
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 12 September 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1252433
 Carla Minoia1*†
Carla Minoia1*† Simonetta Viviani2†
Simonetta Viviani2† Erica Silvestris3
Erica Silvestris3 Simone Palini4
Simone Palini4 Francesca Parissone5
Francesca Parissone5 Giuseppe De Palma6
Giuseppe De Palma6 Anna Fedina7
Anna Fedina7 Gennaro Cormio8
Gennaro Cormio8 Attilio Guarini1
Attilio Guarini1 Guido Gini9
Guido Gini9 Luigi Montano10
Luigi Montano10 Francesco Merli11
Francesco Merli11 Fedro Alessandro Peccatori12
Fedro Alessandro Peccatori12Introduction: Fertility preservation (FP) and monitoring has considerable relevance in the multidisciplinary approach to cancer patients. In these consensus-based practical recommendations, the scientific societies Fondazione Italiana Linfomi (FIL) and Società Italiana della Riproduzione Umana (SIRU) reviewed the main aspects and identified the optimal paths which aim to preserve and monitor fertility in patients diagnosed with lymphoma at the different phases of the disease and during long-term survivorship.
Methods: For the Panel, eleven experts were selected for their expertise in research and clinical practice on onco-fertility and lymphoma. The Panel’s activity was supervised by a chairman. A series of rank-ordering key questions were proposed according to their clinical relevance and discussed among the Panel, focusing on patients diagnosed with non-Hodgkin’s lymphomas and Hodgkin lymphoma. Agreement among all the Panelists on the content and terminology of the statements was evaluated by a web-based questionnaire according to the Delphi methodology.
Results: From the literature review a total of 78 questions or sentences, divided into the 6 areas of interest, were identified. By applying the Gwet's AC, k was: Section 1: 0,934 (Very good); Section 2: 0,958 (Very good); Section 3: 0,863 (Very good); Section 4: 0,649 (Good); Section 5: 0,936 (Very good); Section 6 raw agreement 100%. Two rounds of Delphi allowed to provide the maximum agreement. All statements were newly discussed in a round robin way and confirmed for the drafting of the final recommendations.
Discussion: These recommendations would be useful for onco-hematologists, gynecologists, urologists, and general practice physicians who take care of young lymphoma patients to guarantee an evidence-based oncofertility assessment and treatment during the oncologic pathway.
In 2020, around 627,439 people were diagnosed with lymphoma worldwide; in Europe there were 19,858 new cases of Hodgkin lymphoma (HL) and 86,321 of non-Hodgkin lymphomas (NHL) (1, 2). Among female patients aged 20 to 39 years, HL and NHL accounted for 3,304 and 2,639 new cases, whereas the incidence for male patients was aged 20-59 years was 6,696 and 20,733, respectively (1, 2). Long-term survival of patients diagnosed with lymphoma has significantly improved in the last decades and the population of long survivors has grown substantially (3–6). Among the complications following treatment for lymphoma, permanent loss of fertility in patients of childbearing age is a relevant and potentially quality of life-impairing effect of cancer treatment. In order to estimate the risk of fertility impairment, the following aspects are more relevant than others: patient sex and age, type and dose of chemotherapy regimen, site and dose of radiation therapy, and, in females, ovarian reserve (7–9).
In male patients, cytotoxic treatment targets rapidly dividing cells including spermatocytes, thus disrupting spermatogenesis and potentially leading to infertility after cancer treatment at any age. On the contrary, in females, chemo-and/or radiotherapy induce age-dependent ovarian damage, leading to oocyte depletion and premature ovarian insufficiency (POI). As younger female patients, i.e. those under the age of 30 years, have a higher number of follicles and might have a regular ovulatory cycle even with a small numbers of follicles, less severe gonadal damage is reported in younger compared to older patients, who may enter chemotherapy- induced POI more often (7).
Although over time attention has been paid to aspects of gonadotoxicity for young patients in order to reduce the use of drugs which lead to a risk of permanent infertility for HL and NHL, there are some patients in whom this risk cannot be reduced. An example is cases in which it is necessary to use intensified dose front-line regimens, or in which first-line therapy proves ineffective and a high-dose rescue regimen followed by autologous stem cell transplant (ASCT) is necessary (10). Furthermore, it is important to underline that long-term survivors currently face the late effects of chemotherapy regimens mainly used in the past, especially for the treatment of HL, which have historically demonstrated a significant rate of infertility. Among front line regimens for HL, those containing procarbazine are associated with a significant risk of gonadotoxicity. Some have been given in the past (i.e.MOPP: mechlorethamine, vincristine, procarbazine, prednisone; COPP: cyclophosphamide, vincristine, procarbazine, prednisone), while others are still being used nowadays (escalated BEACOPP: bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) (11, 12). Alkylating agents (mechlorethamine, melphalan, carmustine, lomustine, chlorambucil, busulfan, cyclophosphamide) can cause gonadotoxicity depending on the cumulative dose and age at administration (8, 13, 14). Among salvage regimens, those containing platinum and its derivatives are associated with gonadotoxicity (14, 15). For all these agents, it should be taken into account that, the cumulative risk of gonadal toxic effects are increased by their use in combination regimens and by the number of cycles administered. Recently, the targeted drugs (e.i. brentuximab vedotin, polatuzumab vedotin, checkpoint inhibitors, BCL-2 inhibitor venetoclax, Bruton’s tyrosine kinase inhibitors ibrutinib, zanubrutinib, acalabrutinib, pirtobrutinib and the PI3K inhibitors idelalisib and duvelisib) have been introduced in the clinical practice. Their gonadotoxicity is still unknown, since on the one hand they are mainly used in the relapsed/refractory setting in patients who have already received multiple lines of chemotherapy regimens, and on the other hand they can be administered for a prolonged number of cycles. Their gonadotoxic potential is therefore difficult to be assessed (14–16).
With regard to radiotherapy (RT), the use of more advanced technologies and PET (positron emission tomography)-oriented approaches has made it possible to limit the use of this technique to the abdominal and pelvic regions, progressively reducing the patients at risk. It is known that the testis is extremely sensitive to radiation. Radiation doses as low as 0,1–1,2 Gy can impair spermatogenesis, while doses higher than 4 Gy can cause permanent azoospermia. Gonadotoxicity can occur even if RT is delivered to pelvic nodes without testicular shielding at doses of ≥ 20 Gy, and when it is given concurrently with chemotherapy, doses of 9–10 Gy may induce gonadal dysfunction (7, 17, 18). RT may also induce severe injury to the ovarian reserve. In adult female patients, RT at doses > 6 Gy to the ovaries as well as the whole abdomen or the pelvic nodes will cause ovarian damage, and doses > 30 Gy will also affect uterine function with a raised incidence of spontaneous miscarriage and low fetal intrauterine growth (7, 8, 16, 17).
Considering the aspects presented above, it is of utmost importance to assist clinicians and patients in the recognition of the potential risk of infertility as a consequence of specific treatment modalities, and to provide and effectively implement onco-fertility counseling with an open discussion on the impact of lymphoma and its treatment on reproductive function (9, 19, 20). After histologic diagnosis, available fertility preservation (FP) options should be discussed prior to treatment start at the earliest possible opportunity. The expedited referral for interested patients to reproductive specialists should be facilitated in order to promptly evaluate the risks and benefits of the more appropriate PF procedures based on an accurate assessment of the individual risk of gonadotoxicity. It is also important to allow adequate time to complete FP in order to avoid a delay in the start of cancer treatment. With regard to the options available, it should be emphasized that these also depend on national legislation, in fact some procedures, such as the production and freezing of embryos before anti-cancer treatment, are not permitted in all countries.
The Fondazione Italiana Linfomi (FIL) “Survivorship, Quality of Life and Comorbidity Committee’’ and the Società Italiana della Riproduzione Umana (SIRU) collaborated to review the current evidence on this issue in adult patients with lymphoma and to develop a multidisciplinary consensus paper. Consensus was obtained among the expert Panel by applying the Delphi method. The document presents shared statements of practical help in the management of young patients with HL or NHL about to start therapy and for their short and long-term follow-up.
The multi-disciplinary working group for this consensus-based position paper, hereafter referred to as the Panel, comprised five onco-hematologists belonging to the Fondazione Italiana Linfomi (FIL) – “Survivorship, Quality of Life and Comorbidity Committee” (CM, SV, AG, GG, FM), four gynecologists or andrologists expert in fertility preservation and reproduction affiliated with the Società Italiana della Riproduzione Umana (SIRU) (ES, GC, FP, LM), and one embryologist and one biologist with expertise in tissue preservation (SP, GDP) affiliated with the SIRU. The experts were selected according to their expertise in research and clinical practice in lymphoma, FP, survivorship, and quality of life. The Panel was supervised by an international expert leader on onco- fertility (FAP) (chairman). Data management and analyses were conducted by the FIL data office in the person of AF. The project was carried out following the Declaration of Helsinki’s ethical principles for medical research involving human subjects, and in accordance with the Good Clinical Practice regulations.
The project was presented and discussed at the annual meeting of the FIL in November 2021. Following the experience gained in previous cooperative studies, the Panel designed a series of practical questions on the topic of fertility preservation and follow-up in patients diagnosed with and treated for lymphoma (16, 19). The series of rank-ordering key questions was proposed according to clinical relevance and discussed among the Panel. The questions were discussed and shared by the Panel during a preliminary online meeting in February 2022. The focus of the questions regarded patients diagnosed with NHL and HL, aged 18- 40 years for females and > 18 years for male patients. Specifically, the following histotypes of NHL were considered: Diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBL), Follicular lymphoma (FL), Peripheral T-cell lymphoma (PTCL), Mantle Cell Lymphoma (MCL) and Burkitt lymphoma (BL). Both classical and nodular lymphocyte-predominant HL were included.
Six main key topics were addressed: i) to which categories of patients is this document addressed?; ii) pre-therapy counseling for both female and male patients; iii) the optimal medical treatment for fertility preservation during chemotherapy for female patients; iv) the follow-up: indications on fertility tests to be carried out in the period following chemotherapy (1-5 years and > 5 years from remission); v) safe conception in both female and male patients; vi) disorders of the sexual sphere after anti-tumor therapy. Table 1 summarizes the rank-ordering key topics discussed among the Panel.
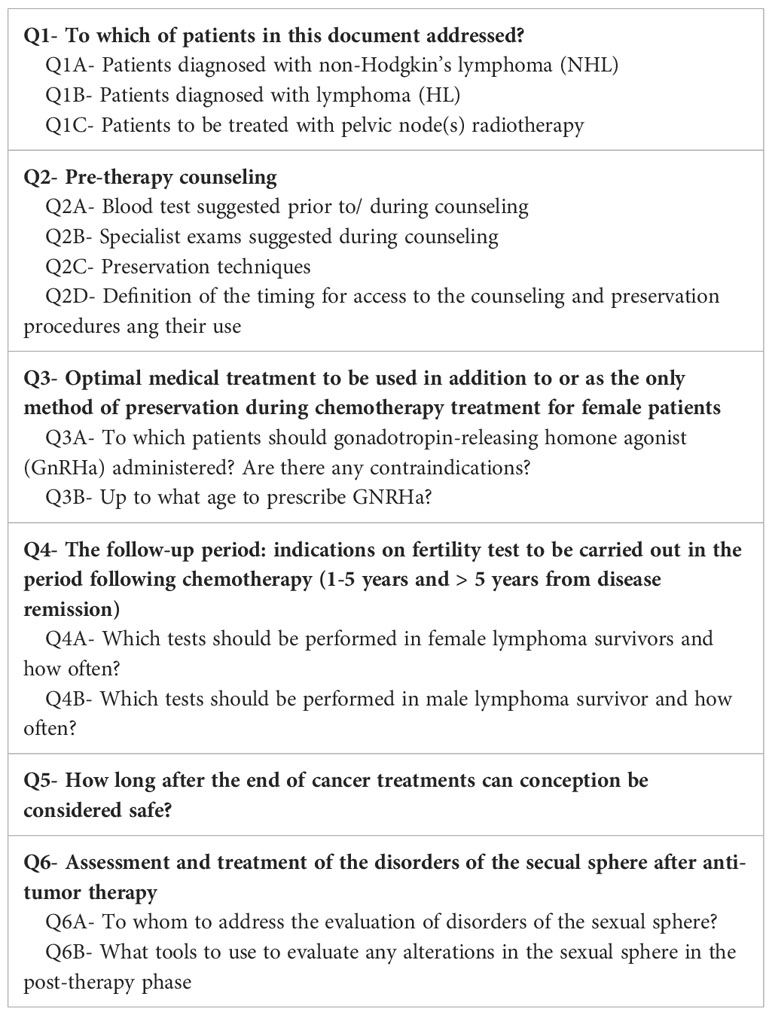
Table 1 Rank-ordering key topics discussed among the Panel on fertility preservation in lymphoma patients.
A qualitative literature review (using Mesh and free text terms) was conducted from January 1990 to January 2022, with no language restrictions. Five panelists (CM, SV, ES, SP, FP) conducted the literature review addressing the selected clinical key questions. For each key topic, the search was conducted by two independent reviewers (title and abstract selection, full text paper reading, data reporting) on the three main search engines: MEDLINE (via PubMed), the Cochrane Library, and EMBASE. The following types of articles were considered eligible: cohort studies, case-control studies, randomized clinical trials (RCT), systematic reviews, and meta-analyses. The search was carried out by combining the conditions (lymphoma, Hodgkin lymphoma, non-Hodgkin’s lymphoma, diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma, primary mediastinal lymphoma, Burkitt lymphoma, female patient, male patient), interventions (e.g., chemotherapy, immunotherapy, radiotherapy, GnRH analogs, GnRH antagonist, GnRH agonist, gonadorelin, leuprorelin, triptorelin, enantone, decapeptyl, buserelin, goserelin) and outcomes of interest (oocyte cryopreservation, ovarian tissue cryopreservation, semen cryopreservation,conception, pregnancy, post-treatment parenthood, post-treatment childhood, acute ovarian failure, premature ovarian insufficiency, primary ovarian insufficiency, premature ovarian failure, azoospermia, infertility, sterility,gonadotoxicity, follicle-stimulating hormone FSH, luteinizing hormone LH, estradiol E2, anti-Müllerian hormone AMH, testosterone TST, prolactin PRL, inhibin B, sexuality, counseling, follow-up). A revision of current oncologic, hematologic, and gynecologic guidelines was also carried out.
The agreement among all the Panelists on the content and terminology of the statements was scored by a (web-based) questionnaire according to the Delphi methodology (21, 22). The methodology is represented in Figure 1. When a consensus for each question of at least ≥ 80% was not obtained, the statement was discussed and suggestions for rephrasing were proposed by the chairman. The inter-rater reliability has been calculated by Gwet’s agreement coefficient (AC). A kappa below 0.2 was indicative for poor agreement and a kappa above 0.8 for very good agreement. The strength of agreement is detailed as follows: kappa < 0.2: Poor; > 0.2 ≤ 0.4: Fair; > 0.4 ≤ 0.6: Moderate; > 0.6 ≤ 0.8: Good; > 0.8 ≤ 1: Very good.
A second round of votes as a web-based questionnaire was then performed for statements not reaching an agreement ≥ 80% (Very good).
At the end of this process, the Panel met in a virtual conference moderated by the chairman held in December 2022. All statements were newly discussed in a round robin way and confirmed for the drafting of the final recommendations. The scientific committees of Fondazione Italiana Linfomi (FIL) and Società Italiana della Riproduzione Umana (SIRU) have revised and approved this consensus paper.
The following outcomes define female infertility in the selected papers: a) Primary ovarian insufficiency (POI) defined as depletion or dysfunction of ovarian follicles with cessation of menses before age 40 years (previously been referred to as premature menopause or primary ovarian failure) (23); POI may comprise acute ovarian failure or premature menopause. Acute ovarian failure (AOF) is defined as the immediate loss of ovarian function after chemotherapy or radiation therapy, which may be transient, or as the permanent loss of ovarian function within 5 years of cancer diagnosis (24). Premature menopause (PM) is defined as the retention of ovarian function for at least five years following cancer diagnosis and the occurrence of non-surgical menopause before age 40 (25). b) Decreased ovarian reserve defined as the reduction in oocyte quantity (oocyte number) by measurements of hormone levels of AMH, FSH, E2 and by transvaginal ultrasonographic measure of the sum of the number of antral follicles (ACF) in both ovaries, and by a reduced response to ovarian stimulation compared with women of comparable age (26, 27) c) Infertility defined as the failure to achieve a successful pregnancy after ≥12 months of regular, unprotected sexual intercourseor due to an impairment of a person’s capacity to reproduce either as an individual or with her/his partner (28).
From the literature review a total of 78 questions or sentences, divided into the 6 areas of interest, were identified. The 1st topic “To which categories of patients is this document addressed?” consisted of 13 statements; the 2nd topic “Pre-therapy counseling” of 2 statements; the 3rd topic “Optimal medical treatment to be used in addition to or as the only method of preservation during chemotherapy treatment for female patients” of 3 statements; the 4th topic “The follow-up period: indications on fertility tests to be carried out in the period following chemotherapy (1-5 years and > 5 years from disease remission)” of 35 statements; the 5th topic “How long after the end of cancer treatments can conception be considered safe?” of 3 sentences; the 6th topic “Assessment and treatment of the disorders of the sexual sphere after anti-tumor therapy” of 3 sentences The complete list of questions of Delphi round 1 is available in Supplementary 1.
In the first round of Delphi, conduced for the 78 statements, an agreement of ≥80% was obtained for 65 questions (83.3%), and a full agreement (100%) for 46 (58.9%) sentences. By applying the Gwet’s AC, k was: Section 1: 0,934 (Very good); Section 2: 0,958 (Very good); Section 3: 0,863 (Very good); Section 4: 0,649 (Good); Section 5: 0,936 (Very good); Section 6 raw agreement 100%. Data are summarized in Table 2.
For Section 4, the statements for which a ≥80% agreement had not been reached were rephrased into 13 additional sentences that made up round 2 of Delphi. At this point a full concordance was reached, with a 100% agreement in 94.4% of cases (Supplementary 2).
The following paragraphs concern the shared statements and the supporting literature. Main statements for the management of fertility preservation in young patients with HL or NHL from diagnosis through survivorship are summarized in Table 3.

Table 3 Statements of practical help in the management of fertility preservation in young patients with HL or NHL of childbearing age from diagnosis through survivorship.
The Panel intended to focus the present document on patients diagnosed with NHL and HL, aged 18- 40 years for females and > 18 years for male patients. Specifically, the following histotypes of NHL were considered: Diffuse large B-cell lymphoma (DLBCL), primary mediastinal diffuse B-cell lymphoma (PM-DLCL), Follicular lymphoma (FL), Peripheral T-cell lymphoma, Mantle Cell Lymphoma (MCL), and Burkitt lymphoma (BL). Both classical and nodular lymphocyte-predominant HL were included.
It is important to note that the majority of studies available on risk of gonadotoxicity after lymphoma treatment are retrospective and related to small patient population, moreover they use different outcomes, in particular for female patients, and different periods of follow-up after the end of treatment.
Chemotherapy-induced gonadotoxicity is influenced by the type of agent (e.g. alkylating agents), the dose intensity, and in women also by age at diagnosis. We can distinguish regimens with a high risk of infertility (≥ 80%), an intermediate risk (40-60%), a low risk (<20%), and of unknown risk (15, 16, 29). Regardless of the risk associated with the specific drug or regimen, patients should receive fertility counseling for the choice of the optimal FP strategy before starting anti-cancer treatment. This concept should be underlined because, especially for diseases with unfavorable prognostic factors at the onset, it could be necessary to initiate a second line therapy and ASCT in the case of an inadequate response.
This document is also addressed to patients who have completed the treatment courses for lymphoma, in order to guide the monitoring of the reproductive sphere.
R-CHOP chemotherapy is considered the gold standard treatment for the majority of patients diagnosed with DLBCL, the most frequent histotype among NHL. R-CHOP given for no more than 6 cycles in females younger than 40 years is associated with a low risk of gonadal dysfunction (<20%) (30, 31). The rate of POI and amenorrhea due to R-CHOP could reach 40-60% (intermediate risk) in female patients treated over 35 years of age (32). The majority of male patients treated with 4-6 cycles of CHOP/R-CHOP will recover spermatogenesis within 2 years from end of treatment, and permanent azoospermia has been documented in about 10% of men (33).
An intermediate risk of infertility (40-60%) is reported also after the intensified regimens used for the treatment of specific histotypes of aggressive NHL: DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) and Hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone, high-doses methotrexate and cytarabine) (34, 35). In these studies, amenorrhea had been the outcome.
High-dose conditioning therapies and ASCT represents a salvage strategy for eligible NHL and a first-line consolidation for some NHL histotypes. It is known that ASCT is associated with a high risk of infertility both in males and females. High-dose chemotherapy appears to be incompatible with a full recovery of spermatogenesis and causes POI and amenorrhea in more than 70% of female patients (10, 36). The most frequent conditioning regimen associated with infertility risk is BEAM (BCNU/carmustine, etoposide, cytarabine, melphalan).
In the setting of both aggressive and indolent NHL, the immunomodulatory agent lenalidomide is used within various lines of therapy. A pregnancy prevention program is routinely applied for this drug, considering its teratogenic risk (37).
In some settings of indolent NHL, patients could be started with anti-CD20 monoclonal antibody monotherapy (rituximab or obinutuzumab). Combining the experiences of rheumatic disorders and lymphomas, data are in favor of avoiding pregnancies within the 12 months after the last administration of rituximab, both for male and female patients (38, 39). There is no evidence on the gonadotoxic potential of obinutuzumab monotherapy in the recorded clinical trials, but it is reasonable to apply the same indications as for rituximab. There is no data about the infertility risk of idelalisib, the PI3K inhibitor approved for relapsed/refractory FL, as well as of the targeted drugs polatuzumab vedotin, the BCL-2 inhibitor venetoclax, and the Bruton’s tyrosine kinase inhibitors ibrutinib, zanubrutinib, acalabrutinib and pirtobrutinib (40).
Regarding the use of CAR-T for the treatment of different histotypes of relapsed or refractory NHL, there is not a homogeneous approach regarding counseling for PF (41).
Poli-chemotherapy ABVD (adriamycin, bleomycin, vinblastine, dacarbazine), the regimen mainly used in the front-line treatment of HL, has a variable risk of infertility according to patient gender and age at administration. ABVD administered to male patients at any age and to female patients treated under the age of 35 years presents a low risk of infertility (< 20%), irrespective of the number of courses (42, 43). Available studies measure the restoration of menses but also the rate of live births (44). It should be emphasized that, when administered to female patients aged over 35 years, the risk of infertility increased and could reach 20-40% (42, 44,–45). In fact, ovarian function reserve measured by recovery of AMH one year after the end of 6 cycles of ABVD/AVD was significantly reduced compared with females younger than 35 years (46).
The intensified dose regimen escalated BEACOPP, which can be employed for the treatment of advanced stage or unfavorable risk classic HL, is generally associated with a high risk of infertility (> 70%) both in men and in women (47–50). The more cycles administered, the higher the risk of oligospermia/azoospermia, amenorrhea and POI (50, 51).
Some patients diagnosed with HL could benefit from treatment with targeted or innovative anti-tumor drugs (e.g. brentuximab vedotin, nivolumab, pembrolizumab). Even in the absence of clinical data, it is suggested to avoid conception for at least 6 months after brentuximab vedotin discontinuation both for male and female patients (40, 52). No clinical data are available on the gonadotoxic profile of the anti-programmed cell death 1 (PD-1) immune checkpoint inhibitor nivolumab and pembrolizumab, considering that the majority of patients who have access to these drugs are heavily pre-treated (53, 54).
Radiotherapy on subdiaphragmatic and pelvic regions is less and less used at fertile age. In anticipation of this treatment, generally performed as a consolidation after chemotherapy, fertility counseling is advised (17, 18, 32).
For female patients, AMH (Anti-Müllerian Hormone) is the more appropriate hormonal ovarian reserve marker; FSH and LH (Luteinizing Hormone) may be additionally useful only if performed on the 3rd-5th day of the menstrual cycle. The usefulness of these tests is intended as a baseline value for the post-treatment ovarian reserve follow-up; if available, they can also be useful during the onco-fertility counseling together with other clinical information (e.g. age, previous obstetric history, AFC/Antral Follicle Count, proposed treatment). AMH, together with AFC, is also recommended for prediction of response to ovarian stimulation in case of oocyte cryopreservation procedure (55, 56). FP procedures should not be delayed if the hormonal balance exams are not yet available.
Infective blood exams to be performed before gamete/tissue cryopreservation are: anti-HCV Ab, HBsAg, anti-HBcAb, anti-HIV and VDRL. They are optional for EU law, but mandatory in Italy (57).
Pelvic ultrasound examination with AFC performed by a gynecologic expert in Reproductive Medicine is recommended before/at the time of fertility counseling; this examination could be performed at any time of the menstrual cycle, but better if performed in the early follicular menstrual phase (58). The aim of this ultrasonographic evaluation is to exclude gynecological co-morbidity (such as ovarian cysts, endometriosis) and to assess the ovarian reserve.
For male patients no specialist exams are requested before cryopreservation. Counseling all males about the reproductive risks of cancer treatment and availability of FP options prior to initiation of cancer therapy and consideration of referral to a reproductive urologist is recommended. Although not mandatory, the assessment of hormonal level measurement (FSH, LH, inhibin B) at the time of semen cryopreservation in order to monitor the spermatogenesis restoration has also been suggested (59).
Oocyte cryopreservation is a well-established FP technique that should be proposed to patients after personalized onco-fertility counseling, as is the possibility to delay treatment of 10-14 days, required to induce the multiple follicular growth. Ovarian stimulation and oocyte cryopreservation should be performed before starting chemotherapy (55, 56, 59–62). Some clinical conditions do not allow the patient to postpone the start of chemotherapy for 10-14 days and are characterized by therapeutic urgency: symptomatic and rapidly progressive disease, bulky mediastinal adenopathies at risk of superior vena cava syndrome, severe thrombocytopenia, disseminated intravascular coagulation, active infectious disease. In these cases, a medical approach with gonadotropin-releasing hormone agonist (GnRHa) following the FP counseling can be considered.
Ovarian tissue cryopreservation (OTC) has recently been considered a non-experimental technique by ASRM (60), but success and safety data are still limited. OTC should be proposed to patients diagnosed with lymphoma and no evidence of pelvic involvement at diagnosis (9, 55, 56). OTC could be proposed as a unique technique in patients with: therapeutic urgency, when chemotherapy has to be started within 10-14 days, when there is a high/moderate gonadotoxic risk, and if the patient’s clinical conditions are feasible for surgery. There is some literature data about OTC performed after a first-line low-gonadotoxic treatment exposure suggesting that it could be proposed in this situation after accurate counseling (55–62). OTC can be combined to both oocyte cryopreservation and ovarian transposition. In these cases, OTC should be performed immediately before starting ovarian stimulation and at the same time as ovarian transposition (9, 55–62).
To improve the safety of ovarian tissue transplantation (OTT) for patients in complete and prolonged survival after lymphoma, the ovarian samples have to be analyzed in order to exclude the presence of neoplastic cells by using molecular and histological analyses prior to graft, especially for aggressive NHL histotypes. Thus, an individual multidisciplinary evaluation of each case is required (55–62). The ovarian cortical strips may be grafted onto orthotopic sites, such as the atrophic ovary or pelvic peritoneum, close to infundibulopelvic ligaments or ovarian fossa, allowing the recovery of ovarian function and spontaneous pregnancy, or in heterotopic sites, such as the subcutaneous space of the forearm or abdominal wall, allowing recovery of endocrine function. So far, no recurrences due to OTT have been reported. Patients requiring pelvic radiation may also benefit from transposition of the ovaries to sites away from maximal radiation exposure (9, 55, 56, 62). Ovarian transposition can be performed at the same time as OTC.
Post-pubertal males should be offered sperm cryopreservation as this is the standard fertility-preservation method (62). Semen collection should be performed prior to the administration of gonadotoxic therapies such as chemotherapy or radiation therapy. It is also important to recognize that men with cancer may have underlying impairment in semen parameters prior to the administration of any oncologic therapy (50). Several factors associated with cancer can negatively impact male reproductive potential favoring azoospermia, including disruption of the normal hypothalamic-pituitary-gonadal axis and injury to the germinal epithelium as a result ofthe cytotoxic immune response to cancer, fever, and malnutrition (9, 33). This issue has been documented in the majority of patients with systemic symptoms of lymphoma (fever, night sweats, weight loss). For young men or for men who are unable to ejaculate, the following therapeutic options can be considered to obtain ejaculated sperm for cryopreservation: use of phosphodiesterase type 5 (PDE-5) inhibitors, vibratory stimulation, electroejaculation, and retrograde ejaculation.
Surgical sperm extraction by testicular biopsy (TESE) or microsurgical TESE (Micro-TESE) is an alternative strategy for males who cannot ejaculate via the aforementioned techniques, or who have azoospermia or insufficient sperm in the ejaculate (63). Testicular tissue cryopreservation is currently the only possibility to preserve fertility potential in children because sperm is not produced until puberty (64). Nonetheless, this technique remains experimental. Other techniques, such as in vitro culture or autologous transplantation of testicular tissue or precursor cells, are still being investigated, and in the future could be alternative strategies for FP. During the counseling, it is important to explain to the patients that the fertility potential of cryopreserved semen as well as semen from testicular biopsy decreases after cryopreservation (42).
The correct timing for onco-fertility counseling is as soon as possible, ideally at time of diagnosis, in order to increase the patient’s awareness and to allow optimization of timing to apply FP techniques (9, 61, 62). An urgent referral pathway needs to be established between the Hematological and The Reproductive Centers with the aim to offer onco-fertility counseling within 24-48 hours (55–62).
Counseling with an expert in Reproductive Medicine should be offered to all patients who desire to know the implications of the disease and its therapy on their reproductive potential; even when application of FP techniques is not possible or the patient is not interested in future childbearing, knowledge about gonadotoxic risk, future implications on fertility, fertility treatments in case of future infertility and other childbearing and parenting options (e.g./adoptions, oocyte/semen donation) could be of interest (55–62).
In order to optimize treatment and time, blood exams (see Q2) such as AMH and infective blood exams should be performed as soon as possible. These exams could be requested by the onco-hematologist prior to the onco-fertility counseling.
Ovarian stimulation should be started as soon as possible. If necessary, a random start protocol for ovarian stimulation is possible in order to minimize delay to oncologic treatments (55, 56, 61, 65). A prompt start to ovarian stimulation could also allow a double stimulation in case of poor ovarian reserve and the possibility of postponing the start of oncologic treatment (61, 62).
Laparoscopy for OT harvesting (OTC) should be performed as soon as clinical assessment of suitability for surgery is available. Usually from the moment of the decision, the organization of the procedure will take 24-48 hours.
Hematologic Centers are advised to establish a multidisciplinary FP team with the nearest Fertility Center (and vice versa) in order to improve referral pathways, reduce time loss and optimize procedures; this will allow an expanded access to FP options (9, 55–62).
The available evidence on the efficacy and safety of administering gonadotropin-releasing hormone agonist (GnRHa) during chemotherapy as an approach for ovarian protection in premenopausal oncologic patients with pathologies other than breast cancer is still limited. In fact, only four small randomized trials have involved women with hematologic malignancies (66). Among the several available meta-analyses, the largest one that groups the results from 3 randomized trials on hemato-oncological diseases included 109 patients diagnosed with HL and NHL (67). The median age for patients receiving chemotherapy regimens with different gonadotoxicity ranging from low (e.g. ABVD) to high (e.g. conditioning regimens for hematopoietic stem cell transplantation) was around 25 years. No significant difference in POI rates was observed between HL patients who received chemotherapy with or without concomitant GnRHa (18.9% vs. 32.1%; OR 0.70, 95% CI 0.20- 2.47), as well as for post-treatment pregnancies (17 vs. 18) (67). In one trial, which was included in the meta-analysis, AMH serum levels were assessed before and after treatment, with significantly higher levels of AMH in patients receiving GnRHa during chemotherapy at one year follow-up (68). Considering the available data and the good tolerability profile, the use of GnRH analogues during chemotherapy could be recommended as an adjunct and not as an alternative to in vitro fertilization and cryopreservation of embryos, oocytes and ovarian tissue for FP (69, 70). GnRHa should be administered every four weeks, starting preferably at least one week before the initiation of the first chemotherapy cycle and should be continued until after the administration of the last chemotherapy cycle (71). All patients who are candidates to receive GnRHa should be informed of the uncertainties regarding the potential role of GnRHa and the association with adverse events like hot flushes, bone and muscle pains, mood changes, and vaginal dryness (69, 70). GnRHa should not be considered as an alternative option to FP with cryopreservation techniques except for women for whom the latter is contraindicated due to treatment start delay or safety issues (55, 56).
Although it has been shown not to be effective as a FP method, hormonal estroprogestinic contraceptives are adopted by 12% of Italian hematologic centers affiliated with the FIL in conjunction with chemotherapy. In the same Italian surveys, the use of GnRHa during chemotherapy was used by 72.5% of the FIL hematologic centers (19).
In order to prevent chemotherapy-induced POI and early menopause-related symptoms, the administration of GnRHa during chemotherapy is the only medical approach available for clinical use (55, 56, 71, 72). The mechanisms underlying the protective role of ovarian suppression with GnRHa during gonadotoxic treatments have not been fully understood (73). However, given the fast-acting effects and the suppressive ovarian function of GnRHa, these drugs may be able to protect the ovarian reserve from toxicity in adolescent girls and pre-menopausal women with ages between 15 and 45 years undergoing chemotherapeutic treatments (74).
Evaluation of ovarian or testicular function after the end of cancer treatment and during the follow-up is not yet routinely performed in Italian hematological and oncological centers (19). While cardiac, pulmonary, and thyroid function as well as the occurrence of second cancers are closely monitored after lymphoma treatment, and several cancer survivorship guidelines recommend appropriate tests and timing of evaluation according to the known toxicities of the administered treatment, gonadal function is not part of these assessments, even though infertility, sexual hormone deficiency and sexual dysfunction have a significant impact on the quality of life of lymphoma survivors (75–78).
The assessment of gonadal function after lymphoma treatment in females includes history of menses, hormonal evaluation of FSH, estradiol and AMH, and transvaginal ovarian ultrasound examination with AFC (62). Asking the patient about the presence or absence of spontaneous regular menses is the first and simplest method to evaluate ongoing ovarian activity. Transient amenorrhea, in particular if lasting longer than 12 months from the end of chemotherapy, has been demonstrated to be a risk factor for subsequent infertility (62, 79). However, regular menses cycles after chemotherapy have been reported also in infertile females, those who were not able to remain pregnant, due to reduced ovarian reserve, and those who developed POI (79).
FSH cut-offs varied among the studies due to the use of different FSH assays and reagents (80). A value of FSH serum concentration greater than 25 UI/L, alongside low estradiol, is the most established diagnostic test for POI (46, 81). Although simple to perform, it is not useful in the diagnosis of poor ovarian reserve until high thresholds are used. In fact, normal FSH levels have been reported in patients with compromised ovarian reserve precluding FSH levels as a good predictive marker of the chance of conception (82, 83). Furthermore, large inter-cycle variations in basal FSH levels have been reported, and although the appropriate timing of FSH measurement should be on day 3 of the menstrual cycle, this timing may be difficult in females with irregular periods of menses after chemotherapy (84). Measurement of cycle day 3 estradiol levels as well as FSH/LH ratio and inhibin-B levels seems not to be useful in the prediction of ovarian reserve (85).
In contrast, serum levels of AMH, which is produced by granulosa cells of the recruited growing follicles in the ovary, were found to be a reliable marker of ovarian reserve and predictive for POI (56, 86, 87). In patients treated for Hodgkin lymphoma, AMH serum levels decreased during chemotherapy, more profoundly after BEACOPP than after ABVD, with a reciprocal increase of FSH (79, 81, 88). After the end of chemotherapy, AMH recovery to pre-treatment baseline values was observed within one year in ABVD-treated patients, whereas in BEACOPP-treated patients, AMH levels remained significantly lower than at baseline (89, 90). Study results suggest that pre-treatment AMH may predict mid- and long-term ovarian function. In female survivors with restored menstrual cycles, AMH recovery may be highly variable. Pregnancy can occur even in the presence of low post-chemotherapy AMH levels, below 1 ng/mL, documenting that AMH levels do not predict short-term fertility, unless associated with amenorrhea (91, 92).
Transvaginal ultrasound analysis is very useful to determine ovarian volume and, most importantly, AFC has been proposed as a surrogate marker for ovarian reserve evaluation (42). The performance of AFC only for predicting failure to achieve pregnancy is poor, mainly because AFC determines the number of oocytes but not the oocyte quality, on which pregnancy outcome also depends (91). Nowadays, no available test may help predicting pregnancy or live birth reliably in women undergoing anticancer therapy. The combination of AMH levels and AFC may be more accurate than a single exam in predicting ovarian reserve and menstrual function, as stated by Loverro G et al. in a small cohort of 29 female patients treated for HL (sensitivity of 83% and specificity of 88%), but this issue deserve further research (92).
Recovery of ovarian function can occur from several months to years after treatment completion and therefore there is no agreement between experts on the optimal time interval between end of lymphoma treatment and first gonadal function assessment, frequency and duration of surveillance. Gonadal function should be tested by measurement of AMH and AFC at the request of the patient or when pregnancy is desired. AMH below 0.5-1.1 ng/ml and AFC 5–7 follicles or less are suggestive for reduced ovarian function (23, 80, 93). The tests should be performed after an interval of at least one year following chemotherapy completion (94–97). In relation to the parameters found during the follow-up evaluation, an Assisted Reproductive Technology (ART) program could be suggested to the patient and the clinical decision would concern the proposal of using autologous or donor gametes in order to achieve maternity (98).
Testicular function should be evaluated at the request of the patient or when paternity is desired. Despite the 75-day duration of the spermatogenesis cycle, it is reasonable to suggest that the first assessment after potential gonadotoxic treatment should be performed not prior to 12 months from the end of therapy. This derives from the results of most of the studies which qualitatively and quantitatively evaluated spermatogenesis starting from 12 months after chemotherapy treatment (81, 99).
The simplest and most reliable method of assessing the effect of treatment on spermatogenesis is semen analysis, collected after 2–7 days of abstinence, recording sperm count, morphology, vitality and motility (7, 100). According to WHO reference limits, the normal sperm concentration is ≥ 15 x 10 (6) spermatozoa/mL, normal forms should account for at least 4%, and normal total motility (progressive and non-progressive) should be 40% (101).
Evaluation of gonadal function in male patients may include a physical examination and hormonal evaluation. Physical examination will be performed by the measurement of testicular size using scrotal ultrasonography or Prader orchidometer, which may reveal a decrease in testicular volume due to impaired sperm production and loss of tubular space (7, 102). Among hormonal evaluation, serum FSH, inhibin B and inhibin B to FSH ratio may be used as surrogate markers of impaired spermatogenesis in patients who decline or are not able to perform semen analysis. High levels of pituitary FSH indicate testicular dysfunction (79, 103). A cut-off level of 10.4 IU/L has been identified to predict azoospermia with specificity 81% (95% CI 76%-86%) and sensitivity 83% (95% CI 76%-89%) in childhood cancer survivors (104, 105). However a confirmatory semen analysis is still required for the diagnosis of infertility (74).
Inhibin B is produced by Sertoli cells andis responsible for the negative feedback regulation of FSH secretion in men (106). Decreased or even undetectable serum levels of Inhibin B and concurrent high levels of FSH have been reported in patients with disrupted spermatogenesis (107–110). Jensen et al. stated that the predictive power in detecting oligospermia, defined as semen concentration below 20 mill/mL, among men with a serum inhibin B below 80 pg/L and a serum FSH above 10 IU/L, was 100% (109). More recently a cutoff value of Inhibin B of 97.1 pg/ml between normospermia and oligospermia, with sensitivity and specificity of 79.2% and 72.7%, respectively, has been reported (111). Inhibin B to FSH ratio levels < 23.5 ng/U is associated to impaired fertility (79). However controversial study results have also been reported showing a poor specificity of FSH and inhibin B in determining spermatogenic capacity in adult male childhood cancer survivors (112). Therefore, although FSH, inhibin B and inhibin B to FSH ratio to some degree indicate spermatogenic capacity, direct evaluation by semen samples, which can be repeated during the follow-up, should be recommended for all patients interested in their fertility potential (107).
Serum testosterone (TST) and luteinising hormone (LH) level measurement are useful to assess testicular Leydig cell function. They remain within the normal range after different chemotherapy regimens, such as escalated BEACOPP, supporting the hypothesis that Leydig cells are more resistant to cytotoxic chemotherapy and are not associated with impaired spermatogenesis (110). TST serum levels below normal ranges indicate hypogonadism which is responsible for sexual dysfunction, fatigue and increased cardiovascular disease risk (113). Therefore, in the event of symptoms of hypogonadism, it is recommended to measure TST levels in order to guide hormonal replacement treatment and ameliorate quality of life.
Based on available study results, no recommendation can be given on the optimal time interval between completion of lymphoma treatment and first gonadal function assessment tests, frequency and duration of surveillance (100). Based on expert opinion, we suggest performing gonadal evaluation during the follow-up at the request of the patient or when paternity is desired, starting 12 months after treatment completion, and repeating abnormal tests annually thereafter.
For female lymphoma survivors no specific data are available in literature (6). Although there are some data from other malignancies suggesting a “safety window” of 6 months after chemotherapy (114), the current indications for female lymphoma survivors are to avoid pregnancy in the period of greatest risk of lymphoma recurrence, corresponding to the first two to three years, calculated from the end of treatment (16). An interval of at least 1 year after the end of chemotherapy is also recommended in order to reduce pregnancy complications, which seem to be higher in this population (55, 56, 62). However, preconception counseling and timing should be personalized in relation to the prognosis of the disease and the patient’s age and desire for parenthood.
For male lymphoma survivors, data from longitudinal, prospective cohort studies are awaited to provide further evidence on the potential risk of congenital abnormalities. The only guidelines currently available, issued by the European Society for Medical Oncology (ESMO), advocate the deferral of childbearing for at least 12 months after cancer therapy, as a Grade C recommendation based on level IV evidence (65, 115, 116).
Patients should be aware that fertility may be resumed a few months after the end of antiblastic treatment, especially for males. Considering the reasons set out in this paragraph, female patients should be informed to adopt efficacious contraception for the first two to three years of follow-up. Male patients should also be informed to employ efficacious contraception for the first 12 months since last anti-cancer treatment.
Cancer treatments have been shown to induce several effects on psychological and interpersonal conditions that can negatively impact sexual function and satisfaction. Data on the likelihood of specific sexual disorders seem to be related to pre-diagnosis function, patient response, and support from the treatment team as well as specific treatments employed and efforts to mitigate potential problems.
For hematologic cancers, sexual function might not be initially crucial for this group of patients as the malignancy is not directly linked to a sexual organ, although high-dose chemotherapy, total-body irradiation and stem cell transplantation can be significantly detrimental to patients’ body image, intimacy, and sexuality (117–120). Studies on sexual function in onco-hematologic patients contrast in their methodological approach, and the literature overall remains limited, although the few studies that document the existence of sexual disorders consider this topic of great interest.
In a study of NHL survivors at least 25% of the sample reported sexual problems (121), as compared to HL survivors with a prevalence range between 12% and 62.5% (122–124). Research on interventions for sexual problems following treatment for hematologic cancers is largely absent, and the clinical management generally follows the same recommendations made to women following breast and gynecologic cancers and to men following prostate cancer. True barriers still exist for patients, providers, and institutions to discuss and address sexual dysfunction during and after cancer treatment. Two approaches are discussed to address the potential barriers of time constraints, providers’ feelings of embarrassment and fear, and assumptions about patients’ level of interest in sexual health concerns or potential reactions to sexual health discussions. These approaches include the implementation of a paper-and-pencil screening of symptoms, a self-report survey during routine clinic visits, and a reference guide to starting the conversation about sexual health with patients and survivors of cancer based on established frameworks for discussing this topic (76). Paper-and-pencil screening tools are available to briefly and preliminarily assess sexual function in patients with cancer and help determine which patients may require further specialized assessment and intervention.
In recent years many efforts have been made to expand the tools available for assessing sexual problems in patients with cancer to include measures other than specific organ function, and research studies on these tools continue to be underway. Two instruments have been established as the most widely used and easily accessible to providers to measure sexual function in research. These instruments are the Female Sexual Function Index and International Index of Erectile Function.
The FSFI is a 19-item self-report measure originally developed to assess female sexual function in women of any age, including perimenopause and post-menopause, in the general population. The FSFI takes approximately 15-20 minutes to complete and assesses function “over the past 4 weeks” in the following specific domains relevant to female sexuality: desire, arousal, lubrication, orgasm, satisfaction, and pain (125, 126). Although the FSFI only takes a few minutes to complete, a recently developed shorter version, not yet validated with oncological patients, is the FSFI 6-item version which takes approximately 3 minutes to complete (127). For the FSFI-19, a maximum score of 36 is foreseen. Different cut-offs have been identified according to the cohorts in which the test was used; a score lower than 26.55 would seem to indicate subjectswith sexual dysfunction, who could benefit from a psychological approach and a specialist gynecological evaluation (128, 129).
The IIEF is a 15-item self-report measure developed to assess erectile function in men in the general population. The IIEF measures function “over the past 4 weeks” in the following domains relevant to male sexuality: erectile function, orgasm, desire, intercourse satisfaction, and overall satisfaction. However, shorter versions of the IIEF are also available. Of these shorter versions, the IIEF 5-item version (IIEF-5 also known as the Sexual Health Inventory for Men/HIM374) has also been validated and used in patients with and survivors of cancer (127, 130). Regarding the IIEF questionnaire, a score below 26 is indicative of male sexual dysfunction (1-10: severe erectile dysfunction; 11-16: moderate erectile dysfunction;17-25: mild erectile dysfunction) (127). Patients with this score may be referred for psychological and urological consultation.
FP and parenthood-planning remain among the most important quality of life issues for young female and male patients with NHL and HL. A large number of publications underscore the relevance of these topics and support the recommendation to offer fertility counseling before starting anti-neoplastic treatment and in the follow-up of these patients, including assessment of sexual function (55, 61, 62, 131).
Statements were elaborated according to a literature search and administered to a panel of experts belonging to Fondazione Italiana Linfomi (FIL) and Società Italiana della Riproduzione Umana (SIRU), using the Delphi methodology to reach a consensus.
Results confirm that a multidisciplinary approach including onco-hematology and human reproduction specialists is needed to offer patients and treating physicians updated guidelines to address these issues. Moreover, controversies still remain on specific topics such as the optimal age to offer ovarian and oocyte cryopreservation to female patients and the efficacy of GnRH analogues to prevent POI. Panelists underlined that sexual issues are rarely addressed during treatment and in the follow-up of patients affected by lymphomas, and that more awareness and adequate assessment is needed.
The shared statements contained in this paper should offer practical guidance to help onco-hematologists, human reproduction specialists and their patients to address fertility and sexual issues during the disease trajectory and obtain a thoroughly informed decision.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization, CM, SV, ES. Writing— original draft preparation, CM, SV, ES, GDP, SP, FP. Validation, AG, GG, FM, GC, LM, FP. Supervision, FP. All authors have read and agreed to the published version of the manuscript.
Supported by the Ministry of Health, Italian Government, R.C. funds 2022, to the IRCCS IstitutoTumori “Giovanni Paolo II,” Bari-Italy.
The authors thank AIL (Associazione Italiana contro leucemie, linfomi e myeloma) for the support to the “Long-term Survivor Committee” of FIL.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1252433/full#supplementary-material
1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer (2020). Available at: https://gco.iarc.fr/ (Accessed 17 Apr 2023).
2. . Available at: https://ecis.jrc.ec.europa.eu/ (Accessed 17 Apr 2023).
3. Connors JM, Cozen W, Steidl C, Carbone A, Hoppe RT, Flechtner HH, et al. Hodgkin lymphoma. Nat Rev Dis Primers. (2020) 6(1):61. doi: 10.1038/s41572-020-0189-6
4. Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019update on diagnosis, risk stratification, and treatment. Am JHematol (2019) 94:604–16. doi: 10.1002/ajh.25460
5. Minoia C, Bari A, Nassi L, Banzi R, Gerardi C, Lenti V, et al. Management of lymphomasurvivorpatients in Italy: an evaluation by Fondazione Italiana Linfomi. Tumori (2021) 107(1):91–4. doi: 10.1177/0300891620905649
6. Ciavarella S, Minoia C, Quinto AM, Oliva S, Carbonara S, Cormio C, et al. Improving provision of care for long-term survivors of lymphoma. ClinLymphomaMyelomaLeuk (2017) 17(12):e1–9. doi: 10.1016/j.clml.2017.08.097
7. Wallace WH, Anderson RA. Irvine DS.Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol (2005) 6(4):209–18. doi: 10.1016/S1470-2045(05)70092-9
8. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol (2006) 24(18):2917–31. doi: 10.1200/JCO.2006.06.5888
9. Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol (2018) 36(19):1994–2001. doi: 10.1200/JCO.2018.78.1914
10. Gerstl B, Sullivan E, Koch J, Wand H, Ives A, Mitchell R, et al. Reproductive outcomes following a stem cell transplant for a haematological Malignancy in female cancer survivors: a systematic review and meta-analysis. Support Care Cancer. (2019) 27(12):4451–60. doi: 10.1007/s00520-019-05020-8
11. Marmor D, Duyck F. Male reproductive potential after MOPP therapy for Hodgkin's disease: a long-term survey. Andrologia (1995) 27(2):99–106. doi: 10.1111/j.1439-0272.1995.tb01078.x
12. Andrieu JM, Ochoa-Molina ME. Menstrual cycle, pregnancies and offspring before and after MOPP therapy for Hodgkin's disease. Cancer (1983) 52(3):435–8. doi: 10.1002/1097-0142(19830801)52:3<435::aid-cncr2820520308>3.0.co;2-1
13. Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating aget exposure: a report from the childhood cancer survivor study. Pediatr Blood Cancer (2014) 61(1):53–67. doi: 10.1002/pbc.24679
14. Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol (2016) 12(20):2333–44. doi: 10.2217/fon-2016-0176
15. Silvestris E, Cormio G, Skrypets T, Dellino M, Paradiso AV, Guarini A, et al. Novelaspects on gonadotoxicity and fertility preservation in lymphoproliferative neoplasms. Crit Rev Oncol Hematol (2020) 151:102981. doi: 10.1016/j.critrevonc.2020.102981
16. Viviani S, Caccavari V, Gerardi C, Ramadan S, Allocati E, Minoia C, et al. Male and female fertility: prevention and monitoring hodgkin' Lymphoma and diffuse large B-cell lymphoma adult survivors. A systematic review by the fondazione italianaLinfomi. Cancers (Basel). (2021) 13(12):2881. doi: 10.3390/cancers13122881
17. Wallace WHB, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod (2003) 18(1):117–21. doi: 10.1093/humrep/deg016
18. Kenney LB, Antral Z, Ginsberg JP, et al. Improving male reproductive health after childhood, adolescent, and young adult cancer: progress and future directions for survivorship research. J Clin Oncol (2018) 36(21):2160–8. doi: 10.1200/JCO.2017.76.3839
19. Viviani S, Dellino M, Ramadan S, Peracchio C, Marcheselli L, Minoia C, et al. Fertility preservation strategies for patients with lymphoma: a real-world practice survey among Fondazione ItalianaLinfomi centers. Tumori (2021) 25:3008916211040556. doi: 10.1177/03008916211040556
20. Available at: http://oncofertility.northwestern.edu/sites/oncofertility.northwestern.edu/files/ced_calculator.xisx.
21. McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm (2016) 38:655–62. doi: 10.1007/s11096-016-0257-x
22. Niederberger M, Spranger J. Delphi technique in health sciences: A map. Front Public Health (2020) 22:457. doi: 10.3389/fpubh.2020.00457
23. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med (2009) 360(6):606–14. doi: 10.1056/NEJMcp0808697
24. Clark RA, Mostoufi-Moab S, Yasui Y, Vu NK, Sklar CA, Motan T, et al. Predicting acute ovarian failure in female survivors of childhood cancer: a cohort study in the Childhood Cancer Survivor Study (CCSS) and the St Jude Lifetime Cohort (SJLIFE). Lancet Oncol (2020) 21(3):436–45. doi: 10.1016/S1470-2045(19)30818-6
25. Levine JM, Whitton JA, Ginsberg JP, Green DM, Leisenring WM, Stovall M, et al. Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer (2018) 124(5):1044–52. doi: 10.1002/cncr.31121
26. Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet (2018) 35(1):17–23. doi: 10.1007/s10815-017-1058-4
27. Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. FertilSteril (2020) 114(6):1151–7. doi: 10.1016/j.fertnstert.2020.09.134
28. Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. (2020) 113(3):533–5. doi: 10.1016/j.fertnstert.2019.11.025
29. Schüring AN, Fehm T, Behringer K, Goeckenjan M, Wimberger P, Henes M, et al. Practical recommendations for fertility preservation in women by the FertiPROTEKT network. Part I: Indications for fertility preservation. Arch Gynecol Obstet. (2018) 297(1):241–55. doi: 10.1007/s00404-017-4594-3
30. Elis A, Tevet A, Yerushalmi R, Blickstein D, Bairy O, Dann EJ, et al. Fertility status among women treated for aggressive non-Hodgkin's lymphoma. Leuk Lymphoma. (2006) 47(4):623–7. doi: 10.1080/10428190500353877
31. Meissner J, Tichy D, Dietrich S, Schmitt T, Ziepert M, Kuhnt E, et al. Parenthood in long-term survivors after CHOP with or without etoposide treatment for aggressive lymphoma. Br J Haematol (2014) 166(4):612–5. doi: 10.1111/bjh.12877
32. Gini G, Annibali O, Lupasco D, Bocci C, Tomarchio V, Sampaolo M, et al. Gonadal function recovery and fertility in women treated with chemo- and/or radiotherapy for hodgkin's and non-hodgkin lymphoma. Chemotherapy (2019) 64(1):36–41. doi: 10.1159/000499535
33. Pallotti F, Pelloni M, Faja F, Di Chiano S, Di Rocco A, Lenzi A, et al. Semen quality in non-Hodgkin lymphoma survivors: a monocentric retrospective study. Hum Reprod (2021) 36(1):16–25. doi: 10.1093/humrep/deaa266
34. Gharwan H, Lai C, Grant C, Dunleavy K, Steinberg SM, Shovlin M, et al. Female fertility following dose-adjusted EPOCH-R chemotherapy in primary mediastinal B-cell lymphomas. Leuk Lymphoma. (2016) 57(7):1616–24. doi: 10.3109/10428194.2015.1118476
35. Seshadri T, Hourigan MJ, Wolf M, Mollee PN, Seymour JF. The effect of the Hyper-CVAD chemotherapy regimen on fertility and ovarian function. Leuk Res (2006) 30(4):483–5. doi: 10.1016/j.leukres.2005.08.014
36. Blumenfeld Z, Patel B, Leiba R, Zuckerman T. Gonadotropin-releasing hormone agonist may minimize premature ovarian failure in young women undergoing autologous stem cell transplantation. FertilSteril (2012) 98(5):1266–70.e1. doi: 10.1016/j.fertnstert.2012.07.1144
37. Bwire R, Freeman J, Houn F. Managing the teratogenic risk of thalidomide and lenalidomide: an industry perspective. Expert Opin Drug Saf. (2011) 10(1):3–8. doi: 10.1517/14740338.2011.527331
38. Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood (2011) 117(5):1499–506. doi: 10.1182/blood-2010-07-295444
39. Mouyis M, Flint JD, Giles IP. Safety of anti-rheumatic drugs in men trying to conceive: A systematic review and analysis of published evidence. Semin Arthritis Rheumatol (2019) 48(5):911–20. doi: 10.1016/j.semarthrit.2018.07.011
40. Traila A, Dima D, Achimas-Cadariu P, Micu R. Fertility preservation in Hodgkin's lymphoma patients that undergo targeted molecular therapies: an important step forward from the chemotherapy era. Cancer Manag Res (2018) 10:1517–26. doi: 10.2147/CMAR.S154819
41. Ligon JA, Fry A, Maher JY, Foley T, Silbert S, Yates B, et al. Fertility and CAR T-cells: Current practice and future directions. Transplant Cell Ther (2022) 28(9):605.e1–8. doi: 10.1016/j.jtct.2022.06.002
42. Anderson RA, Clatot F, Demeestere I, Lambertini M, Morgan A, Nelson SM, et al. Cancer survivorship: Reproductive health outcomes should be included in standard toxicity assessments. Eur J Cancer. (2021) 144:310–6. doi: 10.1016/j.ejca.2020.11.032
43. van der Kaaij MA, Heutte N, Le Stang N, Raemaekers JM, Simons AH, Carde P, et al. Gonadal function in males after chemotherapy for early-stage Hodgkin's lymphoma treated in four subsequent trials by the European Organisation for Research and Treatment of Cancer: EORTC Lymphoma Group and the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol (2007) 25(19):2825–32. doi: 10.1200/JCO.2006.10.2020
44. Hodgson DC, Pintilie M, Gitterman L, Dewitt B, Buckley CA, Ahmed S, et al. Fertility among female hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol (2007) 25(1):11–5. doi: 10.1002/hon.802
45. Machet A, Poudou C, Tomowiak C, Gastinne T, Gardembas MM, Systchenko T, et al. Hodgkin lymphoma and female fertility: a multicenter study in women treated with ABVD. Blood Adv (2022) 21:bloodadvances.2021005557. doi: 10.1182/bloodadvances.2021005557
46. Anderson RA, Remedios R, Kirkwood AA, Patrick P, Stevens L, Clifton-Hadley L, et al. Determinants of ovarian function after response-adapted therapy in patients with advanced Hodgkin’slymphoma (RATHL): A secondary analysis of a randomised phase 3 trial. Lancet Oncol (2018) 19(10):1328–37. doi: 10.1016/S1470-2045(18)30500-X
47. Behringer K, Breuer K, Reineke T, May M, Nogova L, Klimm B, et al. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol (2005) 23(30):7555–64. doi: 10.1200/JCO.2005.08.138
48. Sieniawski M, Reineke T, Nogova L, Josting A, Pfistner B, Diehl V, et al. Fertility in male patients with advanced Hodgkin lymphoma treated with BEACOPP: a report of the German Hodgkin Study Group (GHSG). Blood (2008) 111(1):71–6. doi: 10.1182/blood-2007-02-073544
49. Sieniawski M, Reineke T, Josting A, Nogova L, Behringer K, Halbsguth T, et al. Assessment of male fertility in patients with Hodgkin's lymphoma treated in the German Hodgkin Study Group (GHSG) clinical trials. Ann Oncol (2008) 19(10):1795–801. doi: 10.1093/annonc/mdn376
50. Paoli D, Rizzo F, Fiore G, Pallotti F, Pulsoni A, Annechini G, et al. Spermatogenesis in Hodgkin's lymphoma patients: a retrospective study of semen quality before and after different chemotherapy regimens. Hum Reprod (2016) 31(2):263–72. doi: 10.1093/humrep/dev310
51. Amin MSA, Brunckhorst O, Scott C, Wrench D, Gleeson M, Kazmi M, et al. ABVD and BEACOPP regimens' effects on fertility in young males with Hodgkin lymphoma. Clin Transl Oncol (2021) 23(6):1067–77. doi: 10.1007/s12094-020-02483-8
52. Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood (2003) 102(4):1458–65. doi: 10.1182/blood-2003-01-0039
53. Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol (2016) 17(9):1283–94. doi: 10.1016/S1470-2045(16)30167-X
54. Ramchandren R, Domingo-Domènech E, Rueda A, Trněný M, Feldman TA, Lee HJ, et al. Nivolumab for newly diagnosed advanced-stage classic hodgkin lymphoma: safety and efficacy in the phase II checkMate 205 study. J Clin Oncol (2019) 37(23):1997–2007. doi: 10.1200/JCO.19.00315
55. ESHRE Guideline Group on Female Fertility Preservation, Anderson RA, Amant F, Braat D, D'Angelo A, Chuva de Sousa Lopes SM, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open (2020) 2020(4):hoaa052. doi: 10.1093/hropen/hoaa052
56. Anderson RA, Cameron D, Clatot F, Demeestere I, Lambertini M, Nelson SM. PeccatoriF.Anti-Mullerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: a systematic review. HumReprod (2022) 24:dmac004. doi: 10.1093/humupd/dmac004
57. Commission of the European Communities. Commission Directive 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Official Journal of the European Union: CELEX. (2006). p. 40. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006L0017.
58. Coelho Neto MA, Ludwin A, Borrell A, Benacerraf B, Dewailly D, da Silva Costa F, et al. Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound Obstet Gynecol. (2018) 51(1):10–20. doi: 10.1002/uog.18945
59. Di Bisceglie C, Bertagna A, Composto ER, Lanfranco F, Baldi M, Motta G, et al. Effects of oncological treatments on semen quality in patients with testicular neoplasia or lymphoproliferative disorders. Asian J Androl. (2013) 15(3):425–9. doi: 10.1038/aja.2012.171
60. Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. FertilSteril (2019) 112(6):1022–33. doi: 10.1016/j.fertnstert.2019.09.013
61. Linee Guida AIOM. (Associazione Italiana Oncologia Medica) Preservazione della fertilità nei pazienti oncologici Edizione (2021). Available at: https://snlg.iss.it/wp-content/uploads/2022/01/LG296_Fertilit%C3%A0_PZ_Oncologici_agg2021.pdf.
62. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines†. Ann Oncol (2020) 31(12):1664–78. doi: 10.1016/j.annonc.2020.09.006
63. Gandini L, Lombardo F, Salacone P, Paoli D, Anselmo AP, Culasso F, et al. Testicular cancer and Hodgkin's disease: evaluation of semen quality. Hum Reprod (2003) 18(4):796–801. doi: 10.1093/humrep/deg163
64. Goossens E, Jahnukainen K, Mitchell RT, van Pelt A, Pennings G, Rives N, et al. Fertility preservation in boys: recent developments and new insights †. Hum Reprod Open (2020) 2020(3):hoaa016. doi: 10.1093/hropen/hoaa016
65. Schrader M, Müller M, Sofikitis N, Straub B, Krause H, Miller K. "Onco-tese": testicular sperm extraction in azoospermic cancer patients before chemotherapy-new guidelines? Urology (2003) 61(2):421–5. doi: 10.1016/s0090-4295(02)02264-1
66. von Wolff M, Capp E, Jauckus J, Strowitzki T, Germeyer A. FertiPROTEKT study group. Timing of ovarian stimulation in patients prior to gonadotoxic therapy: an analysis of 684 stimulations. Eur J ObstetGynecolReprod Biol (2016) 199:146–9. doi: 10.1016/j.ejogrb.2016.02.006
67. Lambertini M, Moore HCF, Leonard RCF, Loibl S, Munster P, Bruzzone M, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: A systematic review and meta-analysis of individual patient-level data. J Clin Oncol (2018) 36(19):1981–90. doi: 10.1200/JCO.2018.78.0858
68. Senra JC, Roque M, Talim MCT, Reis FM, Tavares RLC. Gonadotropin-releasing hormone agonists for ovarian protection during cancer chemotherapy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2018) 51(1):77–86. doi: 10.1002/uog.18934
69. Demeestere I, Brice P, Peccatori FA, Kentos A, Dupuis J, Zachee P, et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol (2016) 34(22):2568–74. doi: 10.1200/JCO.2015.65.8864
70. Blumenfeld Z, Katz G, Evron A. ‘An ounce of prevention is worth a pound of cure’: the case for and against GnRH-agonist for fertility preservation. Ann Oncol (2014) 25(9):1719–28. doi: 10.1093/annonc/mdu036
71. Blumenfeld Z. Fertility preservation and GnRHa for chemotherapy: debate. Cancer Manag Res (2014) 6:313–5. doi: 10.2147/CMAR.S66600
72. Garrido-Oyarzún MF, Castelo-Branco C. Controversies over the use of GnRH agonists for reduction of chemotherapy-induced gonadotoxicity. Climacteric (2016) 19(6):522–5. doi: 10.1080/13697137.2016.1225713
73. Kitajima Y, Endo T, Nagasawa K, Manase K, Honnma H, Baba T, et al. Hyperstimulation and a gonadotropin-releasing hormone agonist modulate ovarian vascular permeability by altering expression of the tight junction protein claudin-5. Endocrinology (2006) 147(2):694–9. doi: 10.1210/en.2005-0700
74. Dolmans MM, Taylor HS, Rodriguez-Wallberg KA, Blumenfeld Z, Lambertini M, von Wolff M, et al. Utility of gonadotropin-releasing hormone agonists for fertility preservation in women receiving chemotherapy: pros and cons. FertilSteril (2020) 114(4):725–38. doi: 10.1016/j.fertnstert.2020.08.011
75. Chen H, Xiao L, Li J, Cui L, Huang W. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev (2019) 3(3):CD008018. doi: 10.1002/14651858.CD008018.pub3
76. Tevaarwerk A, Denlinger CS, Sanft T, Ansbaugh SM, Armenian S, Baker KS, et al. Survivorship, version 1.2021. J Natl ComprCancNetw. (2021) 19(6):676–85. doi: 10.6004/jnccn.2021.0028
77. Massarotti C, Scaruffi P, Lambertini M, Sozzi F, Remorgida V, Anserini P. Beyond fertility preservation: role of the oncofertilityunit in the reproductive and gynecological follow-up of youngcancer patients. HumReprod (2019) 34(8):1462e9. doi: 10.1093/humrep/dez108
78. Jacobs LA, Pucci DA. Adult survivors of childhood cancer: the medical and psychosocial late effects of cancer treatment and the impact on sexual and reproductive health. J Sex Med (2013) 10 Suppl 1:120–6. doi: 10.1111/jsm.12050
79. Behringer K, Mueller H, Goergen H, Thielen I, Eibl AD, Stumpf V, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol (2013) 31(2):231–9. doi: 10.1200/JCO.2012.44.3721
80. Jain T, Soules MR, Collins JA. Comparison of basal follicle-stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. FertilSteril (2004) 82(1):180–5. doi: 10.1016/j.fertnstert.2003.11.045
81. Behringer K, Thielen I, Mueller H, Goergen H, Eibl AD, Rosenbrock J, et al. Fertility and gonadal function in female survivors after treatment of early unfavorable Hodgkin lymphoma (HL) within the German Hodgkin Study Group HD14 trial. Ann Oncol (2012) 23:1818–25. doi: 10.1093/annonc/mdr575
82. Maheshwari A, Fowler P, Bhattacharya S. Assessment of ovarian reserve—should we perform tests of ovarian reserve routinely? HumReprod (2006) 21(11):2729–35. doi: 10.1093/humrep/del188
83. Wolff EF, Taylor HS. Value of the day 3 follicle-stimulating hormone measurement. FertilSteril (2004) 81:1486–8. doi: 10.1016/j.fertnstert.2003.10.055
84. Bukulmez O, Arici A. Assessment of ovarian reserve. CurrOpinObstetGynecol (2004) 16:231–7. doi: 10.1097/00001703-200406000-00005
85. Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. HumReprod Update (2014) 20(5):688–701. doi: 10.1093/humupd/dmu020
86. Anderson RA, Su HI. The clinical value and interpretation of anti-Müllerian hormone in women with cancer. Front Endocrinol (Lausanne) (2020) 11:574263. doi: 10.3389/fendo.2020.574263
87. Decanter C, Morschhauser F, Pigny P, Lefebvre C, Gallo C, Dewailly D. Anti-M̈llerian hormone follow-up in young womentreated by chemotherapy for lymphoma: Preliminary results. Reprod Biomed Online (2010) 20:280–5. doi: 10.1016/j.rbmo.2009.11.010
88. Sharara FI, Scott RT, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J ObstetGynecol (1998) 179:804–12. doi: 10.1016/s0002-9378(98)70087-0
89. Decanter C, Delepine J, Behal H, Manier S, Bruno B, Barbatti M, et al. Longitudinal study of AMH variations in 122 Adolescents and Young Adults (AYA) and non-AYA lymphoma patients to evaluate the chemo-induced ovarian toxicity to further personalise fertility preservation counselling. Hum Reprod (2021) 36(10):2743–52. doi: 10.1093/humrep/deab189
90. Hagen CP, Vestergaard S, Juul A, Skakkebæk NE, Andersson AM, Main KM, et al. Low concentration of circulating antimüllerian hormone is not predictive of reduced fecundability in young healthy women: a prospective cohort study. FertilSteril (2012) 98(6):1602–1608.e2. doi: 10.1016/j.fertnstert.2012.08.008
91. Demeestere I, Racape J, Dechene J, Dupuis J, Morschhauser F, De Wilde V, et al. Gonadal function recovery in patients with advanced Hodgkin lymphoma treated with a PET-adapted regimen: prospective analysis of a randomized phase III trial (AHL2011). J Clin Oncol (2021) 39:3251–60. doi: 10.1200/JCO.21.00068
92. Loverro G, Guarini A, Di Naro E, GiacOmantonio L, Lavopa C, Liso V. Ovarianfunctionaftercancer treatment in youngwomenaffected by Hodgkindisease (HD). Hematology (2007) 12(2):141–7. doi: 10.1080/10245330600954072
93. Ferraretti AP, Gianaroli L. The Bologna criteria for the definition of poor ovarian responders: is there a need for revision? Hum Reprod (2014) 29(9):1842–5. doi: 10.1093/humrep/deu139
94. Ji J, Sundquist J, Sundquist K. Stillbirth and neonatal death among female cancer survivors: a national cohort study. Int J Cancer (2016) 139:1046–52. doi: 10.1002/ijc.30156
95. Anderson C, Engel SM, Mersereau JE, Black KZ, Wood WA, Anders CK, et al. Birth outcomes among adolescent and young adult cancer survivors. JAMA Oncol (2017) 3:1078–84. doi: 10.1001/jamaoncol.2017.0029
96. van der Kooi ALF, Brewster DH, Wood R, Nowell S, Fischbacher C, van den Heuvel-Eibrink MM, et al. Perinatal risks in female cancer survivors: a population-based analysis. PLoSOne (2018) 13:e0202805. doi: 10.1371/journal.pone.0202805
97. Hartnett KP, Mertens AC, Kramer MR, Lash TL, Spencer JB, Ward KC, et al. Pregnancy after cancer: does timing of conception affect infant health? Cancer (2018) 124:4401–7. doi: 10.1002/cncr.31732
98. Muñoz E, Fernandez I, Martinez M, Tocino A, Portela S, Pellicer A, et al. Oocyte donation outcome after oncological treatment in cancer survivors. FertilSteril (2015) 103(1):205–13. doi: 10.1016/j.fertnstert.2014.09.027
99. Kulkarni SS, Sastry PS, Saikia TK, Parikh PM, Gopal R, Advani SH. Gonadal function following ABVD therapy for hodgkin’s disease. Am J Clin Oncol (1997) 20:354–7. doi: 10.1097/00000421-199708000-00006
100. Skinner R, Mulder RL, Kremer LC, Hudson MM, Constine LS, Bardi E, et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol (2017) 18(2):e75–90. doi: 10.1016/S1470-2045(17)30026-8
101. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. HumReprod Update (2010) 16(3):231. doi: 10.1093/humupd/dmp048
102. Siimes MA, Rautonen J. Small testicles with impaired production of sperm in adult male survivors of childhood Malignancies. Cancer (1990) 65(6):1303–6. doi: 10.1002/1097-0142(19900315)65:6<1303::aid-cncr2820650608>3.0.co;2-d
103. Viviani S, Santoro A, Ragni G, Bonfante V, Bestetti O, Bonadonna G. Gonadal toxicity after combination chemotherapy for Hodgkin's disease. Comparative results of MOPP vs ABVD. Eur J Cancer Clin Oncol (1985) 21(5):601–5. doi: 10.1016/0277-5379(85)90088-4
104. Kelsey TW, McConville L, Edgar AB, Ungurianu AI, Mitchell RT, Anderson RA, et al. Follicle stimulating hormone is an accurate predictor of azoospermia in childhood cancer survivors. PloS One (2017) 12(7):e0181377. doi: 10.1371/journal.pone.0181377
105. Nieschlag E, Simoni M, Gromoll J, Weinbauer GF. Role of FSHin the regulation of spermatogenesis: clinical aspects. J Endocrinol (1999) 162(3):393–400. doi: 10.1677/joe.0.1620393
106. De Kretser DM, Meinhardt A, Meehan T, Phillips DJ, O’Bryan MK, Loveland KA. The roles of inhibin and related peptides in gonadal function. Mol Cell Endocrinol (2000) 161:43–6. doi: 10.1016/S0303-7207(99)00222-1
107. Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, McNeilly AS, et al. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab (1996) 81:3341–334. doi: 10.1210/jcem.81.9.8784094
108. Petersen PM, Andersson AM, Rorth M, Daugaard G, Skakkebaek NE. Undetectable Inhibin B serum levels in men after testicular irradiation. J Clin Endocrinol Metab (1999) 84:213–5. doi: 10.1210/jcem.84.1.5406
109. Jensen TK, Andersson AM, Hjollund NH, Scheike T, Kolstad H, Giwercman A, et al. Inhibin B as a serum marker of spermatogenesis: Correlation to differences in sperm concentration and follicle-stimulating hormone levels—A study of 349 Danish men. J Clin Endocrinol Metab (1997) 82(12):4059–4063. doi: 10.1210/jcem.82.12.4456
110. Andersson AM, Petersen JH, Jørgensen N, Jensen TK, Skakkebæk NE. Serum inhibin B and follicle-stimulating hormone levels as tools in the evaluation of infertile men: significance of adequate reference values from proven fertile men. J Clin Endocrinol Metab (2004) 89(6):2873–9. doi: 10.1210/jc.2003-032148
111. Kong X, Ye Z, Chen Y, Zhao H, Tu J, Meng T, et al. Clinical application value of Inhibin B alone or in combination with other hormone indicators in subfertile men with different spermatogenesis status: A study of 324 Chinese men. J Clin Lab Anal (2021) 35(8):e23882. doi: 10.1002/jcla.23882
112. Green DM, Zhu L, Zhang N, Sklar CA, Ke RW, Kutteh WH, et al. Lack of specificity of plasma concentrations of inhibin B and follicle-stimulating hormone for identification of azoospermic survivors of childhood cancer: a report from the St Jude lifetime cohort study. J Clin Oncol (2013) 31(10):1324–8. doi: 10.1200/JCO.2012.43.7038
113. Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab (2011) 96(10):3007–19. doi: 10.1210/jc.2011-1137
114. Lawrenz B, Banys M, Henes M, Neunhoeffer E, Grischke EM, Fehm T. Pregnancy after breast cancer: case report and review of the literature. Arch Gynecol Obstet. (2011) 283(4):837–43. doi: 10.1007/s00404-010-1829-y
115. Pentheroudakis G, Orecchia R, Hoekstra HJ, Pavlidis N, Guidelines Working Group ESMO. Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2010) 21 Suppl 5:v266–73. doi: 10.1093/annonc/mdq198
116. van der Kaaij MA, van Echten-Arends J, Heutte N, Meijnders P, Abeilard-Lemoisson E, Spina M, et al. Cryopreservation, semen use and the likelihood of fatherhood in male Hodgkin lymphoma survivors: an EORTC-GELA Lymphoma Group cohort study. Hum Reprod (2014) 29(3):525–33. doi: 10.1093/humrep/det430
117. Chatterjee R, Kottaridis PD, McGarrigle HH, Linch DC. Management of erectile dysfunction by combination therapy with testosterone and sildenafil in recipients of high-dose therapy for haematological Malignancies. Bone Marrow Transplant. (2002) 29(7):607–10. doi: 10.1038/sj.bmt.1703421
118. Yi JC, Syrjala KL. Sexuality after hematopoietic stem cell transplantation. Cancer J (2009) 15(1):57–64. doi: 10.1097/PPO.0b013e318198c758
119. Milroy CL, Jones KP. Gynecologic care in hematopoietic stem cell transplant patients: a review. ObstetGynecolSurv (2010) 65(10):668–79. doi: 10.1097/OGX.0b013e31820955be
120. Møller T, Adamsen L. Hematologic patients' clinical and psychosocial experiences with implanted long-term central venous catheter: self-management versus professionally controlled care. Cancer Nurs. (2010) 33(6):426–35. doi: 10.1097/NCC.0b013e3181dc1908
121. Beckjord EB, Arora NK, Bellizzi K, Hamilton AS, Rowland JH. Sexual well-being among survivors of non-Hodgkin lymphoma. OncolNurs Forum. (2011) 38(5):E351–9. doi: 10.1188/11.ONF.E351-E359
122. Recklitis CJ, Sanchez Varela V, Ng A, Mauch P, Bober S. Sexual functioning in long-term survivors of Hodgkin's lymphoma. Psychooncology (2010) 19(11):1229–33. doi: 10.1002/pon.1679
123. Behringer K, Müller H, Görgen H, Flechtner HH, Brillant C, Halbsguth TV, et al. Sexual quality of life in Hodgkin Lymphoma: a longitudinal analysis by the German Hodgkin Study Group. Br J Cancer. (2013) 108(1):49–57. doi: 10.1038/bjc.2012.550
124. van Tulder MW, Aaronson NK, Bruning PF. The quality of life of long-term survivors of Hodgkin's disease. Ann Oncol (1994) 5(2):153–8. doi: 10.1093/oxfordjournals.annonc.a058768
125. Corona G, Jannini EA, Maggi M. Inventories for male and female sexual dysfunctions. Int J Impot Res (2006) 18(3):236–50. doi: 10.1038/sj.ijir.3901410
126. Isidori AM, Pozza C, Esposito K, Giugliano D, Morano S, Vignozzi L, et al. Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med (2010) 7(3):1139–46. doi: 10.1111/j.1743-6109.2009.01635.x
127. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology (1997) 49(6):822–30. doi: 10.1016/s0090-4295(97)00238-0
128. Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther (2000) 26(2):191–208. doi: 10.1080/009262300278597
129. Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther (2005) 31(1):1–20. doi: 10.1080/00926230590475206
130. Jeffery DD, Tzeng JP, Keefe FJ, Porter LS, Hahn EA, Flynn KE, et al. Initial report of the cancer Patient-Reported Outcomes Measurement Information System (PROMIS) sexual function committee: review of sexual function measures and domains used in oncology. Cancer (2009) 115(6):1142–53. doi: 10.1002/cncr.24134
131. Dal Maso L, Santoro A, Iannelli E, De Paoli P, Minoia C, Pinto M, et al. Alliance against cancer (ACC) survivorship care and nutritional support working group. Cancer cure and consequences on survivorship care: position paper from the italian alliance against cancer (ACC) survivorship care working group. Cancer Manag Res (2022) 14:3105–18. doi: 10.2147/CMAR.S380390
Keywords: fertility preservation, lymphoma, chemotherapy, quality of life, survivorship, sexuality, long-term side effects, consensus-based reccommendations
Citation: Minoia C, Viviani S, Silvestris E, Palini S, Parissone F, De Palma G, Fedina A, Cormio G, Guarini A, Gini G, Montano L, Merli F and Peccatori FA (2023) Fertility preservation and monitoring in adult patients diagnosed with lymphoma: consensus-based practical recommendations by the Fondazione Italiana Linfomi & Società Italiana della Riproduzione Umana. Front. Oncol. 13:1252433. doi: 10.3389/fonc.2023.1252433
Received: 03 July 2023; Accepted: 21 August 2023;
Published: 12 September 2023.
Edited by:
Monica Balzarotti, Humanitas Research Hospital, ItalyReviewed by:
Paola Anserini, San Martino Hospital (IRCCS), ItalyCopyright © 2023 Minoia, Viviani, Silvestris, Palini, Parissone, De Palma, Fedina, Cormio, Guarini, Gini, Montano, Merli and Peccatori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Minoia, Yy5taW5vaWFAb25jb2xvZ2ljby5iYXJpLml0
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.