- 1Department of Thoracic Surgery, Kanagawa Cancer Center, Yokohama, Japan
- 2Department of Genetic Medicine, Kanagawa Cancer Center, Yokohama, Japan
- 3Cancer Prevention and Cancer Control Division, Kanagawa Cancer Center Research Institute, Yokohama, Japan
- 4Department of Surgery, Tokyo Medical University, Tokyo, Japan
- 5Department of Surgical Oncology, Hiroshima University, Hiroshima, Japan
Objectives: We aimed to clarify the differences in prognosis between wedge resection and segmentectomy performed for cN0 non-small cell lung cancer (NSCLC) measuring ≤ 2 cm, with consolidation tumor ratio (CTR) > 0.25.
Methods: This multicenter study included 570 patients with cN0 NSCLC (tumor size ≤ 2 cm, CTR > 0.25) who underwent wedge resection (n = 244) and segmentectomy (n = 326) between January 2010 and December 2018. After propensity score matching (PSM, 1:1 method), 182 patients were matched for clinical characteristics (age, sex, laterality, smoking index, tumor size, CTR, carcinoembryonic antigen value, positron-emission tomography-documented maximum standardized uptake value, clinical stage, and tumor disappearance rate) and intergroup comparison of disease-free survival (DFS) and overall survival (OS). Using Gray’s test, an intergroup comparison of the cumulative incidence of lung cancer-specific mortality was performed.
Results: After PSM, similar DFS (5-year DFS, 79.9% vs. 87.1%, p = 0.103) and OS (5-year OS, 88.7% vs. 88.9%, p = 0.719) rates were observed in the wedge resection and segmentectomy groups. We observed no significant intergroup differences in lung cancer-specific mortality (5-year cumulative incidence: 4.6% vs. 3.5%; p = 0.235). Subgroup analysis revealed no specific subgroup demonstrating improved DFS or OS after undergoing wedge resection or segmentectomy.
Conclusion: DFS, OS, and lung cancer-specific mortality were comparable between wedge resection and segmentectomy of cN0 NSCLC—tumor size ≤ 2 cm and CTR > 0.25. Large-scale prospective clinical trials are warranted to compare the prognoses of wedge resection and segmentectomy for these tumors.
1 Introduction
A randomized clinical trial comparing lobectomy and sublobar resection for early-stage lung cancer by the Lung Cancer Study Group in 1995 established lobectomy as the standard procedure for non-small cell lung cancer (NSCLC) of ≤ 3 cm in size and cN0 (1). However, with the widespread use of computed tomography (CT) in recent years, primary lung cancer has been detected at an earlier stage (2, 3), and the efficacy of sublobar resection for small peripheral lung cancers has been reported (4). Recently, large clinical trials of sublobar resection for early stage NSCLC have been conducted, and sublobar resection has been reported to be an acceptable alternative to lobectomy (5–8).
Sublobar resection can be classified as a segmentectomy or wedge resection. In JCOG0804/WJOG4507L, a single-arm study of sublobar resection for peripheral tumors 2 cm or smaller in diameter with consolidation tumor ratio (CTR) of 0.25 or less, the 5-year relapse-free survival (RFS) was 99.7% (5). In JCOG1211, a single-arm study of segmentectomy for tumor sizes of 2 cm to 3 cm with CTR of 0.50 or less, and tumor size 2 cm or less with CTR of 0.25 to 0.50, the 5-year RFS was 98% (6). In JCOG0802, a randomized clinical trial comparing overall survival (OS) between segmentectomy and lobectomy in patients with NSCLC of 2 cm with CTR > 0.5, segmentectomy was superior to lobectomy in terms of OS (7). Moreover, the recent CALGB140503 (Alliance) randomized clinical trial demonstrated the non-inferiority of sublobar resection for cN0 NSCLC ≤ 2 cm, excluding pure ground-glass nodules. This finding suggests that sublobar resection is a viable and acceptable treatment option for small-sized NSCLC (8). However, it remains unclear whether wedge resection is as effective as segmentectomy for NSCLCs ≤ 2 cm in size with CTR > 0.25.
Due to the longer operative time (9–11), increased blood loss (9, 11), and higher frequency of postoperative complications (9, 10, 12, 13), segmentectomy is a more invasive procedure than wedge resection for patients with lung cancer. Furthermore, segmentectomy is more technically challenging than wedge resection and requires a greater level of surgical expertise (9). However, wedge resection has been reported to have smaller tumor margins than segmentectomy (9, 10, 12, 14) and there is a difficulty in dissecting lymph nodes, especially in the hilar region (9, 11–13). There is concern that wedge resection may be less curative for tumors than segmentectomy (10, 15, 16).
Although there have been many reports comparing the outcomes of wedge resection and segmentectomy (9–11, 13–20), there have been only a few reports comparing the two surgical techniques for cN0 NSCLC ≤ 2 cm in size (4, 19, 21, 22). Therefore, there is still no consensus on the difference in therapeutic efficacy between wedge resection and segmentectomy for small tumors. It is also unclear whether wedge resection can be interpreted as an equivalent surgical technique to segmentectomy in the CALGB140503 study. This multicenter study compared the prognosis of wedge resection and segmentectomy in cN0 NSCLC patients ≤ 2 cm in size with CTR > 0.25 using propensity score matching (PSM) analysis.
2 Materials and methods
2.1 Ethics statement
The institutional review board of the participating institutions has approved this retrospective review of the multicenter database and waived the requirement for informed consent for each patient (Kanagawa Cancer Center, approval 24EKI54 (Approved on June 14, 2021); Tokyo Medical University Hospital, approval SH2969; Hiroshima University Hospital, approval E-1216).
2.2 Patients
Among the 1253 consecutive NSCLC patients who underwent complete resection for tumors ≤ 2 cm in size with CTR > 0.25 and cN0 from January 2010 to December 2018, 244 patients who received wedge resection and 326 patients who received segmentectomy were included in this study (Figure 1). Patients with NSCLC and pure ground-glass nodules were excluded. Segmentectomy was primarily carried out in patients with cN0 lung tumor ≤ 2 cm in size and had a CTR > 0.25. However, the decision to perform wedge resection was influenced by on surgeons’ preferences, considering factors such tumor location, radiological characteristics of the tumor, as well as patients’ age, comorbidities and lung function. TNM staging was performed according to the 8th edition of the TNM classification for lung and pleural tumors (23).
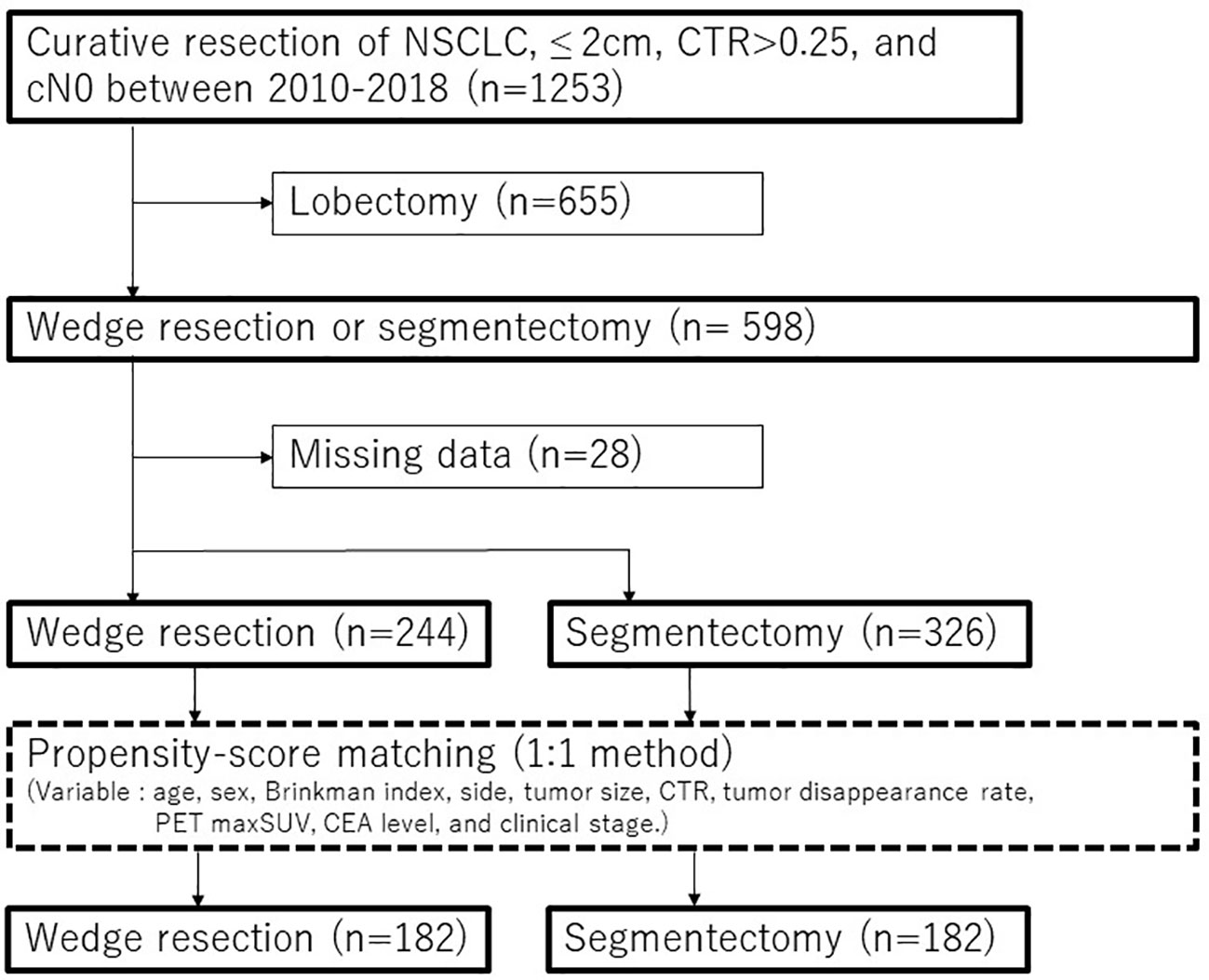
Figure 1 Consort diagram of this study. CEA, carcinoembryonic antigen; CTR, consolidation tumor ratio; NSCLC, non-small cell lung cancer; PET, positron emission tomography; maxSUV, maximum standardized uptake values.
2.3 Word definitions
Patients who underwent a subsegmentectomy or two segmentectomies that were less than a lobectomy were included in the segmentectomy group. The study included patients who underwent segmentectomy, either with or without mediastinal lymph node dissection. The Brinkman index was defined as the number of cigarettes smoked per day multiplied by the number of years of smoking, as previously reported (24). Overall survival (OS) was defined as the period from the date of surgery to the date of death or censoring of patients without events during the last observation period. Disease-free survival (DFS) was defined as the period from the date of surgery to the date of recurrence or death from any cause; patients without recurrence were censored during the last observation period. Lung cancer-specific mortality was defined as death attributable to lung cancer in patients with postoperative recurrence. Intrathoracic recurrence included recurrence in the lungs, mediastinum, hilar and subclavian lymph nodes, and pleura, without extrathoracic recurrence. Extrathoracic recurrence included recurrence at sites other than intrathoracic sites, such as the bone, central nervous system, and abdominal organs.
2.4 Statistical analyses
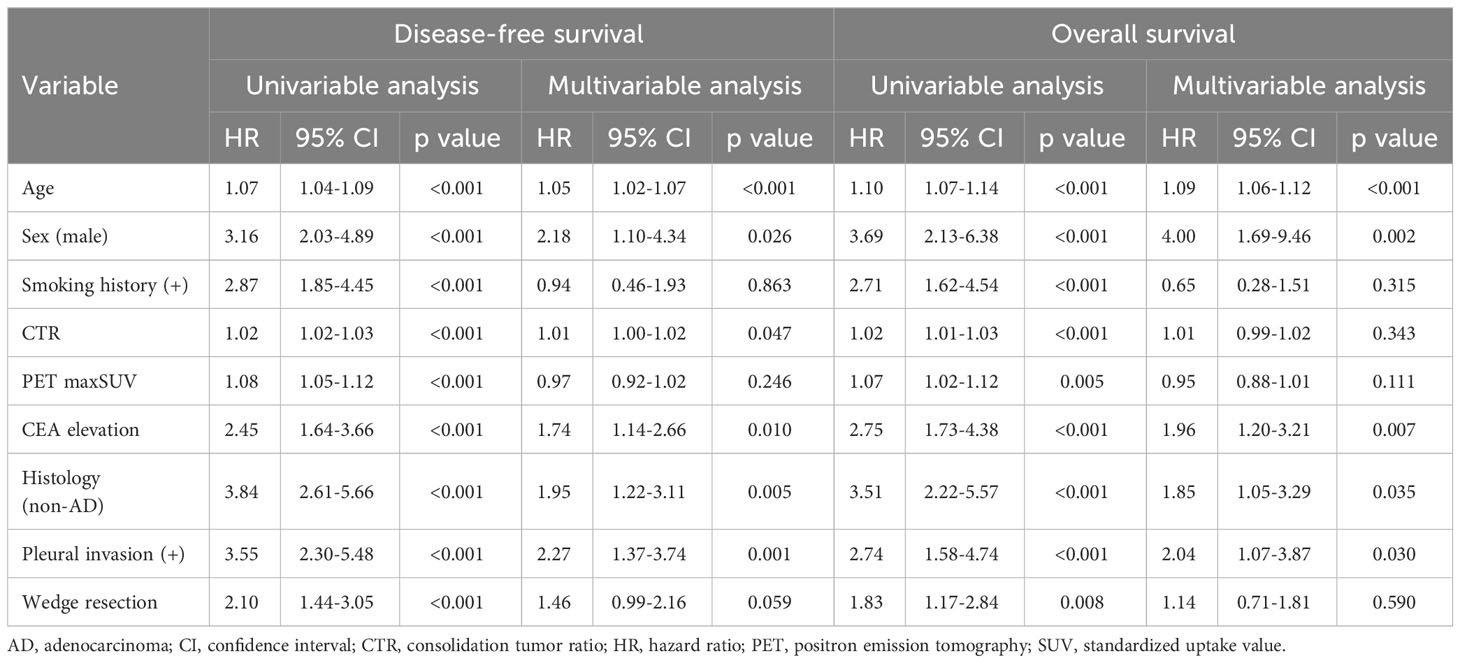
The Mann-Whitney U test was performed to compare continuous variables between the wedge resection and segmentectomy groups, while Fisher’s exact test was used to compare categorical variables. All survival curves were analyzed using the Kaplan-Meier method and compared using the log-rank test. Univariable and multivariable analyses were performed using the Cox proportional hazard regression model to assess the impact of potential prognostic factor for DFS and OS with the following variables: age, sex, smoking history, CTR, positron emission tomography (PET) maximum standardized uptake values (maxSUVs), carcinoembryonic antigen (CEA) level (≤5ng/ml or >5ng/ml), histology (adenocarcinoma or non-adenocarcinoma), pleural invasion, and surgical procedure (wedge resection or segmentectomy).
To reduce selection bias between patients who underwent wedge resection and those who underwent segmentectomy, PSM analysis was conducted using a 1:1 matching method (caliper = 0.001). The patients were matched based on the following preoperative variables: age, sex, Brinkman index, side, CT tumor size, CTR, tumor disappearance rate, PET maxSUV, CEA level, and clinical stage (Figure 1). The cutoff values for age, CT tumor size, tumor disappearance rate, and PET maxSUVs were determined using receiver operating characteristic curve analysis. An inverse probability of treatment weighting (IPTW) analysis based on propensity scoring was also conducted as a supplemental. Multivariable analysis was performed on all variables with a p-value of < 0.05 in the univariable analysis. The cumulative incidence of lung cancer-specific mortality, intrathoracic recurrence, and extrathoracic recurrence was analyzed using Gray’s test. Statistical significance was set at p < 0.05. Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
3 Results
The median observation period was 60.4 (42.1–77.9) months. Before PSM, patients in the wedge resection group were older (72 vs. 69 years, p < 0.001) and had more frequent pleural invasion (14.3% vs. 6.7%, p = 0.004), pathological T1c stage or higher (20.9% vs. 13.2%, p = 0.017), and recurrence (16.8% vs. 5.5%, p < 0.001) (Table 1). The wedge resection group had a worse DFS (5-year DFS 74.9% vs. 88.2%, p < 0.001; Figure 2A) and OS (5-year OS 84.1% vs. 91.1%, p = 0.007; Figure 2B) than the segmentectomy group.

Table 1 Comparison of clinicopathological characteristics between segmentectomy and wedge resection groups before propensity score matching.
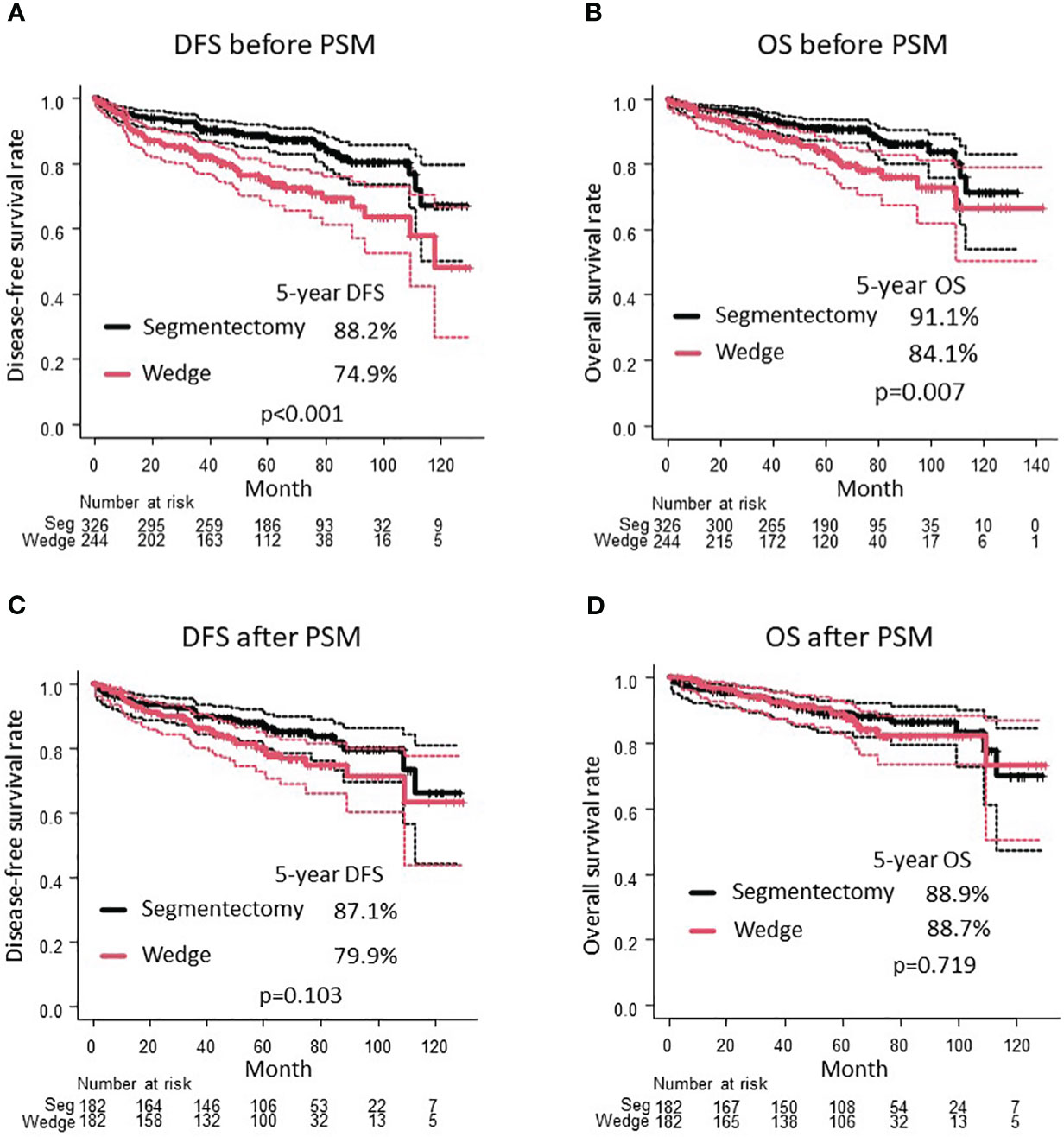
Figure 2 Comparison of DFS (A) and OS (B) between wedge resection group and segmentectomy group before PSM. Comparison of DFS (C) and OS (D) between wedge resection group and segmentectomy group after PSM. DFS, disease-free survival; OS, overall survival; PSM, propensity score matching.
In multivariable analysis using Cox proportional hazard regression model, age, sex, CTR, CEA value, histology, and pleural invasion were independent prognostic factors for DFS; however, wedge resection was not prognostic (hazard ratio [HR], 1.46; 95% confidence interval (CI), 0.99–2.16; p = 0.059) (Table 2). Moreover, age, sex, CEA value, histology, and pleural invasion were independent prognostic factors for OS; however, wedge resection was not prognostic (HR, 1.14; 95% CI, 0.71–1.81; p = 0.590) (Table 2).
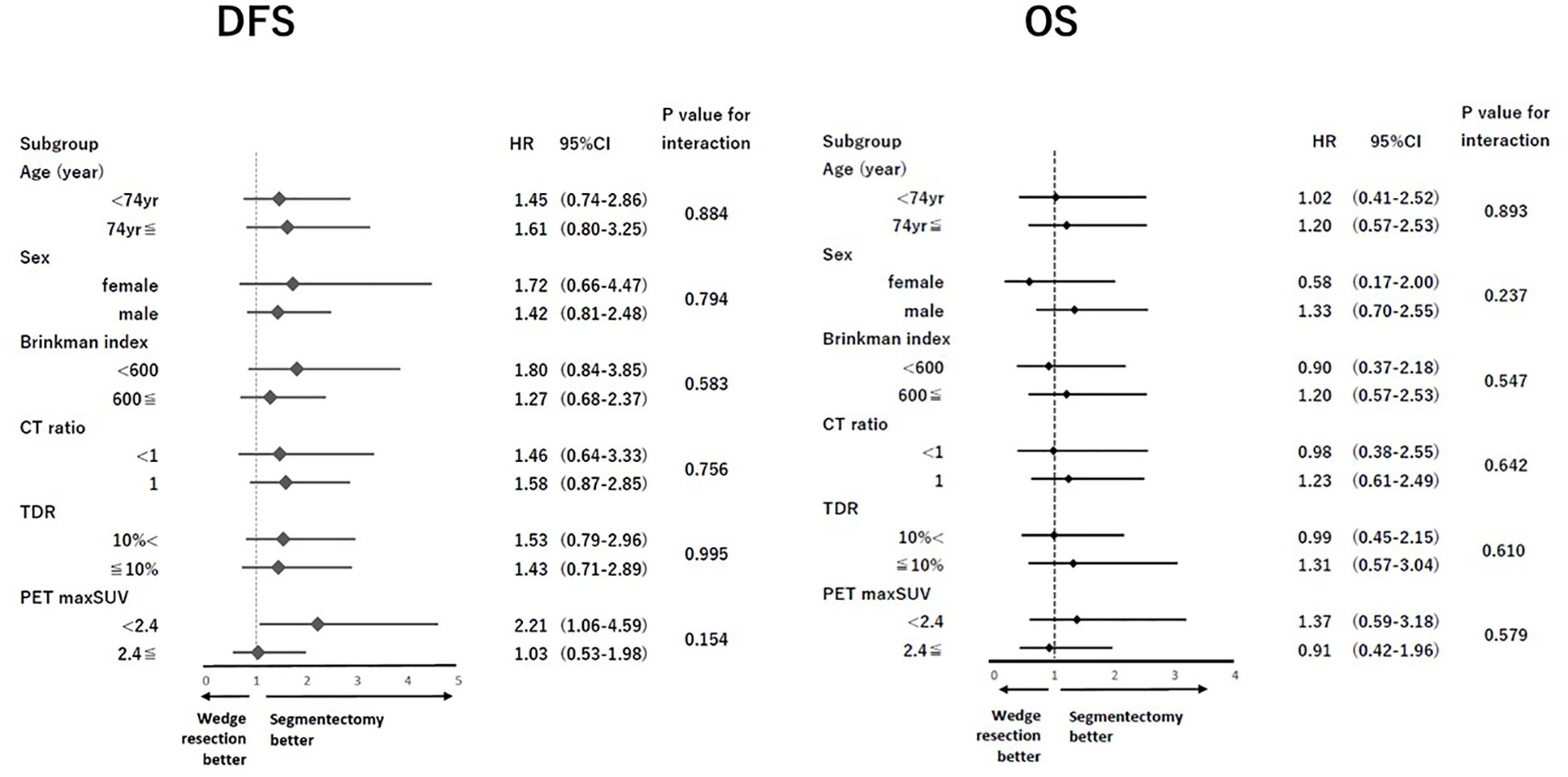
After PSM, the clinical characteristics of the 182 patients in the segmentectomy and wedge resection groups were well matched (Table 3). The pathological characteristics of both groups were generally similar. However, the frequencies of pleural invasion and intrathoracic recurrence appeared to be higher in the wedge resection group compared to the segmentectomy group, through these differences were not statistically significant (Table 3). There was no significant difference in the DFS between patients who underwent wedge resection and segmentectomy (5-year DFS: 79.9% vs. 87.1%, p = 0.103; Figure 2C). Moreover, there was no significant difference in the OS of patients who underwent wedge resection and segmentectomy (5-year OS: 88.7% vs. 88.9%, p = 0.719; Figure 2D). As depicted in Figure 3, no specific subgroups were identified in which wedge resection or segmentectomy significantly improved DFS or OS.
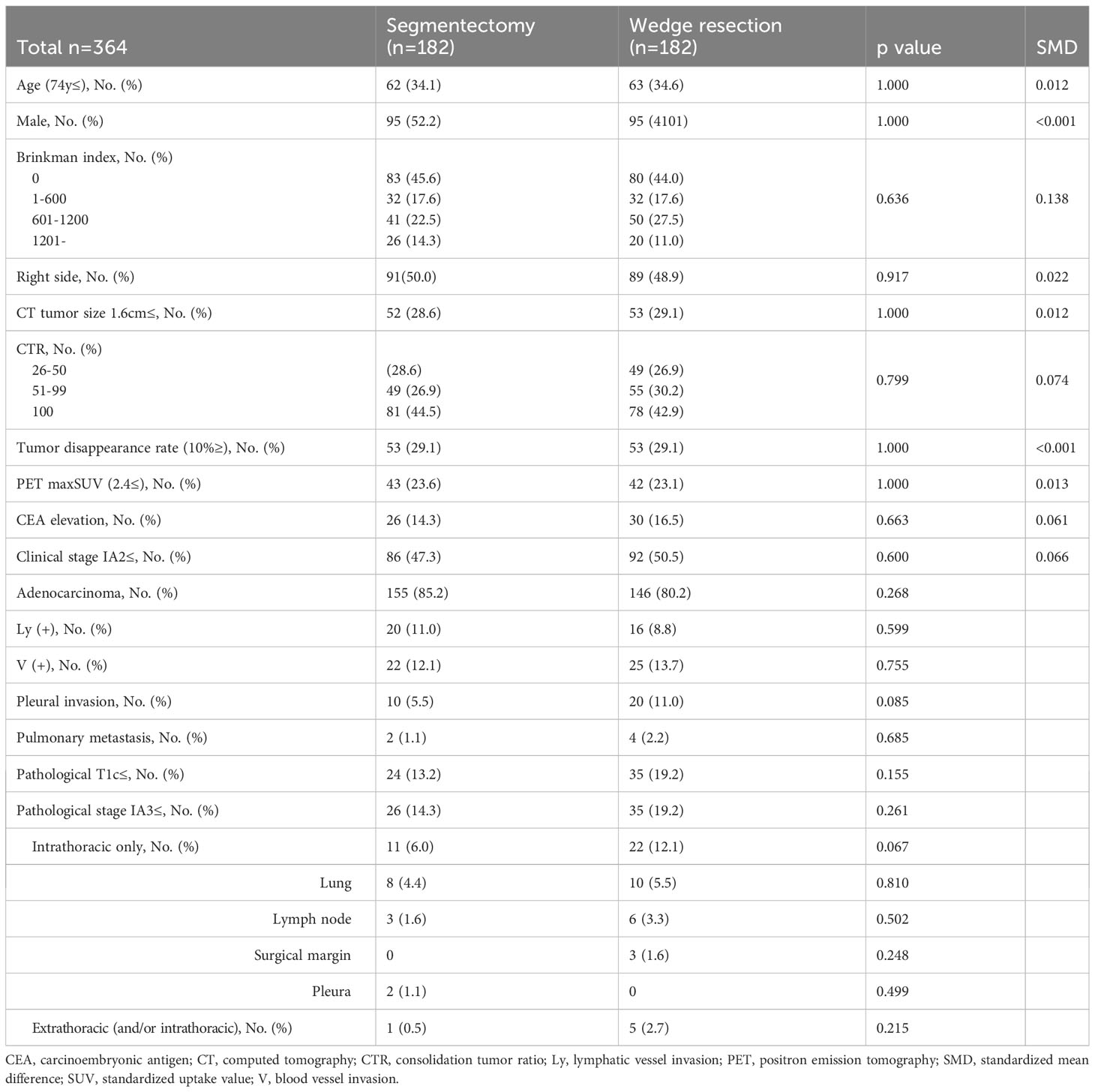
Table 3 Comparison of clinicopathological characteristics between segmentectomy and wedge resection groups after propensity score matching.
As shown in Supplementary Figure B, the OS curves adjusted by IPTW showed comparability between the segmentectomy and wedge resection groups after IPTW adjustment (p = 0.165). The adjusted HR for OS with wedge resection on OS was 1.38 (95% CI, 0.88–2.18; p = 0.200). However, the DFS curves adjusted by IPTW were significantly worse in the wedge resection group than in the segmentectomy group (p = 0.013). The adjusted HR of wedge resection was 1.63 (95% CI, 1.10–2.41; p = 0.020).
Cumulative incidence of lung cancer-specific mortality was not significantly different between wedge resection and segmentectomy groups after PSM (4.6% vs. 3.5% at 5-year, p = 0.235; Figure 4A). The cumulative incidence of intrathoracic recurrence (11.5% vs. 4.8% at 5-year, p = 0.025; Figure 4B) was significantly higher in the wedge resection group than in the segmentectomy group. However, there was no significant difference in the cumulative incidence of extrathoracic recurrence (3.3% vs. 0% at 5-year, p = 0.092; Figure 4C).
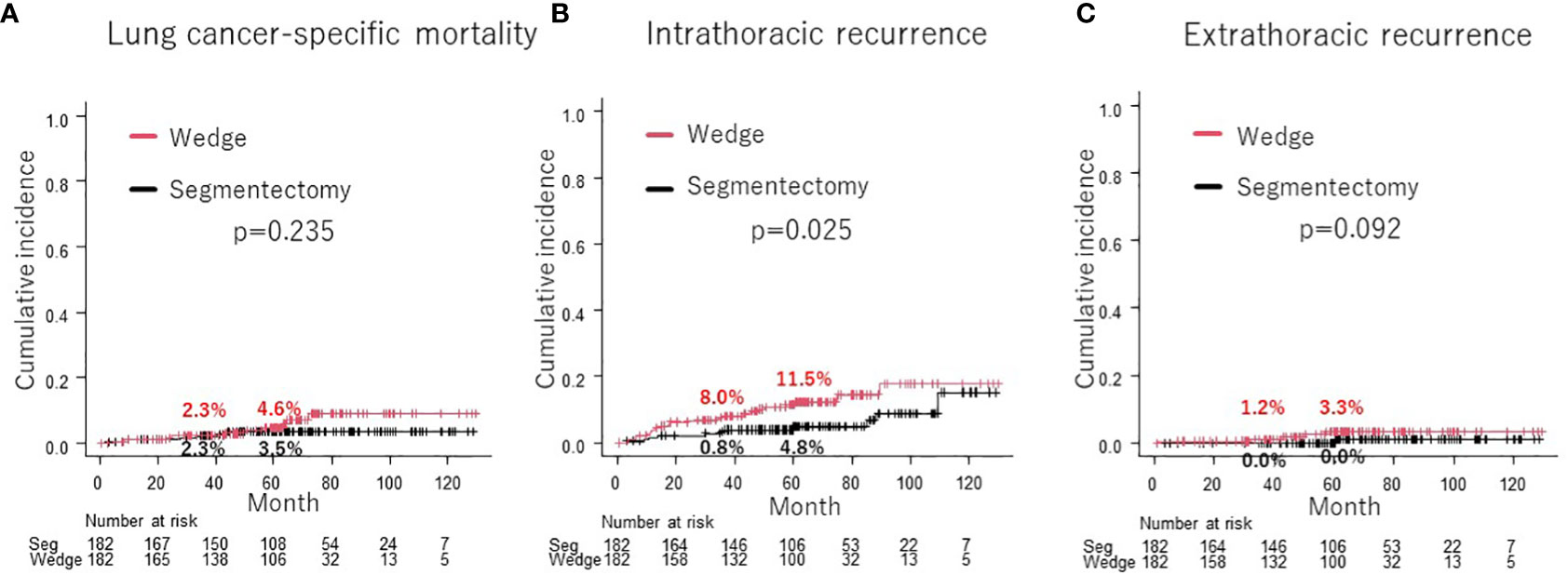
Figure 4 Comparison of cumulative incidence of lung cancer-specific mortality between wedge resection group and segmentectomy group (A). Comparison of cumulative incidence of intrathoracic recurrence (B) and extrathoracic recurrence (C) between wedge resection group and segmentectomy group.
4 Discussion
This large-scale multicenter PSM analysis demonstrated the comparable DFS, OS, and lung cancer-specific mortality of wedge resection and segmentectomy for patients between wedge resection and segmentectomy for cN0 NSCLC ≤ 2 cm in size with CTR > 0.25. Moreover, wedge resection was not a prognostic factor in the multivariable analysis, and there was no specific subgroup with improved DFS or OS by wedge resection or segmentectomy. Although the cumulative incidence of intrathoracic recurrence was higher in the wedge resection group than in the segmentectomy group, that of extrathoracic recurrence was statistically comparable.
A meta-analysis comparing wedge resection and segmentectomy for stage I (7th TNM (25)) reported that segmentectomy resulted in better OS and lung cancer-specific survival (LCSS) (26, 27). In stage IA (7th TNM) NSCLC, patients who underwent segmentectomy had better OS than those who underwent wedge resection (16, 22), although there are reports of comparable OS (9, 11). Moreover, in cN0 NSCLC of ≤ 2 cm in size, a previous study reported that segmentectomy was associated with better OS than wedge resection (22), while others reported that both procedures are comparable in terms of OS (4, 21). The difference in OS efficacy between wedge resection and segmentectomy for early-stage NSCLC remains unclear.
This is the first study to compare the prognosis of patients with cN0 NSCLC ≤ 2 cm in size with CTR > 0.25 who underwent wedge resection versus segmentectomy using PSM analysis, showing no difference in OS between the two techniques. Moreover, no specific subgroup that exhibited enhanced OS following either wedge resection or segmentectomy was found (Figure 3). Only a limited number of studies have conducted comparison of the efficacy of wedge resection and segmentectomy for cN0 NSCLC using PSM, and even fewer for tumors of size ≤ 2 cm (Table 4). Tsutani et al. compared the prognosis of patients with c-stage I (7th TNM) NSCLC who underwent wedge resection and segmentectomy using PSM analysis and demonstrated comparable OS (segmentectomy, HR = 1.21, p = 0.62) between the two procedures (10). Smith CB et al. compared wedge resection with segmentectomy by using PSM analysis for stage IA(7th TNM)NSCLC and found favorable OS of segmentectomy (HR: 0.8, 95% CI: 0.69–0.93) and LCSS (HR: 0.72, 95% CI: 0.59–0.88) (22). Furthermore, they reported segmentectomy was associated with improved OS (HR: 0.81, 95% CI: 0.67–0.99) and LCSS (HR: 0.75, 95% CI: 0.58–0.98) for NSCLC ≤ 2 cm in size (Table 4) (22). Zhou et al. compared the prognosis of 100 paired patients with NSCLC who underwent simple segmentectomy and wedge resection for tumor size 2–3 cm with a solid component less than 2 cm in size using PSM analysis. They reported comparable OS between the two surgical procedures (11). Cao et al. reported comparable OS (HR 1.171, p = 0.541) and LCSS (HR 0.745, p = 0.447) between 126 paired matched patients after wedge resection and segmentectomy for tumors 1 cm or smaller; however, for 429 paired NSCLC patients with tumor of 1.1 cm to 2.0 cm in size, wedge resection was inferior to segmentectomy in terms of OS (HR 1.399, p = 0.005) and LCSS (HR 1.704, p = 0.002) (Table 4) (19).
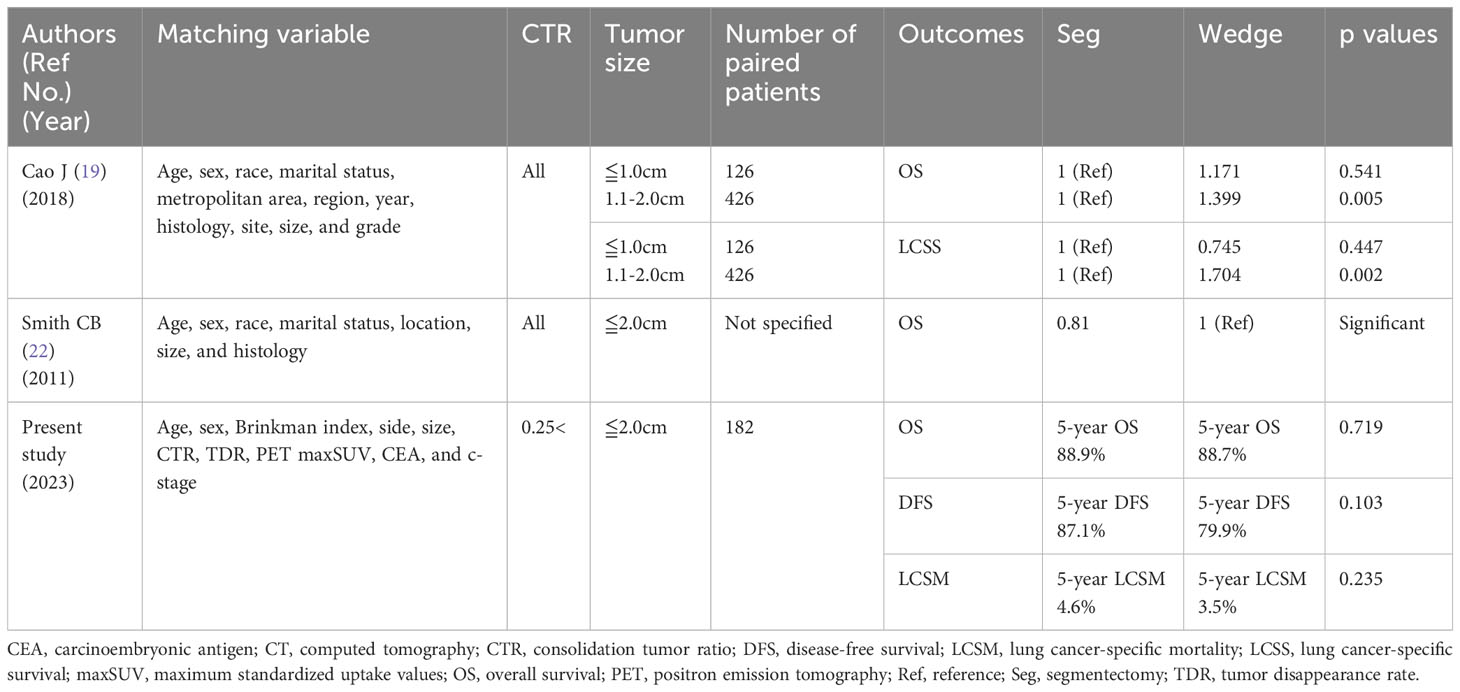
Table 4 Previous studies comparing the prognosis of patients after segmentectomy and wedge resection for cN0 non-small cell lung cancer with tumor size ≤ 2 cm using propensity score matching analysis.
There is no consensus regarding the difference in DFS between wedge resection and segmentectomy. For stage I (7th TNM) NSCLC, previous studies have reported comparable DFS between wedge resection and segmentectomy (10, 26); however, other studies have reported superior DFS in the segmentectomy group compared to the wedge resection group (27). Comparable DFS were reported between wedge resection group and segmentectomy group for stage IA (7th TNM) NSCLC (9), and for NSCLC with 2 cm or smaller in size (4). In the present study with PSM analysis, comparable OS, DFS, and lung cancer-specific mortality were observed between wedge resection and segmentectomy group among cN0 NSCLC patients with ≤ 2 cm in size with CTR > 0.25. JCOG0802 and JCOG1211 suggested that segmentectomy is the standard procedure for cN0 NSCLC patients with ≤ 2 cm in size with a CTR > 0.25; however, future prospective studies comparing wedge resection and segmentectomy for these tumors are necessary.
Wedge resection is associated with less intraoperative blood loss (9, 10) and a shorter operative time (9, 11) than segmentectomy. Moreover, wedge resection has been reported to have a lower frequency of postoperative complications than segmentectomy (9–12). While segmentectomy requires a higher surgeon’s expertise to avoid intraoperative and postoperative complications, wedge resection, a non-anatomical resection, is relatively easy and safe compared to other types of pulmonary resection (9). Furthermore, recovery of pulmonary functions such as forced vital capacity, forced expiratory volume in 1 second, predicted diffusing capacity of the lung of carbon monoxide percentage, and peak expiratory flow has been reported to be better with wedge resection than with segmentectomy (4, 11). Although neither wedge resection nor segmentectomy has been reported to have a 30-day postoperative mortality rate (9, 13), wedge resection for patients with NSCLC is considered a less invasive procedure than segmentectomy in terms of blood loss, operating time, and postoperative complications.
In this study, the subgroup analysis did not identify any specific subgroup with a statistically significant favorable prognosis after wedge resection or segmentectomy. However, nearly all subgroups tended to exhibit a favorable DFS after segmentectomy compared to wedge resection (Figure 3). Moreover, the cumulative incidence of intrathoracic recurrence was higher after wedge resection than after segmentectomy (Figure 4B). Previous studies have reported a higher local recurrence rate for wedge resection than for segmentectomy in patients with stage I NSCLC (7th TNM) (17), stage IA NSCLC (7th TNM) (1), and NSCLC 2 cm in size (13). In contrast, another study reported no difference in the frequency of recurrence or recurrence patterns between Stage IA NSCLC (9) and NSCLC ≦ 2 cm in size (4). In the present study, a trend towards improved DFS after segmentectomy, compared with wedge resection, was observed in the PSM analysis (Figure 2C), and significantly better DFS was evident in the segmentectomy group in the IPTW analysis (Supplementary Figure A). These findings could be attributed to the reduced intrathoracic recurrence post segmentectomy.
There are four possible reasons why intrathoracic recurrence was more common in the wedge resection group. First, although not examined in this study, tumor margins may have been smaller in the wedge resection group than in the segmentectomy group. The incidence of local and intrathoracic recurrence has been reported to be associated with macroscopic parenchymal resection margins (9), and several studies have reported shorter tumor margins with wedge resection than with segmentectomy (9, 10, 12). However, Zhou et al. reported no difference in tumor margins between segmentectomy and wedge resection (19.5 mm vs 22.4 mm, p = 0.7) (11). The appropriate tumor margin to prevent local recurrence via sublobar resection for cN0 NSCLC ≦ 2 cm in size with CTR > 0.25 needs further elucidation. Secondly, wedge resection is more difficult than segmentectomy for obtaining intrathoracic lymph nodes, especially those located in the hilar region (9, 11, 13), which may have resulted in increased intrathoracic recurrence after wedge resection. Third, patients who undergo wedge resection may be understaged more than those who undergo segmentectomy because of the difficulty in obtaining intrathoracic lymph nodes during surgery (28). Therefore, these patients may lose the opportunity to receive postoperative adjuvant therapy, which may increase their risk of recurrence. Fourth, there was a trend toward more positive pathological pleural invasion in the wedge resection group than in the segmentectomy group (5.5% vs. 11.0%, p = 0.086). Tumors located closer to the pleura were more common in the wedge resection group, which may have been one reason for the higher cumulative intrapleural recurrence rate in the wedge resection group. Large prospective randomized clinical trials are needed to compare the recurrence rates between wedge resection and segmentectomy.
It is unclear why there was no difference in the OS between the two groups, even though the cumulative intrathoracic recurrence rate was higher for wedge resection in this study. It has been reported that local recurrence after wedge resection is often caused by the remaining lung parenchyma in the former resection area, and local treatment for local recurrence, such as reoperation or radiation therapy, may result in a favorable prognosis even after recurrence (13). In this study, among patients experiencing intrathoracic recurrence, the occurrence of lone intrathoracic lymph node recurrence or lone lung metastasis (excluding cases of multiple lung metastases) was higher in the wedge resection group (54.5%, n = 12) compared to the segmentectomy group (36.4%, n = 4, respectively). Because post-relapse survival after intrathoracic recurrence, including pulmonary metastases and intrathoracic lymph node recurrence, is reported to be favorable (29), there may have been no difference in OS between the two groups, although more intrathoracic recurrences were observed in the wedge resection group.
This study has several limitations. First, this was a retrospective study, and selection bias may have occurred. Although this study is based on a comprehensive multicenter database, it remains uncertain whether the sample size was sufficiently large to definitively conclude that the prognosis of cN0 NSCLC patients with tumor diameters ≦ 2 cm and CTRs > 0.25 is equivalent between wedge resection and segmentectomy. Additional extensive prospective clinical trials are required to effectively compare the prognoses of patients who undergoing wedge resection and segmentectomy. Second, the absence of information on surgical outcomes such as blood loss, operation time, and surgical complications limits the ability to analyze the substantial invasiveness associated with wedge resection and segmentectomy in this study. Moreover, the patient comorbidities and preoperative respiratory function was lacking for preoperative variables in the PSM analysis. Third, this study did not account for factors such as location (peripheral or central), distance from the margin, or the existence of tumor spread through the air space, which have been associated with local recurrence. Fourth, a description of treatment after recurrence was lacking in this study, and the effect of local therapy on patients with intrathoracic recurrence remains unclear.
5 Conclusion
In conclusion, this large-scale multicenter PSM analysis demonstrated the comparable DFS, OS, and lung cancer-specific mortality of patients who received wedge resection and segmentectomy for cN0 NSCLC ≤ 2 cm in size with CTR > 0.25. Large-scale prospective clinical trials are warranted to compare the prognoses of wedge resection and segmentectomy for these tumors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Kanagawa Cancer Center, Tokyo Medical University Hospital, and Hiroshima University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because The institutional review board of the participating institutions has approved this retrospective review of the multicenter database and waived the requirement for informed consent for each patient (Kanagawa Cancer Center, approval 24EKI54 (Approved on June 14, 2021); Tokyo Medical University Hospital, approval SH2969; Hiroshima University Hospital, approval E-1216).
Author contributions
TI: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. TN: Data curation, Supervision, Writing – review & editing. HA: Data curation, Supervision, Writing – review & editing. HN: Methodology, Software, Supervision, Validation, Writing – review & editing. KM: Data curation, Writing – review & editing. SS: Data curation, Writing – review & editing. NK: Data curation, Writing – review & editing. NS: Data curation, Writing – review & editing. KW: Data curation, Writing – review & editing. YK: Data curation, Writing – review & editing. YM: Data curation, Writing – review & editing. MO: Data curation, Supervision, Writing – review & editing. NI: Data curation, Supervision, Writing – review & editing. HI: Conceptualization, Data curation, Supervision, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1253414/full#supplementary-material
Supplementary Figure | Comparison of DFS (Supplementary Figure A) and OS (Supplementary Figure B) between the wedge resection group and the segmentectomy group after inverse probability of treatment weighting (IPTW) analysis, based on propensity scoring. DFS, disease-free survival; IPTW, inverse probability of treatment weighting; OS, overall survival.
Abbreviations
CI, confidence interval; CT, computed tomography; CTR, consolidation tumor ratio; DFS, disease-free survival; HR, hazard ratio; LCSS, lung cancer-specific survival; maxSUV, maximum standardized uptake values; NSCLC, non-small cell lung cancer; OS, overall survival; PET, positron emission tomography; PSM, propensity score matching; RFS, relapse-free survival.
References
1. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg (1995) 60:615–22. doi: 10.1016/0003-4975(95)00537-u
2. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Me (2011) 365:395–409. doi: 10.1056/NEJMoa1102873
3. Edwards JP, Datta I, Hunt JD, Stefan K, Ball CG, Dixon E, et al. The impact of computed tomographic screening for lung cancer on the thoracic surgery workforce. Ann Thorac Surg (2014) 98:447–52. doi: 10.1016/j.athoracsur.2014.04.076
4. Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg (2006) 132:769–75. doi: 10.1016/j.jtcvs.2006.02.063
5. Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Sur (2022) 163:289–301.e2. doi: 10.1016/j.jtcvs.2020.09.146
6. Aokage K, Suzuki K, Saji H, Wakabayashi M, Kataoka T, Sekino Y, et al. Segmentectomy for ground-glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir Med (2023) S2213-2600(23):00041–3. doi: 10.1016/S2213-2600(23)00041-3
7. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet (2022) 399:1607–17. doi: 10.1016/S0140-6736(21)02333-3
8. Altorki N, Wang X, Kozono D, Watt C, Landrenau R, Wigle D, et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med (2023) 388:489–98. doi: 10.1056/NEJMoa2212083
9. Altorki NK, Kamel MK, Narula N, Ghaly G, Nasar A, Rahouma M, et al. Anatomical segmentectomy and wedge resections are associated with comparable outcomes for patients with small cT1N0 non-small cell lung cancer. J Thorac Oncol (2016) 11:1984–92. doi: 10.1016/j.jtho.2016.06.031
10. Tsutani Y, Kagimoto A, Handa Y, Mimae T, Miyata Y, Okada M. Wedge resection versus segmentectomy in patients with stage I non-small-cell lung cancer unfit for lobectomy. Jpn J Clin Oncol (2019) 49:1134–42. doi: 10.1093/jjco/hyz122
11. Zhou Y, Yu T, Zhang Y, Qian L, Xia Q. Comparison of surgical outcomes and prognosis between wedge resection and simple Segmentectomy for GGO diameter between 2 cm and 3 cm in non-small cell lung cancer: a multicenter and propensity score matching analysis. BMC Cancer (2022) 22:71. doi: 10.1186/s12885-021-09129-0
12. Fernando HC, Landreneau RJ, Mandrekar SJ, Hillman SL, Nichols FC, Meyers B, et al. Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg (2011) 142:1143–51. doi: 10.1016/j.jtcvs.2011.07.051
13. Sienel W, Dango S, Kirschbaum A, Cucuruz B, Hörth W, Stremmel C, et al. Sublobar resections in stage IA non-small cell lung cancer: segmentectomies result in significantly better cancer-related survival than wedge resections. Eur J Cardiothorac Surg (2008) 33:728–34. doi: 10.1016/j.ejcts.2007.12.048
14. Akamine T, Yotsukura M, Yoshida Y, Nakagawa K, Yatabe Y, Watanabe SI. Feasibility and effectiveness of segmentectomy versus wedge resection for clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg (2023) 63:ezad018. doi: 10.1093/ejcts/ezad018
15. Kent M, Landreneau R, Mandrekar S, Hillman S, Nichols F, Jones D, et al. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. Ann Thorac Surg (2013) 96:1747–54. doi: 10.1016/j.athoracsur.2013.05.104
16. Koike T, Koike T, Yoshiya K, Tsuchida M, Toyabe S. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg (2013) 146:372–8. doi: 10.1016/j.jtcvs.2013.02.057
17. Fiorelli A, Caronia FP, Daddi N, Loizzi D, Ampollini L, Ardò N, et al. Sublobar resection versus lobectomy for stage I non-small cell lung cancer: an appropriate choice in elderly patients? Surg Today (2016) 46:1370–82. doi: 10.1007/s00595-016-1314-8
18. Dai C, Shen J, Ren Y, Zhong S, Zheng H, He J, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol (2016) 34:3175–82. doi: 10.1200/JCO.2015.64.6729
19. Cao J, Yuan P, Wang Y, Xu J, Yuan X, Wang Z, et al. Survival rates after lobectomy, segmentectomy, and wedge resection for non-small cell lung cancer. Ann Thorac Surg (2018) 105:1483–91. doi: 10.1016/j.athoracsur.2018.01.032
20. Tsutani Y, Handa Y, Shimada Y, Ito H, Ikeda N, Nakayama H, et al. Comparison of cancer control between segmentectomy and wedge resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg (2021) 162:1244–52.e1. doi: 10.1016/j.jtcvs.2020.10.024
21. Razi SS, John MM, Sainathan S, Stavropoulos C. Sublobar resection is equivalent to lobectomy for T1a non-small cell lung cancer in the elderly: a Surveillance, Epidemiology, and End Results database analysis. J Surg Res (2016) 200:683–9. doi: 10.1016/j.jss.2015.08.045
22. Smith CB, Swanson SJ, Mhango G, Wisnivesky JP. Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol (2013) 8:73–8. doi: 10.1097/JTO.0b013e31827451c4
23. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol (2016) 11:39–51. doi: 10.1016/j.jtho.2015.09.009
24. Brinkman Gl, Coates EO Jr. The effect of bronchitis, smoking, and occupation on ventilation. Am Rev Respir Dis (1963) 87:684–93. doi: 10.1164/arrd.1963.87.5.684
25. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of Malignant tumours. J Thorac Oncol (2007) 2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a
26. Hou B, Deng XF, Zhou D, Liu QX, Dai JG. Segmentectomy versus wedge resection for the treatment of high-risk operable patients with stage I non-small cell lung cancer: a meta-analysis. Ther Adv Respir Dis (2016) 10:435–43. doi: 10.1177/1753465816667121
27. Zhang H, Liu C, Tan Z, Zhang T. Segmentectomy versus wedge resection for stage I non-small cell lung cancer: A meta-analysis. J Surg Res (2019) 243:371–9. doi: 10.1016/j.jss.2019.05.058
28. Khullar OV, Liu Y, Gillespie T, Higgins KA, RaMalingam S, Lipscomb J, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the national cancer data base. J Thorac Oncol (2015) 10:1625–33. doi: 10.1097/JTO.0000000000000664
Keywords: wedge resection, segmentectomy, propensity score matching, prognosis, overall survival, disease-free survival
Citation: Isaka T, Nagashima T, Adachi H, Narimatsu H, Murakami K, Shigefuku S, Kikunishi N, Shigeta N, Watabe K, Kudo Y, Miyata Y, Okada M, Ikeda N and Ito H (2023) Wedge resection vs. segmentectomy for lung cancer measuring ≤ 2 cm with consolidation tumor ratio > 0.25. Front. Oncol. 13:1253414. doi: 10.3389/fonc.2023.1253414
Received: 05 July 2023; Accepted: 15 August 2023;
Published: 28 August 2023.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesReviewed by:
Niccolò Daddi, University of Bologna, ItalyHisashi Saji, St. Marianna University School of Medicine, Japan
Ze-Rui Zhao, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2023 Isaka, Nagashima, Adachi, Narimatsu, Murakami, Shigefuku, Kikunishi, Shigeta, Watabe, Kudo, Miyata, Okada, Ikeda and Ito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tetsuya Isaka, bDQwMTA5MmtAeWFob28uY28uanA=
 Tetsuya Isaka
Tetsuya Isaka Takuya Nagashima1
Takuya Nagashima1 Hiroto Narimatsu
Hiroto Narimatsu Naoko Shigeta
Naoko Shigeta Yujin Kudo
Yujin Kudo Yoshihiro Miyata
Yoshihiro Miyata