
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 22 December 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1260796
This article is part of the Research Topic Recent Advances and New Challenges in Minimally Invasive Surgery and Chemotherapy for Colorectal Cancer View all 7 articles
 Tao Zhang
Tao Zhang Yong chang Miao*
Yong chang Miao*Objective: To investigate the impact of preoperative systemic immune inflammatory index (SII) on the clinical prognosis of patients undergoing colorectal cancer (CRC) surgery.
Methods: One hundred and sixty CRC patients who underwent surgical treatment in our gastrointestinal surgery department from January 2019 to May 2023 were collected. ROC curves were applied to determine the sensitivity and specificity of SII, determine the optimal cut-off value into low SII and high SII groups, compare the clinicopathological data of SII patients in the two groups, and analyze the postoperative survival of patients in the two groups using Kaplan-Meier and Log-rank methods. Univariate and multifactor COX proportional risk regression models were used to analyze clinical prognostic factors.
Results: The ROC curve showed that the area under the curve of SII for the evaluation of OS in CRC patients was 0.859, and the best cut-off value was 513.53. There was statistical significance (P < 0.05) in terms of tissue grading and diabetes mellitus in both groups. The Kaplan-Meier survival curves showed that the overall survival rates of the SII<513.53 group and the SII≥513.53 group were 50.88% (29/57) and 32.04% (33/103), and the overall survival rate of the SII<513.53 group was significantly higher than that of the SII≥513.53 group, and the difference was statistically significance (χ2 = 8.375, P=0.004). COX proportional risk regression showed that TNM stage, lymph node metastases, anastomotic fistula and SII were independent risk factors affecting postoperative survival in patients with CRC.
Conclusion: Preoperative SII is an independent prognostic factor for CRC, which is simple, convenient, and non-invasive, and can be used to predict the prognosis of CRC patients.
Colorectal cancer (CRC) is the third most common malignant tumor and the second leading cause of death in the world (1). In China, due to changes in living standards, lifestyles, and dietary habits in recent years, the incidence of CRC has been increasing, with colorectal cancer ranking third and fifth in terms of incidence and mortality rates, and in 2020, about 9.4 percent of deaths will be due to CRC disease (2). Currently, CRC is mainly surgical, but postoperative recurrence and metastasis severely limit the prognosis of CRC. In clinical practice, pathological type, tissue grading, and TNM stage are the most commonly used methods to predict the prognosis of CRC; however, prognostic heterogeneity still exists in patients with the same TNM stage (3). Inflammatory response is a key component of tumor development and a major cause of prognosis in tumor patients. There has been a marked increase in research on the relationship between inflammation and tumors, and combinations of these systemic inflammatory parameters, such as systemic immune-inflammatory index (SII), neutrophil-to-lymphocyte ratio (NLR), and platelet-lymphocyte ratio (PLR), are markers of active tumor inflammation (4, 5), which play an important role in promoting tumor progression. Generally, an increase in NLR, PLR, and SII is associated with a poor prognosis in tumor patients (6–11), and recent studies (12–14) have shown that the composite inflammatory markers of NLR, PLR, and SII can also be used as prognostic predictors for colorectal cancer patients. Although cancer is strongly associated with inflammation, the mechanisms linking patients with poor prognosis to elevated SII, NLR, and PLR need to be further investigated (15). In this paper, we retrospectively analyzed the clinical data of 160 cases of CRC patients admitted to our hospital from January 2019 to May 2023, and analyzed the effects of preoperative peripheral blood levels of SII, NLR, and PLR on the clinical prognosis of CRC patients, to provide a certain reference for CRC prognosis.
Retrospective analysis of clinicopathologic data of 160 patients who underwent CRC surgical treatment at the Department of Gastrointestinal Surgery of the Second People’s Hospital of Lianyungang City between January 2019 to May 2023. The flow chart is shown in Figure 1. Tumor TNM staging is based on the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging criteria for colorectal cancer.
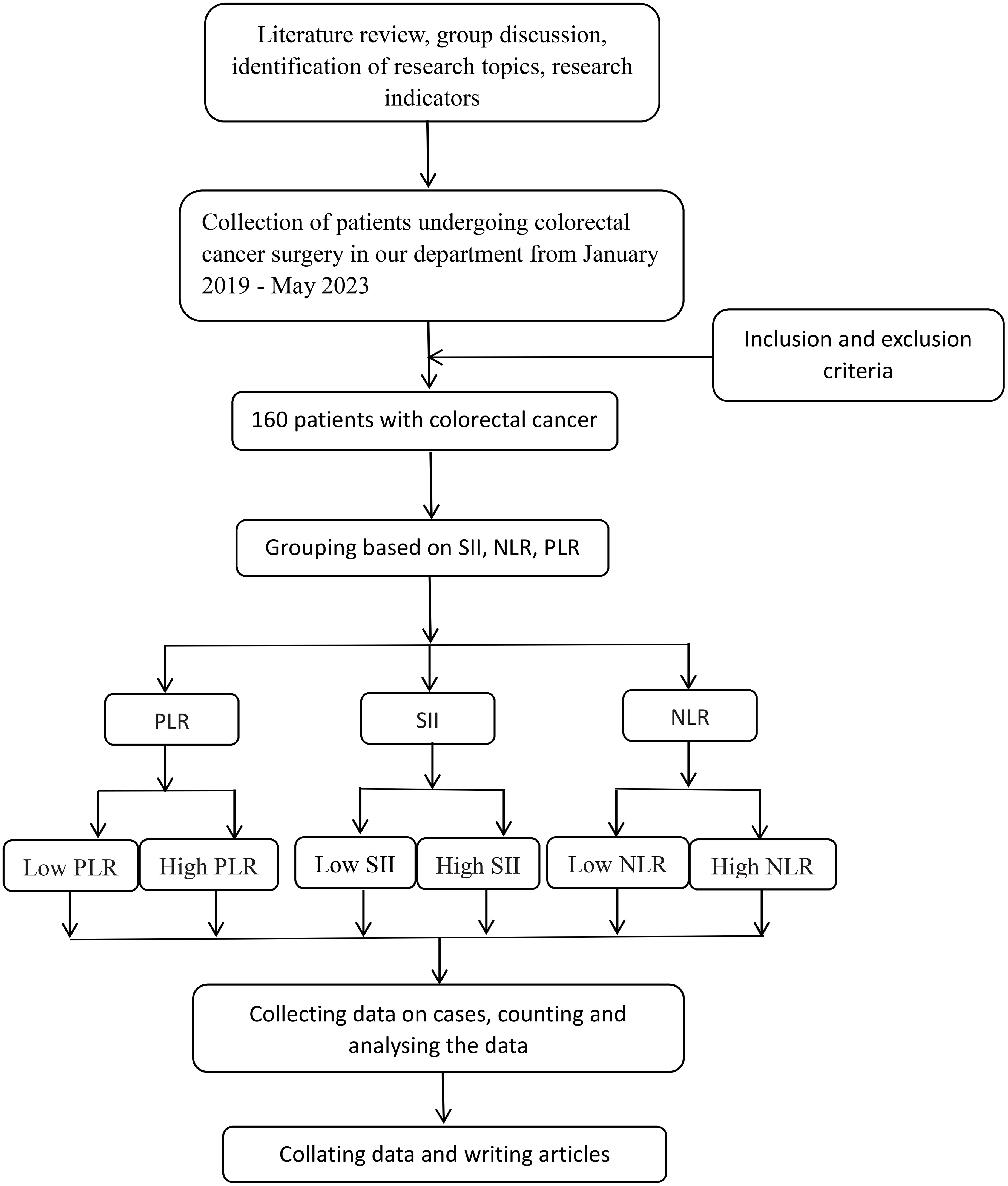
Figure 1 The flowchart of this paper is shown below. NLR, neutrophil-to-lymphocyte ratio; SII: systemic immune inflammatory index; PLR, platelet-to-lymphocyte ratio.
Inclusion criteria: (1) All the selected patients underwent radical CRC surgery; (2) All the postoperative pathologies were CRC; (3) The patients did not undergo radiotherapy, chemotherapy and other immunotherapy before surgery.
Exclusion criteria: (1) Combination of other primary tumors; (2) Other serious infectious diseases, autoimmune diseases, etc. before surgery; (3) Blood diseases or history of trauma, blood transfusion, etc. before surgery; (4) Those who lost the visit after surgery; (5) Those who had missing pathological clinical data.
General clinicopathological data such as age, gender, TNM stage, tumor diameter, diabetes mellitus, and coronary artery disease were collected, and patients were grouped according to the critical values of age and mean tumor diameter;
A combination of outpatient, telephone, and We Chat was used for follow-up, with the first follow-up at 1 month after surgery, every 1 to 3 months during the first year after surgery, every 6 months during the second year after surgery, and annually from the third year after surgery onwards. The follow-up cut-off time was May 2023 or the patient’s death, and the overall survival (OS) was from the date of admission to the final follow-up cut-off time or the time of death.
All data were analyzed using SPSS26.0 software for statistical analysis and processing of data, to establish the receive operating characteristic (ROC) curve, according to the Yo den index (Yo den index = sensitivity + specificity-1) the critical value corresponding to the maximum point was defined as the optimal truncation value of SII, NLR and PLR, and to calculate the sensitivity, sensitivity and area under the curve (AUC). The sensitivity, sensitivities and AUC were also calculated; Patients were grouped according to the SII optimal cutoff value; measurements were expressed using , and counts were expressed as cases (%) using the χ2 test or Fishers exact test; Survival analysis was performed using the Kaplan-Meier method to plot survival curves and the Log-rank method to compare the survival differences between groups, to plot survival curves, and to compare the overall survival Overall survival (OS) of patients in different subgroups; All clinicopathological factors and SII were included in the univariate analysis, and multivariate analysis was performed for variables with meaningful differences, and the COX regression model was used to analyze the effect of each clinicopathological index on prognosis. Hazard ratio (HR) and 95% confidence interval (CI) were used to assess the relative risk, and P < 0.05 indicated a statistically significant difference.
The optimal intercept values for SII, NLR and PLR were selected based on the ROC curves, from which the patients were divided into two groups, high and low SII, high and low NLR, and high and low PLR, Table 1 and Figure 2.
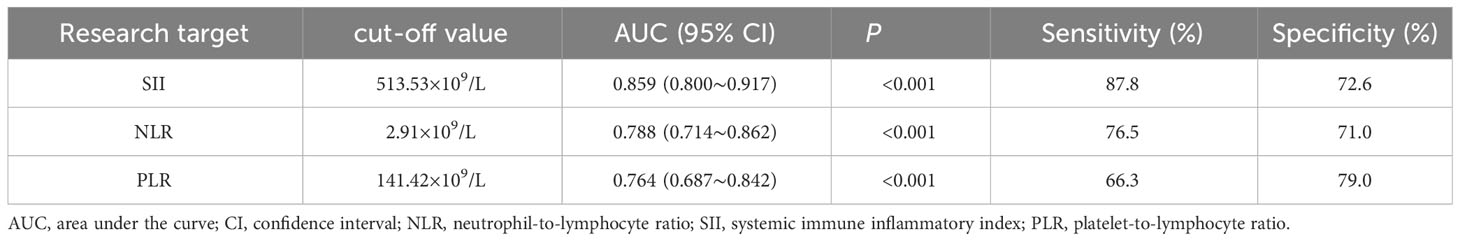
Table 1 Analysis of diagnostic efficacy of SII, NLR and PLR in predicting patients with colorectal cancer.
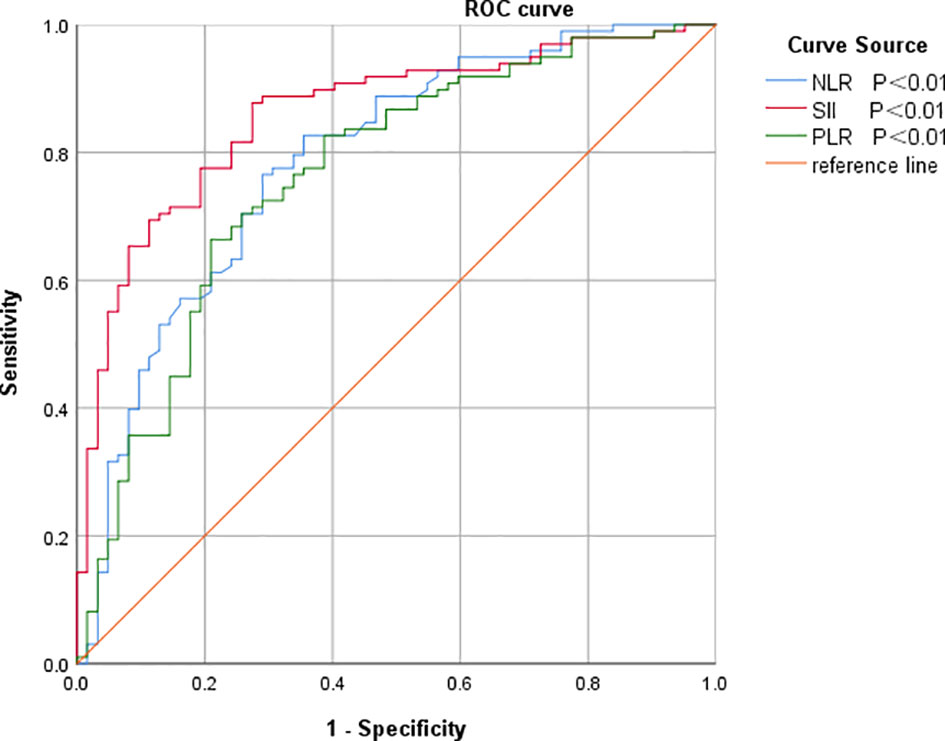
Figure 2 ROC curves of SII, NLR and PLR for predicting prognosis in colorectal cancer patients. NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune inflammatory index; PLR, platelet-to-lymphocyte ratio.
Clinicopathologic data of the whole group of 160 patients, the difference between SII and patients’ tissue grading and whether they had diabetes mellitus was statistically significant (P<0.05), The difference in terms of tissue grading between NLR and patients was statistically significant (P<0.05), The difference between PLR and patients in terms of gender and TNM staging was statistically significant (P<0.05) Table 2.
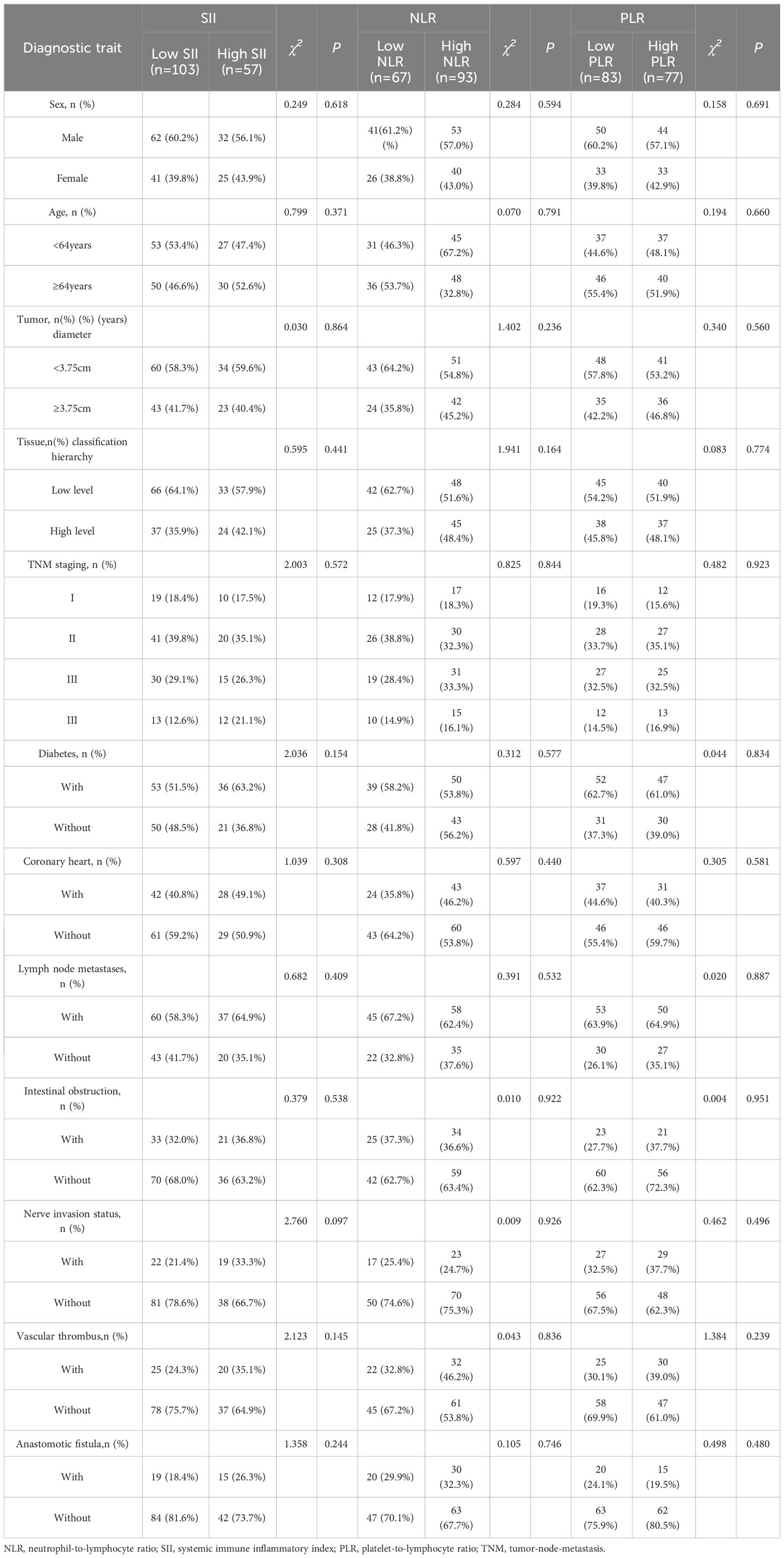
Table 2 Relationship between SII, NLR and PLR and clinicopathologic factors in patients with colorectal cancer.
Survival analysis was complete in 160 patients with a mean follow-up of 29.25 (2-60) months, with 62 (38.75%) survivors and 98 (61.25%) deaths by the time of the last follow-up. The overall survival rates of the SII <513.53 and SII≥513.53 groups were 50.88% (29/57) and 32.04% (33/103), and the overall survival rate of the SII<513.53 group was significantly higher than that of the SII≥513.53 group, and the difference was statistically significant (χ2 = 8.375, P=0.004), Figure 3. The results of univariate analysis showed statistically significant differences in terms of tumor diameter, TNM stage, lymph node metastases, anastomotic fistula, intestinal obstruction, vascular thrombus, nerve invasion status and SII; the inclusion of univariate significant factors in the COX multifactorial analysis showed that patient’s TNM stage, lymph node metastases, anastomotic fistula and SII were independent risk factors affecting the prognosis of CRC patients Table 3.
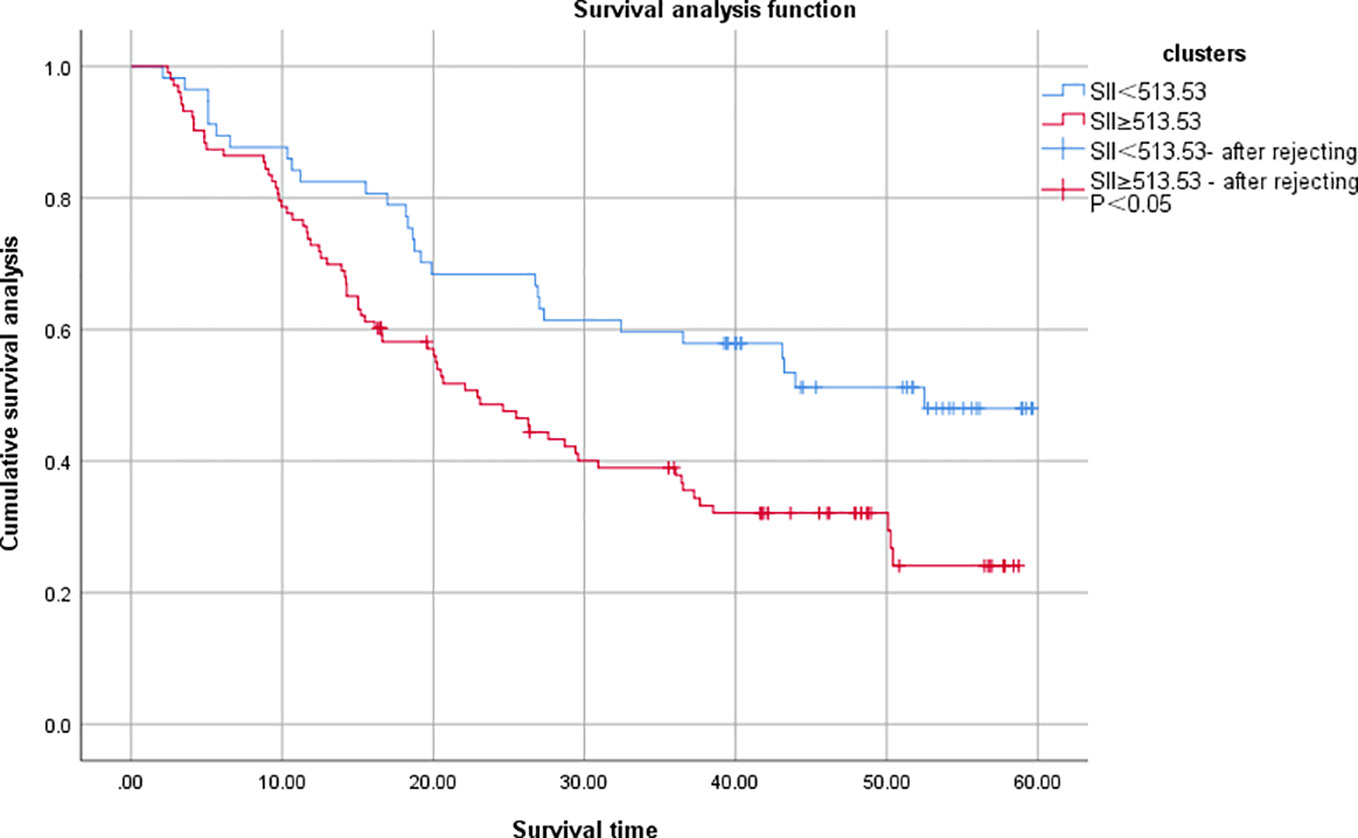
Figure 3 Comparison of OS between two groups of colorectal cancer patients. SII, systemic immune inflammatory index.
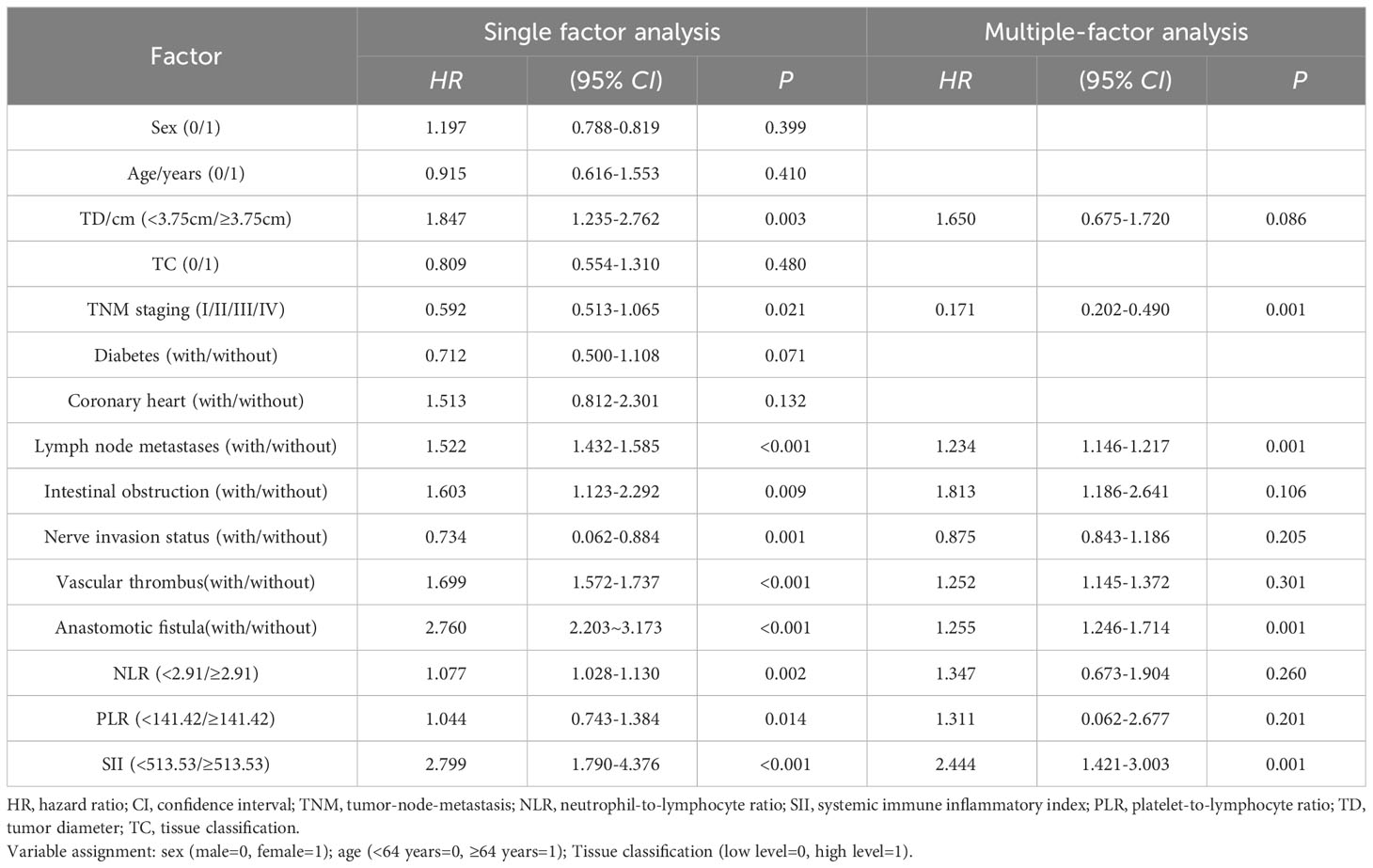
Table 3 Unifactorial and multifactorial analyses affecting the prognosis of patients with colorectal cancer.
CRC is one of the most common malignant tumors of the gastrointestinal tract, with inconspicuous early manifestations, and most of them are already in the middle and late stages when they are detected. In recent years, the incidence of CRC has been increasing year by year, and it is mostly found in middle-aged and elderly people. Due to the highly invasive and malignant nature of the tumor, it leads to poor prognosis, high mortality and short survival time. Currently, surgery is the main treatment for CRC patients, and postoperative adjuvant radiotherapy, targeted therapy and immunotherapy and other comprehensive treatment methods have made great progress in treatment, but the survival time after surgery is still not significantly improved. Therefore, it is very meaningful to actively seek and explore the influencing factors that predict the prognosis of CRC patients.
Inflammation is an important component of the tumor microenvironment, and peripheral blood inflammatory cells can directly or indirectly interact with tumor cells, thereby promoting tumor cell proliferation, migration and invasion, and inhibiting apoptosis (16, 17). In recent years, the role of inflammatory factors in malignant tumors has received increasing attention, including neutrophils, platelets and lymphocytes, which are major factors in angiogenesis, invasion and metastasis in the tumor-associated inflammatory microenvironment (18–20). Neutrophils can directly promote tumor proliferation, metastasis, and local angiogenesis through the secretion of a variety of pro-angiogenic factors, and they also play an important role in the migration and invasion of circulating tumor cells (21). Yang et al. (22) showed that a high serum neutrophil count was associated with OS and progression-free survival in patients with metastatic colorectal cancer Ras wild type. There is growing experimental and clinical evidence that platelet activation can act as a chemotactic agent for cancer cells, inducing optimal conditions for the formation of metastatic foci, and that platelets promote the survival of cells with high metastatic potential during their haemotransport (23). Lymphopenia is usually accompanied by leukocytosis and thrombocytosis, which may help tumor cells evade immune surveillance and prevent damage caused by an autoimmune response of cytotoxic T cells (20). SII first studied by Hu et al. (24) in hepatocellular carcinoma, is a new inflammatory index based on neutrophils, platelets and lymphocytes, which combines the three types of immune-inflammatory cells mentioned above, and can comprehensively reflect the balance between immune and inflammatory responses in patients with tumors. Elevated levels of SII are mostly caused by the elevation of neutrophils and platelets, and the reduction of lymphocyte levels, suggesting that patients have increased inflammatory responses and weakened immune responses, indicating a higher risk of tumor recurrence and a worse prognosis. Elevated SII is mostly caused by elevated neutrophils and platelets and decreased lymphocyte levels, suggesting that the patient’s inflammatory response is enhanced and the immune response is weakened, indicating that the patient has a higher risk of tumor recurrence and a worse prognosis.
The objective of this study was to discuss the clinicopathological and prognostic value of systemic inflammatory markers, including SII, NLR, and PLR, in patients with colorectal cancer, and to compare their predictive accuracy. After analyzing the predictive value of inflammatory indicators for CRC by ROC curve, the best cut-off value was determined, and it was found that the best cut-off values of SII, NLR and PLR for CRC prediction were 513.53, 2.91, and 141.42, with the AUCs of 0.859, 0.788, and 0.764, respectively, which indicated that the three inflammatory indicators had good predictive value for CRC, but the predictive value of SII was higher than that of NLR and PLR. value was higher than NLR and PLR.According to the best cut-off value of SII, all patients were divided into a low SII group and a high SII group.Kaplan-Meier survival analysis found that the OS of the low SII group was significantly better than that of the high SII group, and the survival period was longer, which was consistent with the studies of Chen et al. (25) and Xie et al. (26). The results of univariate and multifactorial analyses showed that patients’ TNM stage, lymph node metastases, anastomotic fistula and SII were independent risk factors affecting the prognosis of CRC patients.
In the present study, the cut-off values of SII, NLR and PLR for predicting CRC were 513.53, 2.91 and 141.42, respectively, which were different from the cut-off values of other studies. There are a number of reasons for the difference regarding the cut-off values. Firstly, the present study was retrospective, single center, small sample size and there may be confounding errors; Secondly, some studies have taken different times for blood routines, resulting in potentially large differences in some indicators of inflammation; Again, some studies have inconsistent inclusion and exclusion criteria, which may also affect the cut off values; Finally, in patients with colorectal cancer, differences in surgical approach and operator experience can also lead to some differences in cut off values. Taken together, these can cause some differences in cut-off values.
The author’s analysis of the predictive value of SII for tumor recurrence may be based on the following aspects. (1) Neutrophils may infiltrate into the tumor microenvironment and become tumor-associated neutrophils, releasing chemical and cytokines associated with tumor proliferation and metastasis, such as vascular endothelial growth factor (VEGF), elastase and matrix metalloproteinases (27). (2) Lymphocytes, as the most important immune cells of the body, when stimulated by antigens, T-lymphocytes will trigger specific immune responses and participate in the immune response to tumors, thus inhibiting tumor growth and improving the prognosis of cancer patients (28). Therefore, the lower the lymphocyte level, the worse the patient’s immune ability; while elevated neutrophils will inhibit the activation of T-lymphocytes, leading to a reduction in the body’s anti-tumor ability. (3) Platelets originate from megakaryocytes in the bone marrow, which not only have the function of coagulation, but are also related to the occurrence and development of tumors. Tumor cells can damage vascular endothelium, activate platelets to release vascular endothelial growth factor to repair endothelial cells, promote the formation of tumor neovascularization and the adhesion of tumor cells to the vascular wall, resulting in tumor cell proliferation and metastasis, and thus forming a vicious cycle (29).Therefore, higher neutrophil and platelet counts and lower lymphocyte counts, i.e. high SII tend to suggest that patients are at higher risk of tumor recurrence and have a worse prognosis.
In conclusion, preoperative SII can be used as an effective predictor for determining the prognosis of CRC patients, which is worth promoting and applying in clinical practice. However, this study still has some shortcomings, firstly it is a single-center, small-sample retrospective study and there may be a case selection bias; Secondly, this study did not analyze whether the occurrence of postoperative complications had an impact on survival; Finally, different surgical experiences of surgeons can also lead to differences in postoperative tumor recurrence. Therefore, prospective, large sample and multi-center studies are still needed to verify the value of SII in predicting tumor recurrence in CRC patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the Second People’s Hospital of Lianyungang. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TZ: Conceptualization, Writing – original draft, Writing – review & editing. YM: Supervision, Writing – review & editing, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received financial support from the “521 High-level Talents Training Project” of Lianyungang City (LYG06521202127).
We wish to express our sincere appreciation to all the reviewers for their contribution to this article and their insightful comments.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health (2023) 11(2):e197–206. doi: 10.1016/S2214-109X(22)00501-0
3. Hari DM, Leung AM, Lee J-H, Sim MS, Vuong B, Chiu CG, et al. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? J Am Coll Surg (2013) 217(2):181–90. doi: 10.1016/j.jamcollsurg.2013.04.018
4. Zhang J, Zhang H-Y, Li J, The elevated NLR. PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget (2017) 8(40):68837–46. doi: 10.18632/oncotarget.18575
5. Guo Y, Cai K, Mao S, Zhang J, Wang L, Zhang Z, et al. Preoperative C-reactive protein/albumin ratio is a significant predictor of survival in bladder cancer patients after radical cystectomy: a retrospective study. Cancer Manag Res (2018) 10. doi: 10.2147/CMAR.S180301
6. Bugada D, Allegri M, Lavand'homme P, De Kock M, Fanelli G. Inflammation-based scores: a new method for patient-targeted strategies and improved perioperative outcome in cancer patients. BioMed Res Int (2014) 2014. doi: 10.1155/2014/142425
7. Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol (2012) 22(1):33–40. doi: 10.1016/j.semcancer.2011.12.005
8. Ahmad J, Grimes N, Farid S, Morris-Stiff G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int (2014) 13(5):474–81. doi: 10.1016/S1499-3872(14)60284-8
9. Yang T, Mao P, Chen X, Niu X, Xu G, Bai X, et al. Inflammatory biomarkers in prognostic analysis for patients with glioma and the establishment of a nomogram. Oncol Lett (2019) 17(2):2516–22. doi: 10.3892/ol.2018.9870
10. Mcmillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care (2009) 12(3):223–6. doi: 10.1097/MCO.0b013e32832a7902
11. Chang X, Zhang F, Liu T, Liu T, Wang W, Guo H. Neutrophil-to-lymphocyte ratio as an independent predictor for survival in patients with localized clear cell renal cell carcinoma after radiofrequency ablation: a propensity score matching analysis. Int Urol Nephrol (2017) 49(6):967–74. doi: 10.1007/s11255-017-1554-6
12. Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med (2021) 10(17):5983–97. doi: 10.1002/cam4.4143
13. Jia W, Yuan L, Ni H, Xu B, Zhao P. Prognostic value of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and lymphocyte-to-white blood cell ratio in colorectal cancer patients who received neoadjuvant chemotherapy. Technol Cancer Res Treat (2021) 20. doi: 10.1177/15330338211034291
14. Dong M, Shi Y, Yang J, Zhou Q, Lian Y, Wang D, et al. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol (2020) 12. doi: 10.1177/1758835920937425
15. Wang S, Ma Y, Sun L, Shi Y, Jiang S, Yu K, et al. Prognostic significance of pretreatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in patients with diffuse large B-cell lymphoma. BioMed Res Int (2018) 2018. doi: 10.1155/2018/9651254
16. Jinushi M. Yin and yang of tumor inflammation: how innate immune suppressors shape the tumor microenvironments. Int J Cancer (2014) 135(6):1277–85. doi: 10.1002/ijc.28626
17. Dmitrieva-Posocco O, Dzutsev A, Posocco DF, Hou V, Yuan W, Thovarai V, et al. Cell-type-specific responses to interleukin-1 control microbial invasion and tumor-elicited inflammation in colorectal cancer. Immunity (2019) 50(1). doi: 10.1016/j.immuni.2018.11.015
18. Felix K, Gaida MM. Neutrophil-derived proteases in the microenvironment of pancreatic cancer -active players in tumor progression. Int J Biol Sci (2016) 12(3):302–13. doi: 10.7150/ijbs.14996
19. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell (2011) 20(5):576–90. doi: 10.1016/j.ccr.2011.09.009
20. Quigley DA, Kristensen V. Predicting prognosis and therapeutic response from interactions between lymphocytes and tumor cells. Mol Oncol (2015) 9(10):2054–62. doi: 10.1016/j.molonc.2015.10.003
21. Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood (2019) 133(20):2159–67. doi: 10.1182/blood-2018-11-844548
22. Yang J, Guo X, Wang M, Ma X, Ye X, Lin P. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep (2017) 7(1):17166. doi: 10.1038/s41598-017-17130-6
23. Lucotti S, Muschel RJ. Platelets and metastasis: new implications of an old interplay. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.01350
24. Hu B, Yang X-R, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20(23):6212–22. doi: 10.1158/1078-0432.CCR-14-0442
25. Chen J-H, Zhai E-T, Yuan Y-J, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol (2017) 23(34):6261–72. doi: 10.3748/wjg.v23.i34.6261
26. Xie Q-K, Chen P, Hu W-M, Sun P, He WZ, Jiang C, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med (2018) 16(1):273. doi: 10.1186/s12967-018-1638-9
27. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2015) 12(12):681–700. doi: 10.1038/nrgastro.2015.173
28. Liakou CI, Narayanan S, Ng Tang D, Logothetis CJ, Sharma P. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun (2007) 7:10.
Keywords: colorectal cancer, systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-lymphocyte ratio, prognosis
Citation: Zhang T and Miao Yc (2023) Prognostic evaluation of preoperative systemic immune inflammatory index in patients with colorectal cancer. Front. Oncol. 13:1260796. doi: 10.3389/fonc.2023.1260796
Received: 18 July 2023; Accepted: 25 October 2023;
Published: 22 December 2023.
Edited by:
Nobu Oshima, Kyoto University, JapanReviewed by:
Sonia López-Cisneros, Instituto Nacional de Geriatría, MexicoCopyright © 2023 Zhang and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong chang Miao, MzIzOTQyMTYyOEBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.