
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 01 December 2023
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1252652
This article is part of the Research TopicDeveloping next-generation therapeutics to defeat mutation-driven cancerView all 5 articles
In the past 15 years, non-small cell lung cancer (NSCLC) treatment has changed with the discovery of mutations and the development of new targeted therapies and immune checkpoint inhibitors. Epidermal growth factor receptor (EGFR) was the first mutation in NSCLC to have a drug that was FDA-approved in 2013. Osimertinib, a third-generation tyrosine kinase inhibitor, is approved as first-line therapy for advanced NSCLC and in the adjuvant setting for Stage IB-IIIA resected NSCLC. However, resistance to osimertinib is inevitably an issue, and thus patterns of resistance to EGFR-mutated NSCLC have been studied, including MET amplification, EGFR C797X-acquired mutation, human epidermal growth factor 2 (HER2) amplification, and transformation to small cell and squamous cell lung cancer. Current management for EGFR-mutated NSCLC upon progression of EGFR TKI is limited at this time to chemotherapy and radiation therapy, sometimes in combination with the continuation of osimertinib. Antibody–drug conjugates (ADCs) are made up of a monoclonal antibody linked to a cytotoxic drug and are an increasingly popular class of drug being studied in NSCLC. Trastuzumab deruxtecan has received accelerated FDA approval in HER2-mutated NSCLC. ADCs offer a possible solution to finding a new treatment that could bypass the intracellular resistance mechanism. In this review article, we summarize the mechanism of ADCs and investigational ADCs for EGFR-mutated NSCLC, which include targets to MET amplification, HER3, Trop2, and EGFR, along with other ADC targets being investigated in NSCLC, and discuss future directions that may arise with ADCs in EGFR-mutated NSCLC.
The development of targeted therapies has changed the systemic treatment landscape of non-small cell lung cancer (NSCLC) (1–9). Erlotinib was the first targeted therapy FDA-approved for epidermal growth factor receptor (EGFR) mutations in 2013 (10). Osimertinib, a third-generation tyrosine kinase inhibitor (TKI), is approved as first-line therapy for advanced NSCLC as well as in the adjuvant setting for stage IB-IIIA-resected NSCLC (1, 11). However, osimertinib and other TKIs develop resistance patterns (12, 13). The most common patterns of resistance in EGFR-mutated NSCLC treated with osimertinib frontline are MET amplification, EGFR C797X-acquired mutation, and human epidermal growth factor 2 (HER2) amplification. Up to 15% of EGFR-mutated NSCLC treated with osimertinib transforms into small cell lung cancer (SCLC) or squamous cell carcinoma (12, 13).
EGFR-mutated NSCLC with progression of disease treatment options on osimertinib is limited, as oligoprogression or CNS metastasis is often treated with radiation therapy while continuing osimertinib (14, 15). Those with visceral metastasis beyond oligoprogression or SCLC transformation are treated with chemotherapy (14, 15). Immune checkpoint inhibitors (ICIs) have little benefit and significant toxicity in EGFR-mutated NSCLC (16, 17). The IMMUNOTARGET registry showed that ICI by itself in EGFR-mutated NSCLC had a shorter median progression-free survival (PFS) of 2.1 months and a lower overall response rate (ORR) of 12% compared to other targetable mutations (18). Attempts to combine EGFR TKIs with ICIs were not successful, as the TATTON study, which involved an EGFR TKI and durvalumab, was discontinued due to toxicity primarily due to interstitial lung disease (ILD) (19). Other combination ICI and EGFR TKI studies (KEYNOTE-021 and Checkmate 012) showed no OS benefit with severe grade 3+ hepatotoxicity in five of seven patients receiving gefitinib in KEYNOTE-021 (19, 20). Furthermore, the use of ICI followed by osimertinib led to severe immune-related adverse events in 15% of patients, particularly within 3 months of osimertinib use (17). KEYNOTE-789, a phase 3 randomized controlled trial of chemotherapy and pembrolizumab in TKI-resistant EGFR-mutated patients, showed no PFS or OS benefit (21). The lack of success of ICIs in EGFR-mutated NSCLC has limited treatment options upon progression of first-line EGFR TKI.
Antibody–drug conjugates (ADCs) consist of a monoclonal antibody attached to a cytotoxic drug (22, 23). These drugs have highly specific targeting abilities and can effectively kill the targeted cells and theoretically avoid toxicity toward other nontargeted cells (22, 23). Gemtuzumab ozogamicin was the first ADC given approval for treatment of patients >60 years with CD33+ acute myeloid leukemia who are not candidates for aggressive therapy (24). In solid tumors, one of the first ADC targets was HER2, a transmembrane protein in the erb-b2 receptor tyrosine kinase 2 (ERBB2). HER2 protein overexpression is observed in about 20% of NSCLC patients (25). Trastuzumab deruxtecan (T-DXd), an ADC-targeting HER2, was the first ADC in NSCLC given FDA-accelerated approval in 2022 (8, 26).
The development of T-DXd has led to more antigens being targeted in NSCLC for ADCs. ADCs could circumvent the EGFR cell signaling pathway and provide new treatment options for EGFR-mutated patients who progress on osimertinib. We will discuss ADC structure, mechanism of action, and targets currently in development in patients with EGFR-mutated NSCLC.
ADC consists of a monoclonal antibody, typically immunoglobulin G, bound to the target antigen of cells in the tumor. The ADC then fuses with the lysosome, leading to cytotoxic “payload” release that causes death of the targeted cancer cell (22, 23). When choosing an antigen, antigens chosen to be targets for designed ADCs are antigens that have much greater expression in cancer cells than in noncancer cells. For example, ERBB2, one of the early targets for ADC development, is expressed 100-fold in tumor tissues more than in nontumor tissues (22, 23, 27). The antibody and payload are connected by the linker and help with ADC stability. Cleavable linkers break down and release the payload based on either a specific pH or a specific lysosomal protease, while noncleavable linkers release the payload following internalization of ADCs by lysosome or proteases (22, 23, 28). The payload is where the ADC acts on the cancer cell and induces cell death. The payloads include agents that affect microtubule stability, inhibit topoisomerase 1, or cleave the DNA (22, 23, 29). There is a bystander effect where the drug, after internalization and degradation, is released across the cell membrane to kill adjacent cells (22, 23, 30). Finally, the drug-to-antibody ratio (DAR) is an important property of ADCs. DAR is the average number of drug molecules (payload) conjugated to the antibody. To calculate DAR, newer ADCs utilize high-performance liquid chromatography-ultraviolet spectroscopy, which adjusts for changes to the antibody or heavy/light chain hydrophobicity due to conjugated payloads, though other approaches include liquid chromatography-quadruple time-of-flight mass spectrometry and UV-Vis (31). Earlier ADCs developed had a DAR of 2–4, but newer ADCs such as trastuzumab deruxtecan have a DAR of 8 (31).
MET amplification is a common resistance mechanism in EGFR-mutated NSCLC, and so ADCs targeting MET-dysregulated NSCLC are being developed to address this (12, 13) (Figure 1). Amivantamab is a bispecific monoclonal antibody targeting MET and EGFR. In the CHRYALSIS study in which amivantamab is given with lazertinib, a third-generation EGFR TKI, the ORR was 36% (95% CI, 23–51) with 39% having a duration of response (DOR) longer than 6 months. Of those patients with disease progression after osimertinib and chemotherapy, the ORR was 29% (95% CI, 17–42) and the median DOR was 8.6 months (95% CI, 4.2–not reached). The most common grade ≥3 treatment-related adverse events was infusion-related reaction (7%) (NCT04077463) (32). The MARIPOSA-2 trial comparing amivantamab–chemotherapy, amivantamab–chemotherapy–lazertinib, and chemotherapy only showed significantly increased PFS results in amivantamab–chemotherapy and amivantamab–lazertinib–chemotherapy versus chemotherapy (median PFS of 8.2 and 8.3, respectively, versus 4.2 months), but with decreased hematologic adverse events in amivantamab–chemotherapy arm versus amivantamab–lazertinib–chemotherapy (NCT04988295) (33). An ADC involving MET dysregulation is telisotuzumab vedotin (Teliso-V), which is an ADC consisting of a c-Met antibody (ABT-700) and a microtubule inhibitor (monomethyl auristatin E) (34). In the LUMINOSITY phase 2 trial involving c-MET overexpressing NSCLC, there was a subcohort of 43 patients in which the ORR was 11% (95% CI, 3.9–25.1). Peripheral sensory neuropathy was the most common adverse event (25.0%), with two grade 5 adverse events possibly related to Teliso-V (sudden death and pneumonitis in one patient each) (NCT03539536) (34). Other ADCs being developed that may be promising in the future for MET-amplified EGFR-mutated NSCLC include ABBV-400, which targets c-MET and topoisomerase, in which there is an ongoing phase 1 study (NCT05029882) along with a biparatropic MET × MET ADC REGN-M114 (NCT04982224) (35, 36). Preclinical activity of REGN-M114 showed activity in EGFR mutant NSCLC with PTEN loss or MET Y1230C mutation cell lines that were pretreated with osimertinib and savolitinib (37). There is currently a phase 1 study (NCT04982224) looking at REGN-5093-M114 in MET-overexpressing advanced solid tumors (Table 1).
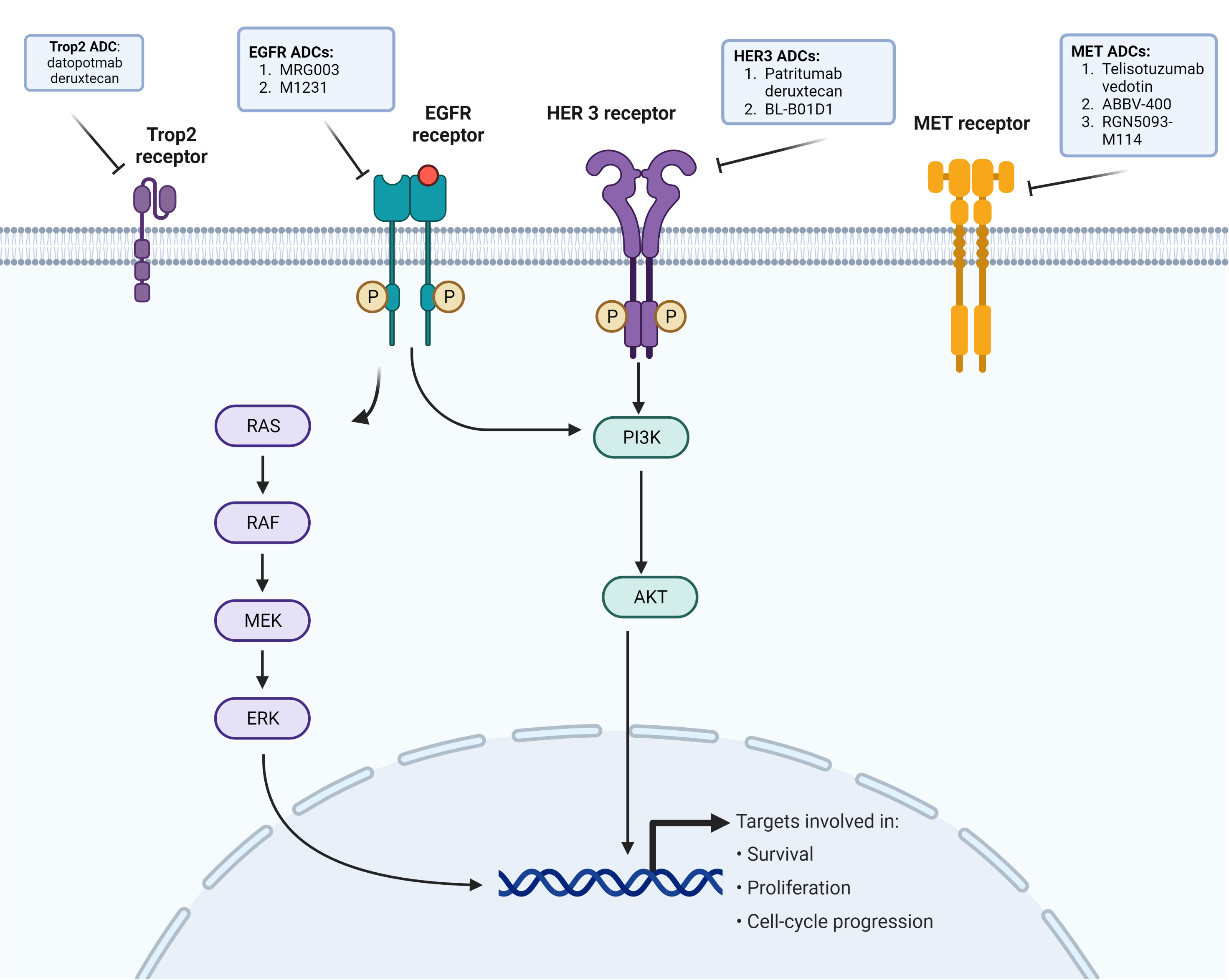
Figure 1 EGFR cellular pathway and purpose, and receptors with ADCs in development against EGFR-mutated NSCLC.
AZD9592 is a bispecific ADC targeting EGFR and cMET with a monovalent bispecific IgG platform with an increased affinity toward c-MET to reduce EGFR-related toxicities, along with a topoisomerase I payload (38). AZD9592 monotherapy in vivo in patient-derived xenograft models showed a 30% reduction of tumor in 41% of EGFR mutant NSCLC tumors at 2 mg/kg and a 73% response rate in those treated at 8 mg/kg (38, 39). Currently, there is a phase 1 trial on AZD9592 monotherapy in combination with osimertinib in EGFR mutant NSCLC (NCT05647122) (40) (Table 1).
There remains a large percentage of EGFR-mutated NSCLC patients receiving EGFR TKI with no known identifiable resistance mechanisms (12, 13). ERBB3 (HER3) is found in 83% of primary NSCLC tumors and is also expressed in other solid tumors (41). HER3 is found in a greater proportion of EGFR mutant NSCLC compared to EGFR wild-type NSCLC (42). Patritumab deruxtecan (HER3-DXd) is a HER3-directed ADC composed of a monoclonal antibody to HER3 covalently linked to a topoisomerase I inhibitor payload with a DAR ratio of 8 and is cell membrane-permeable, thus allowing cell death both at the target and surrounding tumor cells (43) (Figure 1). In a phase 1 study looking at HER3-DXd in metastatic EGFR-mutated NSCLC with prior EGFR TKI therapy, 57 patients received the dose-recommended HER3-DXd of 5.6 mg/kg q3 weeks (44). The ORR was 39% (95% CI, 26.0–62.4) with a median PFS of 8.2 (95% CI, 4.4–8.3) (44). The ORR in 23 of the 57 patients with known EGFR-related resistance mechanisms, excluding T790M, was 35%. HER3-DXd was well tolerated with a low discontinuation rate (9%, 7/81) (44). Treatment-related ILDs in this study occurred in 5% of patients and resolved in all patients after drug discontinuation (44) (Table 1).
HERTHENA-Lung01, a phase II study of HER3-DXd in EGFR-mutated NSCLC patients with disease progression after EGFR TKI and platinum-baseed chemotherapy, demonstrated a promising ORR of 29.8% (95% CI, 23.9–36.2) with a CNS ORR of 33.3% (95% CI, 17.3–52.8) (NCT04619004) (45, 46). Meanwhile, there is a phase III study comparing HER3-DXd to platinum-based chemotherapy in patients with disease progression after EGFR TKI therapy(HERTHENA-Lung02; NCT05338970). Another phase I study involving HER3-DXd in combination with osimertinib in patients who have progressed with osimertinib monotherapy (NCT04676477) is currently recruiting (Table 1).
Meanwhile, BL-B01D1 is a first-in-class EGFR × HER3 bispecific ADC linked to topoisomerase I inhibitor via a cleavable linker. Phase I data from a first-in-human study showed an ORR of 61% (95% CI, 43.6–77.8) and a disease control rate (DCR) of 91.2% (76.3–98.1) in heavily treated NSCLC EGFR-mutated cases (n = 34). All EGFR-mutated patients had previous EGFR TKI exposure, and 88% of these patients had prior third-generation EGFR TKI (NCT05194982) (47). There was an ORR of 40% (25.6–56.7) in NSCLC EGFR wild-type patients, and the most common grade 3 or higher treatment-related adverse events were leukopenia (30%), neutropenia (34%), and anemia (15%). No ILD was observed (47) (Table 1).
Izalontamab (SI-B001) is another first-in-class novel EGFR × HER3 bispecific antibody. A phase II study looking at SI-B001 plus docetaxel in patients who had failed on anti-PD-1/L1 antibody plus platinum-based chemotherapy had an ORR of 31.3% with no drug-related deaths, suggesting that this agent has activity with a manageable safety profile warranting further investigation (NCT05020457) (48).
There have been other ADCs directly targeting EGFR, with mixed results. MRG003 is an anti-EGFR humanized immunoglobulin G1 monoclonal antibody conjugated with monomethyl auristatin E via a valine–citrulline linker (Figure 1). In a phase 1 study of primarily head and neck squamous cell carcinoma, nasopharyngeal carcinoma, and colorectal cancer patients, the ORR was 40%, 44%, and 0%, respectively (NCT04868344) (49). Currently, a phase II study looking at the efficacy and safety of MRG003 is ongoing in EGFR-positive advanced non-small cell lung cancer (NCT04838548) (Table 1).
Depatuxizumab mafodotin (ABT-414), which consists of an EGFR-specific humanized antibody, a non-cleavable malemidocaproyl linker, and monomethyl auristatin F, was tested for patients with EGFR-amplified newly diagnosed glioblastoma but discontinued due to a lack of survival benefit (50). AVID-100, which is another EGFR-targeted ADC failed in phase I/II trial in solid tumors due to a lack of efficacy (NCT03094169) (51). M1231 is another ADC that consists of a bispecific antibody targeting Mucin 1 (MUC1) and EGFR with a hemiasterlin-related payload; a current phase I trial in solid tumors is ongoing (NCT04695847) (52) (Figure 1).
AFM24 is a tetravalent bispecific innate cell engager targeting EGFR on tumor cells and CD16A on natural killer cells to enhance antitumor antibody-dependent cellular cytotoxicity (53). A phase 1/2 monotherapy evaluating AFM24 in 14 heavily treated EGFR mutant NSCLC patients showed a DCR of 50% and a median duration of therapy of 6.7 weeks (1.0–26.1). There was acceptable safety with one incidence of grade 5 pneumonitis (NCT04259450) (54).
Another promising ADC target is trophoblast cell surface antigen (Trop2), which is a transmembrane glycoprotein calcium signal transducer and is overexpressed in over 60% of adenocarcinomas and 75% of squamous cell carcinoma (NSCLC). Datopotamab deruxtecan (Dato-DXd) consists of a Trop2-directed monoclonal antibody conjugated to a topoisomerase I inhibitor via a stable tetrapeptide-based cleavable linker (55) (Figure 1). The TROPION-PanTumor 01 trial included 159 NSCLC mostly pretreated patients and had an ORR of 21%–25% (23% at 4 mg/kg, 21% at 6 mg/kg, and 25% at 8 mg/kg) with a median PFS of 4.3–8.2 months (55). In those with advanced/metastatic NSCLC with actionable mutations (n = 34), which included 29 EGFR-mutated patients, the ORR was 35% (19.7–53.5) with a median DOR of 9.5 months (95% CI, 3.3–NE) (56). The TROPION-Lung05 is looking at patients with actionable genomic alterations previously treated with at least one targeted therapy and one platinum-based chemotherapy, and initial results show ORR of 35.8% (95% CI, 27.8–44.4) and a median DOR of 7.0 months (NCT04484142) (57, 58). Another ADC-targeting Trop2 is sacituzumab govitecan (SG). In the phase I/II IMMU-132 basket trial, there were 54 patients who received 8–12 mg/kg, and the ORR was 16.7% (95% CI, 7.9–29.3), with a median PFS of 4.4 months and a median OS of 7.3 months. About 60% had grade 3 or greater treatment-related adverse events, with neutropenia (42.4%) being most common (59) (Table 1).
In advanced NSCLC targeting HER2, ado-trastuzumab emtansine (TDM-1) had an ORR of 44%. However, the median duration of response (DOR) was 3.5 months with a median PFS of 2.8 months and a median OS of 8.1, and thus no further phase III NSCLC trials were pursued due to the limited efficacy (60, 61). T-DXd had an ORR of 55% in advanced HER2-mutant NSCLC with a median PFS of 8.2 months (95% CI, 6.0–11.9 months) and a median OS of 17.8 months (95% CI, 13.8–22.1 months). Drug-related ILD did occur in 26% of patients (8). Consequently, a phase II trial comparing T-DXd 5.4 mg/kg versus 6.4 mg/kg dosing showed similar efficacy in both doses, with a decrease in the adjudicated drug-related ILD rate in the 5.4-mg/kg dose (NCT04644237) (62). In regard to other ADC targets, carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) is highly expressed in about 25% of lung cancers, and a novel ADC targeting this is tusamitamab ravtansine, comprising a humanized monoclonal antibody and a cytotoxic maytansinoid, DM4. Phase 1 data of tusamitamab ravtansine showed ORR 20.3% (95% CI, 12.3–31.7) in high expression of CEACAM5 with grade 3 or greater TEAE in 48% of patients (63). Finally, B7-H3 protein is seen in 80% of NSCLC cases with high protein levels associated with poor prognosis and could be a considered a target for ADC development (64).
While ADCs provide promise, there are multiple considerations. The first consideration is resistance patterns and treatment upon progression from an ADC. Some mechanisms of ADC resistance include decreased antigen expression after exposure to ADC, as seen in some subjects who had received TDM-1; processing of ADC that may lead to less uptake in the cancer cell; and resistance to the payload (65, 66). As ADCs have an increased risk for ILD toxicity, there may be a risk for pneumonitis if patients were to go back to an EGFR TKI afterward (67). This is important as newer generation of TKIs are being developed, such as BLU-945 to address EGFR C797X resistance, and questions arise as to sequencing between drugs (68, 69).
Another consideration is weighing drug efficacy versus drug toxicity. The incidence of ILD in the key trials has been about 20%, and there are other notable toxicities that can significantly alter the quality of life, such as 20% ocular toxicity seen in TROPION-Lung02 (44, 70). Subsequent analysis of patient-reported outcomes will be vital in determining the benefit of drug efficacy versus drug toxicity.
CNS response rate is an important consideration. EGFR-mutated NSCLC leads to brain metastasis in about 50%–60% of patients, so finding an agent with CNS activity is vital. Earlier ADCs did not have good blood–brain barrier penetration due to a lack of homogeneity of the ADC along with a suboptimal drug-to-antibody ratio (71). The phase 1 HER3-DXd study in NSCLC patients has an ORR of 36.4% in patients with brain metastasis and a median DOR of 7.3 months, suggesting some activity, but more work needs to be done, particularly given the high risk of CNS metastasis in these patients (72).
In addition, up to 15% of EGFR-mutated patients have the risk of small-cell lung cancer transformation on progression (12, 13). There have not been any reported trials on ADCs in these patients, but delta-like ligand 3 (DLL-3) is a target expressed in 80% of SCLCs. Rovalpituzumab tesirine was an ADC in development in SCLC that was discontinued due to lack of benefit, but bispecific T-cell engagers such as tarlatamab have shown promising responses in heavily treated SCLC (73, 74). However, the mechanism of SCLC and squamous cell transformation in these patients needs to be understood so that we can focus on a specific target for this population.
Finally, combinations with ADCs are a consideration. Many trials combine chemotherapy, but with EGFR-mutated NSCLC, it will be interesting to see if a combination of ADC with EGFR TKI as studied in HER3-DXd with osimertinib will prove to be more efficacious and not more toxic than ADC monotherapy, and if so, if this combination will be more effective than ADC with chemotherapy (NCT04676477).
There has been tremendous progress in EGFR-mutated NSCLC since the approval of erlotinib in 2013, but with that come challenges, notably acquired resistance and treatment upon disease progression (10). ADCs are an increasingly studied drug class with more antigen targets being found. With trastuzumab deruxtecan approved for HER2-mutated NSCLC, there are promising developments in other ADCs targeting HER3, MET amplification, and EGFR directly. Future challenges involve the combination of ADCs with other drugs, sequencing of drugs, toxicity, particularly ILD incidence, and controlling brain metastasis in patients with CNS involvement.
RH designed the review and wrote the original manuscript draft. DB produced Figure 1, reviewed, and edited the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported in part by the USC Norris Comprehensive Cancer Center (Core) Grant, P30 CA014089.
RH is a consultant for Targeted Oncology and Takeda and received honoraria from DAVA Oncology and The Dedham Group. DB has received consulting fees from Seagen and is a part of the speakers' bureau for Merck while also receiving travel and accommodations from Merck.
The reviewer FC declared a shared affiliation with the author RH to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ramalingam SS, Yang JC-H, Lee CK, Kurata T, Kim D-W, John T, et al. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J Clin Oncol (2018) 36:841–9. doi: 10.1200/JCO.2017.74.7576
2. Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. New Engl J Med (2021) 384:2371–81. doi: 10.1056/NEJMoa2103695
3. Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol (2017) 18:1307–16. doi: 10.1016/S1470-2045(17)30679-4
4. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim D-W, et al. Alectinib versus crizotinib in untreated ALK -positive non–small-cell lung cancer. N Engl J Med (2017) 377(9):829–38. doi: 10.1056/NEJMoa1704795
5. Shaw AT, Riely GJ, Bang Y-J, Kim D-W, Camidge DR, Solomon BJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol (2019) 30:1121–6. doi: 10.1093/annonc/mdz131
6. Drilon A, Oxnard GR, Tan DSW, Loong HHF, Johnson M, Gainor J, et al. Efficacy of selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med (2020) 383:813–24. doi: 10.1056/NEJMoa2005653
7. Wolf J, Seto T, Han J-Y, Reguart N, Garon EB, Groen HJM, et al. Capmatinib in MET exon 14–mutated or MET -amplified non–small-cell lung cancer. New Engl J Med (2020) 383:944–57. doi: 10.1056/NEJMoa2002787
8. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2 -mutant non–small-cell lung cancer. New Engl J Med (2022) 386:241–51. doi: 10.1056/NEJMoa2112431
9. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol (2020) 21:271–82. doi: 10.1016/S1470-2045(19)30691-6
10. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
11. Wu Y-L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR -mutated non–small-cell lung cancer. New Engl J Med (2020) 383:1711–23. doi: 10.1056/NEJMoa2027071
12. Tumbrink HL, Heimsoeth A, Sos ML. The next tier of EGFR resistance mutations in lung cancer. Oncogene (2021) 40:1–11. doi: 10.1038/s41388-020-01510-w
13. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. (2019) 121:725–37. doi: 10.1038/s41416-019-0573-8
14. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.3.2023. (2023). © National Comprehensive Cancer Network, Inc (Accessed 15 June 2023). All rights reserved.
15. Fu K, Xie F, Wang F, Fu L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J Hematol Oncol (2022) 15:173. doi: 10.1186/s13045-022-01391-4
16. Yang JC-H, Gadgeel SM, Sequist LV, Wu C-L, Papadimitrakopoulou VA, Su W-C, et al. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol (2019) 14:553–9. doi: 10.1016/j.jtho.2018.11.028
17. Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol (2019) 30:839–44. doi: 10.1093/annonc/mdz077
18. Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol (2019) 30:1321–8. doi: 10.1093/annonc/mdz167
19. Oxnard GR, Yang JC-H, Yu H, Kim S-W, Saka H, Horn L, et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol (2020) 31:507–16. doi: 10.1016/j.annonc.2020.01.013
20. Gettinger S, Hellmann MD, Chow LQM, Borghaei H, Antonia S, Brahmer JR, et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol (2018) 13:1363–72. doi: 10.1016/j.jtho.2018.05.015
21. Yang JC-H, Lee DH, Lee J-S, Fan Y, de Marinis F, Okamoto I, et al. Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor (TKI)-resistant, EGFR -mutant, metastatic nonsquamous NSCLC: Phase 3 KEYNOTE-789 study. J Clin Oncol (2023) 41:LBA9000–LBA9000. doi: 10.1200/JCO.2023.41.17_suppl.LBA9000
22. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther (2022) 7:93. doi: 10.1038/s41392-022-00947-7
23. Coleman N, Yap TA, Heymach JV, Meric-Bernstam F, Le X. Antibody-drug conjugates in lung cancer: dawn of a new era? NPJ Precis Oncol (2023) 7:5. doi: 10.1038/s41698-022-00338-9
24. Norsworthy KJ, Ko C-W, Lee JE, Liu J, John CS, Przepiorka D, et al. FDA approval summary: mylotarg for treatment of patients with relapsed or refractory CD33-positive acute myeloid leukemia. Oncologist (2018) 23:1103–8. doi: 10.1634/theoncologist.2017-0604
25. Heinmöller P, Gross C, Beyser K, Schmidtgen C, Maass G, Pedrocchi M, et al. HER2 status in non-small cell lung cancer: results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res (2003) 9:5238–43.
26. Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial☆. Ann Oncol (2020) 31:1350–8. doi: 10.1016/j.annonc.2020.06.020
27. von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New Engl J Med (2019) 380:617–28. doi: 10.1056/NEJMoa1814017
28. Lu J, Jiang F, Lu A, Zhang G. Linkers having a crucial role in antibody-drug conjugates. Int J Mol Sci (2016) 17:561. doi: 10.3390/ijms17040561
29. Ponziani S, Di Vittorio G, Pitari G, Cimini AM, Ardini M, Gentile R, et al. Antibody-drug conjugates: the new frontier of chemotherapy. Int J Mol Sci (2020) 21:5510. doi: 10.3390/ijms21155510
30. Giugliano F, Corti C, Tarantino P, Michelini F, Curigliano G. Bystander effect of antibody–drug conjugates: fact or fiction? Curr Oncol Rep (2022) 24:809–17. doi: 10.1007/s11912-022-01266-4
31. Matsuda Y, Mendelsohn BA. Recent advances in drug-antibody ratio determination of antibody-drug conjugates. Chem Pharm Bull (Tokyo). (2021) 69:976–83. doi: 10.1248/cpb.c21-00258
32. Shu CA, Goto K, Ohe Y, Besse B, Lee S-H, Wang Y, et al. Amivantamab and lazertinib in patients with EGFR-mutant non–small cell lung (NSCLC) after progression on osimertinib and platinum-based chemotherapy: Updated results from CHRYSALIS-2. J Clin Oncol (2022) 40:9006–6. doi: 10.1200/JCO.2022.40.16_suppl.9006
33. Passaro A, Wang J, Wang Y, Lee S-H, Melosky B, Shih JY, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol (2023) 23:S0923-7534(23)04281-3. doi: 10.1016/j.annonc.2023.10.117. [Epub ahead of print].
34. Camidge DR, Bar J, Horinouchi H, Goldman JW, Moiseenko FV, Filippova E, et al. Telisotuzumab vedotin (Teliso-V) monotherapy in patients (pts) with previously treated c-Met–overexpressing (OE) advanced non-small cell lung cancer (NSCLC). J Clin Oncol (2022) 40:9016–6. doi: 10.1200/JCO.2022.40.16_suppl.9016
35. Drilon AE, Awad MM, Gadgeel SM, Villaruz LC, Sabari JK, Perez J, et al. A phase 1/2 study of REGN5093-M114, a METxMET antibody-drug conjugate, in patients with mesenchymal epithelial transition factor (MET)-overexpressing NSCLC. J Clin Oncol (2022) 40:TPS8593–TPS8593. doi: 10.1200/JCO.2022.40.16_suppl.TPS8593
36. Reilly RM, Ji C, Matuszak RP, Anderson MG, Tucker L, Klunder N, et al. Abstract 6311: ABBV-400: An ADC delivering a novel topoisomerase 1 inhibitor to c-Met-positive solid tumors. Cancer Res (2023) 83:6311–1. doi: 10.1158/1538-7445.AM2023-6311
37. Oh SY, Lee YW, Lee EJ, Kim JH, Park Y, Heo SG, et al. Preclinical study of a biparatopic METxMET antibody–drug conjugate, REGN5093-M114, overcomes MET-driven acquired resistance to EGFR TKIs in EGFR-mutant NSCLC. Clin Cancer Res (2023) 29:221–32. doi: 10.1158/1078-0432.CCR-22-2180
38. Comer F, Mazor Y, Hurt E, Yang C, Fleming R, Shandilya H, et al. Abstract 5736: AZD9592: An EGFR-cMET bispecific antibody-drug conjugate (ADC) targeting key oncogenic drivers in non-small-cell lung cancer (NSCLC) and beyond. Cancer Res (2023) 83:5736–6. doi: 10.1158/1538-7445.AM2023-5736
39. McGrath L, Zheng Y, Christ S, Sachs CC, Khelifa S, Windmüller C, et al. Abstract 5737: Evaluation of the relationship between target expression and in vivo anti-tumor efficacy of AZD9592, an EGFR/c-MET targeted bispecific antibody drug conjugate. Cancer Res (2023) 83:5737–7. doi: 10.1158/1538-7445.AM2023-5737
40. Aggarwal C, Azzoli CG, Spira AI, Solomon BJ, Le X, Rolfo C, et al. EGRET: A first-in-human study of the novel antibody-drug conjugate (ADC) AZD9592 as monotherapy or combined with other anticancer agents in patients (pts) with advanced solid tumors. J Clin Oncol (2023) 41:TPS3156–TPS3156. doi: 10.1200/JCO.2023.41.16_suppl.TPS3156
41. Scharpenseel H, Hanssen A, Loges S, Mohme M, Bernreuther C, Peine S, et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci Rep (2019) 9:7406. doi: 10.1038/s41598-019-43678-6
42. Kawano O, Sasaki H, Endo K, Suzuki E, Haneda H, Yukiue H, et al. ErbB3 mRNA expression correlated with specific clinicopathologic features of Japanese lung cancers. J Surg Res (2008) 146:43–8. doi: 10.1016/j.jss.2007.05.030
43. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, A novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res (2016) 22:5097–108. doi: 10.1158/1078-0432.CCR-15-2822
44. Jänne PA, Baik C, Su W-C, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of Patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated non-small cell lung cancer. Cancer Discovery (2022) 12:74–89. doi: 10.1158/2159-8290.CD-21-0715
45. Yu HA, Yang JC, Hayashi H, Goto Y, Felip E, Reck M, et al. HERTHENA-Lung01: a phase II study of patritumab deruxtecan (HER3-DXd) in previously treated metastatic EGFR-mutated NSCLC. Future Oncol (2023) 19(19):1319–29. doi: 10.2217/fon-2022-1250. [Epub ahead of print].
46. Yu HA, Goto Y, Hayashi H, Felip E, Chih-Hsin Yang J, Reck M, et al. HERTHENA-Lung01, a phase II trial of patritumab deruxtecan (HER3-DXd) in epidermal growth factor receptor-mutated non-small-cell lung cancer after epidermal growth factor receptor tyrosine kinase inhibitor therapy and platinum-based chemotherapy. J Clin Oncol (2023) 10:JCO2301476. doi: 10.1200/JCO.23.01476. [Epub ahead of print].
47. Zhang L, Ma Y, Zhao Y, Fang W, Zhao H, Huang Y, et al. BL-B01D1, a first-in-class EGFRxHER3 bispecific antibody-drug conjugate (ADC), in patients with locally advanced or metastatic solid tumor: Results from a first-in-human phase 1 study. J Clin Oncol (2023) 41:3001–1. doi: 10.1200/JCO.2023.41.16_suppl.3001
48. Zhao Y, Zhang L, Fang W, Yang Y, Huang Y, Zou W, et al. SI-B001 plus chemotherapy in patients with locally advanced or metastatic EGFR/ALK wild-type non-small cell lung cancer: A phase II, multicenter, open-label study. J Clin Oncol (2023) 41:9025–5. doi: 10.1200/JCO.2023.41.16_suppl.9025
49. Qiu M-Z, Zhang Y, Guo Y, Guo W, Nian W, Liao W, et al. Evaluation of safety of treatment with anti-epidermal growth factor receptor antibody drug conjugate MRG003 in patients with advanced solid tumors: A phase 1 nonrandomized clinical trial. JAMA Oncol (2022) 8:1042–6. doi: 10.1001/jamaoncol.2022.0503
50. Narita Y, Muragaki Y, Kagawa N, Asai K, Nagane M, Matsuda M, et al. Safety and efficacy of depatuxizumab mafodotin in Japanese patients with Malignant glioma: A nonrandomized, phase 1/2 trial. Cancer Sci (2021) 112:5020–33. doi: 10.1111/cas.15153
51. Thwaites MJ, Figueredo R, Tremblay G, Koropatnick J, Goldmacher V, O’Connor-McCourt M. Abstract 218: AVID100 is an anti-EGFR ADC that promotes DM1-meditated cytotoxicity on cancer cells but not on normal cells. Cancer Res (2019) 79:218–8. doi: 10.1158/1538-7445.AM2019-218
52. Knuehl C, Toleikis L, Dotterweich J, Ma J, Kumar S, Ross E, et al. Abstract 5284: M1231 is a bispecific anti-MUC1xEGFR antibody-drug conjugate designed to treat solid tumors with MUC1 and EGFR co-expression. Cancer Res (2022) 82:5284–4. doi: 10.1158/1538-7445.AM2022-5284
53. Wingert S, Reusch U, Knackmuss S, Kluge M, Damrat M, Pahl J, et al. Preclinical evaluation of AFM24, a novel CD16A-specific innate immune cell engager targeting EGFR-positive tumors. MAbs (2021) 13:1950264. doi: 10.1080/19420862.2021.1950264
54. El-Khoueiry AB, Rivas D, Lee S-H, Thomas JS, Kim YJ, Cervantes A, et al. Leveraging innate immunity with AFM24, a novel CD16A and epidermal growth factor receptor (EGFR) bispecific innate cell engager: Interim results for the non-small cell lung cancer (NSCLC) cohort. J Clin Oncol (2023) 41:2533–3. doi: 10.1200/JCO.2023.41.16_suppl.2533
55. Spira A, Lisberg A, Sands J, Greenberg J, Phillips P, Guevara F, et al. OA03.03 Datopotamab deruxtecan (Dato-DXd; DS-1062), a TROP2 ADC, in patients with advanced NSCLC: updated results of TROPION-panTumor01 phase 1 study. J Thorac Oncol (2021) 16:S106–7. doi: 10.1016/j.jtho.2021.01.280
56. Garon EB, Johnson ML, Lisberg AE, Spira A, Yamamoto N, Heist RS, et al. LBA49 Efficacy of Datopotamab deruxtecan (Dato-DXd) in patients (pts) with advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC) and actionable genomic alterations (AGAs): Preliminary results from the phase I TROPION-PanTumor01 study. Ann Oncol (2021) 32:S1326–7. doi: 10.1016/j.annonc.2021.08.2128
57. Johnson M, Spira A, Yoh K, Heist R, Lisberg A, Greenberg J, et al. P47.05 A phase 2 study of Datopotamab deruxtecan (Dato-DXd) in advanced NSCLC with actionable genomic alterations (TROPION-lung05). J Thorac Oncol (2021) 16:S1098. doi: 10.1016/j.jtho.2021.08.498
58. Paz-Ares L, Ahn M-J, Lisberg AE, Kitazono S, Cho BC, Blumenschein G, et al. 1314MO TROPION-Lung05: Datopotamab deruxtecan (Dato-DXd) in previously treated non-small cell lung cancer (NSCLC) with actionable genomic alterations (AGAs). Ann Oncol (2023) 34:S755–6. doi: 10.1016/j.annonc.2023.09.2348
59. Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol (2021) 32:746–56. doi: 10.1016/j.annonc.2021.03.005
60. Iwama E, Zenke Y, Sugawara S, Daga H, Morise M, Yanagitani N, et al. Trastuzumab emtansine for patients with non-small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations. Eur J Cancer. (2022) 162:99–106. doi: 10.1016/j.ejca.2021.11.021
61. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol (2018) 36:2532–7. doi: 10.1200/JCO.2018.77.9777
62. Goto K, Goto Y, Kubo T, Ninomiya K, Kim S-W, Planchard D, et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-lung02 trial. J Clin Oncol (2023) 41:4852–63. doi: 10.1200/JCO.23.01361
63. Gazzah A, Bedard PL, Hierro C, Kang Y-K, Abdul Razak A, Ryu M-H, et al. Safety, pharmacokinetics, and antitumor activity of the anti-CEACAM5-DM4 antibody–drug conjugate tusamitamab ravtansine (SAR408701) in patients with advanced solid tumors: first-in-human dose-escalation study. Ann Oncol (2022) 33:416–25. doi: 10.1016/j.annonc.2021.12.012
64. Altan M, Pelekanou V, Schalper KA, Toki M, Gaule P, Syrigos K, et al. B7-H3 expression in NSCLC and its association with B7-H4, PD-L1 and tumor-infiltrating lymphocytes. Clin Cancer Res (2017) 23:5202–9. doi: 10.1158/1078-0432.CCR-16-3107
65. Sung M, Tan X, Lu B, Golas J, Hosselet C, Wang F, et al. Caveolae-mediated endocytosis as a novel mechanism of resistance to trastuzumab emtansine (T-DM1). Mol Cancer Ther (2018) 17:243–53. doi: 10.1158/1535-7163.MCT-17-0403
66. Loganzo F, Sung M, Gerber H-P. Mechanisms of resistance to antibody-drug conjugates. Mol Cancer Ther (2016) 15:2825–34. doi: 10.1158/1535-7163.MCT-16-0408
67. Abelman RO, Wu B, Spring LM, Ellisen LW, Bardia A. Mechanisms of resistance to antibody-drug conjugates. Cancers (Basel) (2023) 15(4):1278. doi: 10.3390/cancers15041278
68. Elamin YY, Nagasaka M, Shum E, Bazhenova L, Camidge DR, Cho BC, et al. BLU-945 monotherapy and in combination with osimertinib (OSI) in previously treated patients with advanced EGFR -mutant (EGFRm) NSCLC in the phase 1/2 SYMPHONY study. J Clin Oncol (2023) 41:9011–1. doi: 10.1200/JCO.2023.41.16_suppl.9011
69. Eno MS, Brubaker JD, Campbell JE, De Savi C, Guzi TJ, Williams BD, et al. Discovery of BLU-945, a reversible, potent, and wild-type-sparing next-generation EGFR mutant inhibitor for treatment-resistant non-small-cell lung cancer. J Med Chem (2022) 65:9662–77. doi: 10.1021/acs.jmedchem.2c00704
70. Goto Y, Su W-C, Levy BP, Rixe O, Yang T-Y, Tolcher AW, et al. TROPION-Lung02: Datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). J Clin Oncol (2023) 41:9004–4. doi: 10.1200/JCO.2023.41.16_suppl.9004
71. Anami Y, Otani Y, Xiong W, Ha SYY, Yamaguchi A, Rivera-Caraballo KA, et al. Homogeneity of antibody-drug conjugates critically impacts the therapeutic efficacy in brain tumors. Cell Rep (2022) 39:110839. doi: 10.1016/j.celrep.2022.110839
72. Daiichi-Sankyo. Patritumab deruxtecan continues to show encouraging clinical activity in distinct patient populations with metastatic lung and breast cancer in updated results of two early trials . Available at: https://www.daiichisankyo.com/files/news/pressrelease/pdf/202303/20230320_E.pdf (Accessed 19 June 2023).
73. Uprety D, Remon J, Adjei AA. All that glitters is not gold: the story of Rovalpituzumab tesirine in SCLC. J Thorac Oncol (2021) 16:1429–33. doi: 10.1016/j.jtho.2021.07.012
Keywords: non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR), antibody drug conjugate (ADC), tyrosine kinase inhibitor resistance (TKI resistance), targeted therapy resistance
Citation: Hsu R and Benjamin DJ (2023) A narrative review of antibody–drug conjugates in EGFR-mutated non-small cell lung cancer. Front. Oncol. 13:1252652. doi: 10.3389/fonc.2023.1252652
Received: 04 July 2023; Accepted: 10 November 2023;
Published: 01 December 2023.
Edited by:
Gatien Moriceau, University of California, Los Angeles, United StatesReviewed by:
Frances Elaine Chow, University of Southern California, United StatesCopyright © 2023 Hsu and Benjamin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert Hsu, cm9iZXJ0LmhzdUBtZWQudXNjLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.