
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 31 October 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1253845
Introduction: Data on colorectal cancer (CRC) patients’ thorough management practices and medication-related harms (MRH) are scarce. This study’s aim was to investigate the MRHs in patients receiving CRC chemotherapy at the comprehensive specialized hospital of the University of Gondar (UoGCSH).
Methods: A registry-based retrospective cohort study was conducted on CRC patients at the UoGCSH during 2017–2021. From February to May 2022, medical records were reviewed using a pretested data collection tool to collect socio-demographic and disease-related characteristics, MRHs, and medication regimens. MRHs occurrence and adverse drug reactions (ADRs) severity were assessed using standard guidelines and protocols. Version 16/MP of STATA for Windows was used for the analysis. Independent predictors of MRHs were investigated using logistic regression analysis. A p-value ≤0.05 was used to determine an independent variable’s statistical significance.
Results: One hundred forty three CRC patients were included, with a mean age of 49.9 ± 14.5 years. About 32.9% and 33.6% had stage II and III cancer, respectively. Significant patients had co-morbidities (15.4%) and complications (13.3%). Fluorouracil (5-FU)-based regimens were given to more than half (56%) of the patients. MRHs were found in 53.1% of the patients, with a mean of 2.45 ± 1.37 MRHs. The most common MRHs were the need for additional drug therapy, sub-therapeutic dose, DDIs, and ADRs. Being on stage IV (AOR = 27.7, 95% CI = 3.85–199.38, p = 0.001), having co-morbidity (AOR = 7.42, 95% CI = 1.80–30.59, p = 0.018) and having complication (AOR = 11.04, 95% CI = 1.72–70.95, p = 0.011) and treated with five or more drugs (AOR = 2.54, 95% CI = 1.07–6.07, p = 0.035) were independent predictors of MRHs.
Conclusion: A fluorouracil-based treatment regimen was most frequently used. MRHs were found in nearly half of CRC patients. Furthermore, MRHs were significantly associated with cancer stage, comorbidity and complication status, and the number of medications used. Because MRHs are common, improving clinical pharmacy services is critical for optimizing drug therapy in CRC patients.
Although developed countries have a higher cancer burden, mortality rates in developing countries are much higher (1). A cancer of the large intestine is colorectal cancer (CRC) (2, 3).
CRC is a significant global health concern, with a high incidence and mortality rate. According to recent data from the World Health Organization (WHO) (4), CRC ranks as the third most common cancer worldwide, affecting millions of individuals annually. Furthermore, a study done by Douaiher et al. (1), highlights that CRC is the second leading cause of cancer-related deaths.
The incidence of CRC was found to be 4.04 per 100,000 people in Sub-Saharan Africa, with a male-to-female ratio of 1.2:1 and an estimated 24,711 new cases reported annually (5). In Ethiopia, it affects men the most frequently (6). Unfortunately, a 6-year retrospective cohort study of CRC patients in Ethiopia revealed a mortality rate of 34.8% (7).
Treatment for CRC patients is complex and carries an inherent risk of MRHs, which ultimately influences treatment outcome due to the high prevalence of co-occurring chronic diseases (8). The stage of CRC at diagnosis and the location of the tumor influence treatment. The most typical therapy for early-stage (stage I or II) CRC is surgical removal of the tumor and any adjacent lymph nodes. Chemotherapy alone or in conjunction with radiation therapy is frequently administered prior to or following surgery for individuals with late-stage illnesses (9). The existence and kind of comorbidities, drug therapy problems, screening practices, and treatment accessibility all contribute to worse treatment results (10, 11).
A MRH is described as “an event or scenario involving medication therapy that actually or potentially interferes with anticipated health outcomes” by the Pharmaceutical Care Network of Europe (PCNE) (12). The likelihood of developing MRHs like ADRs, drug interactions, medication errors, and non-compliance increases with drug therapy complexity (13). Up to 25% of hospitalized patients have been documented to have ADRs, which may be made worse by unneeded pharmaceutical therapy, improper drug selection, and untreated conditions. Significant morbidity and mortality can result from MRHs (14). MRHs in cancer chemotherapy can have negative effects due to the anticancer medicines’ high toxicity and narrow therapeutic window (13).
Cancer patients are particularly vulnerable to drug interactions due to their exposure to anticancer drugs and frequently experience comorbid illnesses and tumor-related symptoms like pain, depression, and seizures. Drug pharmacokinetics in cancer patients are altered for a variety of hypothetical reasons, including drug interactions with liver enzymes, impaired drug excretion in patients with renal and/or hepatic dysfunction, hampered drug absorption due to mucositis, malnutrition, and infection, and variation in the volume of drug distribution due to decreased levels of serum binding proteins (15). A more thorough investigation of MRHs in CRC patients might offer invaluable information to healthcare professionals regarding MRH management and/or prevention (16). Several studies have found that DRPs cause significant hospitalizations, with 50% of them being avoidable, and have a significant negative impact on the health of cancer patients (17–19). Studies in Ethiopia (20), India (21) reported the prevalence of MRHs caused by chemotherapy in cancer patients was 48.7%and 58.6%, respectively. Various studies showed that adverse drug reaction (ADR), the need for additional drug therapy, and drug–drug interactions (DDI) are the most prevalent DRPs (20, 22, 23). Several studies have found that MRH development is influenced by sex, age, length of hospital stay, cancer stage, polypharmacy, co-morbidity, and complication status (20, 24, 25). Data on thorough MRHs among CRC patients are, however, rare. The majorities of studies that have been published thus far have either addressed the issue of drug-related hospital admissions or have exclusively looked at ADRs among hospitalized patients. A comprehensive MRH study would provide valuable insight for healthcare providers in reducing the incidence of MRHs and improving treatment outcomes in cancer patients. A systematic review found that MRHs can be prevented and managed with the help of clinical pharmacists (26, 27). Thus, this study aimed to examine the MRHs in patients receiving CRC chemotherapy and management pattern at the UoGCSH.
This study was a registry-based retrospective cohort using the UoGCSH data collected from February to May 2022. UoGCSH is located in the outskirts of the city of Gondar serving more than forty million inhabitants of Amhara Regional State as an oncologic center. The oncology center comprises a range of professionals providing treatment for different cancer types, including CRC and its complications.
All histologically confirmed adult CRC patients’ charts/registries, or logbooks who were receiving chemotherapy at the UoGCSH oncology center from 2017 to 2021 were part of this study.
Medication-related harm: In our study, MRH describes at least one of the following undesirable events: unsafe, ineffective, DDI, medication use without indication, and the need for additional drug therapy (17).
A total survey sampling technique was employed to select 143 medical records with confirmed colorectal cancer. The data collection instrument was created using peer-reviewed published journal findings and the MRH lists of Cipole et als (12, 23, 28). The format contains socio-demographic such as sex, age and residence and disease-related characteristics, including but not limited to: histological types and stages of cancer, presence and types of complications and comorbidities, treatment modalities, and status and list MRHs. The patient’s age was collected as a continuous variable and categorized for analysis purposes.
The European Society for Medical Oncology practice guideline, the NCCN, and the Ethiopian cancer treatment protocols were employed to evaluate the occurrence of MRHs. Stockley’s Drug Interactions Checker and the modified Hartwig and Siegel ADR Severity Assessment Scale were used to assess the occurrence of DDIs and the severity of ADRs, respectively (29).
Six data collectors were hired: three nurses and three pharmacists. The data collectors received pretest training one day before the commencement of data collection, focusing on the data collection tool, research ethics, selection criteria, and confidentiality.
Before starting data collection, a pretest was performed on the medical records of eight CRC patients to ensure that the data abstraction format was understandable. The results of the pretest were used to make changes. Furthermore, the data collection process was closely monitored on-site throughout the data collection period, and the data’s completeness and consistency were checked on a daily basis.
After a week of data collection, data analysis was carried out. EpiData 4.6 for Windows was used to prep, verify, code, and enter the data before exporting it to STATA version 16/MP for analysis. A bivariable logistic regression analysis was performed to investigate whether there is an association between the occurrence of MRH and various independent factors. All variables such as sex, age, co-morbidity and complication status, cancer stage, and number of medications with p<0.2 in the bivariable logistic regression analysis were included in the multivariable logistic regression. A 95% CI was constructed for Adjusted Odds Ratios (AORs) to determine the strength of associations. The statistical significance of each independent variable was determined using a p-value ≤ 0.05.
The study included 143 CRC patients with a mean age of 49.9 ± 14.5 years. Males (61.5%) and urban residents (58.7%) made up nearly two-thirds of study participants. Adenocarcinoma (86.7%) was the most common histological type in terms of clinical characteristics. This study also showed that two-thirds of the participants had stage II or III cancer. Furthermore, one-fourth of the patients had metastases, with the liver and lung being the most common metastatic sites. Less than one-fifth of patients had co-morbidities and complications, with hypertension and anemia being the most common co-morbid conditions and complications, respectively (Table 1).
About two-thirds (67.1%) of the patients were on chemotherapy alone. Metoclopramide with dexamethasone (52, 36.4%) combination were the most commonly used prophylactic antiemetic regimen followed by a combination of ondansetron and dexamethasone (34, 23.8%). However, one-fourth of patients did not receive antiemetic prophylaxis. According to the study’s findings, the most commonly used analgesics were morphine (28%), paracetamol (24%), and diclofenac (20.3%) (Table 2).
The combination of cisplatin, 5-FU and oxaliplatin (50.4%) followed by oxaliplatin and capecitabine (30.8%) were the most widely used treatment regimen. The combination of cisplatin and paclitaxel, however, was the least commonly used treatment regimen (Figure 1).
A total of 186 MRHs were found in 76 colorectal cancer patients, representing 53.1% prevalence. A mean of 2.45 ± 1.37 MRHs per patient occurred. The most common MRHs were the need for additional drug therapy, sub-therapeutic doses, and DDIs, accounting for 29.6%, 22%, and 18.3% of cases, respectively (Table 3).
In terms of the severity of drug interactions, 41% were moderate, necessitating close monitoring of the drug interactions’ outcomes. Ciprofloxacin interacting with oxaliplatin, metoprolol combined with hydrochlorothiazide, and metoprolol in conjunction with ibuprofen were some of the noteworthy drug-drug interactions that were noticed. However, 6% of them were severe and required the use of alternative medications (Figure 2). In our study, we identified serious drug interactions, for example, between ciprofloxacin and ondansetron.
Nausea (61.8%), leucopenia (50%), and vomiting (47.1%) were the most frequent types of ADRs. Conversely, thrombocytopenia (2.9%) was the least frequent ADR (Table 4).
The stage of cancer, co-morbidity and complication status, and the number of medications all had a significant association with the development of MRHs in our study, as identified by multivariable logistic regression analysis. CRC patients in stage IV were 28 times (AOR = 27.7, 95% CI = 3.85–199.38, p = 0.001) more likely to have MRHs than CRC patients in stage I. In addition, when compared to their counterparts, CRC patients with co-morbidity and complications were 7 times (AOR = 7.42, 95% CI = 1.80–30.59, p = 0.018) and 11 times (AOR = 11.04, 95% CI = 1.72-70.95, p = 0.011) more likely to develop MRHs. Patients who had taken five or more medications were three times more likely to develop MRHs (AOR = 2.54, 95% CI = 1.07–6.07, p = 0.035) than those who had taken fewer than five medications (Table 5).
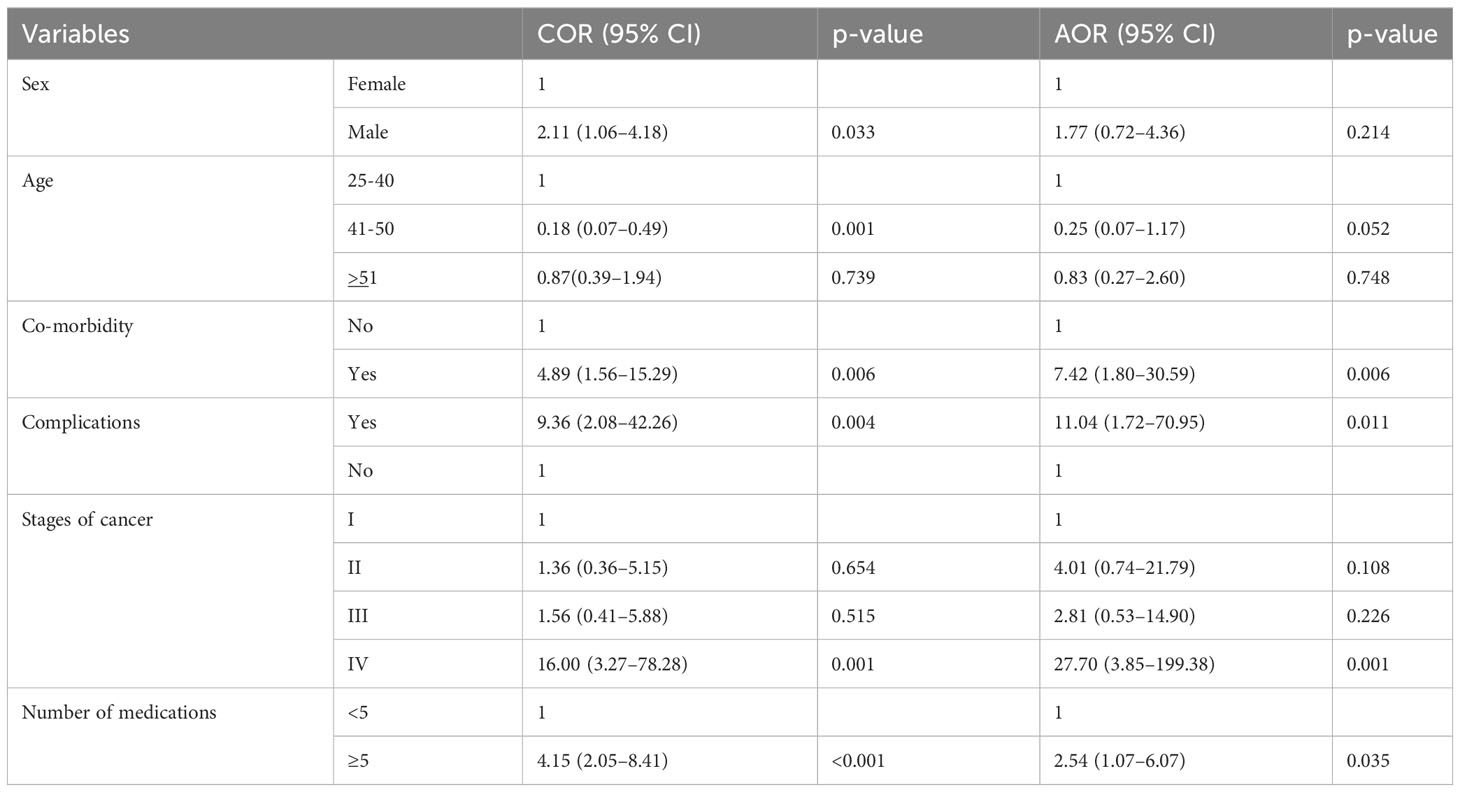
Table 5 Univariable and multivariable binary logistic regression analysis of medication-related harm predictors.
Patients with CRC are at high risk for MRH due to the complexity of the management pattern and the presence of various socio-demographic and clinical factors associated with the development of MRH. In the present study the male-to-female ratio for CRC patients in this study was 1.6:1, which is consistent with studies conducted in Tanzania, the United Kingdom, and China (2, 30–32) but differs from previous studies with a very small male preponderance for CRC (33, 34). Many biological and behavioral factors may contribute to men’s increased susceptibility to CRC (35–38). Moreover, men are more likely to store visceral fat (39), which has been linked to a higher risk of CRC (40–42).
The most prevalent histological type (86.7%) was adenocarcinoma, which was consistent with several findings (30, 33, 43–47). In the current study, there were 58.8% of patients with advanced CRC (stage III, 33.6%, and stage IV, 25.2%), which was greater than what was shown in other studies (33, 46). A typical observation in underdeveloped nations is that the majority of patients are detected at an advanced stage (48, 49). This might be as a result of the fact that most cancer patients in developing nations like Ethiopia seek treatment extremely late in the course of their illness. Early-onset CRC has been found to be on the rise recently in a number of nations (50–52).
Anemia (52.6%) was the most frequent co-occurring complication, affecting about 13.3% of patients. Similar to this, anemia is a condition that affects a significant number of colorectal patients (20, 53, 54) and is associated with a worse prognosis (55).
Regarding management pattern, a three-drug combination (5-FU plus leucovorin plus Oxaliplatin, or FOLFOX), followed by a two-drug combination (Oxaliplatin plus Capecitabine, or XELOX), which is frequently used in Ethiopian cancer centers, was taken by the majority (50.4%) of CRC patients who are receiving only chemotherapy. This is also recommended by the American Cancer Society and the National Comprehensive Cancer Network, and it is consistent with an Ethiopian and Kenyan research (20, 33, 48, 49). Combination chemotherapeutic treatments have been demonstrated to increase quality of life, time to progression, and overall survival while free of illness (9). This might be attributable to the synergistic and additive effects of chemotherapy medication combinations. These medications work in a variety of ways to help kill malignant cells (56).
MRHs are major healthcare problems, and a large proportion of them can be avoided. A total of 186 MRHs were found in 76 CRC patients, resulting in 53.1% prevalence. This finding is in line with another Ethiopian study (20) that demonstrates 48%. Contrastingly, when compared to prospective research conducted in Kenya that revealed 132 MRHs in 71 CRC patients, which was much greater (33). These differences suggest that comparing the results is challenging due to variations in study contexts, measurement methods, and classification systems. The risk of developing MRHs like ADRs, comorbidities, drug interactions, and non-adherence increases with the complexity of the chemotherapy (14). The most frequent MRHs were the need for additional drug therapy (29.6%), sub-therapeutic dose (22%), drug interaction (18.3%), and ADRs (18.3%). On the other side, a study done in Kenya found that ADRs, the need for additional drug therapy, and non-compliance were the most frequent MRHs (33).
Adverse drug events are frequent in chemotherapy patients due to the drug’s pharmacodynamic properties and narrow therapeutic indices (17). Many ADRs seem inevitable because most cytotoxic drugs cannot distinguish between healthy and cancerous cells. Nausea and leucopenia were the most frequently reported adverse drug reactions, at 61.8% and 50%, respectively. Serotonin plays an important role in both acute and delayed chemotherapy-induced nausea and vomiting, involving both peripheral and central nervous system pathways (57).
In our study, taking five or more medications was found to be an independent predictor of the presence of MRHs. Gender and age were not predictors of the presence of MRHs in a study conducted in Singapore and at Tikur Anbessa Specialized Hospital, which is consistent with our findings (17, 58). Puts et al. discovered that being ≥76 years old and taking ≥5 drugs were risk factors for moderate-to-severe potential MRH when they investigated the association between patient factors and the existence of MRH in an older cancer cohort (58). The fact that this study focuses on a specific patient population and involves clinical pharmacists and oncology nurses in data collection, which is critical to ensuring data quality, is one of its strengths. Numerous studies have demonstrated clinical pharmacists’ ability to recognize and prevent clinically important MRHs, as well as physicians’ recognition and responsiveness to clinical pharmacist recommendations for drug-related interventions (59, 60). Our study has two significant limitations: reliance on patient medical charts for retrospective analysis and a single-site design focused on patients at the UoGCSH oncology center. Consequently, the results may not be generalizable to the broader population and should be interpreted cautiously. Thus, multicenter prospective interventional follow-up studies are required.
FOLFOX and XELOX treatment regimen were most frequently used. MRHs were identified in 53.1% of CRC patients. The study revealed that 29.6% of patients experienced the need for additional drug therapy, while 22% received sub-therapeutic doses. Furthermore, the stage of cancer, the presence of comorbidity and complications, and the number of medications taken were all independent predictors of developing MRHs. Given the prevalence of MRHs, it is crucial to enhance clinical pharmacy services and medication review stewardship to maximize the effectiveness of CRC treatment.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Debre Tabor University Ethical Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
BK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ME: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Writing – original draft. DT: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. MZ: Methodology, Software, Validation, Visualization, Writing – review & editing. YK: Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft. CT: Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
This work was supported by Debre Tabor University (grant no. DTU/RE/1/3059/2013).
We would like to thank the oncology department of UoGCSH for their invaluable assistance in providing information and other materials during the data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ADR, Adverse drug reaction; AOR, Adjusted odd ratio; CRC, Colorectal cancer; DDI, Drug-drug interaction; MRH, Medication-related harm; UoGCSH, University of Gondar Comprehensive Specialized Hospital.
1. Douaiher J, Ravipati A, Grams B, et al. Colorectal cancer—global burden, trends, and geographical variations. J Surg Oncol (2017) 115(5):619–30. doi: 10.1002/jso.24578
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Peterse EF, Meester RG, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer (2018) 124(14):2964–73. doi: 10.1002/cncr.31543
4. WHO. International Agency Research on Cancer. Latest global cancer data. Available at: https://www.iarc.fr/wp-content/uploads/2018/09/pr263_E.pdf (Accessed January 21, 2023).
5. Graham A, Adeloye D, Grant L, Theodoratou E, Campbell H. Estimating the incidence of colorectal cancer in Sub–Saharan Africa: A systematic analysis. J Global Health (2012) 2(2). doi: 10.7189/jogh.02.020404
6. Woldu M, Legese D, Abamecha F, Berha A. The prevalence of cancer and its associated risk factors among patients visiting oncology unit, Tikur Anbessa Specialized Hospital, Addis Ababa-Ethiopia. J Cancer Sci Ther (2017) 9(10.4172):1948–5956.
7. Atinafu BT, Kebede WM, Demlew TM, Aynalem YA, Shiferaw WS, Tarekegn FN, et al. Mortality rate and its determinants among colorectal cancer patients in comprehensive specialized hospitals, Ethiopia: a retrospective cohort study. PAMJ-One Health (2022) 7(26). doi: 10.11604/pamj-oh.2022.7.26.29412
8. Galvin R, Moriarty F, Cousins G, Cahir C, Motterlini N, Bradley M, et al. Prevalence of potentially inappropriate prescribing and prescribing omissions in older Irish adults: findings from T he I rish L ongitu D inal Study on A geing study (TILDA). Eur J Clin Pharmacol (2014) 70:599–606. doi: 10.1007/s00228-014-1651-8
9. Akhtar R, Chandel S, Sarotra P, Medhi B. Current status of pharmacological treatment of colorectal cancer. World J Gastrointest Oncol (2014) 6(6):177. doi: 10.4251/wjgo.v6.i6.177
10. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The global burden of cancer 2013. JAMA Oncol (2015) 1(4):505–27. doi: 10.1001/jamaoncol.2015.0735
11. Carmona-Bayonas A, Jiménez-Fonseca P, Castañón E, Ramchandani-Vaswani A, Sánchez-Bayona R, Custodio A, et al. Chronic opioid therapy in long-term cancer survivors. Clin Trans Oncol (2017) 19:236–50. doi: 10.1007/s12094-016-1529-6
12. Vm J, Horvat N, Tommy W. Pharmaceutical care network europe foundation. Classification Drug related problems revised. (2018) 2(2):15–6.
13. Jaehde U, Liekweg A, Simons S, Westfeld M. Minimising treatment-associated risks in systemic cancer therapy. Pharm World Sci (2008) 30:161–8. doi: 10.1007/s11096-007-9157-4
14. Mustapha S, Mohammed M, Mustapha L, Yunusa I, Basgut B. A survey on drug related problems in cervical cancer patients receiving chemotherapy in ahmadu bello university teaching hospital zaria. Bayero J Pure Appl Sci (2017) 10(1):489–92.
15. Riechelmann RP, Saad ED. A systematic review on drug interactions in oncology. Cancer Invest (2006) 24(7):704–12. doi: 10.1080/07357900601063766
16. Koh Y, Kutty FBM, Li SC. Drug-related problems in hospitalized patients on polypharmacy: the influence of age and gender. Ther Clin Risk Manage (2005) 1(1):39–48. doi: 10.2147/tcrm.1.1.39.53597
17. Yeoh TT, Tay XY, Si P, Chew L. Drug-related problems in elderly patients with cancer receiving outpatient chemotherapy. J Geriatric Oncol (2015) 6(4):280–7. doi: 10.1016/j.jgo.2015.05.001
18. Pereira KG, Peres MA, Iop D, Boing AC, Boing AF, Aziz M, et al. Polypharmacy among the elderly: a population-based study. Rev Bras Epidemiologia (2017) 20:335–44. doi: 10.1590/1980-5497201700020013
19. Kim J, Parish AL. Polypharmacy and medication management in older adults. Nurs Clin (2017) 52(3):457–68. doi: 10.1016/j.cnur.2017.04.007
20. Kefale B, Engidaw MT, Tesfa D, Yazie TS, Molla M, Yismaw MB. Clinical pattern and drug-related problems among colorectal cancer patients at oncology center in Ethiopia: A hospital-based study. SAGE Open Med (2022) 10:20503121221131691. doi: 10.1177/20503121221131691
21. Agrawal R, Nagpure S. A study on polypharmacy and drug interactions among elderly hypertensive patients admitted in a tertiary care hospital. Int J Health Allied Sci (2018) 7(4):222–.
22. Kefale B, Engidaw MT, Tesfa D, Molla M, Tegegne GT. Medication-related problems among patients with cervical cancers at oncology centers of University of Gondar comprehensive specialized hospital: A hospital-based retrospective study. J Oncol Pharm Pract (2023) 1:0781552231174589. doi: 10.1177/10781552231174589
23. Kefale B, Engidaw MT, Tesfa D, Molla M, Yismaw MB. Management practice and drug related problems and its contributing factors among cervical cancer patients at oncologic center in Ethiopia: A hospital-based retrospective study. Ther Clin Risk Manage (2022) 18:643–55. doi: 10.2147/TCRM.S364923
24. Lund JL, Sanoff HK, Hinton SP, Muss HB, Pate V, Stürmer T. Potential medication-related problems in older breast, colon, and lung cancer patients in the United States. Cancer Epidemiol Prev Biomarkers (2018) 27(1):41–9. doi: 10.1158/1055-9965.EPI-17-0523
25. Neugut AI, Zhong X, Lebwohl B, Hillyer GC, Accordino MK, Wright JD, et al. Adherence to colonoscopy at 1 year following resection of localized colon cancer: a retrospective cohort study. Ther Adv Gastroenterol (2018) 11:1756284818765920. doi: 10.1177/1756284818765920
26. Tezcan S, İzzettin FV, Sancar M, Turhal NS, Yumuk PF. Role of clinical oncology pharmacist in determination of pharmaceutical care needs in patients with colorectal cancer. Eur J Hosp Pharm (2018) 25(e1):e17–20. doi: 10.1136/ejhpharm-2016-001188
27. Colombo L, Aguiar PM, Lima T, Storpirtis S. The effects of pharmacist interventions on adult outpatients with cancer: A systematic review. J Clin Pharm Ther (2017) 42(4):414–24. doi: 10.1111/jcpt.12562
28. Cipolle RJ, Strand L, Morley PC, Morley P. Pharmaceutical Care Practice: The Clinician's Guide: The Clinician's Guide: McGraw-Hill Medical. US: McGraw-Hill Medical (2004).
29. Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm (1992) 49(9):2229–32. doi: 10.1093/ajhp/49.9.2229
30. Chalya PL, Mchembe MD, Mabula JB, Rambau PF, Jaka H, Koy M, et al. Clinicopathological patterns and challenges of management of colorectal cancer in a resource-limited setting: a Tanzanian experience. World J Surg Oncol (2013) 11(1):1–9. doi: 10.1186/1477-7819-11-88
31. White A, Ironmonger L, Steele RJ, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC cancer (2018) 18(1):1–11. doi: 10.1186/s12885-018-4786-7
32. Xie Y, Shi L, He X, Luo Y. Gastrointestinal cancers in China, the USA, and europe. Gastroenterol Rep (2021) 9(2):91–104. doi: 10.1093/gastro/goab010
33. Kabiru CM, Karimi PN, Nyamu DG, Weru IW. Drug therapy problems and health related quality of life among patients with colorectal cancer in a Kenyan tertiary health facility. J Oncol Pharm Practice (2021) 27(2):428–34. doi: 10.1177/1078155220971024
34. Keighley MR, Williams NS. Surgery of the Anus, Rectum and Colon. Philadelphia: W.B. Saunders (1999).
35. Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev (2009) 18(4):1174–82. doi: 10.1158/1055-9965.EPI-08-1118
36. White A, Thomson C, Howard T, Shelton J. Excess Cancer Burden in Men. UK London: Cancer Research (2013).
37. Edgren G, Liang L, Adami H-O, Chang ET. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol (2012) 27:187–96. doi: 10.1007/s10654-011-9647-5
38. Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survivalSex disparities in cancer mortality. Cancer epidemiol Biomarkers Prev (2011) 20(8):1629–37. doi: 10.1158/1055-9965.EPI-11-0246
39. Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev (2013). doi: 10.1152/physrev.00033.2011
40. Bassett JK, Severi G, English DR, Baglietto L, Krishnan K, Hopper JL, et al. Body size, weight change, and risk of colon cancer. Cancer epidemiol Biomarkers Prev (2010) 19(11):2978–86. doi: 10.1158/1055-9965.EPI-10-0543
41. Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev (2007) 16(12):2533–47. doi: 10.1158/1055-9965.EPI-07-0708
42. Teka MA, Yesuf A, Hussien FM, Hassen HY. Histological characteristics, survival pattern and prognostic determinants among colorectal cancer patients in Ethiopia: A retrospective cohort study. Heliyon (2021) 7(2):e06366. doi: 10.1016/j.heliyon.2021.e06366
43. Zemenfes D, Kotisso B. A two-year review of colorectal cancer at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. East Cent Afr J Surg (2015) 20(2):10–6.
44. Atinafu BT, Bulti FA, Demelew TM. Survival status and predictors of mortality among colorectal cancer patients in Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia: a retrospective followup study. J Cancer Prev (2020) 25(1):38. doi: 10.15430/JCP.2020.25.1.38
45. Fang L, Yang Z, Zhang M, Meng M, Feng J, Chen C. Clinical characteristics and survival analysis of colorectal cancer in China: a retrospective cohort study with 13,328 patients from southern China. Gastroenterol Rep (2021) 9(6):571–82. doi: 10.1093/gastro/goab048
46. Etissa EK, Assefa M, Ayele BT. Prognosis of colorectal cancer in Tikur Anbessa Specialized Hospital, the only oncology center in Ethiopia. PloS One (2021) 16(2):e0246424. doi: 10.1371/journal.pone.0246424
47. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(3):329–59.
48. Ozols RF, Herbst RS, Colson YL, Gralow J, Bonner J, Curran WJ, et al. Clinical cancer advances 2006: major research advances in cancer treatment, prevention, and screening-a report from the American Society of Clinical Oncology. J Clin Oncol (2007) 25(1):146–62. doi: 10.1200/JCO.2006.09.7030
49. Arani SH, Kerachian MA. Rising rates of colorectal cancer among younger Iranians: is diet to blame? Curr Oncol (2017) 24(2):131–7. doi: 10.3747/co.23.3226
50. Troeung L, Sodhi-Berry N, Martini A, Malacova E, Ee H, O’Leary P, et al. Increasing incidence of colorectal cancer in adolescents and young adults aged 15–39 years in Western Australia 1982–2007: examination of colonoscopy history. Front Public Health (2017) 5:179. doi: 10.3389/fpubh.2017.00179
51. Bailey CE, Hu C-Y, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg (2015) 150(1):17–22. doi: 10.1001/jamasurg.2014.1756
52. Edna T-H, Karlsen V, Jullumstro E, Lydersen S. Prevalence of anaemia at diagnosis of colorectal cancer: assessment of associated risk factors. Hepato-gastroenterology (2012) 59(115):713–6.
53. Khanbhai M, Shah M, Cantanhede G, Ilyas S, Richards T. The problem of anaemia in patients with colorectal cancer. Int Scholarly Res Notices (2014) 2014. doi: 10.1155/2014/547914
54. Gvirtzman R, Livovsky DM, Tahover E, Goldin E, Koslowsky B. Anemia can predict the prognosis of colorectal cancer in the pre-operative stage: A retrospective analysis. World J Surg Oncol (2021) 19:1–7. doi: 10.1186/s12957-021-02452-7
55. MacDonald DA, Chang H, Wei Y, Hager KD. Drug therapy problem identification and resolution by clinical pharmacists in a family medicine residency clinic. Innov Pharm (2018) 9(2):1. doi: 10.24926/iip.v9i2.971
56. Adel N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. Am J managed Care (2017) 23(14 Suppl):S259–S65.
57. Sisay EA, Engidawork E, Yesuf T, Ketema E. Drug related problems in chemotherapy of cancer patients. J Cancer Sci Ther (2015) 7(2):55–9.
58. Puts MT, Costa-Lima B, Monette J, Girre V, Wolfson C, Batist G, et al. Medication problems in older, newly diagnosed cancer patients in Canada: how common are they? A prospective pilot study. Drugs aging (2009) 26:519–36. doi: 10.2165/00002512-200926060-00008
59. Viktil KK, Blix HS. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol toxicol (2008) 102(3):275–80. doi: 10.1111/j.1742-7843.2007.00206.x
Keywords: colorectal cancer, medication related harm, oncology center, predictors, ethiopia
Citation: Kefale B, Engidaw MT, Tesfa D, Molla M, Kefale Y and Tafere C (2023) Management pattern and medication-related harms and its predictors in colorectal cancer patients: an institutional-based retrospective study. Front. Oncol. 13:1253845. doi: 10.3389/fonc.2023.1253845
Received: 06 July 2023; Accepted: 17 October 2023;
Published: 31 October 2023.
Edited by:
Aditi Banerjee, University of Maryland, United StatesReviewed by:
Mesnad Alyabsi, King Abdullah International Medical Research Center (KAIMRC), Saudi ArabiaCopyright © 2023 Kefale, Engidaw, Tesfa, Molla, Kefale and Tafere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Belayneh Kefale, YmtlZmFsZTVAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.