
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 20 December 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1264281
 Toshimitsu Tanaka1,2
Toshimitsu Tanaka1,2 Sachiko Nagasu1,3
Sachiko Nagasu1,3 Takuya Furuta4
Takuya Furuta4 Mizuki Gobaru5
Mizuki Gobaru5 Hiroyuki Suzuki2
Hiroyuki Suzuki2 Yasutaka Shimotsuura1,2
Yasutaka Shimotsuura1,2 Jun Akiba4
Jun Akiba4 Masatoshi Nomura5
Masatoshi Nomura5 Fumihiko Fujita3
Fumihiko Fujita3 Takumi Kawaguchi2
Takumi Kawaguchi2 Keisuke Miwa1*
Keisuke Miwa1*The occurrence of fulminant type 1 diabetes mellitus as an adverse event during cancer immunotherapy has been previously reported. However, little is known about the causal relationship between the coronavirus disease 2019 (COVID-19) vaccination and fulminant type 1 diabetes mellitus. A 60-year-old man with advanced gastric cancer, receiving S-1 + oxaliplatin and nivolumab therapy, followed by nab-paclitaxel + ramucirumab as a second-line treatment, with steroid supplementation for complications of hypopituitarism-induced hypoadrenocorticism, was administered a COVID-19 vaccine after three cycles of nab-paclitaxel + ramucirumab. Two days later, he developed severe malaise and anorexia, which required emergency admission to our hospital for suspected adrenal insufficiency. Despite increasing steroids, his general condition changed suddenly after 12 hours leading to his death. Histopathological analysis of autopsy samples revealed loss of the islets of Langerhans, indicating fulminant type 1 diabetes mellitus. We failed to recognize the onset of fulminant type 1 diabetes mellitus because its symptoms were similar to those of adrenal insufficiency. The number of reports on the onset of fulminant type 1 diabetes mellitus after COVID-19 vaccination has been increasing, and in this case, the onset occurred on the second day after COVID-19 vaccination, suggesting an association between vaccination and fulminant type 1 diabetes mellitus. Clinicians should be aware of the risk of fulminant type 1 diabetes mellitus, although rare, after COVID-19 vaccination.
In treatment for advanced gastric cancer, recently, the approval of combination therapy involving, nivolumab, an immune checkpoint inhibitor (ICI) that blocks programmed cell death-1, and chemotherapy as first-line treatment has resulted in the increased overall survival and progression-free survival of patients (1, 2). ICIs, such as nivolumab, can cause imbalances in the immunological tolerance of patients and inflammatory side effects known as immune-related adverse events (irAEs), including pneumonitis, colitis, hepatitis, pancreatitis, and hypophysitis (3). While appropriate management can ameliorate most irAEs, severe irAEs can be fatal (4).
The COVID-19 pandemic is now a global public health problem; as the COVID-19 pandemic continues, COVID-19 vaccine development has progressed rapidly and several COVID-19 vaccines are now approved worldwide (5). Many cancer patients and healthy people have been vaccinated. In recent years, there have been increasing reports of patients developing fulminant type 1 diabetes mellitus (DM) after receiving the COVID-19 vaccine (6–12). However, no report has yet clarified the pathological findings of fulminant type 1 DM.
Here, we report the case of a patient who received the COVID-19 vaccine during steroid replacement therapy for hypopituitarism induced by immunotherapy for advanced gastric cancer and subsequently developed fulminant type 1 DM, resulting in death.
A 60-year-old Japanese man with unresectable advanced gastric cancer with multiple liver metastases (TNM cT3N3M1, stage IVB, well-differentiated adenocarcinoma) presented to our hospital in December 202X. The patient was initially treated with the SOX (S-1 + oxaliplatin) + nivolumab regimen (consisting of oral S-1 40 mg/m2 twice daily on days 1–14, intravenous oxaliplatin 130 mg/m² on day 1, and nivolumab 360 mg/body every 3 weeks) as first-line chemotherapy + immunotherapy. However, during the first cycle, the patient experienced grade 3 (CTCAE, version 5.0) diarrhea as an adverse event related to S-1; therefore, the chemotherapy regimen was changed to CAPOX (consisting of oral capecitabine 1000 mg/m² twice daily on days 1–14 and intravenous oxaliplatin 130 mg/m² on day 1, every 3 weeks). However, 4 months later, computed tomography revealed progressive disease due to primary tumor growth. The chemotherapy regimen was thus switched to nab-paclitaxel (100 mg/m² intravenously on days 1, 8, and 15) + ramucirumab (8 mg/kg intravenously on days 1 and 15) as second-line chemotherapy in April 202X+1. After the second cycle of treatment was initiated, anorexia, fatigue, and hyponatremia (116 mmol/L, reference interval: 138–145 mmol/L) were observed. His serum cortisol (0.36 μg/dL, reference interval: 6.24–18.00 μg/dL) and adrenocorticotropic hormone (ACTH) levels (<1.5 pg/mL, reference interval: 7.2–63.3 pg/mL) had decreased. The patient was referred to an endocrinology and metabolism specialist, who conducted a loading test and diagnosed him with hypopituitarism-induced adrenocortical hypofunction as an irAE at 5 months after the initial administration of nivolumab. Steroid therapy (hydrocortisone 20 mg/day) was immediately initiated, and his anorexia and fatigue improved. Consequently, steroid and nab-paclitaxel + ramucirumab therapy were continued. During the treatment course, the patient received the third dose of the COVID-19 vaccine (Pfizer-BioNTech, NY, USA). However, on the second day after vaccination, he suddenly developed severe anorexia and fatigue, requiring urgent admission to our hospital in June 202X+1 (6 months after the initial administration of nivolumab). The laboratory and genetic data obtained upon admission are detailed in Table 1.
The symptoms of appetite loss and fatigue and laboratory findings indicating hyponatremia meant that he was in a state of adrenal insufficiency. We, therefore, increased the hydrocortisone dose to 50 mg/day and initiated infusions to correct the hyponatremia. However, the patient’s symptoms did not improve and his fatigue worsened. Twelve hours after starting the treatment, sudden decline in his consciousness level and blood pressure was observed. His blood glucose level at this time was markedly elevated, exceeding 1,000 mg/dL. Insulin was immediately administered, but he soon experienced cardiopulmonary arrest. Despite attempting cardiopulmonary resuscitation, his heartbeat did not resume and he died (Figure 1). With the family’s consent, an autopsy was performed to determine the cause of death.
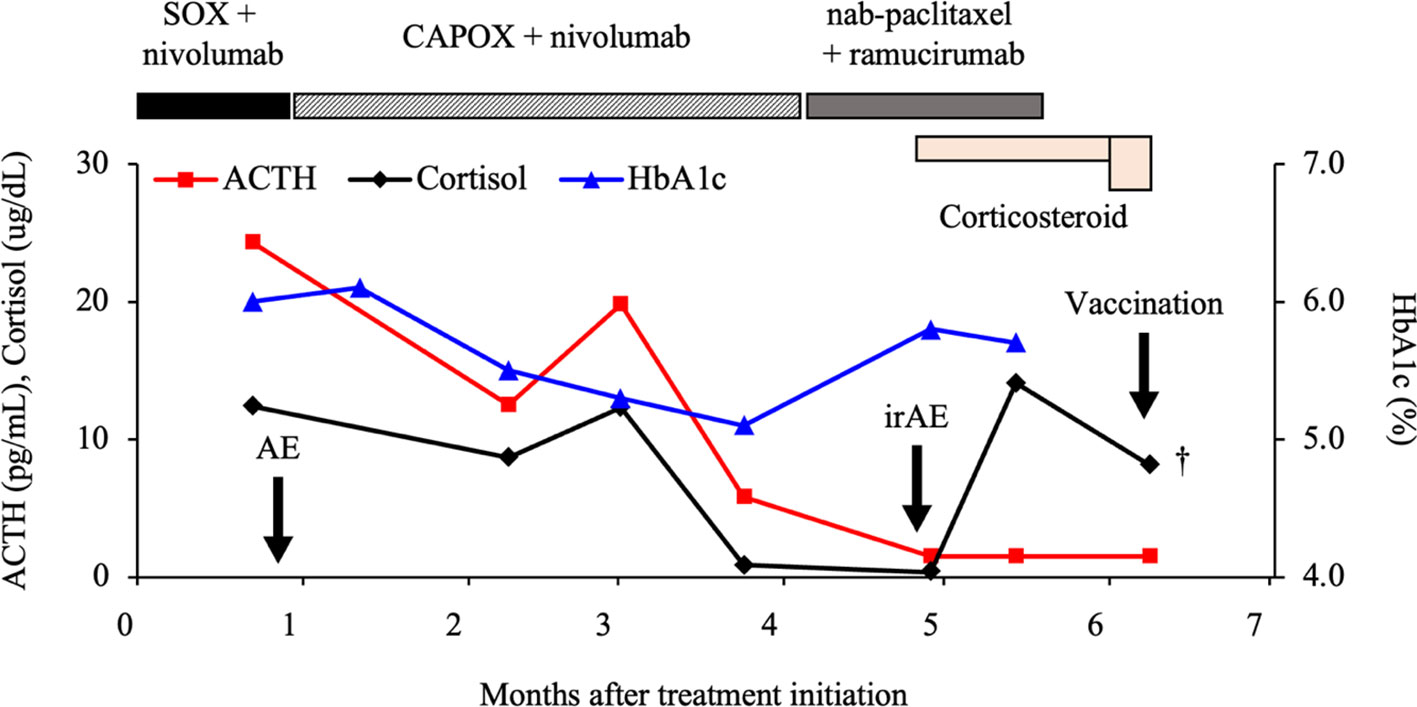
Figure 1 Clinical course of the patient. ACTH, adrenocorticotropic hormone; SOX, S-1 + oxaliplatin; CAPOX, capecitabine + oxaliplatin; HbA1c, hemoglobin A1c; AE, adverse event; irAE, immune-related AE.
Histopathological examination of the anterior pituitary gland revealed the presence of a focal lymphocytic infiltrate comprising CD3+ T cells (CD4<CD8). Negative periodic acid-Schiff staining and negative T-Pit expression indicated a decrease in ACTH-producing cells (13). Hence, the diagnosis of hypopituitarism due to selective disruption of ACTH-producing cells, an irAE induced by nivolumab, was established (Figure 2A). However, the absence of severe inflammation and necrosis indicated that it was not the direct cause of death. The structure of the adrenal cortex was intact, no inflammatory cells were observed, and mild lymphocytic infiltration was observed in the adrenal medulla; these were considered physiological occurrences and not the cause of death. The pancreas exhibited marked interstitial fibrosis, moderate CD3+ T-cell infiltration, with a similar pattern to that of the pituitary gland (CD4<CD8), and absence of synaptophysin- and insulin-positive cells and the islets of Langerhans (Figure 2B). More than two-thirds of the cancer cells in the primary gastric lesion were pathologically necrotic, and the liver metastases were completely necrotic. Therefore, the patient was diagnosed with fulminant type 1 DM. Considering the histopathological findings and clinical course, we concluded that the patient developed severe diabetic acidosis due to rapid hyperglycemia that led to circulatory failure and death.
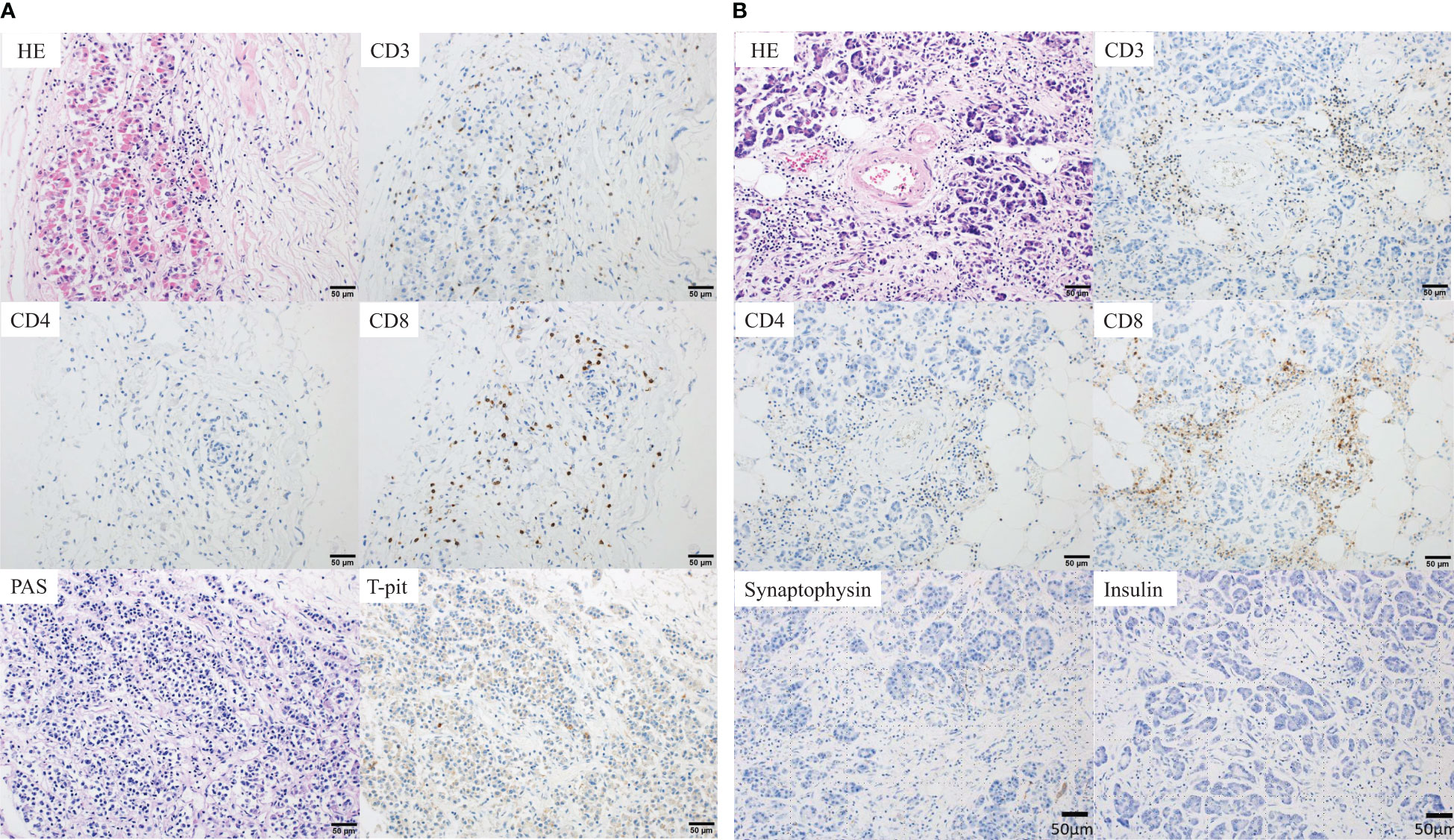
Figure 2 Pathological findings at autopsy. (A) Anterior pituitary gland. Histopathological findings showing focal lymphocytic infiltration comprising CD3+ T cells, suggesting cellular damage to the anterior pituitary gland. The number of infiltrating cells was 1, 0 and 1 per three high power fields (HPFs) for CD4+ T-cells and 62, 44, and 57 three HPFs for CD8+ T-cells. In addition, the negative periodic acid-Schiff (PAS) staining and negative T-Pit expression suggested that the number of ACTH-producing cells was reduced. (B) Pancreas. The pancreas showing marked interstitial fibrosis and moderate CD3+ T-cell infiltration (CD4<CD8), suggesting cellular damage to the pancreas. The number of infiltrating cells was 28, 14, and 13 per three HPFs for CD4+ T-cells and 107, 52 and 71 per three HPFs for CD8+ T-cells. Moreover, there were no synaptophysin- and insulin-positive cells, and the islets of Langerhans were completely absent. ACTH, adrenocorticotropic hormone; HE, hematoxylin and eosin staining.
Nivolumab + chemotherapy has become the standard first-line treatment for advanced gastric cancer after improved outcomes were observed in the CheckMate 649 and ATTRACTION-4 trials (1, 2). However, ICIs such as nivolumab can cause immune activation in non-target tissues, leading to irAEs in some patients (3). In the ATTRACTION-4 study, irAEs involving endocrine disturbance were reported in 11.4% of the patients, with 2.2% experiencing irAEs of grade 3–4 severity (2). Pituitary insufficiency, often presenting as ACTH deficiency, occurs in approximately 6% of the patients on ICIs (14, 15). The most severe form of pituitary insufficiency is ACTH insufficiency; it can lead to acute adrenal insufficiency and be fatal if treatment is delayed. Further, the prevalence of type 1 DM as an endocrine-related irAE due to nivolumab is <0.2% (16). Therefore, type 1 DM is considered a rare irAE. Although there are several reports of hypopituitarism and DM as irAEs caused by ICI (17, 18), to our knowledge, there is no report of hypopituitarism-induced adrenal hypofunction combined with fulminant type 1 DM.
Fulminant type 1 DM, a subtype of type 1 DM, was first identified in Japan and is commonly diagnosed in Asian populations (19). It is characterized by severe hyperglycemia with ketosis, nearly normal hemoglobin A1c (HbA1c) level, and lack of insulin secretion at onset. Other features include absence of detectable levels of islet autoantibodies, elevated levels of pancreatic enzymes, and frequent influenza-like symptoms before DM onset (14). In our patient’s case, although the HbA1c level was within the normal range, since the tests for acidosis and insulin secretory capacity were not performed and the diagnostic criteria for fulminant type 1 DM (16) were not met, hyperglycemia was observed just before death. Pathological findings from the autopsy revealed the near-complete disappearance of the islets of Langerhans, leading to the diagnosis of fulminant type 1 DM. In addition, no insulin antibodies were present in the islets of Langerhans, suggesting endogenous insulin deficiency. The presence of CD3+ T cells and predominance of CD8+ cells in the pancreatic tissue suggest that an immune response contributed to the destruction of pancreatic beta cells and development of fulminant type 1 DM. However, cases of fulminant type 1 DM after COVID-19 vaccination even without prior ICI treatment has been reported (Table 2), and these findings alone cannot distinguish between fulminant type 1 DM due to irAE and fulminant type 1 DM as a pure adverse event of COVID-19 vaccination. In the future, the histopathology of fulminant type 1 DM should be carefully monitored.
The initial irAE was adrenal insufficiency due to hypopituitarism, which showed improvement with hydrocortisone administration. The patient’s chief complaint was anorexia and fatigue, and blood tests revealed decreased cortisol, ACTH, and sodium levels, supporting the diagnosis of severe adrenal insufficiency. In addition, we did not suspect type 1 DM, which is an infrequent complication, as there were no findings suggestive of metabolic ketoacidosis, such as Kussmaul breathing or acetone odor. However, pathological findings from the autopsy revealed no adrenal insufficiency, and hypopituitarism caused by irAEs was mild and not a direct cause of death. Consequently, the increased hydrocortisone dose may have aggravated the type 1 DM, leading to death.
As shown in Table 2, an increasing number of reports has recently suggested an association between COVID-19 vaccination and fulminant type 1 DM (6–12). Hence, the disease may develop relatively early and with a variety of symptoms, regardless of the type of vaccine, number of vaccinations, history of DM, and prior treatment. The vaccine may have triggered the disease in this case as well. Although recent reports suggest that the COVID-19 mRNA vaccine sometimes exacerbates autoimmune diseases (15), the mechanism by which the COVID-19 vaccination causes fulminant type 1 DM remains unclear.
Our patient who received the COVID-19 vaccine during steroid replacement therapy for advanced gastric cancer subsequently developed fulminant type 1 DM. Had we been aware of the possibility of fulminant type 1 DM, we could have detected it early and saved the patient. Clinicians should keep in mind the possibility of their patient developing fulminant type 1 DM, although rare, after COVID-19 vaccination.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the studies involving humans because, since this is a case report of a specific subject for publication in a journal, our hospital does not require Ethics Committee approval, provided that personal information is kept in mind and written informed consent is obtained from the subject or the subject’s family. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patient's next of kin for the publication of this case report.
TT: Writing – original draft. SN: Data curation, Writing – review & editing. TF: Investigation, Writing – review & editing. MG: Investigation, Writing – review & editing. HS: Visualization, Writing – review & editing. YS: Data curation, Writing – review & editing. JA: Investigation, Writing – review & editing. MN: Investigation, Writing – review & editing. FF: Supervision, Writing – review & editing. TK: Supervision, Writing – review & editing. KM: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We extend our deepest sympathies to the patient’s family and friends and our sincere gratitude to the family for consenting to the autopsy and publication of the case report. We also thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (Checkmate 649): A randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
2. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with her2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (Attraction-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(2):234–47. doi: 10.1016/S1470-2045(21)00692-6
3. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: A prospective cohort study. J Thorac Oncol (2017) 12(12):1798–805. doi: 10.1016/j.jtho.2017.08.022
4. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol (2016) 2(10):1346–53. doi: 10.1001/jamaoncol.2016.1051
5. Creech CB, Walker SC, Samuels RJ. Sars-Cov-2 vaccines. JAMA (2021) 325(13):1318–20. doi: 10.1001/jama.2021.3199
6. Kobayashi T, Yakou F, Saburi M, Hirose A, Akaoka H, Hirota Y, et al. New-onset atypical fulminant type 1 diabetes after Covid-19 vaccination: A case report. Clin Case Rep (2022) 10(10):e6473. doi: 10.1002/ccr3.6473
7. Ohuchi K, Amagai R, Tamabuchi E, Kambayashi Y, Fujimura T. Fulminant type 1 diabetes mellitus triggered by coronavirus disease 2019 vaccination in an advanced melanoma patient given adjuvant nivolumab therapy. J Dermatol (2022) 49(5):e167–e8. doi: 10.1111/1346-8138.16304
8. Sakurai K, Narita D, Saito N, Ueno T, Sato R, Niitsuma S, et al. Type 1 diabetes mellitus following Covid-19 Rna-based vaccine. J Diabetes Investig (2022) 13(7):1290–2. doi: 10.1111/jdi.13781
9. Sasaki K, Morioka T, Okada N, Natsuki Y, Kakutani Y, Ochi A, et al. New-onset fulminant type 1 diabetes after severe acute respiratory syndrome Coronavirus 2 vaccination: A case report. J Diabetes Investig (2022) 13(7):1286–9. doi: 10.1111/jdi.13771
10. Tang X, He B, Liu Z, Zhou Z, Li X. Fulminant type 1 diabetes after Covid-19 vaccination. Diabetes Metab (2022) 48(2):101324. doi: 10.1016/j.diabet.2022.101324
11. Huang L, Liang M, He Y. New-onset fulminant type 1 diabetes following Sars-Cov-2 protein subunit vaccine: A case report and literature review. J Korean Med Sci (2023) 38(24):e209. doi: 10.3346/jkms.2023.38.e209
12. Nishino K, Nakagawa K, Yase E, Terashima M, Murata T. Diabetic ketoacidosis after the second dose of Sars-Cov-2 Mrna vaccination in a patient with pembrolizumab-induced fulminant type 1 diabetes. Diabetol Int (2023) 14(2):206–10. doi: 10.1007/s13340-022-00614-w
13. Sjostedt E, Bollerslev J, Mulder J, Lindskog C, Ponten F, Casar-Borota O. A specific antibody to detect transcription factor T-pit: A reliable marker of corticotroph cell differentiation and a tool to improve the classification of pituitary neuroendocrine tumours. Acta Neuropathol (2017) 134(4):675–7. doi: 10.1007/s00401-017-1768-9
14. Iwama S, Arima H. [Clinical practice and mechanism of endocrinological adverse events associated with immune checkpoint inhibitors]. Nihon Rinsho Meneki Gakkai Kaishi (2017) 40(2):90–4. doi: 10.2177/jsci.40.90
15. Kobayashi T, Iwama S, Yasuda Y, Okada N, Okuji T, Ito M, et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both Malignant melanoma and non-small cell lung carcinoma: A prospective study. J Immunother Cancer (2020) 8(2):1–4. doi: 10.1136/jitc-2020-000779
16. Imagawa A, Hanafusa T, Awata T, Ikegami H, Uchigata Y, Osawa H, et al. Report of the committee of the Japan diabetes society on the research of fulminant and acute-onset type 1 diabetes mellitus: new diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig (2012) 3(6):536–9. doi: 10.1111/jdi.12024
17. Marchand L, Paulus V, Fabien N, Perol M, Thivolet C, Vouillarmet J, et al. Nivolumab-induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response. J Thorac Oncol (2017) 12(11):e182–e4. doi: 10.1016/j.jtho.2017.07.021
18. Boswell L, Casals G, Blanco J, Jimenez A, Aya F, de Hollanda A, et al. Onset of fulminant type 1 diabetes mellitus following hypophysitis after discontinuation of combined immunotherapy. A case report. J Diabetes Investig (2021) 12(12):2263–6. doi: 10.1111/jdi.13604
Keywords: Fulminant type 1 diabetes mellitus, gastric cancer, immune-related adverse event, nivolumab, COVID-19 vaccination, Autopsy
Citation: Tanaka T, Nagasu S, Furuta T, Gobaru M, Suzuki H, Shimotsuura Y, Akiba J, Nomura M, Fujita F, Kawaguchi T and Miwa K (2023) Case report: A case of fulminant type 1 diabetes mellitus after COVID-19 vaccination during treatment of advanced gastric cancer: pitfall in managing immune-related adverse events. Front. Oncol. 13:1264281. doi: 10.3389/fonc.2023.1264281
Received: 20 July 2023; Accepted: 14 November 2023;
Published: 20 December 2023.
Edited by:
Xiangsheng Zuo, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Yasunori Deguchi, Ijinkai Takeda General Hospital, JapanCopyright © 2023 Tanaka, Nagasu, Furuta, Gobaru, Suzuki, Shimotsuura, Akiba, Nomura, Fujita, Kawaguchi and Miwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keisuke Miwa, bWl3YV9rZWlzdWtlQG1lZC5rdXJ1bWUtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.