- 1State Key Laboratory of Experimental Hematology, National Clinical Research Center for Blood Diseases, Haihe Laboratory of Cell Ecosystem, Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjin, China
- 2Tianjin Institutes of Health Science, Tianjin, China
SET-NUP214 fusion gene, also known as TAF-1-CAN and SET-CAN, is observed in acute myeloid leukemia (AML) and T-cell lymphoblastic leukemia (T-ALL). SET-NUP214 fusion in T-cell lymphoblastic leukemia is associated with chemotherapy resistance, but the prognosis of patients with AML with SET-NUP214 has rarely been reported. In the present study, we retrospectively analyzed all patients with acute leukemia including AML and T-ALL patients with SET-NUP214 fusion who underwent allogeneic stem cell transplantation (alloHSCT) in our center from July 2017 to November 2022. Of the total 11 patients, 5 patients were diagnosed with AML and 6 patients were diagnosed with T-ALL de novo. All patients received myeloablative regimens in CR1, and there were three (60%) AML patients who relapsed post-alloHSCT and three T-ALL (50%) patients who relapsed post-alloHSCT. Only one patient with AML who relapsed post-alloHSCT responded to subsequent chemotherapy plus donor lymphocyte infusion and survived the last follow-up. The estimated 1-year overall survival and 3-year overall survival for all these 11 patients were 69.3% and 38.5%, respectively. The estimated 1-year leukemia-free survival and 3-year leukemia-free survival for all patients were 69.3% and 38.5%, respectively. The research shows a high incidence of relapse for patients with acute leukemia with the SET-NUP214 fusion gene, even after alloHSCT. More clinical trials or research with larger samples are urgently needed for this group of patients.
Introduction
The SET-NUP214 fusion gene, which results from either cryptic t(9;9)(q34;q34) or del(9)(q34.11q34.13), was first described in a patient with acute undifferentiated leukemia (1). Subsequently, several researchers reported that the fusion gene was also found in patients with acute myeloid leukemia (AML) and T-cell lymphoblastic leukemia (T-ALL) (2–4). SET-NUP214 fusion in patients with T-ALL is associated with corticosteroid/chemotherapy resistance but may respond to hematopoietic stem cell transplantation (HSCT) (5). The impact of SET-NUP214 fusion on patients with AML has rarely been reported. Due to the limited occurrence of SET-NUP214, with the reporting occurrence varying from 4.9 to 6% in T-ALL (5, 6), the outcome of acute leukemia patients with SET-NUP214 after hematopoietic stem cell transplantation has rarely been reported. In the present study, we retrospectively analyzed 11 acute leukemia patients with positive SET-NUP214 fusion gene who underwent allogeneic stem cell transplantation in our center from July 2017 to Nov 2022. These patients’ prognoses were rather poor despite the utilization of myeloablative conditioning.
Methods
Patient characteristics
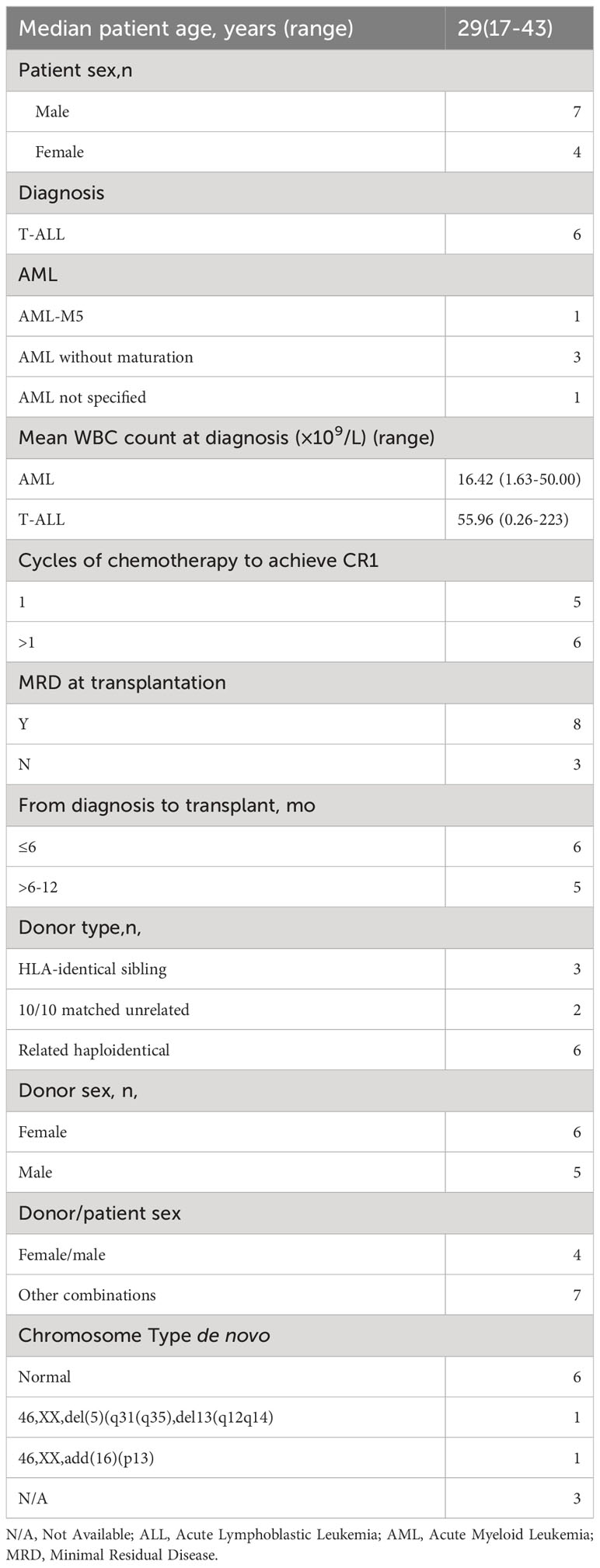
Between July 2017 and Nov 2022, 11 patients with acute leukemia presenting SET-NUP214 who underwent allogeneic stem cell transplantation (alloHSCT) were enrolled in this study. Patients had a median age of 29 years (ranging from 17-43 years) at transplant. Seven of the patients were men and four were women. Five patients were diagnosed with AML, and six patients were diagnosed with T-ALL de nova, according to the fifth edition of the WHO classification. Of the 11 patients, 6 achieved hematological complete remission after one cycle of induction chemotherapy. Only 1 of the 11 patients achieved molecular complete remission after one cycle of induction chemotherapy. The median cycle of chemotherapy to achieve CR1 was one (ranging from one to four). All the patients received a median of four cycles (ranging from three to six) of chemotherapy before alloHSCT. The median time from diagnosis to transplant was 6 months (ranging from 6 to 8 months). All the patients underwent alloHSCT in CR1, but only three patients achieved negative SET-NUP214 fusion gene before alloHSCT. One patient with T-ALL presented with central nervous system leukemia involvement before HSCT and was in remission at transplant. The details of the patients before alloHSCT are summarized in Tables 1, 2.
Transplant procedures
All 11 patients received myeloablative conditioning and allogeneic stem cell transplantation. The conditioning regimens for patients with AML were as follows: four patients with AML received conditioning regimens consisting of Busulfan (Bu, 3.2 mg/kg, days -9 to -7), Cyclophosphamide (Cy, 40 mg/kg, days -3 to -2), and Cytarabine(Ara-C, 2 g/m2, days -6 to -4) or Idarubicin (IDA, 10mg/kg, days -6 to -4) and Fludarabine (Flu, 30 mg/m2, days -6 to -4), or Cladribine (Clad, 5mg/m2, days -6 to -4). One patient with AML received TBI instead of Bu. Patients with T-ALL mostly received conditioning regimens as follows: TBI (3.3 Gy, days −9 to −7), Cy (40mg/kg, days −6, −5), Flu (30 mg/m2, days −4 to −2), and Ara-C (2 g/m2, days −4 to −2) as previously described (n=5) (7). One patient with T-ALL received etoposide instead of fludarabine and cytarabine. Patients who received haploidentical donor and matched unrelated donor HSCT received an additional 2.5mg/kg/day anti-T lymphocyte globulin (ATG) on days -5 to -2 (n=9). ATG was also given to the patient who was older than 40 years old (n=1). Calcineurin inhibitors plus short-term methotrexate along with or without mycophenolate mofetil were used for acute graft versus host disease prophylaxis as previously described (7, 8). Three patients received stem cells from matched-sibling donors, six from related haploidentical donors, and two from matched unrelated donors. More details are shown in Table 2.
Statistical analysis
The primary endpoint was leukemia-free survival (LFS). Relapse or death were considered events. Overall survival (OS) was defined as the duration from stem cell administration to the last follow-up or death due to any cause. Transplant-related mortality (TRM) was defined as death in complete remission of leukemia after HSCT. Relapse was defined as any kind of morphological, cytogenetic, molecular disease recurrence, or extramedullary relapse. Minimal residual disease (MRD) was defined as any kind of molecular disease present without hematological relapse as follows: 1. Positive detection by real-time PCR of the SET-NUP214 fusion gene (ABL copies>104, target gene/ABL >0%), 2. 0%<morphological leukemia blast cells <5%, and 3. leukemia cells/mononuclear cells >0% by flow cytometry analysis (capture 500,000 total events). Neutrophil engraftment was defined as the first date of neutrophil count ≥0.5×109/L for three consecutive days. Platelet engraftment was defined as the first date of platelet count ≥20×109/L and sustained for seven consecutive days independent of transfusion. Acute graft-versus-host disease (GVHD) was based on a previous standard (9). All dates were calculated from the first day of stem cell infusion to the day of the event or censored at the last follow-up. The Kaplan–Meier curve was calculated using SPSS 22.0. P<0.05 were considered statistically significant.
Results
Patient, disease, and transplant characteristics
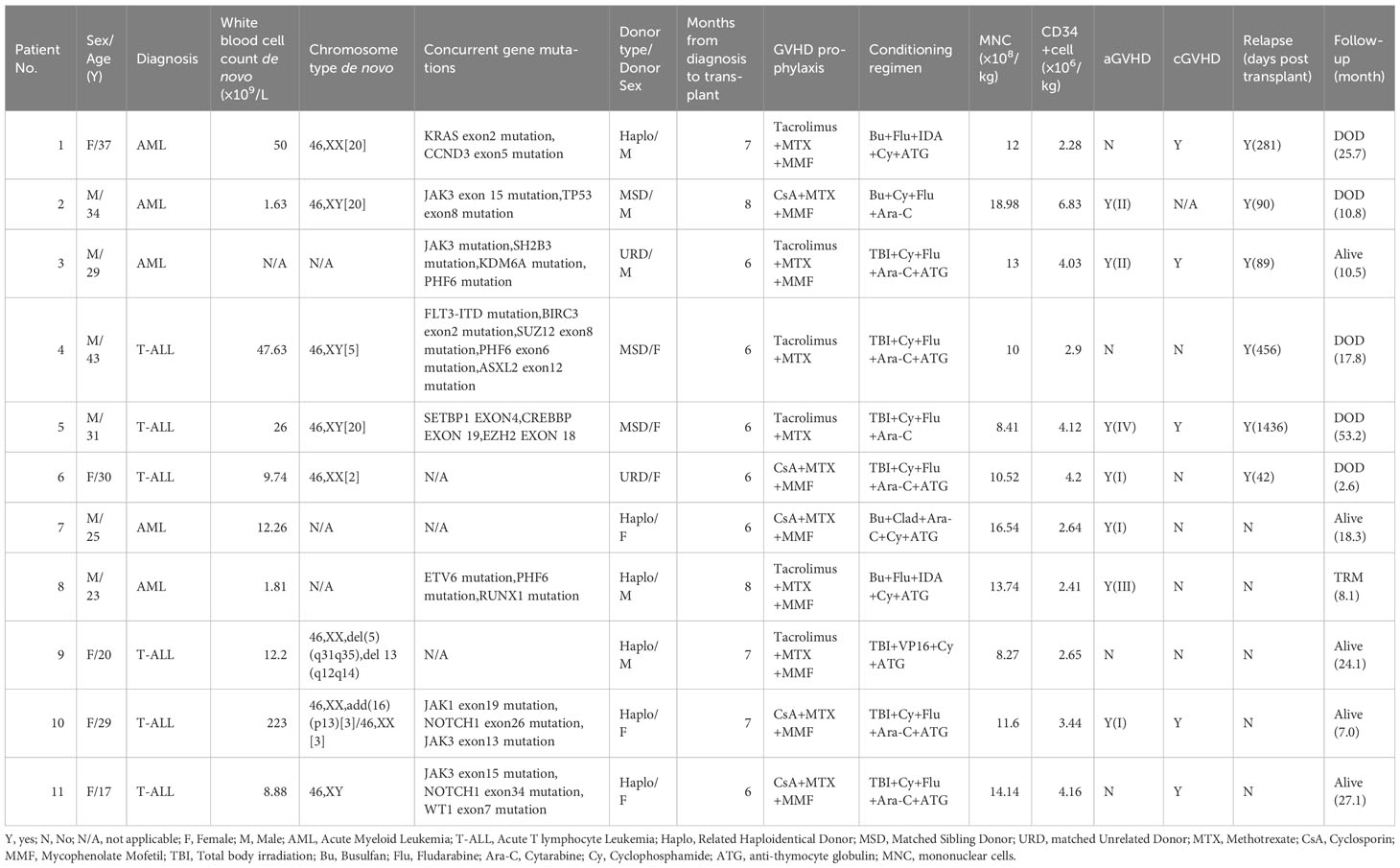
The SET-NUP214 fusion gene could be detected in both T-ALL and AML patients in our single center. Normal chromosome phenotypes were mostly seen in these patients (n=6) (Table 1). JAK mutation (n=4) and PHF6 mutation (n=3) were mostly observed in these patients as concurrent mutations. Of all the patients with AML, three were categorized as AML without maturation. One patient was categorized as acute monocytic leukemia, and one was not otherwise specified de novo, according to the fifth edition of the WHO classification. One patient with T-ALL was categorized as ETP-ALL. Of all the 11 patients with acute leukemia, only 1 patient achieved complete molecular remission after one cycle of chemotherapy, indicating that this cohort of patients is somehow resistant to chemotherapy and should take HSCT as a treatment option, which is consistent with a previous report (5). All the patients underwent alloHSCT in CR1 and received peripheral stem cell infusion with a median positive CD34 count of 3.44 (range 2.28-6.82) ×106/kg and median mononuclear cell count of 12.00 (range 8.27-18.98) ×108/kg. Median times of neutrophil and platelet recovery were 14 days (range 11-17 days) and 15 days (range 11-33 days), respectively. All the patients achieved hematopoietic engraftment and complete remission at the molecular level after alloHSCT.
OS and LFS
The estimated 1-year overall survival (OS) was 53.3% for patients with AML and the 3-year OS was 0% (Figure 1A). The estimated 1-year OS for patients with T-ALL was 83.3% and the 3-year OS was 62.5% (Figure 1A). There were no statistical differences between the two groups (p=0.676). The estimated 1-year and 3-year OS for all these 11 patients were 69.3% and 38.5%, respectively (Figure 1B). The 1-year and 3-year leukemia-free survival (LFS) for all patients were 69.3% and 38.5%, respectively. Of note, only one patient with T-ALL received regular chidamide as maintenance chemotherapy post-HSCT, and none of the others received any kind of maintenance chemotherapy post-HSCT. The patient taking chidamide was in LFS 814 days post-HSCT to the last follow-up.
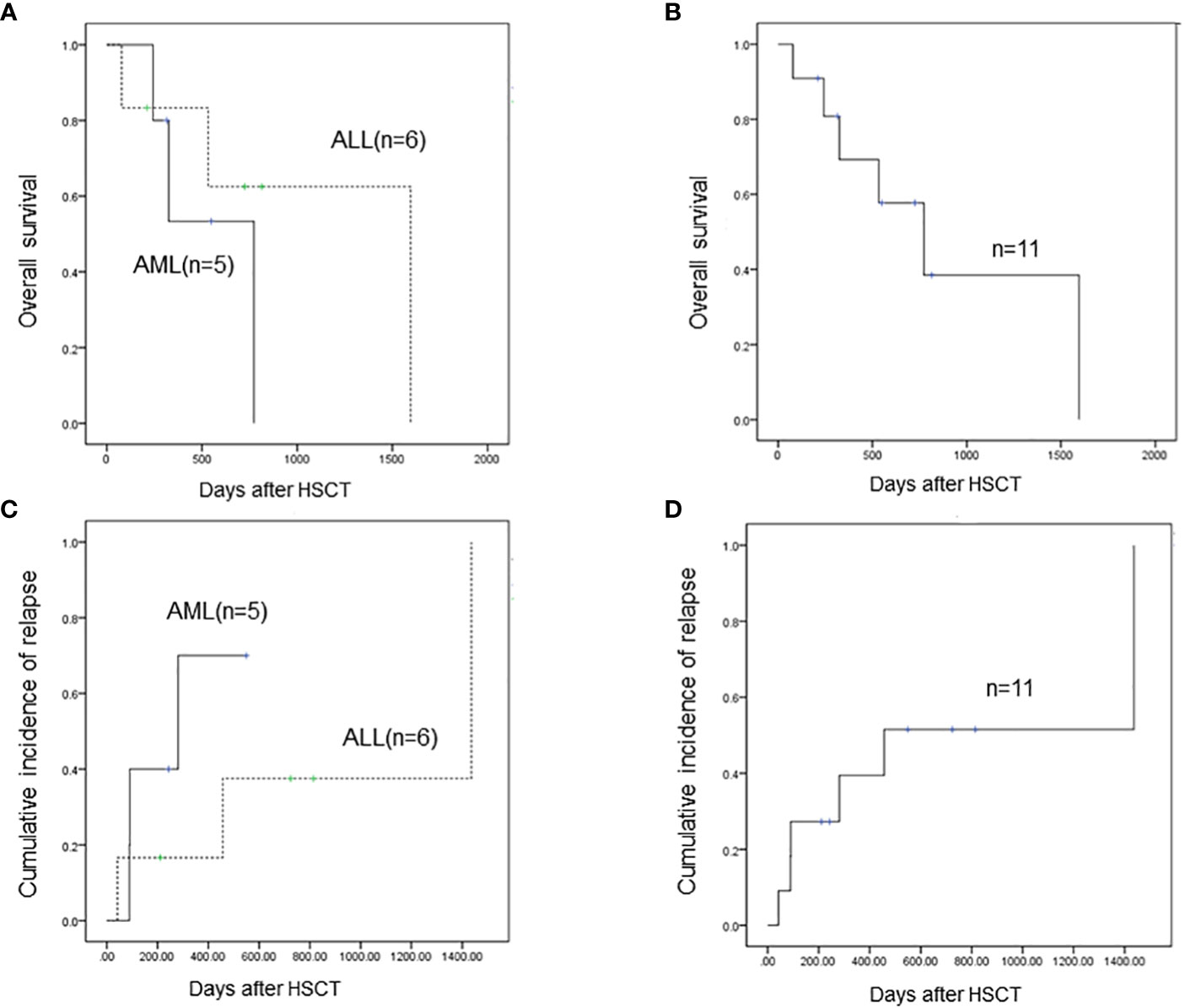
Figure 1 (A) The estimated 1-year overall survival (OS) is 53.3% for AML patients with SET-NUP214 fusion and the 3-year OS is 0%. The estimated 1-year OS for T-ALL patients with SET-NUP214 fusion is 83.3% and the 3-year OS is 62.5%. There are no statistical differences between the two groups (p=0.676). (B) The estimated 1-year and 3-year OS for all these 11 patients are 69.3% and 38.5%, respectively (C) Three of five (60%) patients with AML relapsed post-HSCT, and three of six patients with T-ALL (50%) relapsed post-HSCT. (D) For all 11 patients, the 1-year and 3-year cumulative incidence of relapse is 39.4% and 51.5%, respectively.
Relapse incidence and non-relapse mortality
In total, six patients relapsed after HSCT and five patients died of leukemia at the last follow-up. Three out of five (60%) patients with AML relapsed post-HSCT (Figure 1C): one patient had extramedullary involvement, one patient relapsed in bone marrow, and both patients died due to leukemia. One patient relapsed presenting as MRD and received combined chemotherapy including venetoclax and donor lymphocyte infusion; the patient achieved CR and was alive with chronic graft-versus-host disease to the last follow-up, which was 226 days after relapse post-HSCT. This patient remains the only one who survived after relapse post-HSCT. Three out of six patients with T-ALL (50%) relapsed post-HSCT and died of leukemia (Figure 1C). Two patients received intensive chemotherapy and subsequent DLI but did not respond to treatment. One patient chose palliative care and died 1 month after relapse. One patient with AML died of infection and was the only patient who died of transplant in this cohort. The TRM rate was 9.1%. Of note, the only patient who achieved molecular complete remission after one cycle of chemotherapy was in leukemia-free survival 211 days post-HSCT to the last follow-up. The six patients who relapsed post-HSCT never reached negative detection of the SET-NUP214 fusion gene before HSCT. In patients who did not relapse post-HSCT to the last follow-up, three out of five reached molecular remission before HSCT, and the number of cycles of chemotherapy to achieve negative fusion gene detection was 1,3, and 4, respectively. This indicates that patients with molecular remission before HSCT may achieve long survival after HSCT. Patients who never reach molecular remission have a high incidence of relapse rate (75%) post-HSCT but may still respond to HSCT. Thus, alloHSCT could be a salvage treatment option for these patients who are resistant to conventional chemotherapy. For all 11 patients, the 1-year and 3-year cumulative incidence of relapse was 39.4% and 51.5%, respectively (Figure 1D).
Discussion
The NUP214 mapping at chromosome 9q34 has been reported as significant to genes in leukemogenesis (10). SET was reported as an oncogene that plays a role in transcription by modulating chromatin organization (11). The SET-NUP214, also known as TAF-1-CAN and SET-CAN, as a gene fusion has previously been described as a result of a chromosomal translocation t (9;9)(q34;q34) and del(9)(q34.11q34.13) (2–4). The fusion gene regulated leukemogenesis partly by upregulating the HOXA gene (2, 6). This fusion can be found in patients with T-ALL. The incidence of this fusion in patients with T-ALL is 4.6%-6%, according to data from different centers (5, 6). SET-NUP214 fusion has also been reported in cell lines of AML and single clinical cases of AML and AUL (1, 12, 13). In our study, AML with SET-NUP214 fusion was mostly present as AML without maturation, which may lead to poor survival even after alloHSCT. In patients with T-ALL, SET-NUP214 was reported to be strongly associated with corticosteroid and chemotherapy resistance but did not negatively influence clinical outcomes after HSCT (5). It is indicated that mutations of PHF6 and JAK1 are associated with the rearrangement of SET-NUP214 in T-ALL. In our cohort, concurrent mutations including JAK (n=4) and PHF6 (n=3) were mostly observed in both T-ALL and AML patients. Of note, JAK3 (n=3) and PHF6 (n=2) mutations were also observed in patients with AML. Regarding the prognosis of T-ALL patients with SET-NUP214, a Korean study showed that among four adult patients with T-ALL who presented with the fusion gene, only one patient who underwent HSCT survived (4). Song Y reported that in 17 AML and T-ALL patients with SET-NUP214, the median OS of 6 patients in chemotherapy was 10.5 (3-41) months, indicating none of the patients could survive without further alloHSCT. The OS and relapse-free survival of patients who underwent alloHSCT were better than those of the chemotherapy group (p=0.038) (14). In another study enrolling 11 T-ALL patients with SET-NUP214 fusion, the LFS and OS at 3 years of SET-NUP214–positive patients were 45% and 73%, respectively (5), which is somehow consistent with the prognosis of patients with T-ALL in our center. The present study and previous research all show disappointing LFS of these patients. The two patients with AML who were alive at the last follow-up did not exceed 3 years post-HSCT, thus we have no patients with AML who survived more than 3 years post-HSCT. However, regarding the high incidence of relapse, AML patients with SET-NUP214 fusion have a very poor prognosis even after alloHSCT. Bcl2 inhibitors such as venetoclax may be effective in patients with AML, as shown in our research but there is a need for further clinical trials with larger samples.
Recent research from Oka M linked NUP214 and NUP98, demonstrating that these two fusion proteins share some characteristics, including their nuclear bodies co-localized with CRM1 (also known as XPO1), and are both associated with aberrant activation of HOX genes. In addition, they are both physically and functionally associated with MLL1, which is also known as KMT2A (15). This suggests that treatment options for NUP98 rearranged acute myeloid leukemia may be adaptable to NUP214 rearranged acute myeloid leukemia patients but further evidence is needed. For patients with T-ALL, our previous research shows that maintenance of chidamide after alloHSCT did not significantly reduce the 1-year CIR of high-risk T-ALL but may improve the event-free survival (16). The one patient taking chidamide in our study is somehow in LFS to the last follow-up; there is a need for further investigation of the impact of chidamide on SET-NUP214 positive T-ALL.
Our research shows a very poor prognosis of acute leukemia patients with SET-NUP214 fusion even after alloHSCT. Despite all these patients undergoing alloHSCT in CR1 and achieving molecular remission shortly after HSCT, the major cause of death for these patients is still leukemia relapse. With very limited cases in our study, it is highly recommended that physicians should consider novel treatment strategies for these patients, including a stronger conditioning regimen and proper maintenance chemotherapy. Physicians should be alert of the high incidence of relapse for acute leukemia patients with SET-NUP214, even after alloHSCT. There is an urgent need for more clinical trials or research with larger samples for this group of patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YS: Conceptualization, Data curation, Writing – original draft. DY: Validation, Writing – review & editing. RZ: Validation, Writing – review & editing. XC: Validation, Writing – review & editing. QM: Validation, Writing – review & editing. JW: Validation, Writing – review & editing. WZ: Validation, Writing – review & editing. AP: Validation, Writing – review & editing. YH: Validation, Writing – review & editing. EJ: Supervision, Validation, Writing – review & editing. SF: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by Tianjin Municipal Science and Technology Commission Grant (21JCZDJC01170), the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021-I2M-C&T-B-080,2021-I2M-1-017), and the Haihe Laboratory of Cell Ecosystem Innovation Fund (22HHXBSS00036) to SF.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. von Lindern M, Breems D, van Baal S, Adriaansen H, Grosveld G. Characterization of the translocation breakpoint sequences of two DEK-CAN fusion genes present in t(6;9) acute myeloid leukemia and a SET-CAN fusion gene found in a case of acute undifferentiated leukemia. Genes Chromosomes Cancer (1992) 5:227e34. doi: 10.1002/gcc.2870050309
2. Van Vlierberghe P, van Grotel M, Tchinda J, Lee C, Beverloo HB, van der Spek PJ, et al. The recurrent SET-NUP214 fusion as a new HOXA activation mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood (2008) 111:4668e80. doi: 10.1182/blood-2007-09-111872
3. Lee SG, Park TS, Cho SY, Lim G, Park GJ, Oh SH, et al. T-cell acute lymphoblastic leukemia associated with complex karyotype and SET-NUP214 rearrangement: a case study and review of the literature. Ann Clin Lab Sci (2011) 41(3):267–72.
4. Chae H, Lim J, Kim M, Park J, Kim Y, Han K, et al. Phenotypic and genetic characterization of adult T-cell acute lymphoblastic leukemia with del(9)(q34);SET-NUP214 rearrangement. Ann Hematol (2012) 91(2):193–201. doi: 10.1007/s00277-011-1289-x
5. Ben Abdelali R, Roggy A, Leguay T, Cieslak A, Renneville A, Touzart A, et al. SET-NUP214 is a recurrent γδ lineage-specific fusion transcript associated with corticosteroid/chemotherapy resistance in adult T-ALL. Blood 123(12):1860–3. doi: 10.1182/blood-2013-08-521518
6. Prokopiou C, Koumas S, Neokleous N, Seimeni O, Barmpouti A. SET-NUP214 rearrangement in isolation is insufficient to induce leukemia: a single center experience. Leuk Lymphoma (2016) 57(2):451–2. doi: 10.3109/10428194.2015.1049169
7. Lv M, Liu L, He Y, Yang D, Ma Q, Pang A, et al. Outcomes of allogeneic or autologous stem cell transplantation followed by maintenance chemotherapy in adult patient with B-ALL in CR1 with no detectable minimal residual disease. Br J Haematol (2023) 202(2):369–378. doi: 10.1111/bjh.18846
8. Cao YG, He Y, Zhang SD, Liu ZX, Zhai WH, Ma QL, et al. Conditioning regimen of 5-day decitabine administration for allogeneic stem cell transplantation in patients with myelodysplastic syndrome and myeloproliferative neoplasms. Biol Blood Marrow Transplant (2020) 26(2):285–91. doi: 10.1016/j.bbmt.2019.09.001
9. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant (1995) 15(6):825–8.
10. Xu S, Powers MA. Nuclear pore proteins and cancer. Semin Cell Dev Biol (2009) 20:620e30. doi: 10.1016/j.semcdb.2009.03.003
11. Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell (2001) 104:119e30. doi: 10.1016/S0092-8674(01)00196-9
12. Zhang H, Zhang L, Li Y, Gu H, Wang X. SET-CAN fusion gene in acute leukemia and myeloid neoplasms: report of three cases and a literature review. Onco Targets Ther (2020) 13:7665–81. doi: 10.2147/OTT.S258365.eCollection
13. Quentmeier H, Schneider Björn, Röhrs S, Romani J, Zaborski M, Macleod RA, et al. SET-NUP214 fusion in acute myeloid leukemia- and T-cell acute lymphoblastic leukemia-derived cell lines. J Hematol Oncol (2009) 2:3. doi: 10.1186/1756-8722-2-3
14. Song Y, Gong X-Y, Wei S-N, Li QH, Zhang GJ, Wang Y, et al. Clinical analysis of SET-NUP214 fusion gene positive patients with acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi (2023) 31(2):352–7. doi: 10.19746/j.cnki.issn.1009-2137.2023.02.007
15. Oka M, Otani M, Miyamoto Y, Oshima R, Adachi J, Tomonaga T, et al. Phase-separated nuclear bodies of nucleoporin fusions promote condensation of MLL1/CRM1 and rearrangement of 3D genome structure. Cell Rep (2023) 42(8):112884. doi: 10.1016/j.celrep.2023.112884
Keywords: acute myeloid leukemia, acute T lymphoblastic leukemia, allogeneic stem cell transplanation, SET-CAN/NUP214 fusion, del(9q)
Citation: Shen Y, Yang D, Zhang R, Chen X, Ma Q, Wei J, Zhai W, Pang A, He Y, Jiang E and Feng S (2023) The outcome of acute leukemia patients with SET-NUP214 fusion after allogeneic stem cell transplantation. Front. Oncol. 13:1256043. doi: 10.3389/fonc.2023.1256043
Received: 10 July 2023; Accepted: 25 September 2023;
Published: 13 October 2023.
Edited by:
Jacopo Mariotti, Humanitas Research Hospital, ItalyReviewed by:
Xiao-Dong Mo, Peking University People’s Hospital, ChinaGusheng Tang, Naval Medical University, China
Yifan Pang, Levine Cancer Institute, United States
Copyright © 2023 Shen, Yang, Zhang, Chen, Ma, Wei, Zhai, Pang, He, Jiang and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sizhou Feng, c3pmZW5nQGloY2Ftcy5hYy5jbg==
 Yuyan Shen
Yuyan Shen Donglin Yang
Donglin Yang Rongli Zhang1,2
Rongli Zhang1,2 Aiming Pang
Aiming Pang Yi He
Yi He Erlie Jiang
Erlie Jiang Sizhou Feng
Sizhou Feng