
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol. , 04 January 2024
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1265022
This article is part of the Research Topic Myeloproliferative Neoplasms: Biology and Treatment View all 11 articles
 Zhuanghui Hao1
Zhuanghui Hao1 Juan Li2
Juan Li2 Feng Gao3
Feng Gao3 Weixiao Ren1
Weixiao Ren1 Xiaomei Lu1
Xiaomei Lu1 Jinyi Feng1
Jinyi Feng1 Chen Zhang1
Chen Zhang1 Sicheng Bian1
Sicheng Bian1 Juan Xie1
Juan Xie1 Ming Luo1
Ming Luo1 Jianmei Chang1
Jianmei Chang1 Wanfang Yang1
Wanfang Yang1 Ruixia Hou1
Ruixia Hou1 Daniel Muteb Muyey1
Daniel Muteb Muyey1 Jing Xu1
Jing Xu1 Jiangxia Cui1
Jiangxia Cui1 Xiuhua Chen1*
Xiuhua Chen1* Hongwei Wang1*
Hongwei Wang1*Background: It has been discovered that Janus kinase 2 (JAK2) exon12 mutations lead to the polycythemia vera (PV) phenotype, while somatic mutations of calreticulin (CALR) are associated with essential thrombocythemia (ET) or primary myelofibrosis. In this article, we report a case of ET with coexistence of JAK2 exon12 and CALR mutations. The objective of this study was to elucidate the pathogenicity mechanism of a JAK2 exon12 mutation (JAK2N533S) and the role of the coexistence of mutations on the hematological phenotype.
Methods: We designed a colony analysis of tumor cells obtained from this patient, and attempted to identify mutant genes using DNA from hair follicles. Mutation impairment prediction and conservative analysis were conducted to predict the mutation impairment and structure of JAK2N533S. In addition, we conducted a functional analysis of JAK2N533S by constructing Ba/F3 cell models.
Results: Three distinct tumor subclones, namely JAK2N533Shet+/CALRtype1het+, JAK2N533Shet+/CALRwt, and JAK2N533Shet+/CALRtype1hom+, were identified from the 17 selected erythroid and 21 selected granulocyte colonies. The analysis of hair follicles yielded positive results for JAK2N533S. According to the bioinformatics analysis, JAK2N533S may exert only a minor effect on protein function. Functional studies showed that JAK2N533S did not have a significant effect on the proliferation of Ba/F3 cells in the absence of interleukin-3 (IL-3), similar to wild-type JAK2. Notably, there were no increased phosphorylation levels of JAK2-downstream signaling proteins, including signal transducer and activator of transcription 3 (STAT3) and STAT5, in Ba/F3 cells harboring the JAK2N533S.
Conclusion: Our study revealed that the JAK2N533Shet+/CALRtype1het+ subclone was linked to a significant expansion advantage in this patient, indicating that it may contribute to the development of the ET phenotype. We further demonstrated that JAK2N533S, as a noncanonical JAK2 exon12 mutation, is a germline mutation that may not exert an effect on cell proliferation and protein function. These results and the present body of available data imply that certain noncanonical JAK2 mutations are not gain-of-function mutations leading to the development of myeloproliferative neoplasms.
The classic Philadelphia-negative myeloproliferative neoplasms (MPN), which include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis, are a diverse group of clonal disorders characterized by the excessive production of mature cells in the peripheral blood (1–3). Somatic mutations in genes, such as V617F mutation in Janus kinase 2 (JAK2), JAK2V617F, JAK2 exon12, calreticulin (CALR) exon9, or MPL exon10, exhibit a high prevalence (almost 90%) among patients with MPN, and are the underlying etiology of these disorders (4–8). Initially, researchers thought that these mutations were mutually exclusive in patients with MPN. However, a few cases of MPN associated with multiple driver mutations, most commonly the coexistence of JAK2V617F and CALR mutations, have been reported (9–12).
In this study, we present the case of a patient with ET who carries both JAK2 exon12 mutation (JAK2N533S) and CALR mutation (CALRtype1). Clinical observations revealed that JAK2 exon12 mutations are found in PV (13). It is puzzling that this patient presents with a JAK2 exon12 mutation and exhibits an ET phenotype, but lacks the characteristic PV phenotype. Additionally, the biological function of JAK2N533S, an atypical JAK2 mutation, remains largely unexplored. Therefore, further investigation is warranted to elucidate the mechanism by which the coexistence of mutations contributes to the hematological phenotype in this case.
The patient was diagnosed with MPN according to the 2016 World Health Organization criteria in the Second Hospital of Shanxi Medical University. Mutational investigation in this patient was performed using a DNA sample from fresh bone marrow or peripheral blood samples. Patient history and clinical data were extracted from the medical records. This patient provided written informed consent, and this study was conducted in accordance with the tenets stipulated in the Declaration of Helsinki.
A peripheral blood sample of this patient was collected, and peripheral blood mononuclear cells were isolated. The cells were plated at a density of 1×105cells/mL, which provided an optimal density for colony selection without the risk of contamination by neighboring colonies. Erythroid colony-forming units were cultured in methylcellulose-based medium (STEMCELL Technologies,VAN,CAN) containing additional 3 U/mL erythropoietin for 15 days. Granulocyte colony-forming units were incubated in methylcellulose-based medium containing an additional 100 ng/mL granulocyte colony-stimulating factor for 15 days. Erythroid and granulocyte colonies were selected, and DNA was extracted, amplified using a 2×STA Master Mix Kit (BBI Life Science,HK,China), and sequenced for JAK2 exon12 (sense 5’TGGGCCGAAGTCTGA CCCTTT 3’ and antisense 5’ ACAGAGCGAACCAATGC 3’) and CALR exon9 (sense 5’ TGGGGCGTAACAAAGGTG AG 3’ and antisense 5’ TGAAAGTTC TCGAGTCTCA CAGA 3’). After purifying the polymerase chain reaction product, Sanger sequencing (The Beijing Genomics Institute,SZX,China) was used to identify mutation sites.
We performed pathogenicity analyses for the novel missense variant using Mutation Taster score (https://www.mutationtster.org/ ), E-SNPs&GO (https://esnpsandgo.biocomp.unibo.it/), Polymorphism Phenotyping version 2 (PolyPhen2; http://genetics.bwh.harvard.edu/pph2/), and Sorting Intolerant From Tolerant (SIFT; https://sift.bii.a-star.edu.sg/). Alignment of homologous sequences of JAK2 protein from various species was performed using the Unipro UGENE software (49.1version, http://ugene.net/) to assess the conservation of amino acid residues at different sites. Protein three-dimensional (3D) model of wild-type JAK2 (JAK2wt) was used the homodimeric JAK2 pseudokinase-protein tyrosine kinase (PK-PTK) model by JAK2 PK-PTK model (ma-evjj8). The five models of JAK2S533 was constructed based on the above ma-evjj8 by AlphaFold (https://alphafold.com/) and ColabFold (https://github.com/sokrypton/ColabFold) (14, 15). For further analysis, we used the JAK2S533 model with the highest per-residue confidence score (predicted local distance difference test) compared with the other models. The PyMOL system (4.5.0 vision) was used to visualize the results of the protein model. Detailed data of the model and score are provided in Supplementary Material 1.
Complementary DNAs (cDNAs) for human JAK2N533S and control genes (JAK2wt, JAK2 K539L, and JAK2N542-E543del) were synthesized by GenScript (NKG,CN). All cDNAs were cloned into a pCDH-CMV- MCS-EF1-CopGFP-Puro lentiviral-vector. Lentiviral particles were produced in 293T cells to infect Ba/F3 cells. The efficiency of gene transfer into Ba/F3 cells was assessed by green fluorescent protein (GFP) using laser confocal microscopy (Olympus, Tokyo, Japan) and flow cytometry (Beckman, CA,USA).
Ba/F3 cells were cultured in RPMI 1640 medium (Gibco, USA) containing 15% fetal calf serum (Thermo Fisher Scientific,NK,USA) and 10 ng/mL interleukin-3 (PeproTech,NJ, USA). Cell viability assay was performed using a CCK8 Assay Kit (DOJINDO,kumamato, Japan). For the CCK8 assay, 3,000 cells were seeded in each well of a 96-well plate. Each cell line was cultured for 5 days in the absence of IL-3.
Ba/F3 cells were deprived of IL-3 for 2 days. Total protein was extracted, processed using radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, NK,USA), and supplemented with protease and phosphatase inhibitor mixture tablets (Thermo Fisher Scientific,NK,USA). Western blotting was performed by Simple Western (Protein Simple Technology,SV, USA). Antibodies against signal transducer and activator of transcription 3 (STAT3), STAT5, phospho-STAT3 (pSTAT3), and pSTAT5 were obtained from Cell Signaling Technologies (MA,USA).
A 75-year-old female patient presented with a gradual increase in platelet counts over 3 years. In December 2016, she was referred to the Second Hospital of Shanxi Medical University for evaluation of this marked thrombocytosis (Figure 1A). Despite the elevated platelet count, the patient was in good physical condition and did not experience any clinical symptoms, such as fever, itchy skin, facial flushing, bone pain, splenomegaly, or others. Furthermore, she did not have any complications, such as bleeding, thrombosis, or cerebrovascular disease.
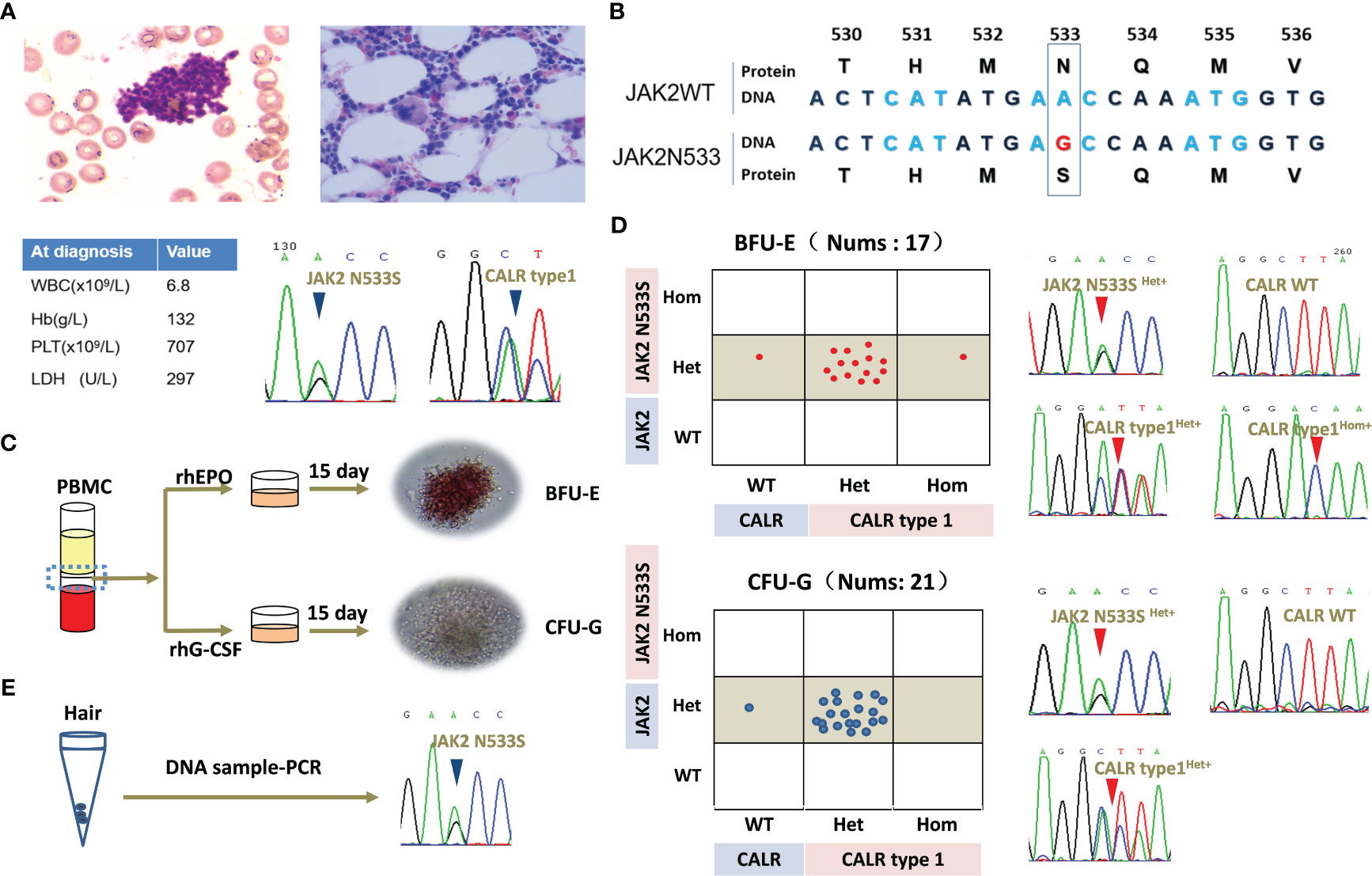
Figure 1 Clinical data of the patient and clonal evolution analysis for JAK2N533S and CALRtype1. (A) Representative image of bone marrow biopsy and blood counts at diagnosis. Sanger Sequencing Charts of heterozygous JAK2N533S and CALRtype1 using a peripheral blood samples at diagnosis. (B) Diagram of JAK2N533S. The substitution of base A with base G results in the change of asparagine to serine. (C) Schematic representation of erythroid and granulocyte colony-forming units. (D) Analysis of single colonies and sequencing chromatograms for JAK2N533S and CALRtype1. (E) Sequencing chromatograms for JAK2N533S using DNA obtained from hair follicles. CALR, calreticulin; JAK2, Janus kinase 2.
A bone marrow biopsy was conducted to investigate the underlying reason for the thrombocytosis. The findings indicated normal cellularity with a normal ratio of myeloid to erythroid cells without fibrosis. However, there was a significant increase in megakaryopoiesis. Chromosomal analysis of 20 karyotypes revealed that the patient had a normal karyotype of 46, XX. Mutational analysis using peripheral blood revealed that the patient carried a JAK2 exon12 mutation (JAK2N533S) and a CALR mutation (CALRtype1). The patient was diagnosed with ET based on the platelet count (707 per mm3), proliferation mainly of the megakaryocytic lineage, and the presence of CALRtype1. However, there was no evidence of PV apart from the presence of JAK2N533S. The patient’s blood routine returned to normal and remained stable after treatment with hydroxyurea. Currently, she remains in good health despite carrying JAK2N533S and CALRtype1 (Figures 1A, B).
The clonal relationship between JAK2N533S and CALRtype1 was further investigated in this study. Peripheral blood mononuclear cells were isolated, and colony-forming assays were performed for erythroid cells and granulocytes. All 17 selected erythroid colonies exhibited a heterozygous JAK2N533S mutation (JAK2N533Shet+). Of those, 14 colonies (82%) also carried a heterozygous CALRtype1 mutation (CALRtype1het+), one colony (6%) harbored a homozygous CALRtype1 mutation (CALRtype1hom+), and two colonies (12%) exhibited a wild-type CALR (CALRwt) genotype. In addition, all 21 selected granulocyte colonies exhibited a JAK2N533Shet+. Among those, 20 colonies (95%) had a CALRtype1het+, and one colony (5%) exhibited a CALRwt genotype (Figures 1C, D).
Because JAK2N533Shet+ was present in all colonies, JAK2N533S was further tested using DNA extracted from hair follicles (Figure 1E). This analysis yielded positive results for the presence of JAK2N533Shet+, indicating that this is a germline mutation. In contrast, among these colonies, 20 (95%) exhibited a CALRtype1het+, while one (5%) had a CALRwt genotype. This evidence further indicated that CALRtype1 was a late somatic event and occurred in a multipotent hematopoietic stem cell compartment capable of generating both myeloid and erythroid progeny.
Individual colony analysis revealed three distinct tumor subclones, namely JAK2 N533Shet+/CALRtype1het+, JAK2N533Shet+/CALRwt, and JAK2N533Shet+/CALRtype1hom+. Notably, significant expansion was observed in the JAK2N533Shet+/CALRtype1het+ clone compared with the JAK2N533Shet+/CALRwt clone. This finding suggested that the acquisition of CALR mutation provides a growth advantage over the presence of JAK2N533S alone (Figures 1D, E).
We sought to better understand the pathogenicity of JAK2N533S. Hence, a bioinformatics analysis was conducted to determine the function of JAK2N533S. In this analysis, various mutation impairment prediction tools were utilized to predict the impact of Asn533Ser substitution on the JAK2 protein. The Mutation Taster score indicated that Asn533Ser substitution was pathogenic, suggesting a detrimental effect on protein function. However, other tools (e.g., E-SNPs&GO, PolyPhen-2, and SIFT) classified the N533S variant as benign, indicating that it is unlikely to significantly impact the protein function. The contradictory results obtained from the pathogenic prediction analysis of JAK2N533S are perplexing (Figure 2A). It is important to consider that different prediction methods may be characterized by inherent biases and limitations, which could contribute to discordant conclusions.
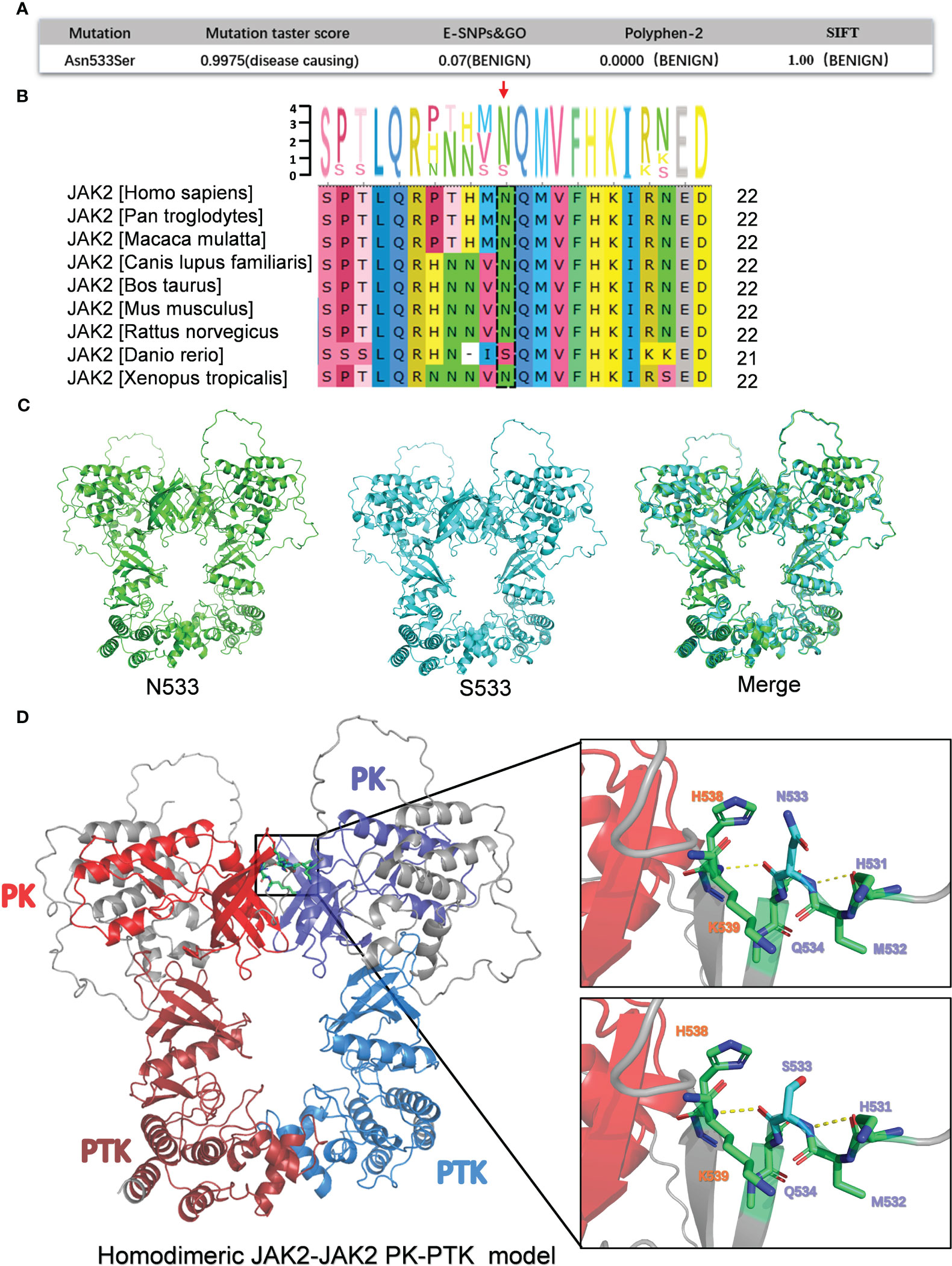
Figure 2 Bioinformatics analysis of the pathogenicity of JAK2N533S. (A) Prediction results for the pathogenicity of N533S based on the Mutation Taster score, E-SNPs&GO, PolyPhen-2, and SIFT. (B) Conservative analysis diagrams of the JAK2 533 site across different species. (C) Homodimeric JAK2 PK-PTK model of JAK2N533 (green) and JAK2S533 (blue). (D) Left, homodimeric JAK2 PK-PTK model of JAK2S533; the PK domain and PTK domain are marked using different colors. Right, zoom-in image showing the amino acids within 6 Å of N533 or S533. JAK2, Janus kinase 2; PK, pseudokinase; PTK, protein tyrosine kinase.
Additionally, a conservation analysis was performed to assess the level of conservation of Asn533 across different species. It was found that Asn533 is conserved in the JAK2 gene among species, such as humans, Pan troglodytes, Macaca mulatta, etc. However, of note, in Danio rerio, Asn533 can naturally be replaced with Ser (Figure 2B).
Furthermore, a comparison of the three-dimensional model of the homodimeric JAK2 PK-PTK model containing N533 and S533 was conducted (Figures 2C, D). The S533 mutation does not appear to significantly alter the structure of the JAK2 protein based on the merge of the JAK2 PK-PTK model of N533 and S533 (Figure 2C). The substitution of Asn with Ser could not trigger significant structural changes due to the slightly shorter length of Ser compared with Asn and their similar polar nature. Hence, it may cause subtle and confined alterations in JAK2 structure.
Previous research has determined that the JAK2K539L mutation in JAK2 exon12 is a pathogenic mutation that leads to JAK2 constitutive activation. This activation occurs by disrupting the highly charged region of D620 and altering the salt bridge interaction between residues D620 and E621. Moreover, the change of salt bridging among Lys539, Glu592, and Glu596 in the periphery of the hydrophobic interface could potentially lead to the stabilization of a constitutive active dimer (15–18). Could the N533S mutation influence them by establishing or disrupting the surrounding salt bridging and hydrogen bonds to promote JAK2 activation? There were no changes observed in the surrounding hydrogen bonds, and the N533S mutation did not establish a salt bridge interaction with residues D620, E621, and K539, as the distance between them exceeded 5 Å.
Collectively, based on the results of the bioinformatics analysis, JAK2N533S may exert only a minor effect on protein function. Further investigation and experimental studies are required to fully understand the functional consequences of this substitution.
To investigate the functional effects of JAK2N533S, we cloned the cDNAs of JAK2N533S and control genes (JAK2wt, JAK2K539L, and JAK2N542-E543del) into a lentiviral expression vector with an GFP and puromycin; of note, JAK2K539L and JAK2N542-E543del are the most common gain-of-function mutations in JAK2 exon12 (Figure 3A). We used a lentiviral transduction to transfect the JAK2 gene with different mutation sites into Ba/F3 cells. We sorted the transgene-positive cells utilizing puromycin (2 ng/mL) and detected the positive rate of GFP by flow cytometry (Figure 3B). Next, we measured the IL-3-independent proliferation of cells. Each cell line was cultured for 5 days in the absence of IL-3. The experiment was conducted in triplicate. Cell viability assay was performed using CCK8. The control cells expressing JAK2K539L and JAK2N542-E543del showed significant accumulation, whereas there was no difference observed between cells carrying JAK2N533S and control cells expressing JAK2wt, empty-vector, and untransfected Ba/F3 cells (Figure 3C). We further examined the levels of phosphorylated JAK2-downstream signal proteins, including STAT5 and STAT3. Cells were deprived of IL-3 cytokine for 2 days and analyzed by western blotting (ProteinSimple Technology) for phosphorylation of STAT5 and STAT3. Control cells carrying JAK2K539L and JAK2N542-E543del presented increased phosphorylation of STAT5(Tyr694) and STAT3(Tyr705), whereas those harboring JAK2N533S, JAK2wt, empty-vector, and untransfected Ba/F3 cells did not show obvious activation of STAT5 and STAT3 (Figures 3D, E). The data obtained from the functional analysis indicated that JAK2N533S did not alter the function of JAK2 protein.
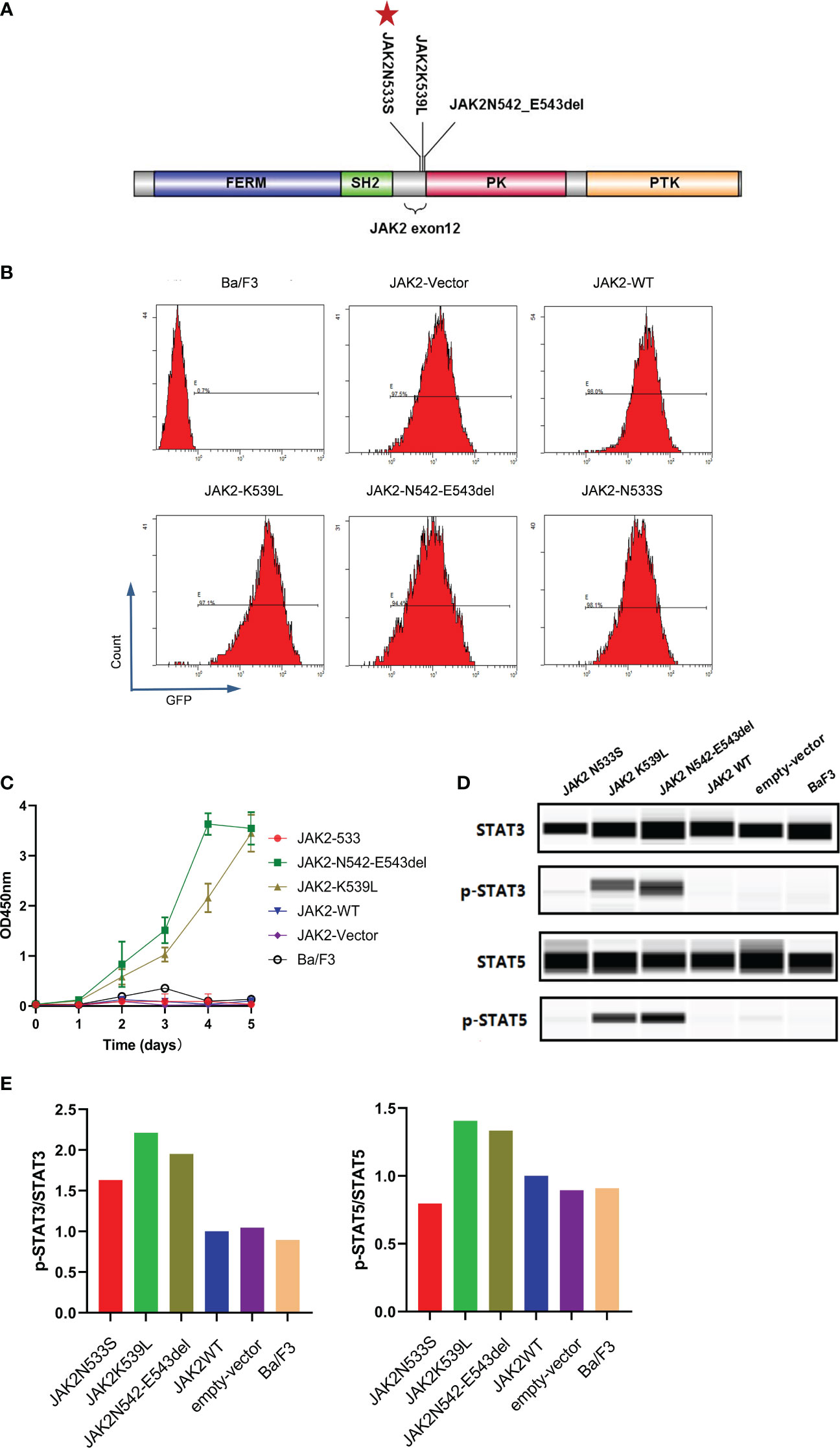
Figure 3 Functional analysis of JAK2N533S. (A) Schematic representation of the JAK2 domain and JAK2 exon12 mutations. Red stars in the panel indicate the positions of JAK2N533S. (B) Detection of GFP in different cell lines through flow cytometry. (C) IL-3-independent proliferation of JAK2 mutation Ba/F3 models, detected by CCK8 assay. Data are presented as the mean ± standard deviation (SD) of three independent experiments; error bars indicate SD. (D) Lower phosphorylation levels of STAT3 and STAT3 in cells carrying JAK2N533S compared with cells harboring JAK2K539L and JAK2N542-E543del, detected by Simple Western. (E) Quantification of the expression of phospho-STAT3 (p-STAT3) and p-STAT5. Left, levels of p-STAT3 determined by densitometry of protein bands and normalized to those of STAT3. Right, levels of p-STAT5 determined by densitometry of protein bands and normalized to those of STAT5. CCK8, Cell Counting Kit-8; GFP, green fluorescent protein; IL-3, interleukin-3; STAT3/5, signal transducer and activator of transcription 3/5.
In this article, we have described a case with coexistence of JAK2N533S and CALRtype1. Mutational analysis of a single colony allowed us to distinguish three distinct tumor subclones in this patient. Among these subclones, the JAK2N533Shet+/CALRtype1het+ clone had a significant expansion advantage over the other two clones. Thus, the JAK2N533Shet+/CALRtype1het+ clone was significantly associated with the ET phenotype of this patient. In contrast, we observed a small number of JAK2N533Shet+/CALRtype1hom+ subclones. It is unlikely that two independent mutation of CALR occurred at the exact same position (CALRtype1). Therefore, we inferred that CALRtype1hom+ could arise from the original JAK2N533Shet+/CALRtype1het+ clone by the loss of the CALRwt allele through deletion or uniparental disomy. The percentage of such homozygous colonies was very low. A possible reason is that loss of CALRwt may not provide a competitive advantage. In contrast, an alternative likely could be explained by the fact that the CALRtype1hom+ subclone emerged shortly before the diagnosis. Therefore, the number of JAK2N533Shet+/CALRtype1hom+ clones was relatively low.
Furthermore, considering that only part of the selected colonies carried CALRtype1, we inferred that CALRtype1 is an acquired somatic mutation. All selected colonies carried JAK2N533S, indicating that this may be a germline mutation. We further tested this mutation using DNA samples obtained from hair follicles, indicating its potential emergence as a germline mutation. Regrettably, it was not possible to locate samples obtained from the parents of this patient. Hence, we were unable to strictly determine that JAK2N533S is a germline mutation.
According to the currently available data, CALRtype1 is sufficient to induce the ET phenotype. Thus far, JAK2 exon12 mutations have only been associated with the PV phenotype; JAK2K539L and JAK2N542-E543del are the most common gain-of-function mutations in JAK2 exon12. In contrast, JAK2N533S (as a noncanonical JAK2 exon12 mutation) has been rarely reported. In this investigation, we demonstrated that JAK2N533S did not exert an effect on the proliferation of cells through protein function prediction and cellular model function assays. The current data indicated that JAK2 N533S did not contribute to MPN in this patient. Nevertheless, it is possible that this variant serves as a basis for CALR driver mutations. Moreover, the coexistence of CALRtype1 and JAK2N533S could induce a stronger growth advantage to promote proliferation. Future research should focus on the effects of this coexistence.
In recent years, an increasing number of other noncanonical JAK2 mutations have been identified in patients with MPN through next-generation sequencing (19–22). However, it has been observed that some of these JAK2 mutations (e.g., G335D, F556W, Y590E,G571S, and Y613F) do not play a role in the pathogenesis of MPN (6, 20, 21, 23, 24). Our findings and other available data suggest that certain noncanonical JAK2 mutations are not gain-of-function mutations leading to the development of MPN (1, 23–25). Therefore, it is recommended to assess the functional impact of noncanonical JAK2 mutations in MPN cases at the time of diagnosis. This study on JAK2N533S can be used a reference in clinical practice.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of the Second Hospital of Shanxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. The manuscript presents research on animals that do not require ethical approval for their study.
ZH: Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. JL: Formal analysis, Conceptualization, Writing – review & editing. FG: Investigation, Methodology, Conceptualization, Writing – review & editing. WR: Methodology, Writing – review & editing. XL: Methodology, Software, Writing – review & editing. JF: Methodology, Software, Writing – review & editing. CZ: Formal analysis, Investigation, Software, Writing – review & editing. SB: Methodology, Writing – review & editing. JXi: Methodology, Writing – review & editing. ML: Data curation, Methodology, Writing – review & editing. JMC: Data curation, Formal analysis, Methodology, Writing – review & editing. WY: Data curation, Formal analysis, Methodology, Writing – review & editing. RH: Data curation, Methodology, Validation, Writing – review & editing. DM: Writing – review & editing. JXu: Formal analysis, Methodology, Writing – review & editing. JXC: Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. XC: Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. HW: Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NO.81670126; NO.81500104), HW, professor, the Second Hospital of Shanxi Medical University; the Shanxi Natural Science Foundation of China (NO.202203021221271; the Science and Technology Innovation Fund of Shanxi Medical University (NO.01201413), XC, Associate chief physician, the Second Hospital of Shanxi Medical University; the Shanxi Natural Science Foundation of China (NO.202203021222391), JXC, Associate chief physician, the Second Hospital of Shanxi Medical University; and the Graduate Research and Innovation Projects of Shanxi Province (2023KY356), ZH, the Second Hospital of Shanxi Medical University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1265022/full#supplementary-material
1. Gerds AT, Gotlib J, Ali H, Bose P, Dunbar A, Elshoury A, et al. Myeloproliferative neoplasms, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(9):1033–62. doi: 10.6004/jnccn.2022.0046
2. Godfrey AL. Myeloproliferative neoplasms (MPNs). Blood Rev (2020) 42:100717. doi: 10.1016/j.blre.2020.100717
3. Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol (2020) 95(12):1599–613. doi: 10.1002/ajh.26008
4. Nangalia J, Green AR. Myeloproliferative neoplasms: from origins to outcomes. Blood (2017) 130(23):2475–83. doi: 10.1182/blood-2017-06-782037
5. Greenfield G, McMullin MF, Mills K. Molecular pathogenesis of the myeloproliferative neoplasms. J Hematol Oncol (2021) 14(1):103. doi: 10.1186/s13045-021-01116-z
6. Grinfeld J, Nangalia J, Green AR. Molecular determinants of pathogenesis and clinical phenotype in myeloproliferative neoplasms. Haematologica (2017) 102(1):7–17. doi: 10.3324/haematol.2014.113845
7. James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature (2005) 434(7037):1144–8. doi: 10.1038/nature03546
8. Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PloS Med (2006) 3(7):e270. doi: 10.1371/journal.pmed.0030270
9. Xing CY, Li HY, Wu JB, Gao SM. Co-occurrence of JAK2 V617F and an uncommon CALR del (p.K368fs*51) mutation facilitates JAK2/STAT signaling in polycythemia vera. Leuk Lymphoma (2016) 57(7):1743–5. doi: 10.3109/10428194.2015.1115031
10. Kang MG, Choi HW, Lee JH, Choi YJ, Choi HJ, Shin JH, et al. Coexistence of JAK2 and CALR mutations and their clinical implications in patients with essential thrombocythemia. Oncotarget (2016) 7(35):57036–49. doi: 10.18632/oncotarget.10958
11. Ahmed RZ, Rashid M, Ahmed N, Nadeem M, Shamsi TS. Coexisting JAK2V617F and CALR exon 9 mutations in myeloproliferative neoplasms - do they designate a new subtype. Asian Pac J Cancer Prev (2016) 17(3):923–6. doi: 10.7314/apjcp.2016.17.3.923
12. Zamora L, Xicoy B, Cabezón M, Fernandez C, Marcé S, Velez P, et al. Co-existence of JAK2 V617F and CALR mutations in primary myelofibrosis. Leuk Lymphoma (2015) 56(10):2973–4. doi: 10.3109/10428194.2015.1015124
13. Levine RL, Pardanani A, Tefferi A, Gilliland DG. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer (2007) 7(9):673–83. doi: 10.1038/nrc2210
14. Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold: making protein folding accessible to all. Nat Methods (2022) 19(6):679–82. doi: 10.1038/s41592-022-01488-1
15. Caveney NA, Saxton RA, Waghray D, Glassman CR, Tsutsumi N, Hubbard SR, et al. Structural basis of Janus kinase trans-activation. Cell Rep (2023) 42(3):112201. doi: 10.1016/j.celrep.2023.112201
16. Gnanasambandan K, Magis AT, Sayeski PP. A shift in the salt bridge interaction of residues D620 and E621 mediates the constitutive activation of Jak2-H538Q/K539L. Mol Cell Biochem (2012) 367(1-2):125–40. doi: 10.1007/s11010-012-1326-7
17. Lee TS, Ma W, Zhang X, Kantarjian H, Albitar M. Structural effects of clinically observed mutations in JAK2 exons 13-15: comparison with V617F and exon 12 mutations. BMC Struct Biol (2009) 9:58. doi: 10.1186/1472-6807-9-58
18. Glassman CR, Tsutsumi N, Saxton RA, Lupardus PJ, Jude KM, Garcia KC. Structure of a Janus kinase cytokine receptor complex reveals the basis for dimeric activation. Science (2022) 376(6589):163–9. doi: 10.1126/science.abn8933
19. Benton CB, Boddu PC, DiNardo CD, Bose P, Wang F, Assi R, et al. Janus kinase 2 variants associated with the transformation of myeloproliferative neoplasms into acute myeloid leukemia. Cancer (2019) 125(11):1855–66. doi: 10.1002/cncr.31986
20. Milosevic Feenstra JD, Nivarthi H, Gisslinger H, Leroy E, Rumi E, Chachoua I, et al. Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood (2016) 127(3):325–32. doi: 10.1182/blood-2015-07-661835
21. Wu QY, Ma MM, Tong YX, Zhu YY, Liu Y, Cao J, et al. Effects of JAK2 V556F mutation on the JAK2’s activity, structural stability and the transformation of Ba/F3 cells. Int J Biol Macromol (2018) 117:271–9. doi: 10.1016/j.ijbiomac.2018.05.185
22. Patel AB, Franzini A, Leroy E, Kim SJ, Pomicter AD, Genet L, et al. JAK2 ex13InDel drives oncogenic transformation and is associated with chronic eosinophilic leukemia and polycythemia vera. Blood (2019) 134(26):2388–98. doi: 10.1182/blood.2019001385
23. Aral B, Courtois M, Ragot S, Bourgeois V, Bottolier-Lemallaz E, Briandet C, et al. Germline JAK2 L611S mutation in a child with thrombocytosis. Haematologica (2018) 103(8):e372–372e373. doi: 10.3324/haematol.2018.188995
24. Funakoshi-Tago M, Pelletier S, Moritake H, Parganas E, Ihle JN. Jak2 FERM domain interaction with the erythropoietin receptor regulates Jak2 kinase activity. Mol Cell Biol (2008) 28(5):1792–801. doi: 10.1128/MCB.01447-07
Keywords: essential thrombocythemia, JAK2 exon12 mutation, CALR type1 mutation, distinct phenotype, myeloproliferative neoplasms
Citation: Hao Z, Li J, Gao F, Ren W, Lu X, Feng J, Zhang C, Bian S, Xie J, Luo M, Chang J, Yang W, Hou R, Muyey DM, Xu J, Cui J, Chen X and Wang H (2024) A germline JAK2 exon12 mutation and a late somatic CALR mutation in a patient with essential thrombocythemia. Front. Oncol. 13:1265022. doi: 10.3389/fonc.2023.1265022
Received: 21 July 2023; Accepted: 11 December 2023;
Published: 04 January 2024.
Edited by:
Gabriela Baerlocher, University of Bern, SwitzerlandReviewed by:
Emilie Leroy, Dana–Farber Cancer Institute, United StatesCopyright © 2024 Hao, Li, Gao, Ren, Lu, Feng, Zhang, Bian, Xie, Luo, Chang, Yang, Hou, Muyey, Xu, Cui, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Wang, d2FuZ2h3NjhAaG90bWFpbC5jb20=; Xiuhua Chen, Y2hlbnhpdWh1YTE5OTZAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.