
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol., 19 September 2023
Sec. Radiation Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1253629
This article is part of the Research TopicNeoadjuvant Therapy in Non-Small Cell Lung Cancer: Clinical, Pathological and Translational Research - Volume IIView all 5 articles
 Emily S. Lebow1,2
Emily S. Lebow1,2 Narek Shaverdian1
Narek Shaverdian1 Jordan E. Eichholz1
Jordan E. Eichholz1 Leah B. Kratochvil1
Leah B. Kratochvil1 Megan McCune1
Megan McCune1 Yonina R. Murciano-Goroff1,3
Yonina R. Murciano-Goroff1,3 Justin Jee1,3
Justin Jee1,3 Juliana Eng1,3
Juliana Eng1,3 Jamie E. Chaft3
Jamie E. Chaft3 Mark G. Kris1,3
Mark G. Kris1,3 Ekaterina Kalashnikova4
Ekaterina Kalashnikova4 Jordan Feeney4
Jordan Feeney4 Carly Bess Scalise4
Carly Bess Scalise4 Sumedha Sudhaman4
Sumedha Sudhaman4 Charuta C. Palsuledesai4
Charuta C. Palsuledesai4 Meenakshi Malhotra4
Meenakshi Malhotra4 Michael Krainock4
Michael Krainock4 Himanshu Sethi4
Himanshu Sethi4 Alexey Aleshin4
Alexey Aleshin4 Minetta C. Liu4
Minetta C. Liu4 Annemarie F. Shepherd1
Annemarie F. Shepherd1 Abraham J. Wu1
Abraham J. Wu1 Charles B. Simone II1
Charles B. Simone II1 Daphna Y. Gelblum1
Daphna Y. Gelblum1 Kaylie A. Johnson1
Kaylie A. Johnson1 Charles M. Rudin1,3
Charles M. Rudin1,3 Daniel R. Gomez1
Daniel R. Gomez1 Pedram Razavi1,3
Pedram Razavi1,3 Jorge S. Reis-Filho1
Jorge S. Reis-Filho1 James M. Isbell1,3
James M. Isbell1,3 Bob T. Li1,3
Bob T. Li1,3 Andreas Rimner1*
Andreas Rimner1*Background: Sensitive and reliable biomarkers for early detection of recurrence are needed to improve post-definitive radiation risk stratification, disease management, and outcomes for patients with unresectable early-stage or locally advanced non-small cell lung cancer (NSCLC) who are treated with definitive radiation therapy (RT). This prospective, multistate single-center, cohort study investigated the association of circulating tumor DNA (ctDNA) status with recurrence in patients with unresectable stage I-III NSCLC who underwent definitive RT.
Methods: A total of 70 serial plasma samples from 17 NSCLC patients were collected before, during, and after treatment. A personalized, tumor-informed ctDNA assay was used to track a set of up to 16 somatic, single nucleotide variants in the associated patient’s plasma samples.
Results: Pre-treatment ctDNA detection rate was 82% (14/17) and varied based on histology and stage. ctDNA was detected in 35% (6/17) of patients at the first post-RT timepoint (median of 1.66 months following the completion of RT), all of whom subsequently developed clinical progression. At this first post-RT time point, patients with ctDNA-positivity had significantly worse progression-free survival (PFS) [hazard ratio (HR): 24.2, p=0.004], and ctDNA-positivity was the only significant prognostic factor associated with PFS (HR: 13.4, p=0.02) in a multivariate analysis. All patients who developed clinical recurrence had detectable ctDNA with an average lead time over radiographic progression of 5.4 months, and post-RT ctDNA positivity was significantly associated with poor PFS (p<0.0001).
Conclusion: Personalized, longitudinal ctDNA monitoring can detect recurrence early in patients with unresectable NSCLC patients undergoing curative radiation and potentially risk-stratify patients who might benefit most from treatment intensification.
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer-related diagnoses and deaths (1). The current standard-of-care for patients with inoperable NSCLC is definitive curative radiotherapy (RT) including stereotactic body radiotherapy (SBRT) for early-stage disease and concurrent chemoradiation (CRT) followed by durvalumab for locally-advanced disease (2).
For early-stage disease, SBRT has shown excellent long-term primary tumor control rates, with nodal and distant recurrences representing the most common failure pattern (3). In patients with locally advanced NSCLC, consolidation durvalumab significantly improves the progression-free survival (PFS) compared to CRT alone, yet in-field recurrence and distant metastases present a challenge post-CRT and durvalumab. Thus, careful long-term surveillance is necessary for early detection of recurrence before the onset of disease-related symptoms and at a time when therapy might provide greater clinical benefit. The current surveillance protocol includes computed tomography (CT) of the chest every 3 months for 2 years, every 6 months during years 3 and 4, and annually thereafter (2). Radiographic surveillance is associated with several challenges such as low sensitivity, detection of macroscopic disease, and difficulties in interpretation of results due to post-treatment effects, such as inflammatory changes, radiation fibrosis, or reactive lymph nodes in cases with local recurrences (4). Therefore, a need exists for a sensitive, blood-based biomarker for early detection of molecular residual disease (MRD), post-definitive therapy.
Circulating tumor DNA (ctDNA) has emerged as a prognostic biomarker to assess MRD and predict recurrence (5, 6). In this study, we assessed the prognostic value of a tumor-informed ctDNA assay for longitudinal monitoring of patients with stage I-III NSCLC undergoing definitive radiotherapy to detect recurrence and identify patients who might benefit from intensification of systemic therapy.
All patients had a pathologically confirmed diagnosis of lung cancer. Blood samples (n=70) serially collected (before and after SBRT as well as before, during, and after conventional RT with/without concurrent systemic therapy and adjuvant durvalumab) from a prospective clinical cohort of patients (N=17) with stage I-III NSCLC diagnosed between 2017 and 2020 were used for ctDNA analysis. All patients were staged according to American Joint Committee on Cancer (AJCC) 8th Edition. Patients with stage I disease were treated with SBRT in 4 or 5 fractions (10 – 12 Gy per fraction). Patients with stage II and stage III disease were treated with conventional fractionation (2 Gy per fraction) with or without concurrent and adjuvant systemic therapy. Post-treatment plasma was collected generally concurrently with the standard-of-care imaging at the discretion of the treating clinician. Light, moderate, and heavy smokers were defined as <20 packs/year, 20-40 packs/year, and >40 packs/year, respectively. The longitudinal setting was defined as serial ctDNA testing of patients after discontinuation of RT (during systemic therapy, if given, and during surveillance), wherein patients had sample collection for ctDNA tests at regular intervals or as determined by the treating physician. This study was approved by the Memorial Sloan Kettering Institutional Review Board. The study was conducted in accordance with the principles of the Declaration of Helsinki 2013. All patients provided informed consent.
Personalized, tumor-informed ctDNA assays were designed for all patients as previously described (7). Briefly, a set of 16 high-confidence, patient-specific, somatic, clonal single nucleotide variants (SNVs) were selected for multiplex polymerase chain reaction (mPCR) testing from whole-exome sequencing of formalin-fixed paraffin-embedded (FFPE) tumor tissue and matched normal blood samples. The mPCR primers targeting the patient-specific SNVs were designed, synthesized, and used for tracking ctDNA in patients’ longitudinal plasma samples. Plasma samples were considered ctDNA-positive when at least 2 SNVs were detected above a predefined confidence threshold. ctDNA concentration was expressed as mean tumor molecules (MTM)/mL of plasma.
Fisher’s exact test was used to evaluate the statistical significance of the association between ctDNA detection rates at baseline and categorical variables. Using the Kaplan-Meier method, PFS was assessed as the primary outcome between the date of RT initiation and clinical recurrence using post-RT ctDNA status for patient stratification. Log-rank test or Cox proportional hazards model was used for comparing two survival distributions with p ≤ 0.05 being considered significant. Statistical analyses were carried out in STATA v16.1.
Patient demographics, baseline characteristics, and treatment regimens are presented in Table 1. Patients were followed for a median of 26 months (range: 4-54). ctDNA assays were successfully designed for all patients.
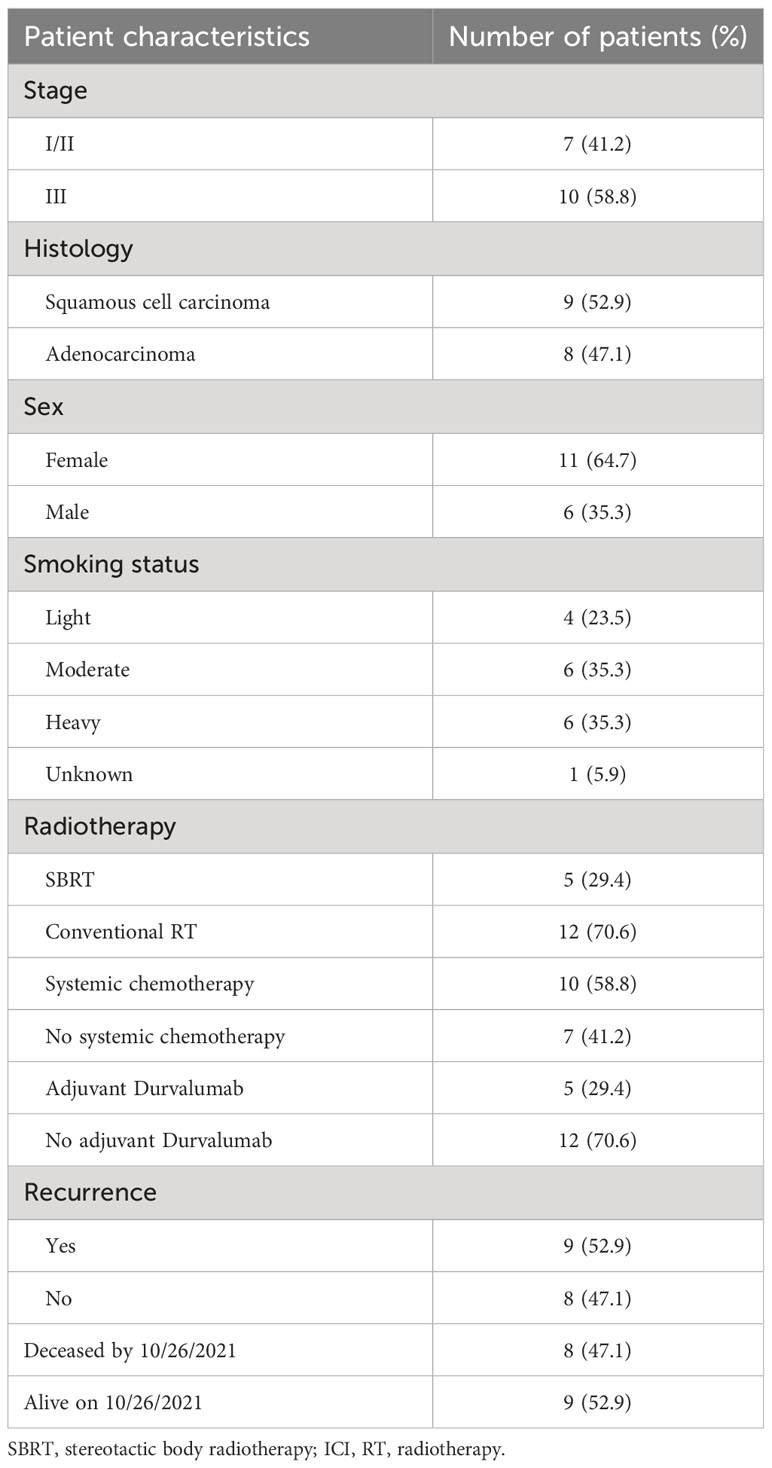
Table 1 Patient demographics, baseline characteristics, treatment regimen, and outcome at the last follow-up.
At baseline (pre-RT time point), the ctDNA detection rate was 82% (14/17; Figure 1A) and varied based on histology and stage (Figures 1B–E). All patients with squamous cell carcinoma (9/9) were ctDNA-positive, whereas 63% (5/8) of patients with adenocarcinoma harbored detectable ctDNA (Figure 1B). All patients (10/10) with stage III disease were ctDNA-positive (Figure 1C) and presented with higher ctDNA levels, compared to patients with stage I/II disease (4/7; 57%) (Figure 1E). All patients with baseline ctDNA-negativity remained progression-free.
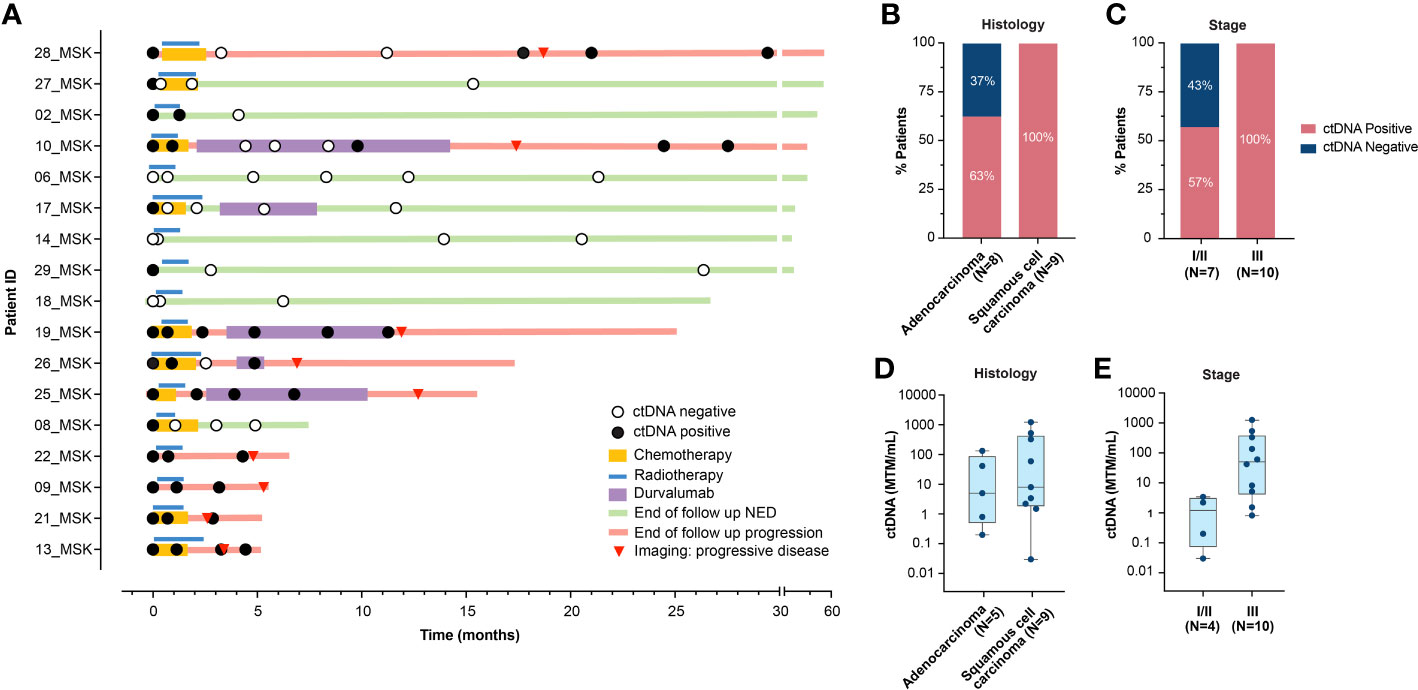
Figure 1 (A) Overview plot depicting treatment regimen, longitudinal ctDNA analysis and clinical outcomes for each patient in the cohort. (B, C). Pre-RT ctDNA detection rate based on (B) histology, and (C) stage. (D, E). Pre-RT ctDNA levels (MTM/mL) in 14 patients with detectable ctDNA based on (D) histology, and (E) stage. RT, radiotherapy; ctDNA, circulating tumor DNA; NED, no evidence of disease; MTM, mean tumor molecules.
ctDNA detection rate at the first post-RT timepoint, collected at a median of 1.66 months (range: 0.4-13.7) was 35.3% (6/17), with all ctDNA-positive patients showing confirmed clinical progression. Additional three patients with transient ctDNA clearance eventually recurred. Compared to ctDNA-negativity at the first post-RT timepoint, patients with ctDNA-positivity had significantly worse PFS [hazard ratio (HR): 24.2, 95% confidence interval (CI): 2.8-208.6, p=0.004; Figure 2A].
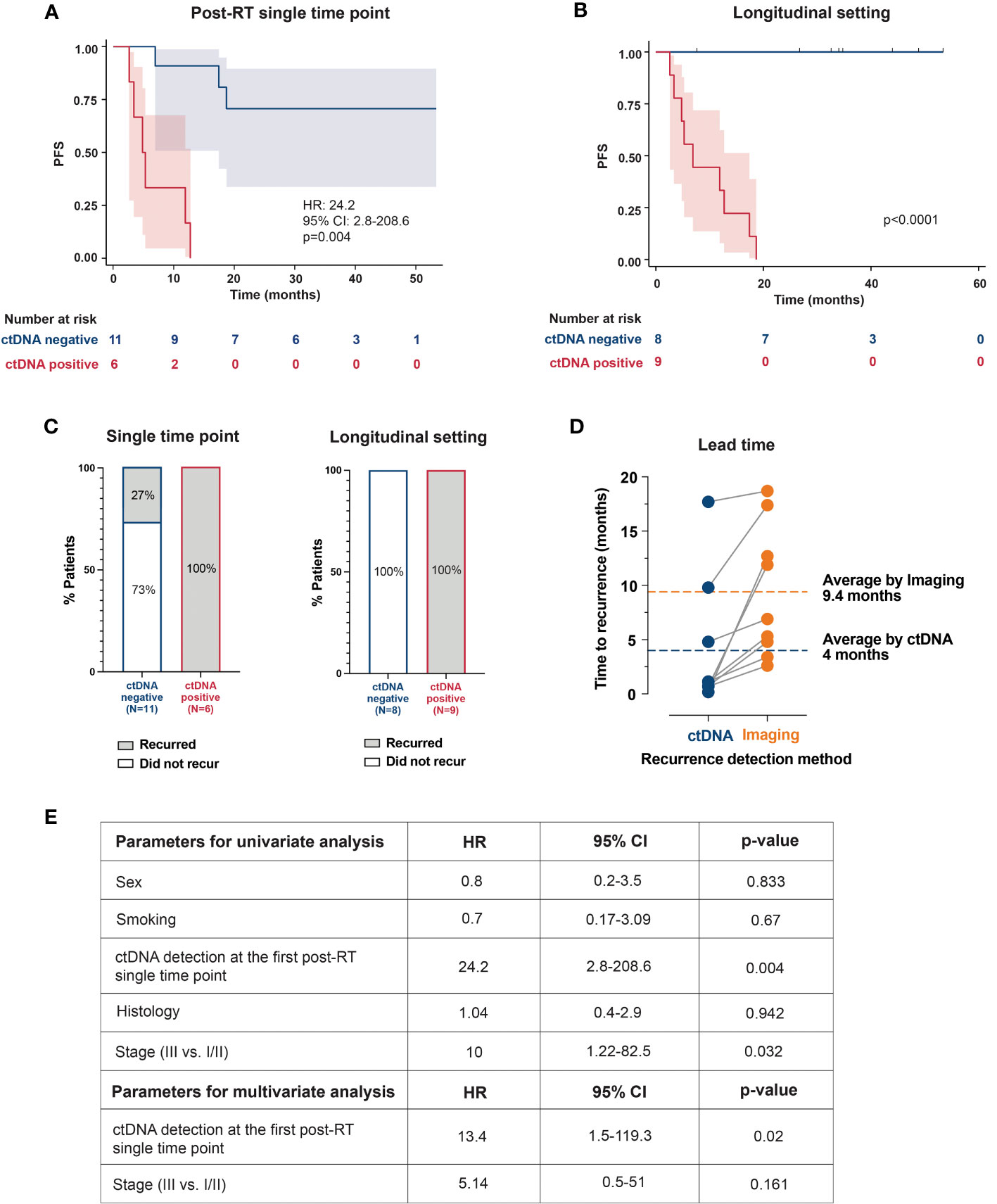
Figure 2 Kaplan–Meier plots representing the association of ctDNA positivity with PFS: (A) at the first available post-RT timepoint (B) and in longitudinal setting (C) The bar plots show recurrence rates in ctDNA-positive and ctDNA-negative patients at a single timepoint and longitudinally. (D) Comparison between ctDNA and radiographic imaging. Lead time indicates the number of months by which the ctDNA detected molecular recurrence ahead of radiological progression. (E) Univariate and multivariate analyses of prognostic factors and their association with PFS. RT, radiotherapy; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval.
In the longitudinal setting with serial ctDNA testing, post-RT ctDNA-positivity was associated with shorter PFS (p<0.0001; Figure 2B). Furthermore, all patients with confirmed clinical progression (9/17, 53%) were ctDNA-positive (sensitivity=100%; 9/9), and all ctDNA-negative patients remained progression-free (specificity=100%; 8/8; Figure 2C). Notably, ctDNA-based MRD detection had an average lead-time of 5.4 months over radiographic progression (Figure 2D). Supplementary Figure 1 highlights clinical courses of 3 patients, depicting ctDNA dynamics during and after definitive RT and their correlation with radiographic response/progression.
In both univariate and multivariate analysis, ctDNA-positivity at the first post-RT timepoint was the strongest prognostic factor associated with PFS (HR: 24.2, p=0.004, and HR: 13.4, p=0.02), followed by stage (HR: 10, p=0.032; univariate analysis; Figure 2E).
Definitive radiation is the standard-of-care for patients with inoperable localized lung cancer. Our study demonstrates that tumor-informed ctDNA monitoring is an effective tool to detect MRD among patients treated with definitive RT. ctDNA monitoring preceded clinical recurrence by a median of 5.4 months, providing a critical window for early therapeutic intervention.
ctDNA monitoring is a promising technology to personalize therapy selection among patients with localized lung cancer. In the early-stage setting, the determination of adjuvant therapy after definitive RT is based on high-risk clinical and pathologic features (2). We observed that all patients with detectable MRD developed clinical recurrence, suggesting utilization of ctDNA may identify a group of patients at high risk of relapse who are likely to derive benefit from adjuvant therapy. In the locally advanced setting, one year of adjuvant durvalumab is standard-of-care for patients following definitive chemoradiation. MRD monitoring may allow for personalization of adjuvant therapy by identifying patients benefiting from consolidative durvalumab versus those who may benefit from an alternative approach (6). For example, in our cohort, patients who failed to clear ctDNA while receiving adjuvant durvalumab developed clinical recurrence. It is possible this cohort would have benefited from an alternative or intensified systemic therapy. An ongoing study (NCT04585490) evaluating personalized escalation of therapy for patients with locally advanced lung cancer treated with chemoradiation therapy will be an important contribution to patient care and demonstration of the clinical utility of ctDNA-based MRD analysis.
MRD monitoring may also identify patients with a low risk of recurrence for whom adjuvant therapy could be de-intensified. For example, among patients receiving adjuvant durvalumab in the PACIFIC trial, 19% who received placebo remained disease-free at 5 years, suggesting there are patients who do not derive clinical benefit but are exposed to toxicity of one year of adjuvant durvalumab (8). In a study by Monding et al, one patient with undetectable ctDNA died from pneumonitis related to immune checkpoint inhibition, highlighting the importance of identifying patients most likely to benefit from a therapy which poses a risk of high-grade toxicities (6).
Other studies in locally-advanced NSCLC utilizing different ctDNA technologies have demonstrated the utility of MRD detection at first timepoint (9), 1-month (10), or within 2 weeks to 4 months (11) of post-definitive treatment to be prognostic of clinical outcomes. In our study, ctDNA-positive patients at first post-RT timepoint were 24 times more likely to experience disease progression. Nonetheless, sensitivity was improved with longitudinal monitoring, which has been reported in lung cancer (9, 12) and other solid tumors (7, 13, 14).
In our cohort, we observed a baseline (pre-RT) ctDNA detection rate of 82% which was associated with stage and histology, consistent with prior analyses (6, 10–12). ctDNA detection is challenging in low-volume disease with limited ctDNA shedding, and further efforts are required to optimize detection in this patient population; however, it is important to note that the patients with baseline ctDNA-negativity had favorable outcomes.
Although blood samples were collected prospectively in this study, the correlation between ctDNA status and PFS was analyzed retrospectively, which precluded real-time assessment of risk of progression based on ctDNA status of patient at a given time point. Additionally, our study is limited by the small cohort size, heterogeneous disease stages and treatment regimens, and limited clinical follow-up for some patients. Nonetheless, our study demonstrated high sensitivity and specificity for ctDNA with for detection of recurrence with serial monitoring after completion of RT. Currently, the determination of adjuvant therapy in early-stage NSCLC patients after definitive RT is based on the presence of high-risk pathologic features (poorly differentiated tumor, vascular invasion, and visceral pleural involvement). However, our results highlight the potential utility of a personalized and tumor-informed ctDNA testing approach to risk-stratify patients for treatment decision-making. Prospective studies with larger cohorts are warranted to establish the clinical utility of ctDNA, particularly to determine the optimal interval for ctDNA testing, to validate the prognostic performance of longitudinal ctDNA monitoring, and to evaluate the benefits and risks of ctDNA-guided adjuvant treatment decision-making in NSCLC patients receiving curative RT.
The authors declare that all relevant data used to conduct the analyses are available within the article. To protect the privacy and confidentiality of patients in this study, clinical data cannot be made publicly available.
The studies involving humans were approved by Memorial Sloan Kettering Cancer Center IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
EL: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. NS: Conceptualization, Writing – review & editing. JE: Conceptualization, Data curation, Writing – review & editing. LK: Data curation, Writing – review & editing. MMc: Data curation, Writing – review & editing. YM-G: Conceptualization, Writing – review & editing. JJ: Conceptualization, Writing – review & editing. JE: Conceptualization, Writing – review & editing. JC: Conceptualization, Writing – review & editing. MGK: Conceptualization, Writing – review & editing. EK: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. JF: Writing – review & editing, Data curation, Formal Analysis, Investigation, Methodology, Visualization. CBS: Writing – review & editing, Data curation, Formal Analysis, Investigation, Methodology. SS: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Methodology. CCP: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. MMa: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Methodology. MGK: Writing – review & editing, Conceptualization, Data curation, Formal Analysis, Methodology. HS: Methodology, Writing – review & editing, Conceptualization, Data curation, Formal Analysis. AA: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review & editing. MCL: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review & editing. AS: Conceptualization, Writing – review & editing. AW: Conceptualization, Writing – review & editing. CSi: Conceptualization, Writing – review & editing. DG: Conceptualization, Writing – review & editing. KJ: Conceptualization, Project administration, Writing – review & editing. CR: Conceptualization, Writing – review & editing. DG: Concepualization, Writing – review & editing. PR: Conceptualization, Writing – review & editing. JR: Conceptualization, Writing – review & editing. JI: Conceptualization, Writing – review & editing. BL: Conceptualization, Investigation, Methodology, Visualization, Writing – review & editing. AR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. EL, EK, HS, and AR had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This research was funded in part through the National Institute of Health/National Cancer Institute Cancer Center support grant (P30 CA008748).
NS reports research funding from Novartis. DG reports research funding from Astra Zeneca and Varian and receives Honoraria from Johnson and Johnson, MedLearning Gropu, and GRAIL. YM-G reports travel, accommodation, and expenses from AstraZeneca and Loxo Oncology/Eli Lilly. She acknowledges honoraria from Virology Education and Projects in Knowledge for a CME program funded by an educational grant from Amgen. She acknowledges associated research funding to the institution from Mirati Therapeutics, Loxo Oncology at Eli Lilly, Elucida Oncology, Taiho Oncology, Hengrui USA, Ltd/Jiangsu Hengrui Pharmaceuticals, Luzsana Biotechnology, and Endeavor Biomedicines. She acknowledges royalties from Rutgers University Press and Wolters Kluwer. She acknowledges food/beverages from Endeavor Biomedicines. YM-G acknowledges receipt of training through an institutional K30 grant from the NIH CTSA UL1TR00457. She has received funding from a Kristina M. Day Young Investigator Award from Conquer Cancer, the ASCO Foundation, endowed by Dr. Charles M. Baum and Carol A. Baum. She is also funded by the Fiona and Stanley Druckenmiller Center for Lung Cancer Research, the Andrew Sabin Family Foundation, the Society for MSK, and a Paul Calabresi Career Development Award for Clinical Oncology NIH/NCI K12 CA184746. JJ has a patent licensed by MDSeq Inc. JC has served as a consultant for Astra Zeneca, Bristol-Myers Squib, Genentech, Merck, Flame Biosciences, Novartis, Regeneron-Sanofi, Guardant Health, and Janssen as well as received research funding to her institution from Astra Zeneca, Bristol-Myers Squib, Genentech, and Merck. MGK receives personal fees from Novartis, Sanofi-Genzyme, AstraZeneca, Pfizer, Janssen, and Daiichi-Sankyo. He has also received honoraria for participation in educational programs from WebMD, OncLive, Physicians Education Resources, Prime Oncology, Intellisphere, Creative Educational Concepts, Peerview, i3 Health, Paradigm Medical Communications, AXIS, Carvive Systems, and AstraZeneca. MGK has received travel support from AstraZeneca, Pfizer, and Genentech as well as editorial support from Hoffman La-Roche. EK, JF, CBS, SS, CCP, MMa, MK, HS, AA, and MCL reported employment and stock ownership or option at Natera, Inc outside the submitted work. MCL reported receiving grants funding to Mayo Clinic from Eisai, Exact Sciences, Genentech, Genomic Health, GRAIL, Menarini Silicon Biosystems, Merck, Novartis, Seattle Genetics and Tesaro, and travel support from AstraZeneca, Genomic Health and Ionis, outside the submitted work. AS has stock ownership in ArcellX and Doximity. AW reports research support from CivaTech Oncology, Inc, Honoraria from Nanovi A/S, serves on the Scientific Advisory Board of Simphotek, Inc, and has stock in Simphotek, Inc. CS reports Honoraria from Varian and Novocure and serves in a Leadership position for the Proton Collaborative Group, American Society for Radiation Oncology, NRG Oncology, American Radium Society, and Annals of Palliative Medicine. DG reports research funding to her institution from Merck. CR reports personal fees from AbbVie, Amgen, Ascentage, AstraZeneca, Bicycle, Celgene, Daiichi Sankyo, Genentech/Roche, Ipsen, Jansen, Jazz, Lilly/Loxo, Pfizer, PharmaMar, Syros, Vavotek, Bridge Medicines, and Harpoon Therapeutics, outside the submitted work. DG reports grants to his institutions from Varian, AstraZeneca, Merck, and Bristol Myers Squibb, personal fees from Varia, AstraZeneca, Merck, US Oncology, Bristol Myers Squibb, Relfexion, WebMD, Vindico, and Medscape; and has served on the advisory board for AstraZeneca; he has also received Honoraria from Johnson and Johnson, MedLearning Group, and GRAIL. PR received institutional grant/funding from Grail, Illumina, Novartis, Epic Sciences, ArcherDx and Consultation/Ad board/Honoraria from Novartis, Foundation Medicine, AstraZeneca, Epic Sciences, Inivata, Natera, and Tempus. JR-F is a paid consultant of Goldman Sachs, Paige.AI and REPARE Therapeutics, a member of the Scientific Advisory Board of Goldman Sachs, Paige.AI and Volition RX, and ad hoc member of the scientific advisory board of Roche, Genetech, Roche Tissue Diagnostics, Ventana, Novartis, InVicro and GRAIL. JR-F reports receiving personal/consultancy fees from Goldman Sachs, Bain Capital, REPARE Therapeutics, Saga Diagnostics and Paige.AI, membership of the scientific advisory boards of VolitionRx, REPARE Therapeutics and Paige.AI, membership of the Board of Directors of Grupo Oncoclinicas, and ad hoc membership of the scientific advisory boards of Astrazeneca, Merck, Daiichi Sankyo, Roche Tissue Diagnostics and Personalis, outside the scope of this study. JR-F is funded in part by the Breast Cancer Research Foundation, by a Susan G Komen Leadership grant, and by the NIH/NCI P50 CA247749 01 grant. JI reports equity in LumaCyte, LLC and has served as an uncompensated member of a steering committee for Genentech. BL has served as an uncompensated advisor and consultant to Amgen, Genentech, Boehringer Ingelheim, Lilly, AstraZeneca, Daiichi Sankyo. He has received research grants to his institution from Amgen, Genentech, AstraZeneca, Daiichi Sankyo, Lilly, Illumina, GRAIL, Guardant Health, Hengrui Therapeutics, MORE Health and Bolt Biotherapeutics. He has received academic travel support from MORE Health, and Jiangsu Hengrui Medicine. He is an inventor on two institutional patents at MSK US62/685,057, US62/514,661 and has intellectual property rights as a book author at Karger Publishers and Shanghai Jiao Tong University Press. AR reports research funding from Varian Medical Systems, Boehringer Ingelheim, Astra Zeneca, Merck, and Pfizer and serves in Leadership Positions for the International Thymic Malignancies Group and International Mesothelioma Interest group.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1253629/full#supplementary-material
Supplementary Figure 1 | Patient-specific changes in ctDNA levels in response to treatment and radiographic imaging data. (A) Patient 17_MSK: ctDNA clearance indicated response to CRT. Pre-treatment chest CT scan showed left suprahilar lung mass and mediastinal adenopathy (in blue with white arrow). Post-treatment chest CT showed NED. (B) Patient 22_MSK: ctDNA increase after SBRT correlated with PD on imaging. Pre-treatment CT chest showed right middle lobe lung nodule (blue and indicated by white arrow), which was treated with SBRT. Post-SBRT CT scan showed new consolidative opacities (indicated by orange arrow) in the right lower lung lobe consistent with post-radiation changes. PET/CT after treatment showed metastatic disease involving the bone and cervical lymph nodes (white arrows). (C) Patient 10_MSK: ctDNA clearance after CRT correlated with NED. ctDNA detection during follow-up period preceded radiographic disease recurrence by 5.3 months. The pre-treatment CT scan showed a very low volume disease at baseline (in blue with white arrow), which cleared after CRT. The subsequent radiographic imaging (image showed NED in the initially involved areas of the lung not shown) showed new subcentimeter lung nodules. After the initial recurrence (lung nodules), the following radiographic imaging showed new brain metastases (white arrows), which corresponded to an increase in ctDNA levels. Abbreviations: ctDNA, circulating tumor DNA; CRT, chemoradiation; CT, computed tomography; SBRT, stereotactic body radiotherapy; PET, positron emission tomography; PD, progressive disease; RT, radiotherapy; NED, no evidence of disease; MTM, mean tumor molecules.
1. Ulrich B, Pradines A, Mazieres J, Guibert N. Detection of tumor recurrence via circulating tumor DNA profiling in patients with localized lung cancer: clinical considerations and challenges. Cancers (Basel). (2021) 13(15):3759. doi: 10.3390/cancers13153759
2. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer Version 2.2023 (2023). © National Comprehensive Cancer Network, Inc (Accessed March 8, 2023). To view the most recent and complete version of the guideline, go online to NCCN.org.
3. Spratt DE, Wu AJ, Adeseye V, Din SU, Shaikh F, Woo KM, et al. Recurrence patterns and second primary lung cancers after stereotactic body radiation therapy for early-stage non-small-cell lung cancer: implications for surveillance. Clin Lung Cancer. (2016) 17(3):177–83 e2. doi: 10.1016/j.cllc.2015.09.006
4. Zhang X, Liu H, Balter P, Allen PK, Komaki R, Pan T, et al. Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (2012) 83(5):1558–65. doi: 10.1016/j.ijrobp.2011.10.035
5. Kasi PM, Fehringer G, Taniguchi H, Starling N, Nakamura Y, Kotani D, et al. Impact of circulating tumor DNA-based detection of molecular residual disease on the conduct and design of clinical trials for solid tumors. JCO Precis Oncol (2022) 6:e2100181. doi: 10.1200/PO.21.00181
6. Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat Cancer. (2020) 1(2):176–83. doi: 10.1038/s43018-019-0011-0
7. Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol (2019) 5(8):1124–31. doi: 10.1001/jamaoncol.2019.0528
8. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol (2022) 40(12):1301–11. doi: 10.1200/JCO.21.01308
9. Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discovery (2017) 7(12):1394–403. doi: 10.1158/2159-8290.CD-17-0716
10. Yang Y, Zhang T, Wang J, Wang J, Xu Y, Zhao X, et al. The clinical utility of dynamic ctDNA monitoring in inoperable localized NSCLC patients. Mol Cancer. (2022) 21(1):117. doi: 10.1186/s12943-022-01590-0
11. Gale D, Heider K, Ruiz-Valdepenas A, Hackinger S, Perry M, Marsico G, et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol (2022) 33(5):500–10. doi: 10.1016/j.annonc.2022.02.007
12. Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. (2017) 545(7655):446–51. doi: 10.1038/nature22364
13. Christensen E, Birkenkamp-Demtroder K, Sethi H, Shchegrova S, Salari R, Nordentoft I, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol (2019) 37(18):1547–57. doi: 10.1200/JCO.18.02052
Keywords: circulating tumor DNA (ctDNA), tumor-informed, molecular residual disease (MRD), non-small cell lung cancer (NSCLC), definitive radiation, prognostic biomarker
Citation: Lebow ES, Shaverdian N, Eichholz JE, Kratochvil LB, McCune M, Murciano-Goroff YR, Jee J, Eng J, Chaft JE, Kris MG, Kalashnikova E, Feeney J, Scalise CB, Sudhaman S, Palsuledesai CC, Malhotra M, Krainock M, Sethi H, Aleshin A, Liu MC, Shepherd AF, Wu AJ, Simone CB II, Gelblum DY, Johnson KA, Rudin CM, Gomez DR, Razavi P, Reis-Filho JS, Isbell JM, Li BT and Rimner A (2023) ctDNA-based detection of molecular residual disease in stage I-III non-small cell lung cancer patients treated with definitive radiotherapy. Front. Oncol. 13:1253629. doi: 10.3389/fonc.2023.1253629
Received: 05 July 2023; Accepted: 24 August 2023;
Published: 19 September 2023.
Edited by:
Valerio Nardone, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Adam Kole, University of Alabama at Birmingham, United StatesCopyright © 2023 Lebow, Shaverdian, Eichholz, Kratochvil, McCune, Murciano-Goroff, Jee, Eng, Chaft, Kris, Kalashnikova, Feeney, Scalise, Sudhaman, Palsuledesai, Malhotra, Krainock, Sethi, Aleshin, Liu, Shepherd, Wu, Simone, Gelblum, Johnson, Rudin, Gomez, Razavi, Reis-Filho, Isbell, Li and Rimner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Rimner, cmltbmVyYUBtc2tjYy5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.