- 1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Geneplus-Beijing Institute, Beijing, China
Non-small cell lung cancers (NSCLCs) with anaplastic lymphoma kinase (ALK)-rearrangement have favorable responses to ALK inhibitors. However, ALK fusion mutations harbored approximately 90 distinct fusion partners. Patients with different ALK fusions might respond distinctly to different-generation ALK inhibitors. In this case report, we identified a novel non-reciprocal ALK fusion, ALK-C2orf91(intergenic) (A19: intergenic) and PPFIA1-ALK (P2:A20), by next-generation DNA sequencing in an advanced lung adenocarcinoma patient. After 2 months of alectinib, the targeted lung lesion regressed significantly, and evaluation of therapeutic efficiency indicated partial response. To date, the patient had achieved 12 months of progression-free survival from alectinib treatment. Our study extended the spectrum of ALK fusion partners in ALK-positive NSCLC, and we reported a new ALK fusion, PPFIA1-ALK and ALK-C2orf91(intergenic), and its sensitivity to alectinib firstly in lung cancer. We believe that this case report has an important clinical reference.
Introduction
ALK gene rearrangement is one of the most important driver mutations in non-small cell lung cancer (NSCLC) occurring in 3%–7% of the cases (1). Echinoderm microtubule-associated protein-like 4 (EML4)-ALK is the most common fusion variant, accounting for over 80% of fusion partners. With the increasing coverage of next-generation DNA sequencing, more than 90 rare ALK fusion subtypes have been discovered in NSCLC, such as kinesin family member 5B (KIF5B)-ALK and striatin gene (STRN)-ALK (2). First- to third-generation anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs), including crizotinib, alectinib, and ceritinib, have brought outstanding efficacy and tolerability to patients (3–5). However, for the newly discovered fusion variants, especially those missing EML4-ALK fusion mutations, it is necessary to evaluate the clinical effectiveness of ALK-TKI in the first line or even the posterior line, and it is helpful to infer possible new therapeutic measures in the face of diverse rare fusion targets. Here, an advanced lung adenocarcinoma patient with PPFIA1-ALK and ALK-C2orf91(intergenic) double-fusion variants is sensitive to first-line alectinib.
Case presentation
A 46-year-old non-smoking Chinese man, without a family history of genetic disease, presented to another hospital in June 2022, with a cough for over 1 month and shortness of breath for approximately 10 days. A computed tomography (CT) scan revealed hydropneumothorax, compression atelectasis (approximately 80% collapse) and tumor mass of the right lung, and multiple nodules in the left lung (Figure 1A). He received pleural space drainage, and malignant cells were found in the pleural effusion. Then, he visited our hospital for further treatment. The pathologic results of ultrasound-guided percutaneous lung biopsy showed infiltrating growth of the allotypic glandular duct (Figure 2A), and the lesions tend to be pulmonary adenocarcinoma, with strongly positive expression for thyroid CK7, TTF-1, and Napsin A and negative for CK5/6, P40, and P60 as assayed by immunohistochemical staining. Bone scan, brain CT scan, and ultrasonography of the abdominal system, urinary system, and cardiovascular system identified no evidence of metastatic disease. Consequently, the patient was diagnosed with right lung adenocarcinoma with pleural metastasis (cT4N0M1a, stage IVa). To identify targetable mutations, the tumor biopsy specimen was sequenced by capture-based next-generation DNA sequencing with a panel containing 73 cancer-related genes ( (6) gene list is shown in Supplementary Table 1, Beijing Geneplus Technology Co., Ltd.). The mean effective depth of coverage of the sequence was 2,032×. Non-reciprocal ALK fusion PPFIA1-ALK (P2:A20) (abundance, 10%) and ALK-C2orf91 (A19, intergenic) (abundance, 13%) was identified in tissue (Figure 3). No other gene mutation was detected. Immunohistochemistry staining revealed that the tumor had positive expression for ALK with the Ventana D5F3 (Figure 2B). Therefore, based on guidelines and clinical studies, combined with the patient’s wishes, he received alectinib (600 mg twice daily) as first-line therapy on July 15, 2022.
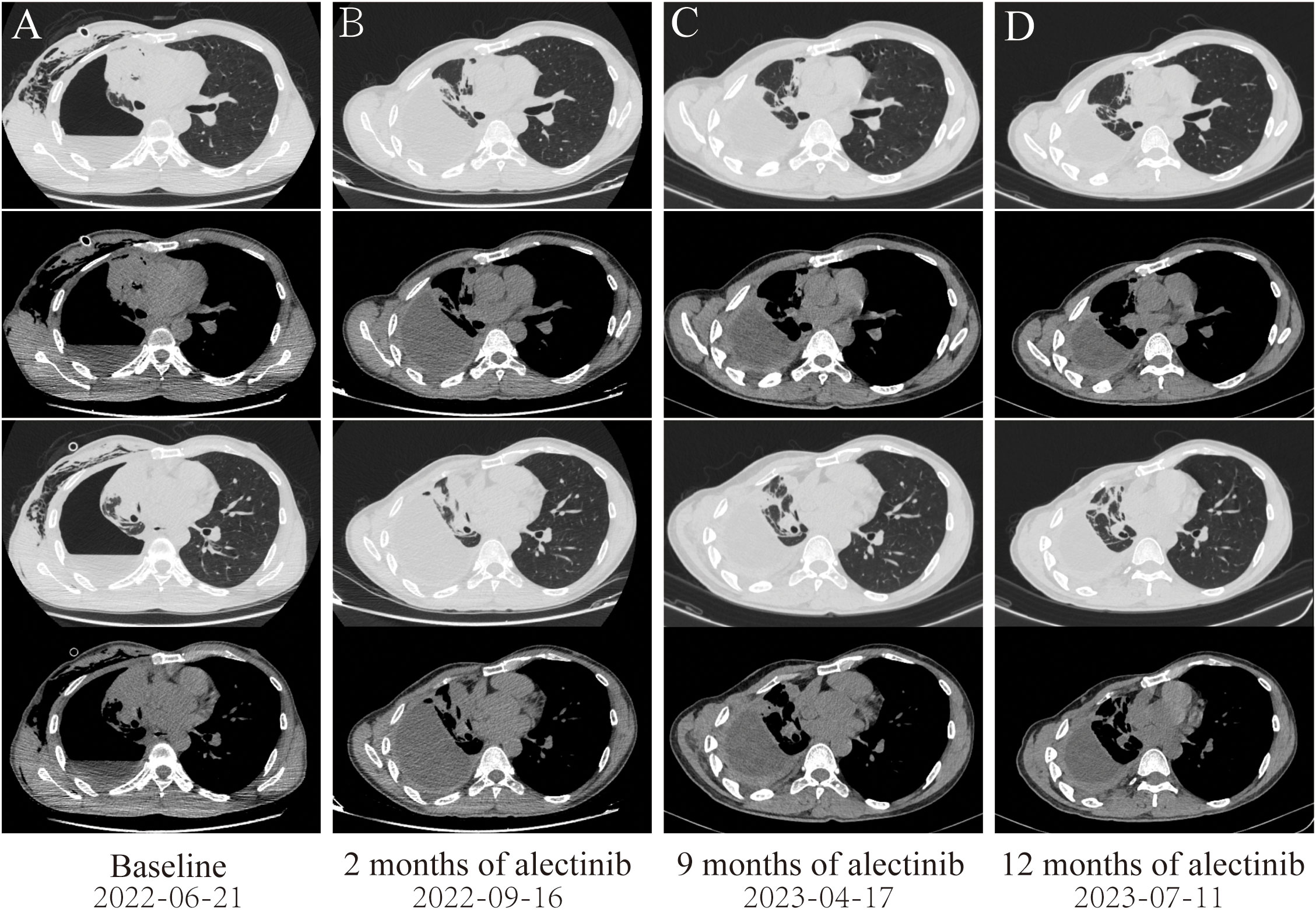
Figure 1 Dynamic imaging of lung lesions at different stages of treatment. (A) Lung lesions at diagnosis. (B) After targeted therapy with alectinib for 2 months, the right lung tumor was significantly reduced, resulting in partial response (PR). (C) After targeted therapy with alectinib for 9 months, the tumor in the right lung continued to shrink. (D) After targeted therapy with alectinib for 12 months, the right lung tumor tends to be stable.
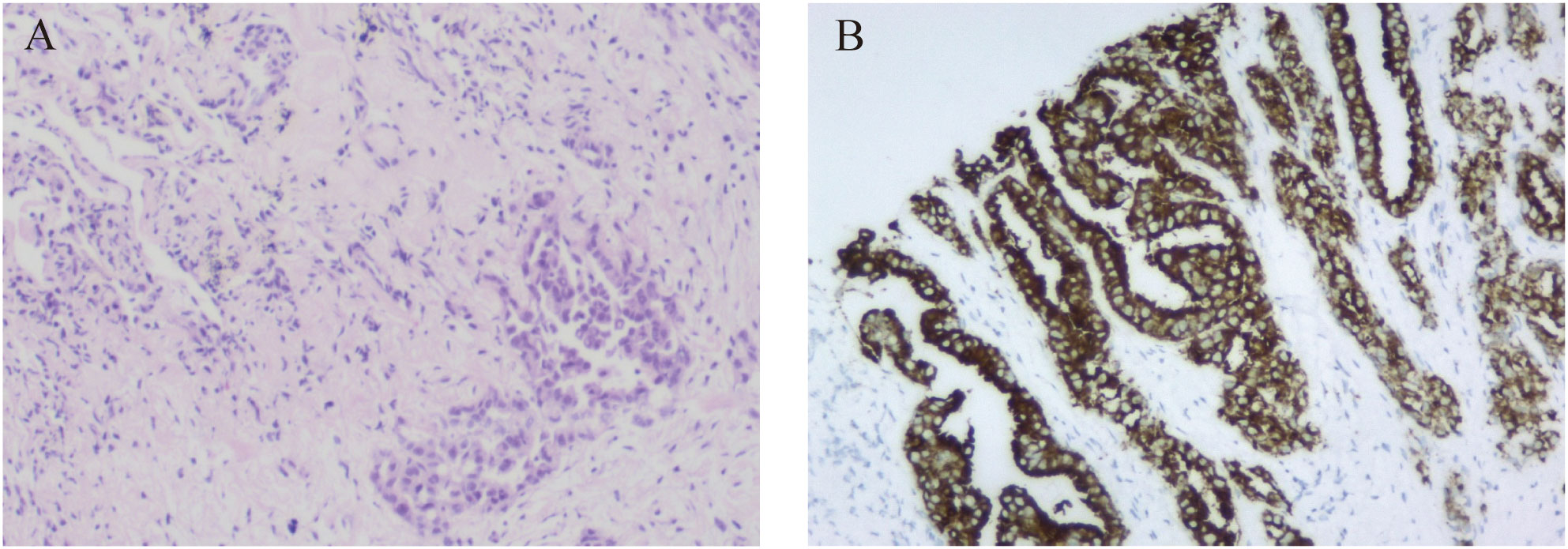
Figure 2 Pathological examination of the patient. (A) Lung tissue biopsy specimen (hematoxylin and eosin staining, magnification ×100). (B) The immunohistochemistry demonstrated positive expression for ALK (D5F3 Ventana).
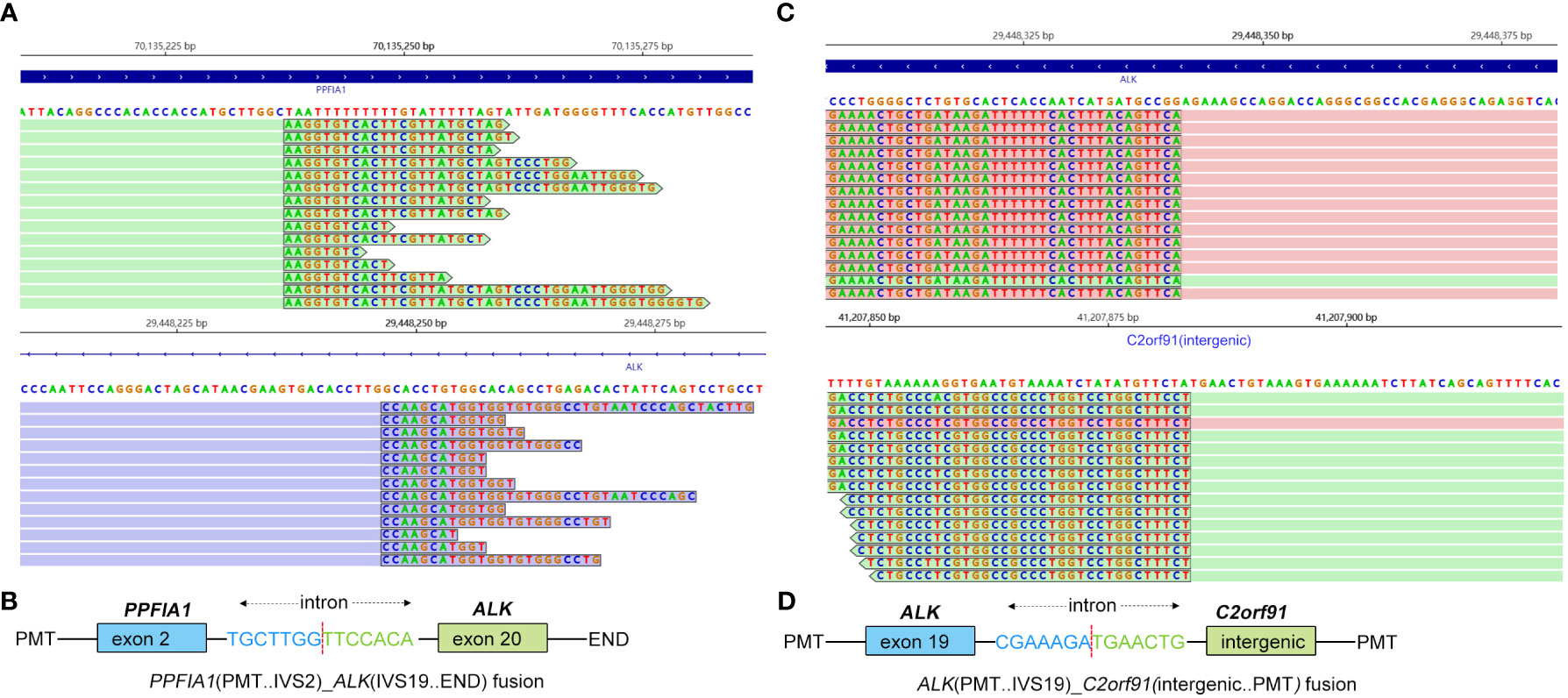
Figure 3 Identification of PPFIA1-ALK and ALK-C2orf91(intergenic) double-fusion variants by next-generation sequencing. (A) Sequencing reads of PPFIA1-ALK were visualized by the Integrative Genomics Viewer (IGV). (B) The schematic structure of the genomic DNA sequence shows the PPFIA1-ALK fusion points. (C) Sequencing reads of ALK-C2orf91(intergenic) were visualized by the IGV. (D) The schematic structure of the genomic DNA sequence shows the ALK-C2orf91(intergenic) fusion points.
After 2 months of alectinib therapy, a follow-up CT scan found the right lung tumor remarkably reduced, thus achieving a partial response based on Response Evaluation Criteria in Solid Tumors [RECIST] version 1.1 (Figure 1B). Follow-up CT scans at 9 months and 12 months showed reduced stable disease (Figures 1C, D). At the cutoff date of this study, the patient had been receiving alectinib for 12 months with no complaints of discomfort and no adverse effects.
Discussion
In summary, this is the first case to describe a novel PPFIA1-ALK and ALK-C2orf91 (intergenic) double-fusion lung adenocarcinoma patient who is sensitive to alectinib. Multiple ALK fusion types have been reported in NSCLC patients, among which different fusion partners and different breakpoint variants may affect the response to ALK-TKIs (7, 8). Previous reports described that HIP1-ALK (H21:A20) and HIP1-ALK (H30:A20) responded well to crizotinib (9, 10), while HIP1-ALK (H19:A20) showed resistance to crizotinib with progression of pleural effusion (8). Therefore, determining the efficacy of different ALK fusions to different ALK inhibitors is vital for personalized therapeutic decision-making.
As far as we know, PPFIA1-ALK fusion has not been reported in lung cancer before. PPFIA1 is a putative invasion suppressor gene located in the 11q13 region, while ALK is located in the short arm of chromosome 2. Amplification and overexpression of PPFIA1 were reported to be associated with poor prognosis in breast cancer (11). In the current case, exon 2 of the PPFIA1 gene rearranged with exon 20 of the ALK gene to form a new fusion gene, PPFIA1-ALK. Although there is no direct evidence to support PPFIA1-ALK as a driver mutation, considering that PPFIA1 has been reported to be highly expressed in lung tissues (12), there is a possibility that PPFIA1‐ALK rearrangement is a driver mutation.
According to the global ALEX study, alectinib showed superior progression-free survival (PFS) versus crizotinib in untreated EML4-ALK fusion NSCLC, irrespective of the EML4-ALK variant (13). For patients with 3′-ALK fusion, the retention of 5′-ALK fusion was defined as non-reciprocal fusion, which was predictive for worse PFS at first-line crizotinib (14). Therefore, ALK-C2orf91 (A19: intergenic) as 5′-ALK and PPFIA1-ALK (P2:A20) as 3′-ALK in our case were defined together as a non-reciprocal ALK fusion. Zeng reported an NSCLC patient with non-reciprocal ALK fusion after resistance to first-line gefitinib who responded to alectinib and had a PFS of more than 26 months. Similar to the previous report, the patient in the present case was also sensitive to alectinib and had been receiving alectinib for 12 months.
Conclusions
In summary, this case presented a rare novel non-reciprocal PPFIA1-ALK and ALK-C2orf91 (intergenic) double-fusion mutation sensitive to first-line alectinib.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of First Affiliated Hospital of Guangxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY: Data curation, Writing – original draft, Writing – review & editing. JZ: Data curation, Writing – review & editing. QP: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. PY: Investigation, Writing – original draft, Writing – review & editing. QC: Funding acquisition, Supervision, Writing – review & editing.
Funding
This work was supported by the Key project S2021104 of Guangxi Medical and Health Appropriate Technology Development, Promotion and Application, and "Medical Excellence Award" funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1264820/full#supplementary-material
References
1. Gainor JF, Varghese AM, Ou SH, Kabraji S, Awad MM, Katayama R, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res (2013) 19:4273–81. doi: 10.1158/1078-0432.CCR-13-0318
2. Ou SI. VW zhuM nagasaka catalog of 5' Fusion partners in ALK-positive NSCLC circa 2020. JTO Clin Res Rep (2020) 1:100015. doi: 10.1016/j.jtocrr.2020.100015
3. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med (2014) 371:2167–77. doi: 10.1056/NEJMoa1408440
4. Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet (2017) 389:917–29. doi: 10.1016/S0140-6736(17)30123-X
5. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med (2017) 377:829–38. doi: 10.1056/NEJMoa1704795
6. Xu Y, Yan J, Zhou C, Wu L, Wang H, Zhao J, et al. Genomic characterisation of de novo EGFR copy number gain and its impact on the efficacy of first-line EGFR-tyrosine kinase inhibitors for EGFR mutated non-small cell lung cancer. Eur J Cancer (2023) 188:81–9. doi: 10.1016/j.ejca.2023.04.009
7. Yoshida T, Oya Y, Tanaka K, Shimizu J, Horio Y, Kuroda H, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non-small-cell lung cancer. J Clin Oncol (2016) 34:3383–9. doi: 10.1200/JCO.2015.65.8732
8. Li M, Tang Q, Chen S, Wang Y. A novel HIP1-ALK fusion variant in lung adenocarcinoma showing resistance to Crizotinib. Lung Cancer (2021) 151:98–100. doi: 10.1016/j.lungcan.2020.11.014
9. Hong M, Kim RN, Song JY, Choi SJ, Oh E, Lira ME, et al. HIP1-ALK, a novel fusion protein identified in lung adenocarcinoma. J Thorac Oncol (2014) 9:419–22. doi: 10.1097/JTO.0000000000000061
10. Ou SH, Klempner SJ, Greenbowe JR, Azada M, Schrock AB, Ali SM, et al. Identification of a novel HIP1-ALK fusion variant in Non-Small-Cell Lung Cancer (NSCLC) and discovery of ALK I1171 (I1171N/S) mutations in two ALK-rearranged NSCLC patients with resistance to Alectinib. J Thorac Oncol (2014) 9:1821–5. doi: 10.1097/JTO.0000000000000368
11. Alfarsi LH, El Ansari R, Craze ML, Masisi BK, Ellis IO, Rakha EA, et al. PPFIA1 expression associates with poor response to endocrine treatment in luminal breast cancer. BMC Cancer (2020) 20:425. doi: 10.1186/s12885-020-06939-6
12. Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics (2014) 13:397–406. doi: 10.1074/mcp.M113.035600
13. Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol (2019) 14:1233–43. doi: 10.1016/j.jtho.2019.03.007
Keywords: PPFIA1-ALK, alectinib, NSCLC, NGS, non-reciprocal fusion
Citation: Yan L, Zheng J, Pan Q, Liang Y, Yu P and Chen Q (2023) Novel PPFIA1-ALK, ALK-C2orf91(intergenic) double-fusion responded well to alectinib in an advanced lung adenocarcinoma patient: a case report. Front. Oncol. 13:1264820. doi: 10.3389/fonc.2023.1264820
Received: 21 July 2023; Accepted: 08 August 2023;
Published: 29 August 2023.
Edited by:
James CM Ho, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Roland Leung, Queen Mary Hospital, Hong Kong SAR, ChinaWang Chun Kwok, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2023 Yan, Zheng, Pan, Liang, Yu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quanfang Chen, Y2hlbnF1YW5mYW5nNTU1QDE2My5jb20=
 Lingxin Yan1
Lingxin Yan1 Pengli Yu
Pengli Yu