26). Appropriate counselling and data-driven risk management with appropriate plans for risk-reducing intervention or surveillance for patients with breast cancer and unaffected individuals, are highly needed (64–67).
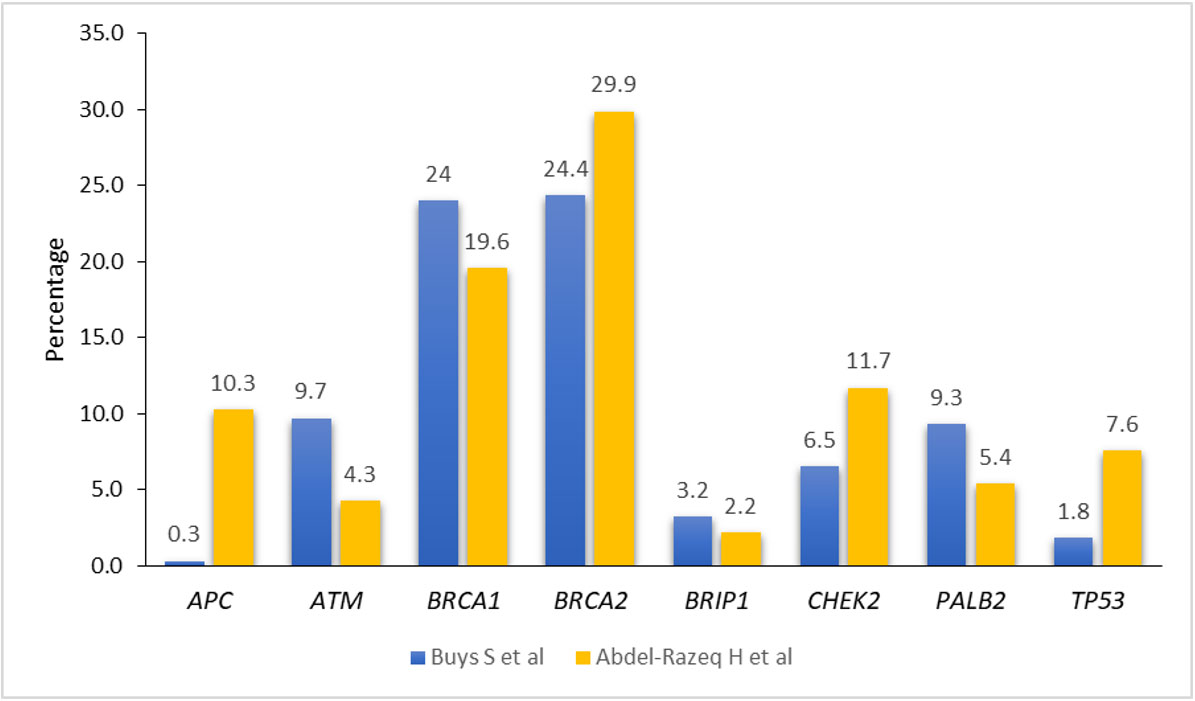
Figure 1 Prevalence of pathogenic/likely pathogenic variants among breast cancer patients in different ethnic groups.
6.1 PALB2
Pathogenic/likely pathogenic PALB2 variants is associated with high risk for breast cancer, with studies showing a life-time risk of 40-60% (68). One multi-national study that analyzed data from 524 families with PALB2 PVs in 21 countries concluded that the estimated relative risk (RR) of breast cancer was 7.18 (95% CI, 5.82- 8.85; p=6.5×10-76) (69). A large family-based study reached similar conclusions (70). Additionally, patients harboring PVs of PALB2 are at higher risk for ovarian cancer and Fanconi anemia which is inherited in an autosomal recessive manner (71). The NCCN guidelines recommend annual mammogram beginning at age 30 years with consideration for breast MRI. Risk-reducing surgery should also be discussed with the patient.
6.2 CHEK2
The rate of CHEK2 germline mutation is higher in certain ethnic groups like the Northern European countries. Certain variants in the CHEK2 gene (I157T and c.1100delC) are associated with higher risk for breast cancer (72). The cumulative lifetime risk ranges from 28% to 37% (73). While no data available on the benefit of RRM, annual mammogram and breast MRI once a year starting at 40 years of age, are highly recommended. Carriers of CHEK2 pathogenic variants are at higher risk for colon, prostate, bladder, kidney and thyroid cancers, more so with c1100delC variant (74).
6.3 TP53
The P53 is a tumor suppressor gene that prevents the development of cancer. Patients with germline mutation, Li-Fraumeni syndrome, are at risk for early-onset breast cancer, sarcomas, and other cancers in children and young adults (75, 76). Following cellular stress, like radiation therapy (RT)-associated cell injury, P53 provides the cell with ability to repair DNA damage through multiple downstream repair pathways. In a small series of 8 patients with breast cancer and germline TP53 pathogenic variant, 6 of them were treated with radiation therapy following surgery, ipsilateral breast recurrences were reported in three and contralateral breast cancers in three more. RT-induced cancers were reported in two, in addition to three new primary cancers. On the other hand, only one contralateral breast cancer occurred among patients who had not received radiation therapy (77). Several other case reports of RT-associated malignancies supported the recommendation against RT in patients with TP53 (78–82). As such, mastectomy should be recommended to possibly avoid radiation therapy following BCS.
6.4 ATM
Heterozygous pathogenic variant in ATM is associated with a 13-33% cumulative lifetime risk for breast cancer (83, 84). Risk-reducing mastectomy is not recommended for carriers; however, it might be considered based on personal and family history. No apparent risk of post-surgery radiation therapy on patients with pathogenic variant. Mammogram with consideration of breast MRI is recommended yearly starting at age 40 years.
7 Conclusions
Germline genetic testing is currently offered for majority of patients with breast cancer, as it informs both preventive and treatment decisions. Available data support the oncologic safety of more conservative surgical approaches in breast cancer patients even with the highest penetrant germline variants like BRCA1 and BRCA2. Unaffected carriers may also be offered active surveillance should they choose so. However, evidence to guide clinical decisions on less frequent, mild to moderate risk variants, is lacking.
Author contributions
HA-R: Conceptualization, Data curation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The author would like to acknowledge Mrs. Hira Bani Hani, Mrs. Alice Haddadin and Ms. Doaa J. AlSadi for their help in preparing the manuscript.
Conflict of interest
The author declares no commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Breast Cancer Research Foundation. . Available at: https://www.bcrf.org/breast-cancer-statistics-and-resources/ (Accessed 04 Jun 2023).
3. Ho PJ, Lim EH, Hartman M, Wong FY, Li J. Breast cancer risk stratification using genetic and non-genetic risk assessment tools for 246,142 women in the UK Biobank. Genet Med (2023) 25(10):100917. doi: 10.1016/j.gim.2023.100917
4. Zhang Y, Wang Q-L, Zeng E, He W, Czene K. Analysis of breast cancer family history, estrogen receptor status, and breast cancer outcomes in Sweden. JAMA Netw Open (2023) 6(6). doi: 10.1001/jamanetworkopen.2023.18053
5. Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet (2001) 358(9291):1389–99. doi: 10.1016/s0140-6736(01)06524-2
6. Edaily S, Abdel-Razeq H. Management strategies of breast cancer patients with BRCA1 and BRCA2 pathogenic germline variants. OncoTargets Ther (2022) 15:815–26. doi: 10.2147/ott.s369844
7. Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol (2007) 25(11):1329–33. doi: 10.1200/jco.2006.09.1066
8. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips K-A, Mooij TM, Roos-Blom M-J, et al. Risks of breast, ovarian, and contralateral breast cancer for brca1 and brca2 mutation carriers. JAMA (2017) 317(23):2402. doi: 10.1001/jama.2017.7112
9. Seal S, Thompson D, Renwick A, Elliott A, Kelly P, Barfoot R, et al. Truncating mutations in the fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet (2006) 38(11):1239–41. doi: 10.1038/ng1902
10. Rahman N, Seal S, Thompson D, Kelly P, Renwick A, Elliott A, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet (2006) 39(2):165–7. doi: 10.1038/ng1959
11. Yoshida R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and Prognosis. Breast Cancer (2020) 28(6):1167–80. doi: 10.1007/s12282-020-01148-2
12. Zelli V, Compagnoni C, Cannita K, Capelli R, Capalbo C, Di Vito Nolfi M, et al. Applications of next generation sequencing to the analysis of Familial Breast/ovarian cancer. High-Throughput (2020) 9(1):1. doi: 10.3390/ht9010001
13. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med (2002) 347(16):1227–32. doi: 10.1056/nejmoa020989
14. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomized trials. Lancet (2005) 366(9503):2087–106. doi: 10.1016/s0140-6736(05)67887-7
15. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med (2002) 347(16):1233–41. doi: 10.1056/nejmoa022152
16. Litière S, Werutsky G, Fentiman IS, Rutgers E, Christiaens M-R, Van Limbergen E, et al. Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20-year follow-up of the EORTC 10801 Phase 3 randomized trial. Lancet Oncol (2012) 13(4):412–9. doi: 10.1016/s1470-2045(12)70042-6
17. Wrubel E, Natwick R, Wright GP. Breast-conserving therapy is associated with improved survival compared with mastectomy for early-stage breast cancer: A propensity score matched comparison using the National Cancer Database. Ann Surg Oncol (2020) 28(2):914–9. doi: 10.1245/s10434-020-08829-4
18. De Boniface J, Frisell J, Bergkvist L, Andersson Y. Breast-conserving surgery followed by whole-breast irradiation offers survival benefits over mastectomy without irradiation. Br J Surg (2018) 105(12):1607–14. doi: 10.1002/bjs.10889
19. Hofvind S, Holen Å, Aas T, ROman M, Sebuødegård S, Akslen LA. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol (EJSO) (2015) 41(10):1417–22. doi: 10.1016/j.ejso.2015.07.002
20. van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, et al. 10-year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: A population-based study. Lancet Oncol (2016) 17(8):1158–70. doi: 10.1016/s1470-2045(16)30067-5
21. Ke S, Wang W, Li B, Feng X, Yan D, Liu J. Superior survival for breast-conserving therapy over mastectomy in patients with breast cancer: A population-based SEER database analysis across 30 years. Front Oncol (2023) 12:1032063. doi: 10.3389/fonc.2022.1032063
22. Xiang W, Wu C, Wu H, Fang S, Liu N, Yu H. Survival comparisons between breast conservation surgery and mastectomy followed by postoperative radiotherapy in stage I–III breast cancer patients: Analysis of the surveillance, epidemiology, and end results (SEER) program database. Curr Oncol (2022) 29(8):5731–47. doi: 10.3390/curroncol29080452
23. Vohra LM, Javed SM, Jabeen D, Abidi SS, Tahseen MU. Quality of life of breast cancer survivors: A comparison of breast conserving surgery versus total mastectomy with and without immediate reconstruction – a prospective cohort study. Ann Med Surg (Lond) (2023) 85(5):1513–7. doi: 10.1097/ms9.0000000000000607
24. Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, Brown KL, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer (2017) 123(10):1721–30. doi: 10.1002/cncr.30498
25. Kurian AW, Ward KC, Howlader N, Deapen D, Hamilton AS, Mariotto A, et al. Genetic testing and results in a population-based cohort of breast cancer patients and Ovarian Cancer patients. J Clin Oncol (2019) 37(15):1305–15. doi: 10.1200/jco.18.01854
26. Abdel-Razeq H, Abujamous L, Al-Azzam K, Abu-Fares H, Bani Hani H, Alkyam M, et al. Guideline-based, multi-gene panel germline genetic testing for at-risk patients with breast cancer. Breast Cancer: Targets Ther (2023) 15:1–10. doi: 10.2147/bctt.s394092
27. Tung NM, Boughey JC, Pierce LJ, Robson ME, Bedrosian I, Dietz JR, et al. Management of hereditary breast cancer: American society of clinical oncology, American society for radiation oncology, and society of surgical oncology guideline. J Clin Oncol (2020) 38(18):2080–106. doi: 10.1200/jco.20.00299
28. National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Available at: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503 (Accessed 18 July, 2023).
29. Trombetta MG, Dragun A, Mayr NA, Pierce LJ. Astro radiation therapy summary of the ASCO-ASTRO-SSO guideline on management of hereditary breast cancer. Pract Radiat Oncol (2020) 10(4):235–42. doi: 10.1016/j.prro.2020.04.003
30. Sessa C, Balmaña J, Bober SL, Cardoso MJ, Colombo N, Curigliano G, et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO clinical practice guideline. Ann Oncol (2023) 34(1):33–47. doi: 10.1016/j.annonc.2022.10.004
31. Robson ME, Tung N, Conte P, Im S-A, Senkus E, Xu B, et al. Olympiad final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol (2019) 30(4):558–66. doi: 10.1093/annonc/mdz012
32. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee K-H, Gonçalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann Oncol (2020) 31(11):1526–35. doi: 10.1016/j.annonc.2020.08.2098
33. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant Olaparib for patients with brca1- or brca2-mutated breast cancer. N Engl J Med (2021) 384(25):2394–405. doi: 10.1056/nejmoa2105215
34. Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol (2017) 35(34):3800–6. doi: 10.1200/jco.2017.73.6314
35. Manchanda R, Blyuss O, Gaba F, Gordeev VS, Jacobs C, Burnell M, et al. Current detection rates and time-to-detection of all identifiable BRCA carriers in the Greater London population. J Med Genet (2018) 55(8):538–45. doi: 10.1136/jmedgenet-2017-105195
36. Beitsch PD, Whitworth PW, Hughes K, Patel R, Rosen B, Compagnoni G, et al. Underdiagnosis of hereditary breast cancer: Are genetic testing guidelines a tool or an obstacle? J Clin Oncol (2019) 37(6):453–60. doi: 10.1200/jco.18.01631
37. Sibilio A, Curcio A, Toesca A, Rossi EM, Corso G. Local treatment in patients with hereditary breast cancer: Decision-making process in low-, moderate-, high-penetrance pathogenic germline mutation carriers. Curr Opin Oncol (2022) 34(6):614–22. doi: 10.1097/cco.0000000000000872
38. Davey MG, Davey CM, Ryan ÉJ, Lowery AJ, Kerin MJ. Combined breast conservation therapy versus mastectomy for BRCA mutation carriers – a systematic review and meta-analysis. Breast (2021) 56:26–34. doi: 10.1016/j.breast.2021.02.001
39. Co M, Liu T, Leung J, Li CH, Tse T, Wong M, et al. Breast conserving surgery for BRCA mutation carriers—a systematic review. Clin Breast Cancer (2020) 20(3):e244-e250. doi: 10.1016/j.clbc.2019.07.014
40. Ye F, Huang L, Lang G, Hu X, Di G, Shao Z, et al. Outcomes and risk of subsequent breast events in breast-conserving surgery patients with BRCA1 and BRCA2 mutation. Cancer Med (2020) 9(5):1903–10. doi: 10.1002/cam4.2836
41. Cao W, Xie Y, He Y, Li J, Wang T, Fan Z, et al. Risk of ipsilateral breast tumor recurrence in primary invasive breast cancer following breast-conserving surgery with BRCA1 and BRCA2 mutation in China. Breast Cancer Res Treat (2019) 175(3):749–54. doi: 10.1007/s10549-019-05199-8
42. Garcia-Etienne CA, Barile M, Gentilini OD, Botteri E, Rotmensz N, Sagona A, et al. Breast-conserving surgery in BRCA1/2 mutation carriers: Are we approaching an answer? Ann Surg Oncol (2009) 16(12):3380–7. doi: 10.1245/s10434-009-0638-7
43. Hartmann LC, Lindor NM. The role of risk-reducing surgery in hereditary breast and ovarian cancer. N Engl J Med (2016) 374(5):454–68. doi: 10.1056/nejmra1503523
44. Franceschini G, Masetti R. What the surgeons should know about the bilateral prophylactic mastectomy in BRCA mutation carriers. Eur J Breast Health (2019) 15(2):135–6. doi: 10.5152/ejbh.2019.4651
45. Galimberti V, Vicini E, Corso G, Morigi C, Fontana S, Sacchini V, et al. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast (2017) 34 Suppl 1(Suppl 1):S82-S84. doi: 10.1016/j.breast.2017.06.034
46. Sisco M, Yao KA. Nipple-sparing mastectomy: A contemporary perspective. J Surg Oncol (2016) 113(8):883–90. doi: 10.1002/jso.24209
47. Jabor MA, Shayani P, Collins DR, Karas T, Cohen BE. Nipple-areola reconstruction: Satisfaction and clinical determinants. Plast Reconstr Surg (2002) 110(2):457–63. doi: 10.1097/00006534-200208000-00013
48. Wei CH, Scott AM, Price AN, Miller HC, Klassen AF, Jhanwar SM, et al. Psychosocial and sexual well-being following nipple-sparing mastectomy and Reconstruction. Breast J (2016) 22(1):10–7. doi: 10.1111/tbj.12542
49. van Verschuer VMT, Mureau MAM, Gopie JP, Vos EL, Verhoef C, Menke-Pluijmers MBE, et al. Patient satisfaction and nipple-areola sensitivity after bilateral prophylactic mastectomy and immediate implant breast reconstruction in a high breast cancer risk population. Ann Plast Surg (2016) 77(2):145–52. doi: 10.1097/sap.0000000000000366
50. Didier F, Radice D, Gandini S, Bedolis R, Rotmensz N, Maldifassi A, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, Body Image and sexuality? Breast Cancer Res Treat (2008) 118(3):623–33. doi: 10.1007/s10549-008-0238-4
51. Mesdag V, Régis C, Tresch E, Chauvet M-P, Boulanger L, Collinet P, et al. Nipple sparing mastectomy for breast cancer is associated with high patient satisfaction and safe oncological outcomes. J Gynecol Obstet Hum Reprod (2017) 46(8):637–42. doi: 10.1016/j.jogoh.2017.07.003
52. Mota BS, Riera R, Ricci MD, Barrett J, de Castria TB, Atallah ÁN, et al. Nipple- and areola-sparing mastectomy for the treatment of breast cancer. Cochrane Database Syst Rev (2016) 11(11):CD008932. doi: 10.1002/14651858.cd008932.pub3
53. Lanitis S, Tekkis PP, Sgourakis G, Dimopoulos N, Al Mufti R, Hadjiminas DJ. Comparison of skin-sparing mastectomy versus non–skin-sparing mastectomy for breast cancer. Ann Surg (2010) 251(4):632–9. doi: 10.1097/sla.0b013e3181d35bf8
54. De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple–areolar recurrence in the setting of nipple-sparing mastectomy: A meta-analysis and systematic review. Ann Surg Oncol (2015) 22(10):3241–9. doi: 10.1245/s10434-015-4739-1
55. Zaborowski AM, Roe S, Rothwell J, Evoy D, Geraghty J, McCartan D, et al. A systematic review of oncological outcomes after nipple-sparing mastectomy for breast cancer. J Surg Oncol (2022) 127(3):361–8. doi: 10.1002/jso.27115
56. Wu Z-Y, Kim H-J, Lee J-W, Chung I-Y, Kim J-S, Lee S-B, et al. Breast cancer recurrence in the nipple-areola complex after nipple-sparing mastectomy with immediate breast reconstruction for invasive breast cancer. JAMA Surg (2019) 154(11):1030. doi: 10.1001/jamasurg.2019.2959
57. Reynolds C, Davidson JA, Lindor NM, Glazebrook KN, Jakub JW, Degnim AC, et al. Prophylactic and therapeutic mastectomy in BRCA mutation carriers: Can the nipple be preserved? Ann Surg Oncol (2011) 18(11):3102–9. doi: 10.1245/s10434-011-1908-8
58. Rocco N, Montagna G, Criscitiello C, Nava MB, Privitera F, Taher W, et al. Nipple sparing mastectomy as a risk-reducing procedure for BRCA-mutated patients. Genes (2021) 12(2):253. doi: 10.3390/genes12020253
59. Webster AJ, Shanno JN, Santa Cruz HS, Kelly BN, Garstka M, Henriquez A, et al. Oncologic safety of nipple-sparing mastectomy for breast cancer in BRCA gene mutation carriers: Outcomes at 70 months median follow-up. Ann Surg Oncol (2023) 30(6):3215–22. doi: 10.1245/s10434-022-13006-w
60. Jakub JW, Peled AW, Gray RJ, Greenup RA, Kiluk JV, Sacchini V, et al. Oncologic safety of prophylactic nipple-sparing mastectomy in a population with BRCA mutations. JAMA Surg (2018) 153(2):123. doi: 10.1001/jamasurg.2017.3422
61. Subaşıoğlu A, Güç ZG, Gür EÖ, Tekindal MA, Atahan MK. Genetic, surgical and oncological approach to breast cancer, with BRCA1, BRCA2, CDH1, PALB2, PTEN and TP53 variants. Eur J Breast Health (2022) 19(1):55–69. doi: 10.4274/ejbh.galenos.2022.2022-7-2
62. Graffeo R, Rana HQ, Conforti F, Bonanni B, Cardoso MJ, Paluch-Shimon S, et al. Moderate penetrance genes complicate genetic testing for breast cancer diagnosis: ATM, CHEK2, bard1 and RAD51D. Breast (2022) 65:32–40. doi: 10.1016/j.breast.2022.06.003
63. Robson M. Management of women with breast cancer and pathogenic variants in genes other than brca1 or brca2. J Clin Oncol (2021) 39(23):2528–34. doi: 10.1200/jco.21.00999
64. Piombino C, Cortesi L, Lambertini M, Punie K, Grandi G, Toss A. Secondary prevention in hereditary breast and/or ovarian cancer syndromes other than BRCA. J Oncol (2020) 2020:1–10. doi: 10.1155/2020/6384190
65. Sokolova A, Johnstone KJ, McCart Reed AE, Simpson PT, Lakhani SR. Hereditary breast cancer: Syndromes, tumor pathology and molecular testing. Histopathology (2022) 82(1):70–82. doi: 10.1111/his.14808
66. Fencer MG, Krupa KA, Bleich GC, Grumet S, Eladoumikdachi FG, Kumar S, et al. Diagnosis, management, and surveillance for patients with PALB2, CHEK2, and ATM gene mutations. Clin Breast Cancer (2023) 23(4):e194-e199. doi: 10.1016/j.clbc.2023.02.004
67. Bergstrom C, Pence C, Berg J, Partain N, Sadeghi N, Mauer C, et al. Clinicopathological features and outcomes in individuals with breast cancer and ATM, CHEK2, or PALB2 mutations. Ann Surg Oncol (2020) 28(6):3383–93. doi: 10.1245/s10434-020-09158-2
68. Hu C, Polley EC, Yadav S, Lilyquist J, Shimelis H, Na J, et al. The contribution of germline predisposition gene mutations to clinical subtypes of invasive breast cancer from a clinical genetic testing cohort. JNCI: J Natl Cancer Inst (2020) 112(12):1231–41. doi: 10.1093/jnci/djaa023
69. Yang X, Leslie G, Doroszuk A, Schneider S, Allen J, Decker B, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol (2020) 38(7):674–85. doi: 10.1200/JCO.19.01907
70. Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkäs K, Roberts J, et al. Breast-cancer risk in families with mutations in palb2. N Engl J Med (2014) 371(6):497–506. doi: 10.1056/nejmoa1400382
71. Tischkowitz M, Xia B. Palb2/FANCN: recombining cancer and fanconi anemia. Cancer Res (2010) 70(19):7353–9. doi: 10.1158/0008-5472.can-10-1012
72. Weischer M, Bojesen SE, Tybjærg-Hansen A, Axelsson CK, Nordestgaard BG. Increased risk of breast cancer associated with chek2*1100delc. J Clin Oncol (2007) 25(1):57–63. doi: 10.1200/jco.2005.05.5160
73. Cybulski C, Wokołorczyk D, Jakubowska A, Huzarski T, Byrski T, Gronwald J, et al. Risk of breast cancer in women with a chek2 mutation with and without a family history of breast cancer. J Clin Oncol (2011) 29(28):3747–52. doi: 10.1200/jco.2010.34.0778
74. Weischer M, Bojesen SE, Ellervik C, Tybjærg-Hansen A, Nordestgaard BG. chek2*1100delc genotyping for clinical assessment of Breast Cancer Risk: Meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol (2008) 26(4):542–8. doi: 10.1200/jco.2007.12.5922
75. Ruijs MW, Verhoef S, Rookus MA, Pruntel R, van der Hout AH, Hogervorst FB, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: Mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet (2010) 47(6):421–8. doi: 10.1136/jmg.2009.073429
76. Varley JM. Germlinetp53 mutations and Li-Fraumeni syndrome. Hum Mutation (2003) 21(3):313–20. doi: 10.1002/humu.10185
77. Heymann S, Delaloge S, Rahal A, Caron O, Frebourg T, Barreau L, et al. Radio-induced Malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat Oncol (2010) 5(1). doi: 10.1186/1748-717x-5-104
78. Limacher J-M, Frebourg T, Natarajan-Ame S, Bergerat J-P. Two metachronous tumors in the radiotherapy fields of a patient with Li-Fraumeni syndrome. Int J Cancer (2001) 96(4):238–42. doi: 10.1002/ijc.1021
79. Hisada M, Garber JE, Li FP, Fung CY, Fraumeni JF. Multiple primary cancers in families with Li-Fraumeni syndrome. JNCI: J Natl Cancer Inst (1998) 90(8):606–11. doi: 10.1093/jnci/90.8.606
80. Henry E, Villalobos V, Million L, Jensen KC, West R, Ganjoo K, et al. Chest wall leiomyosarcoma after breast-conservative therapy for early-stage breast cancer in a young wOman with Li-Fraumeni syndrome. J Natl Compr Cancer Netw (2012) 10(8):939–42. doi: 10.6004/jnccn.2012.0097
81. Salmon A, Amikam D, Sodha N, Davidson S, Basel-Vanagaite L, Eeles RA, et al. Rapid development of post-radiotherapy sarcoma and breast cancer in a patient with a novel germline ‘de-novo’ TP53 mutation. Clin Oncol (2007) 19(7):490–3. doi: 10.1016/j.clon.2007.05.001
82. Ferrarini A, Auteri-Kaczmarek A, Pica A, Boesch N, Heinimann K, Schäfer SC, et al. Early occurrence of lung adenocarcinoma and breast cancer after radiotherapy of a chest wall sarcoma in a patient with a de novo germline mutation in TP53. Familial Cancer (2011) 10(2):187–92. doi: 10.1007/s10689-010-9415-9
83. Marabelli M, Cheng S-C, Parmigiani G. Penetrance of ATM gene mutations in breast cancer: A meta-analysis of different measures of risk. Epidemiology (2016) 40(5):425–31. doi: 10.1002/gepi.21971
Keywords: hereditary breast cancer, risk-reducing surgery, ipsilateral breast tumor recurrence, ATM, CHEK2, BRCA, PALB2, TP53
Citation: Abdel-Razeq H (2023) Surgical options for patients with early-stage breast cancer and pathogenic germline variants: an oncologist perspectives. Front. Oncol. 13:1265197. doi: 10.3389/fonc.2023.1265197
Received: 22 July 2023; Accepted: 29 August 2023;
Published: 14 September 2023.
Edited by:
Maria Rosaria De Miglio, University of Sassari, ItalyReviewed by:
Sandhya Pruthi, Mayo Clinic, United StatesDrakoulis Yannoukakos, National Centre of Scientific Research Demokritos, Greece
Copyright © 2023 Abdel-Razeq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hikmat Abdel-Razeq, SGFiZGVscmF6ZXFAa2hjYy5qbw==
 Hikmat Abdel-Razeq
Hikmat Abdel-Razeq