- 1Department of Public Health, Shaoxing Seventh People's Hospital, Shaoxing, China
- 2Department of Non-communicable Diseases Control and Prevention, Shaoxing Center for Disease Control and Prevention, Shaoxing, China
- 3Dispatch Division of Shaoxing Emergency Medical Services, Shaoxing Center for Emergency, Shaoxing, China
Background & aims: Evidence regarding the prevalence of pre-treatment sarcopenia and its impact on survival in patients with hematological malignancies (HM) varies across studies. We conducted a systematic review and meta-analysis to summarize this discrepancy.
Methods: PubMed, Embase and Cochrane library were systematically searched for relevant studies. Outcomes assessed were: prevalence of pre-treatment sarcopenia, overall survival (OS), progression-free survival (PFS) and complete response (CR). Weighted mean proportion, odds ratios (ORs) and hazard ratios (HRs) were estimated using a fixed-effects and a random-effects model.
Results: A total of 27 retrospective cohort studies involving 4,991 patients were included in this study. The prevalence of pre-treatment sarcopenia was 37.0% (95% CI: 32.0%-42.0%) in HM patients <60 years and 51.0% (95% CI: 45.0%-57.0%) in≥60 years. Patients with leukemia had the lowest prevalence, compared with those with other HM (38.0%; 95% CI: 33.0%-43.0%; P = 0.010). The presence of sarcopenia was independently associated with poor OS (HR = 1.57, 95% CI = 1.41-1.75) and PFS (HR = 1.50, 95% CI = 1.22-1.83) throughout treatment period, which may be partially attributed to decreased CR (OR = 0.54, 95% CI = 0.41-0.72), particularly for BMI ≥ 25 (P = 0.020) and males (P = 0.020).
Conclusion: Sarcopenia is highly prevalent in patients with HM and an adverse prognostic factor for both survival and treatment efficacy. HM and sarcopenia can aggravate each other. We suggest that in future clinical work, incorporating sarcopenia into risk scores will contribute to guide patient stratification and therapeutic strategy, particularly for the elderly.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier (CRD42023392550).
Introduction
Hematological malignancies (HM) represent a mixed group of tumors arising in the blood, bone marrow, lymph, and lymphatic system. These malignancies are classified into four main types: Leukemia, Hodgkin Lymphoma, Non-Hodgkin Lymphoma and Multiple Myeloma. In 2018, HM accounted for approximately 6.6% and 7.2% of all cancer diagnoses and deaths globally, respectively (1). Specifically, the number of new cases and deaths for each HM were as follows: Non-Hodgkin’s Lymphoma (509,590 new cases and 248,724 deaths); Leukemia (437,033 and 309,006); Multiple myeloma (159,985 and 106,105); and Hodgkin Lymphoma (79,990 and 26,167) (1). Of these, more than 60% of HM patients were aged≥60 years and as life expectancy improves, this proportion will increase in the future (2). So far, numerous efforts such as molecular targeted therapy have been developed, leading to great advances observed in almost all HM. However, a substantial proportion of patients fail to achieve disease control. For example, nearly 40% of patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors fail to achieve an optimal response throughout 5-year treatment period, or later relapse (3, 4). Increasing evidence has demonstrated that the prognosis of HM relies not only on HM biology-related factors, such as disease stage, but also on host factors, such as loss of skeletal muscle mass (sarcopenia).
Sarcopenia, caused by aging, disease, inactivity and malnutrition, is a progressive and generalized skeletal muscle disorder (5). It is commonly associated with increased likelihood of poor human health, including physical disability (6), chronic disease states (7, 8), and lowered quality of life (9). Peterson et al. (10) showed that patients with cancer were at an increased risk for sarcopenia, ranging from 15% to 50%. Furthermore, sarcopenia was also shown to have the prognostic value in several cancers, such as lung cancer (11), gastric cancer (12), and esophageal cancer (13).
As two common diseases in the elderly, whether sarcopenia has the predictive value in patients with HM is an active field of current research (14–40). However, the results derived from most of the studies are inconsistent and more than half are even controversial. For example, Besutti et al. (27) showed that sarcopenia did not affect survival in patients with HM. On the other hand, Leone et al. (20) reported that sarcopenia was independently associated with poor survival in patients with HM.
In 2019, Surov et al. (41) conducted a meta-analysis of 7 studies to summarize the impact of sarcopenia on malignant hematological diseases. However, this review assessed the outcome for overall survival only. In addition, as the interest continues to expand in the prognostic value of sarcopenia in patients with HM, the relevant studies have approximately tripled in size from 2019. In view of huge amounts of data obtained from new studies, the sarcopenia prevalence and evidence base of correlation between sarcopenia and HM need to be further updated. Most recently, Xu et al. (42) conducted another meta-analysis to investigate prognostic value of sarcopenia, but the study was restricted to diffuse large B-cell lymphoma. Furthermore, 3 studies included in their meta-analysis of 12 studies were from the same dataset (30, 43, 44), which could lead to biased results. Therefore, we conducted a systematic review and meta-analysis to explore the prevalence of pre-treatment sarcopenia in patients with HM and to ascertain the impact of sarcopenia on clinical outcomes in this population.
Methods
This meta-analysis was performed according to the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (45). The research protocol was registered and approved in PROSPERO (CRD42023392550).
Data sources
The electronic databases (PubMed, Embase and Cochrane library) were screened from inception to January 08, 2023 by using the text words (i) “sarcopenia” or “muscle mass” or “muscle index” or “muscle strength” or “muscle quality” or “muscle quantity” or “body composition” and (ii) “lymphoma” or “leukemia” or “myeloma” to identify published studies evaluating the impact of pre-treatment sarcopenia on clinical outcomes in patients with various HM. The detailed search strategies are shown in Table S1. Reference lists of included studies were also manually searched to identify any relevant studies that did not come up in the initial search. Only English publications were considered.
Selection criteria
Studies were included if they met the following criteria: (1) patients: adult patients with HM; (2) exposure: pre-treatment sarcopenia measured by computed tomography (CT) or positron emission tomography/CT; (3) comparison: non-sarcopenia arm; and (4) outcome: prevalence of pre-treatment sarcopenia, overall survival (OS), progression-free survival (PFS) and complete response (CR). Exclusion criteria were as follows: (1) reviews, conference proceedings, short reports, abstracts or case reports; and (2) duplicate studies from the same database (only the most recent study was included in the analysis). Two researchers (Y.W. and M.H.) independently screened the titles and abstracts to evaluate the potential studies. If a study was relevant, the full article was obtained for further reviewed by two independent reviewers (K.C. and F.C.). Any disagreements were resolved in a consensus meeting with a third researcher (Q.C.) as a referee.
Data extraction and risk of bias assessments
The following data were extracted: lead author, publication year, type of HM, sample size, patient characteristics (including age, sex ratio, BMI and revised international prognostic index), method to measure sarcopenia and their cut-off values, prevalence of sarcopenia, median follow-up, risk of bias, and data on outcomes. The extracted data were checked for accuracy by a third researcher (W.H). Any disagreements were resolved by consensus.
The Newcastle-Ottawa Scale (NOS) for observational studies was used to evaluate the risk of bias of included studies. Two researchers (Y.W. and M.H.) individually evaluated study quality by examining nine items: 1) Representativeness of the sarcopenia cohort, 2) Selection of the non-sarcopenia cohort, 3) Ascertainment of sarcopenia, 4) Demonstration that outcome of interest was not present at start of study, 5) Study controls for age, 6) Study controls for any additional factor, 7) Assessment of outcome, 8) Was follow-up long enough for outcomes to occur, and 9) Adequacy of follow up of cohorts. Each item was scored from 0 to 1, for a total maximum of 9 points. The overall methodological quality of each study was divided into high quality (7-9 points), medium quality (4-6 points) and low quality (≤ 3 points). Any disagreements were resolved in a consensus meeting with a third researcher (W.H.) as a referee.
Statistical analysis
The required data were extracted from each study. Heterogeneity between summary data was assessed using the I2 statistic. I2 < 50% reflected mild to moderate heterogeneity, and > 50% severe heterogeneity. A random effects model was used to calculate the weighted mean proportion, pooled odds ratios (ORs), hazard ratios (HRs) and 95% confidence intervals (CIs) unless we detected mild to moderate heterogeneity, then a fixed effects model was used. To ascertain robustness of findings, sensitivity analyses were performed by repeating with the random-effect method for mild to moderate heterogeneity, or by removing each study one by one for severe heterogeneity. To identify predictors and explore sources of heterogeneity, exploratory sub-analyses were conducted based on prognostic variables which has been reported in other single studies, including clinical characteristics (HM types, revised international prognostic index, prevalence of sarcopenia, method to measure muscle, SMI, age, sex ratio, BMI and follow-up period) and study characteristics (year of publication and sample size). For variables without appropriate threshold to categorize patients, the medians were calculated according to values reported in each study. Publication bias was estimated using funnel plots and Egger’s regression intercept analysis. Analyses were performed with Stata version 16 (Stata Corp., College Station, TX, USA). All tests were 2-tailed, and P < 0.05 was considered statistically significant.
Results
Studies retrieved and characteristics
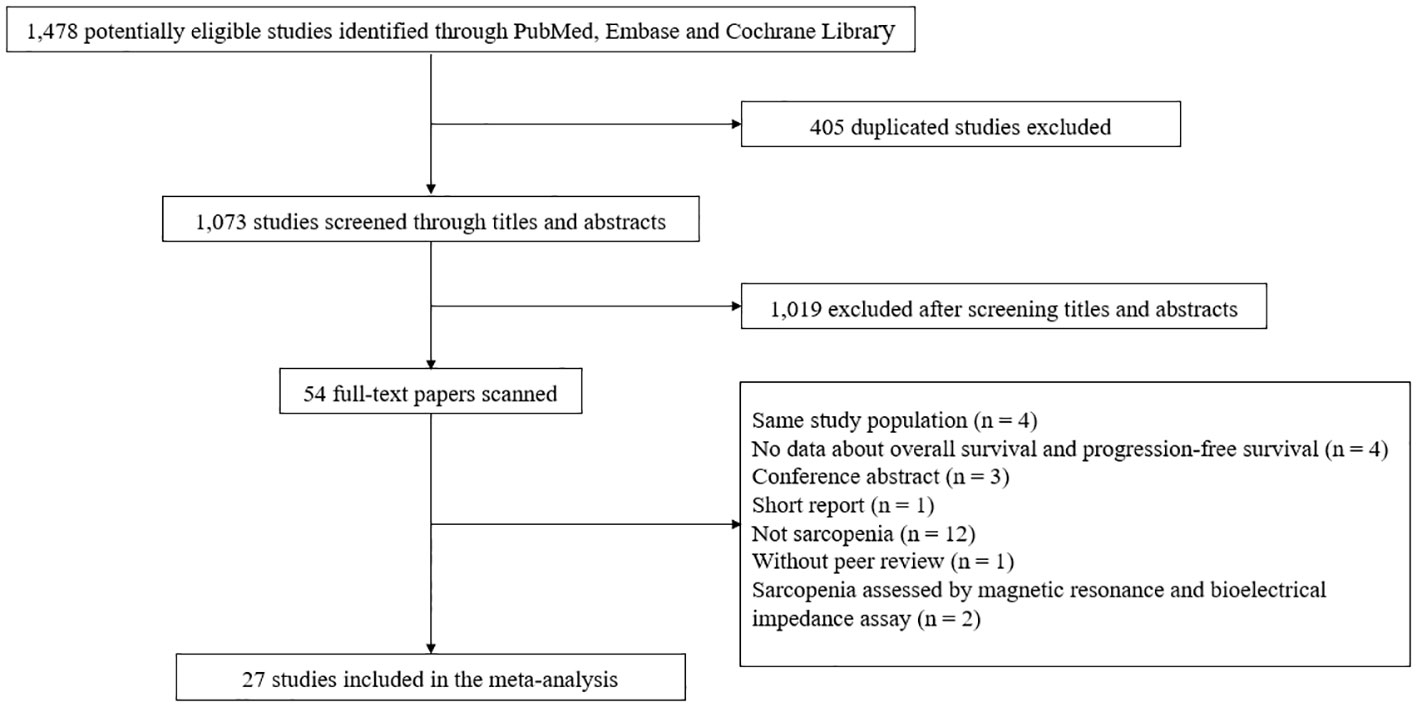
A total of 1,073 studies were identified after removing duplicates. After screening titles and abstracts, the full text was retrieved for 54 studies. Of these, 27 articles were excluded: 12 did not assess sarcopenia; 4 included the same study population; 4 did not provide HRs for OS and PFS; 3 were conference abstracts; 1 was a short report; 1 has not yet been peer-reviewed; 1 study each used magnetic resonance and bioelectrical impedance assay as a modality to diagnose sarcopenia (Table S2). Finally, a total of 27 studies involving 4,991 patients were selected for the final analysis (Figure 1).
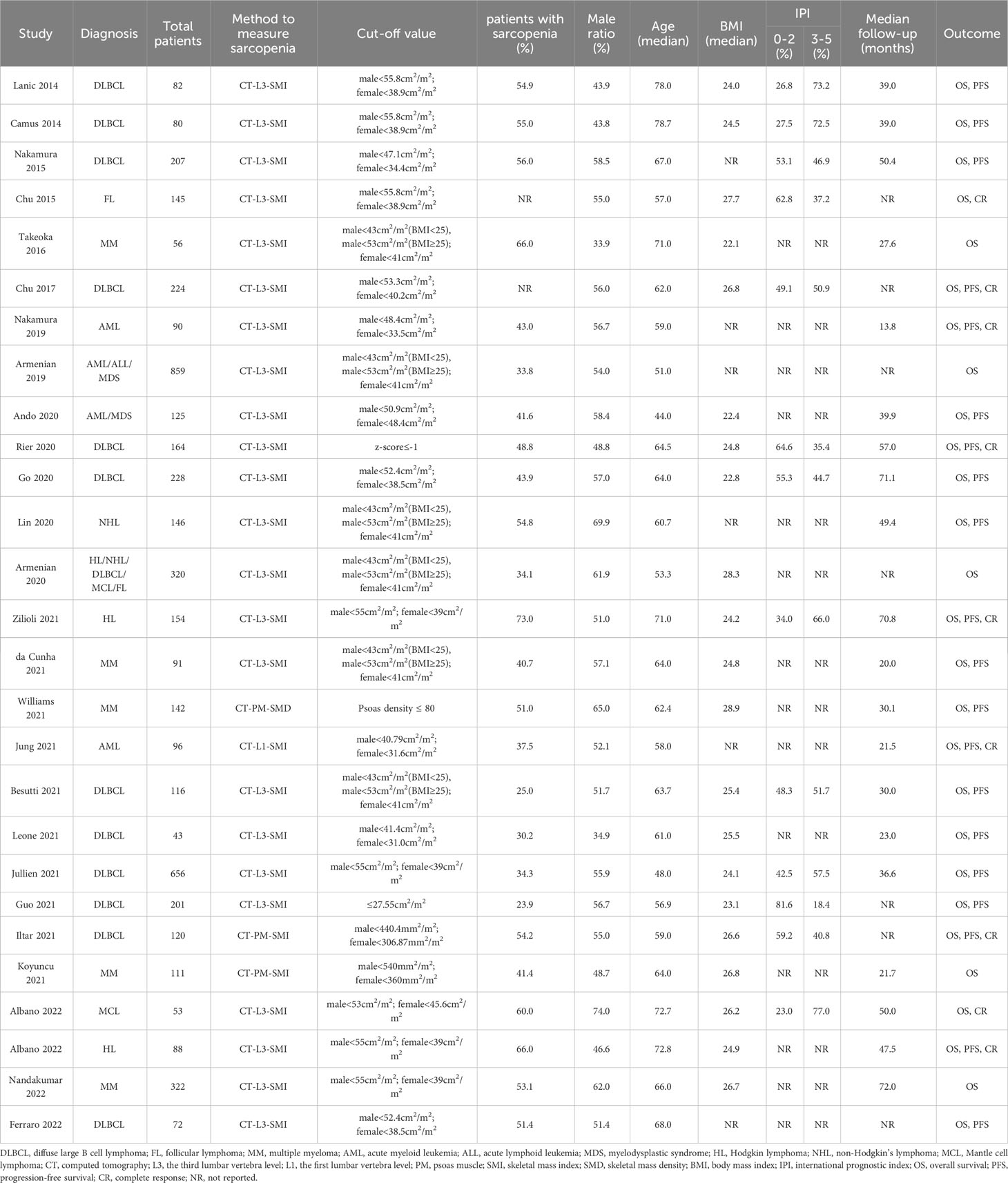
Among the 27 studies, 18 investigated the impact of sarcopenia in patients with lymphoma (15–18, 20, 23–25, 27–31, 35, 37–40), 4 in patients with leukemia (22, 32–34) and 5 in patients with myeloma (14, 19, 21, 26, 36). Of the 27 CT examinations, the skeletal mass index (SMI) at the third lumbar vertebra level (L3) (CT-L3-SMI) was the most commonly used for the measurement of sarcopenia (14–18, 20, 23, 25–40), followed by the psoas muscle index (PMI) (21, 24), psoas muscle density (PMD) (19) and the SMI at the first lumbar level (L1) (CT-L1-SMI) (22). Of note, although the diagnostic methods to measure sarcopenia were identical across 23 studies, their cut-off values are quite different. The detailed characteristics of the studies and patients are given in Table 1. The methodological quality of the included studies was moderate (9 of 27) to high (18 of 27) according to the NOS (Table S3). No significant publication bias was observed for OS (P = 0.883), PFS (P = 0.143) and CR (P = 0.346) (Figure S1).
Prevalence of sarcopenia prior to treatment
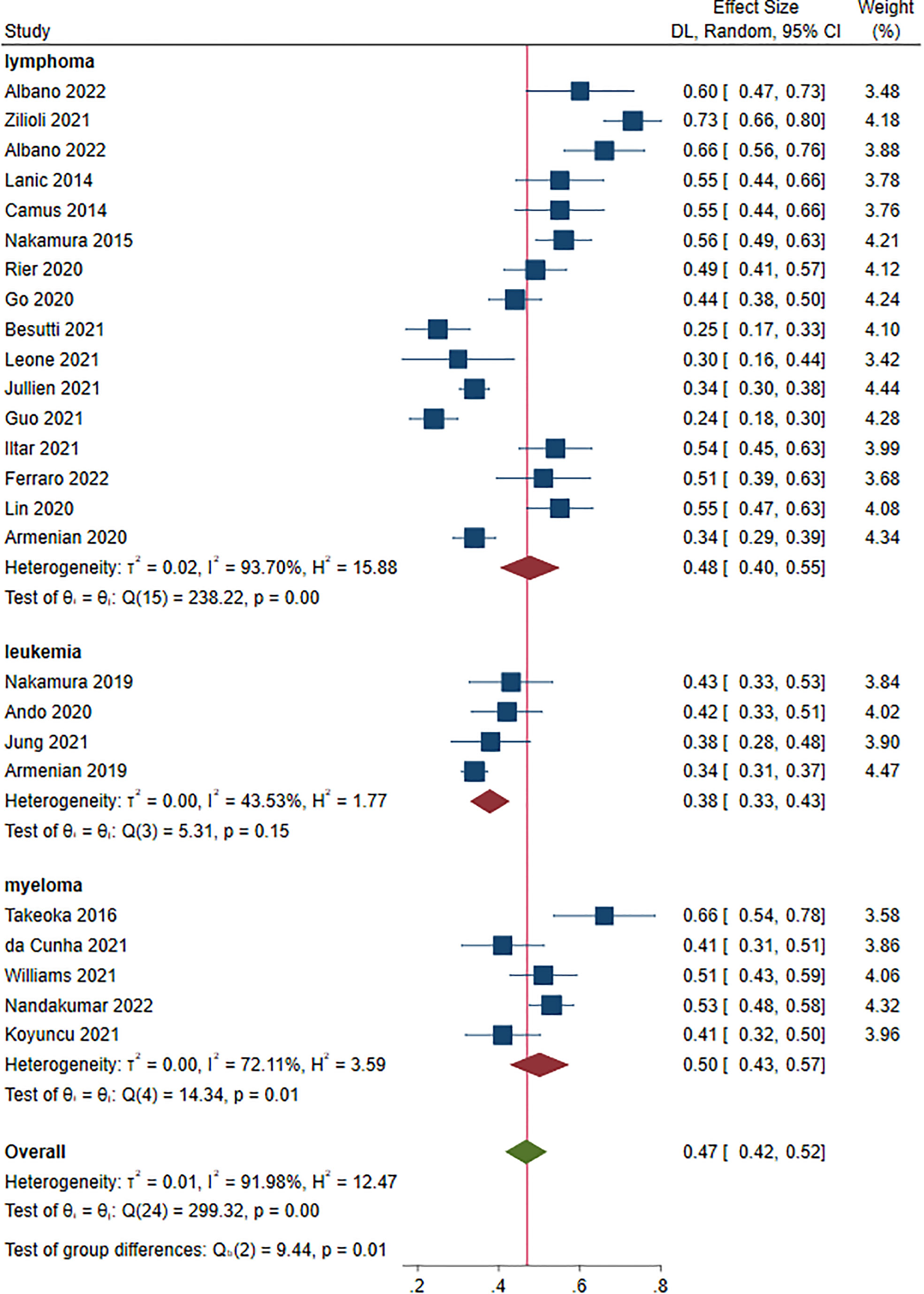
Twenty-five studies, 4,622 patients, were included in this analysis (14–34, 36, 37, 39, 40). The prevalence of sarcopenia ranged from 23.9% to 73.4% prior to treatment. The overall prevalence of sarcopenia was 47.0% (95% CI: 42.0%-52.0%) at a random-effect model (Figure 2). The sensitivity analysis showed that excluding these studies 1 by 1, the overall prevalence did not change (Figure S2).
The prevalence for each disease was as follows: lymphoma: 48.0% (95% CI: 40.0%-55.0%, 16 studies) (15–18, 20, 23–25, 27–31, 35, 37–40); leukemia: 38.0% (95% CI: 33.0%-43.0%, 4 studies) (22, 32–34); myeloma: 50.0% (95% CI: 43.0%-57.0%, 5 studies) (14, 19, 21, 26, 36). A significant difference was found in terms of sarcopenia prevalence in patients with different HM (P = 0.010)(Figure 2).
Subgroup analysis was also classified based on the median age of the patients as < 60 and ≥ 60 years. The estimated prevalence of sarcopenia was 37.0% (95% CI: 32.0%-42.0%, 8 studies) (22–25, 31–34) and 51.0% (95% CI: 45.0%-57.0%, 17 studies) (14–21, 26–30, 36, 37, 39, 40) in studies with patient median age < 60 and ≥ 60 years, respectively (P < 0.001) (Figure 3A).
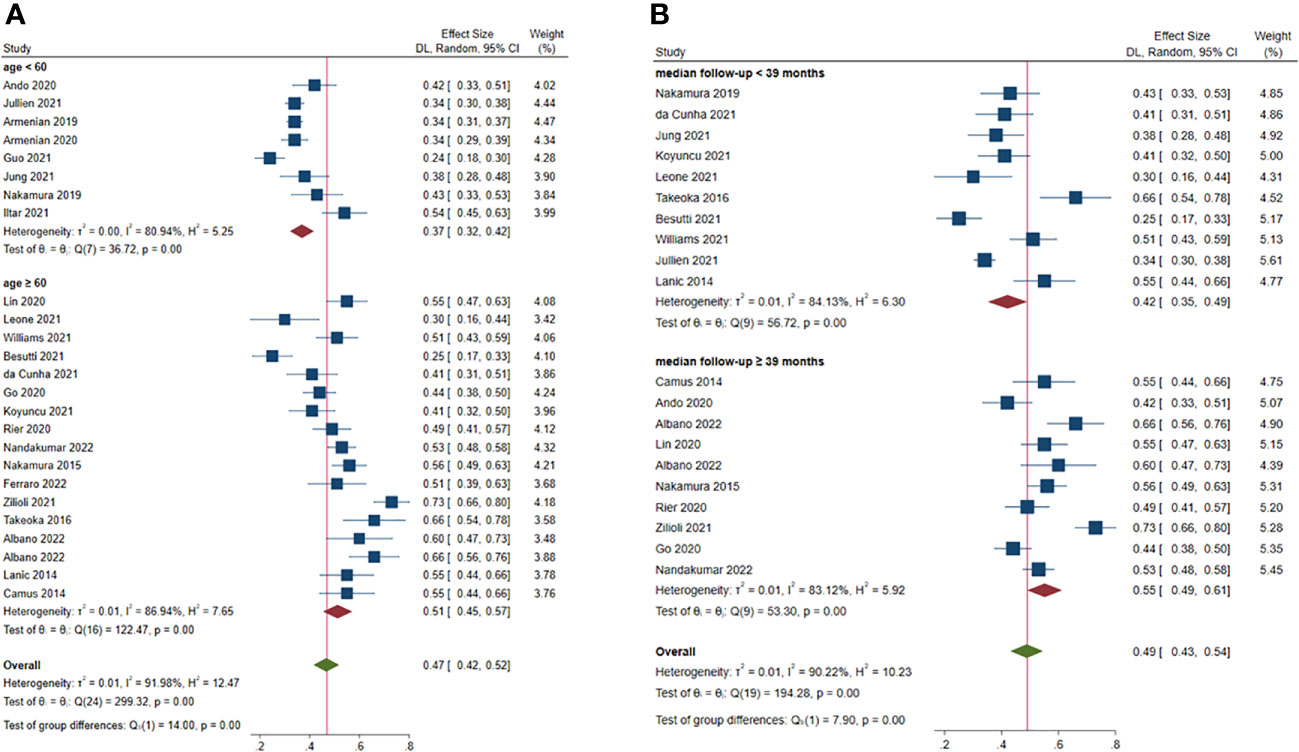
Figure 3 Subgroup analysis of factors contributing to increased prevalence of pre-treatment sarcopenia. (A) age; (B) median follow-up.
Median of the mean follow-up of the studies was 39 months. The estimated prevalence of sarcopenia was 41% (95% CI: 34.0%-47.0%, 9 studies) (19–23, 26, 27, 33, 36) and 55% (95% CI: 49.0%-61.0%, 11 studies) (14, 16–18, 28–30, 32, 37, 39, 40) in studies with follow-up < 39 and ≥ 39 months, respectively (P < 0.001) (Figure 3B).
In addition to types of disease, age and follow-up period, we found no significant differences in prevalence of sarcopenia in subgroup analyses of sex ratio, BMI, revised IPI, method to measure sarcopenia, sample size and publication year (data not shown).
Overall survival and sarcopenia
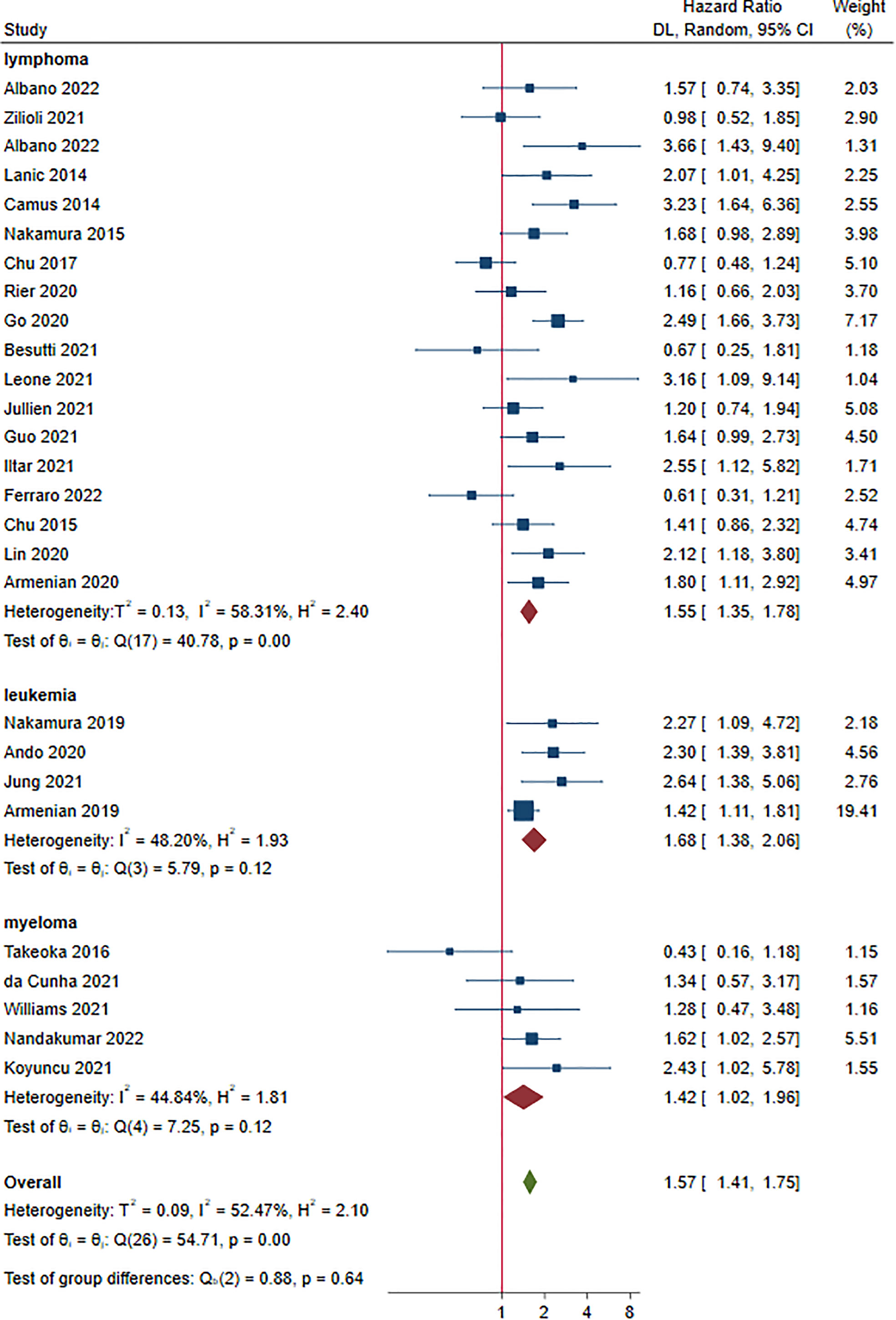
Twenty-seven studies reported OS as an outcome (14–40). The HRs for OS ranged from 0.43 to 3.66. Multivariate Cox regression was performed in 17 studies (14, 16, 19, 20, 22, 24, 26–29, 31–33, 36, 37, 39, 40). As shown in Figure 4, sarcopenia prior to treatment was shown to have a shorter OS in patients with HM (HR 1.57, 95% CI 1.41-1.75, I2 = 52.47%). Lymphoma, leukemia and myeloma were reported in eighteen (15–18, 20, 23–25, 27–31, 35, 37–40), four (22, 32–34) and five studies (14, 19, 21, 26, 36), respectively. The pooled HRs (95% CIs) were 1.55 (1.35-1.78) for lymphoma, 1.68 (1.38-2.06) for leukemia and 1.42 (1.02-1.96) for myeloma. There were no significant differences among the different malignant hematological diseases (P = 0.640). The sensitivity analysis showed that removing studies 1 by 1 did not change the overall results (Figure S3).
Subgroup analyses showed that HRs for OS favored non-sarcopenia in almost all variables and no significant difference between HRs was found for each variable (Table S4).
Progression-free survival and sarcopenia
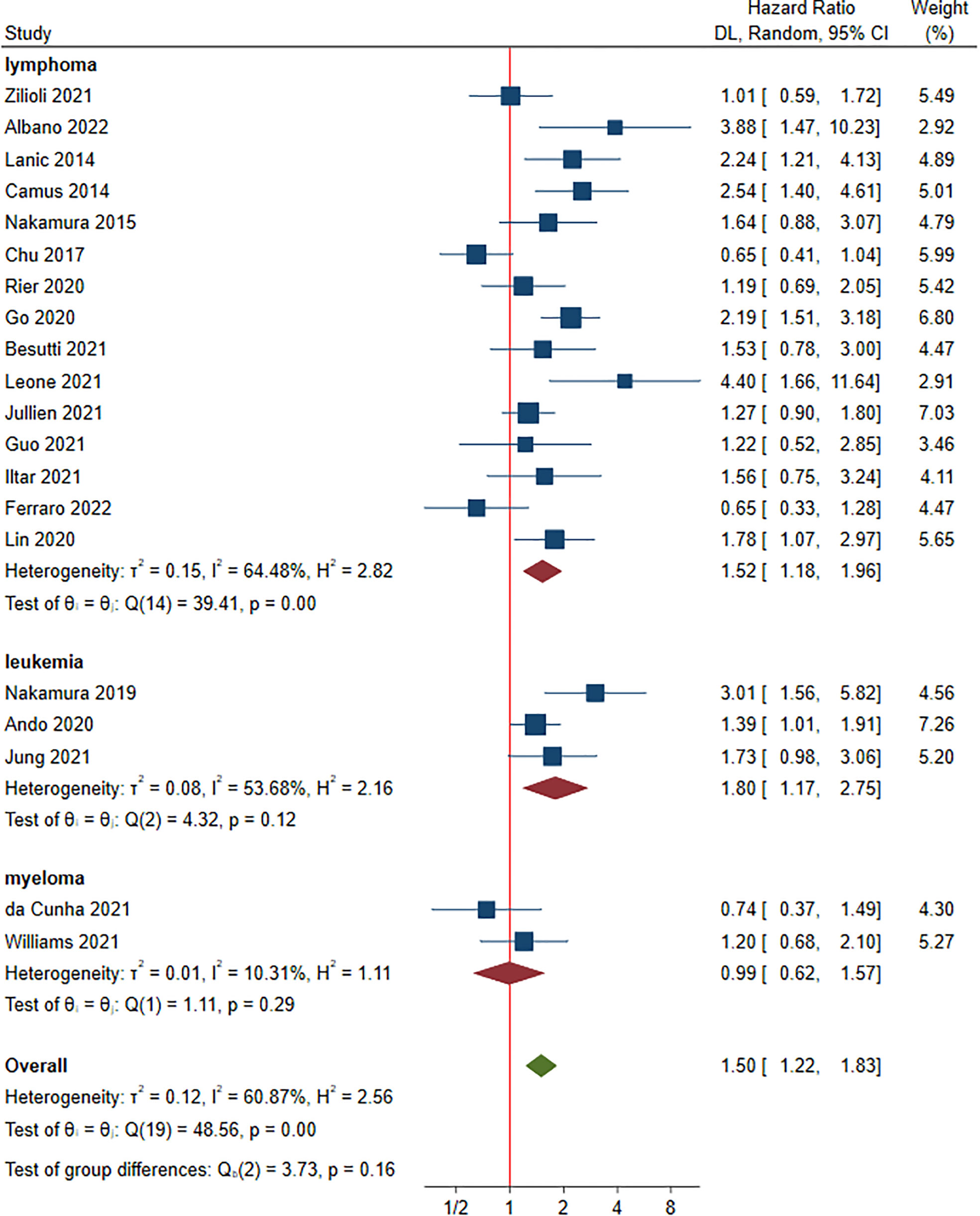
A total of 20 studies involving 3,125 patients were included in this part (15, 17–20, 22–30, 32, 33, 35, 37, 39, 40). Of these, the HRs for PFS ranged from 0.65 to 4.40. Multivariate analysis was performed in 14 studies (17, 19, 20, 22, 24–29, 32, 33, 37, 39). Compared to non-sarcopenia, the patients with sarcopenia had a negative effect on PFS, with a HR of 1.50 (95% CI: 1.22-1.83, I2 = 60.87%) (Figure 5). Lymphoma, leukemia and myeloma were reported in fifteen (15, 17, 18, 20, 23–25, 27–30, 35, 37, 39, 40), four (22, 32, 33) and two studies (19, 26), respectively. The pooled HRs (95% CIs) were 1.52 (1.18-1.96) for lymphoma, 1.80 (1.17-2.75) for leukemia and 0.99 (0.62-1.57) for myeloma. No significant difference in HR was found between different malignant hematological diseases (P = 0.160). The sensitivity analysis showed that removing studies 1 by 1 did not change the overall results (Figure S4).
Subgroup analyses showed that HRs for PFS favored non-sarcopenia in almost all variables and no significant difference between HRs was found for each variable (Table S5).
Complete response and sarcopenia
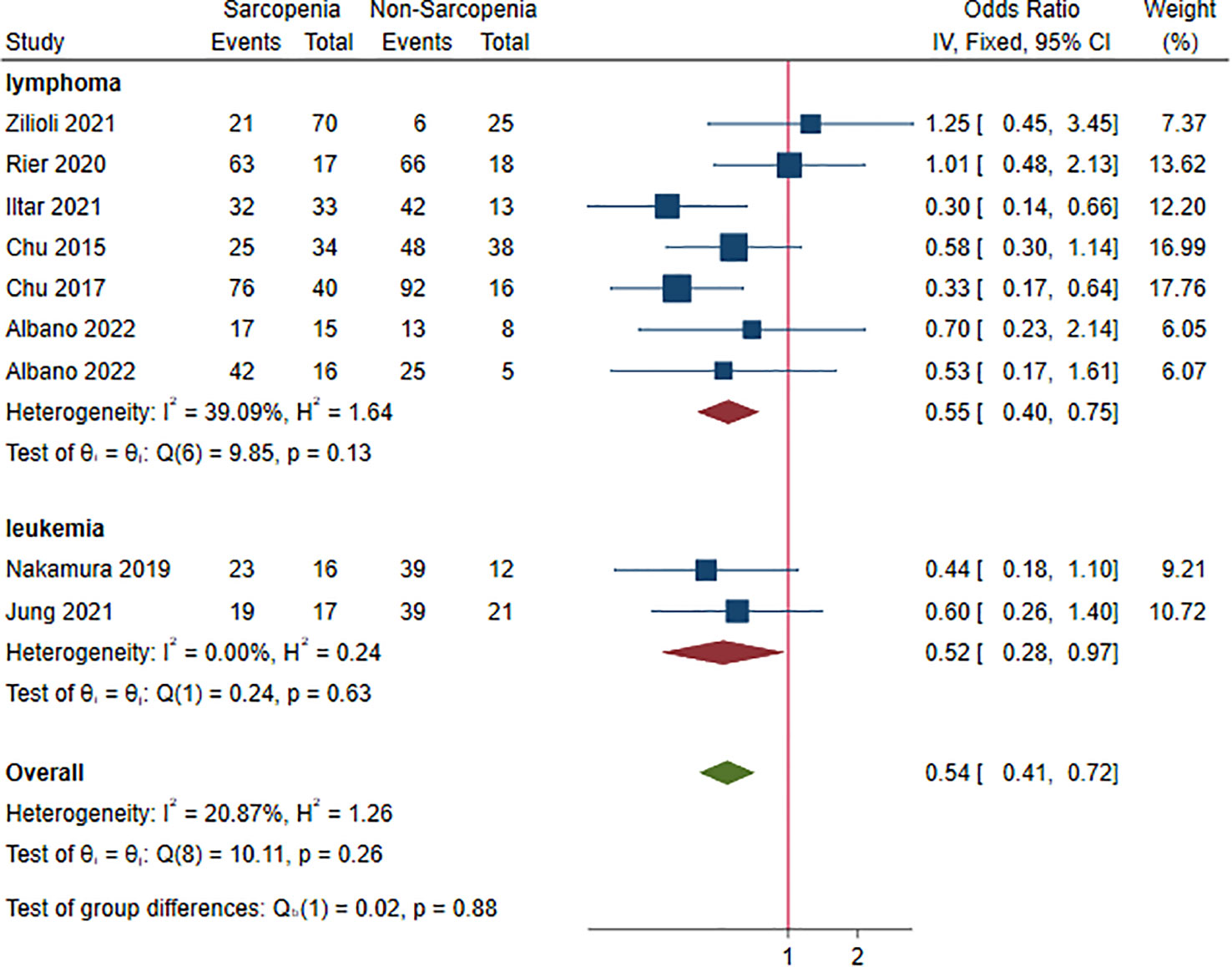
Nine studies, 1,102 patients, were included in this analysis (16–18, 22, 24, 28, 33, 35, 38). The ORs for CR ranged from 0.30 to 1.25. Sarcopenia decreased the rate of CR (OR 0.54, 95% CI 0.41-0.72, I2 = 20.87%; Figure 6). Lymphoma and leukemia were reported in seven (16–18, 24, 28, 35, 38) and two (22, 33) studies, respectively. The pooled ORs (95% CIs) were 0.55 (0.40-0.75) for lymphoma and 0.52 (0.28-0.97) for leukemia. There was no significant difference between the two HM (P = 0.880). In sensitivity analysis, the random-effect method did not change the result, suggesting robustness of analysis to the fixed-effect model (Figure S5).
Table S6 summarizes results of subgroup analysis with respect to potential risk factors for failing to achieve CR. Statistically significant differences were observed within subgroups based on sex ratio (P = 0.020) and BMI (P = 0.020). Sarcopenia was associated with poor CR in patients being male (OR 0.42, 95% CI 0.30-0.60) and BMI≥25(OR 0.42, 95% CI 0.29-0.62) (Table S6).
Discussion
This meta-analysis of retrospective studies in patients with HM focused on the impact of sarcopenia on survival and efficacy but also on the prevalence of sarcopenia prior to treatment. CT has been accepted as a gold standard to assess muscle quantity/mass and was routinely used before treating HM (46). Considering comparability between the instruments used to measure muscle mass, studies in which sarcopenia was diagnosed by CT only were included in our analysis. The overall prevalence of sarcopenia was 47.0% in patients with HM prior to treatment. Subgroup analyses suggested that advanced age (≥60 years) and long-term follow-up was shown to have a significantly higher prevalence, while a significantly lower prevalence was observed in leukemia. Importantly, the presence of sarcopenia was independently associated with poor OS and PFS throughout treatment period, which can be partially attributed to decreased CR, specific for BMI ≥ 25 and males.
Recently, a meta-analysis conducted by Petermann-Rocha et al. (47) estimated the prevalence of sarcopenia across the globe. Based on 151 studies, the global prevalence of sarcopenia ranged from 10.0% to 27.0% in total population. The result did not change in the elderly as the average age in approximately 92.3% of studies was more than 60 years. Obviously, the sarcopenia prevalence was very high in patients with HM (47.0%) as compared to the general population, and even higher in HM patients older than 60 years (51.0%). The causes of HM-related sarcopenia have not yet been elucidated, and the possible mechanisms are as follows: systemic inflammatory (48), metabolic derangement (49) and mitochondrial function (50). Besides these, limited physical activity and poor nutrition intake can also be observed in patients with HM, resulting in non-cancer biology-related decrease in muscle mass. It has been recognized that aging was a significant risk factor for sarcopenia (51), which was also proved to be applicable to HM patients in our subgroup analysis by age (51.0% vs 37.0%). Thus, our study suggested that people with HM and/or advanced age had more likelihood of sarcopenia. One problem we have not yet solved is the effect size of the pathological condition resulted from HM alone and physiological condition resulted from advanced age alone on sarcopenia. Also, whether HM and aging act synergistically to increase the risk of developing sarcopenia is worthy of being investigated in the future. Our study provided pooled prevalence of sarcopenia which can be regarded as a reference for the calculation of sample size for future intervention studies.
The similar difference between the general and HM populations in terms of increased sarcopenia was also observed in non-hematological solid tumors, such as lung cancer, renal cell carcinoma, hepatocellular carcinoma, melanoma, etc. (11, 52, 53) However, our study found that prevalence of sarcopenia was significantly lower for patients with leukemia. It is possible that the lower prevalence observed could be as a result of young patients, since the median age in all studies included in the leukemia subgroup analysis was less than 60 years compared with other cohorts.
Importantly, our study suggested that sarcopenia may serve as an adverse prognostic factor for both OS and PFS in patients with HM. But the HR for PFS in myeloma was no statistical difference. The result may be due to the limited number of studies included (2 studies comprising only 233 patients). The poor survival profiles in patients with HM and sarcopenia can be explained by the drug-related adverse effects. Previous studies demonstrated that lower muscle mass and the resulting reduction in the clearance of anti-tumor drugs were associated with serious toxicities during or after chemotherapy (54, 55). For example, Guo et al. (25) demonstrated that for every 5 cm2/m2 decrease in SMI, the risk of any grade 3-4 toxicity was increased by 34%. Nakamura et al. (33) even found that all sarcopenia patients older than 60 years died within 1 year after induction chemotherapy. Besides, dose reductions or interruption of treatment resulted from toxicities of therapy may also add to the poor prognosis for sarcopenic patients (56). Another potential explanation for the favorable survival observed in non-sarcopenia patients was that numerous cytokines (e.g., myokines) released by skeletal muscle cells can inhibit cancer cell viability and proliferation (57).
Although a substantial proportion of studies (7 of 9) denied the role of sarcopenia in terms of CR, the pooled CR was significant. This result is reasonable because the mechanisms mentioned above influencing OS and PFS can also influence the treatment efficacy such as early discontinuation of treatment due to increased toxicities. Interestingly, the high proportion of males and BMI ≥ 25 were linked to poor CR. Currently, BMI is the most common indicator used for diagnosing overweight and obesity. BMI ≥ 25 means that increased adipose tissue may decrease muscle mass, thereby increasing treatment-related toxicities. However, males tend to have a high fat-free mass. It is opposite to the role of BMI on CR, which was described as the ‘obesity paradox’ (58, 59), and needs further investigation.
The overall findings of this study demonstrated that HM and sarcopenia can interact and aggravate each other. Of note, medical treatment can stimulate muscle wasting. For example, Albano et al. (17) found that the rate of sarcopenia patients increased from 66% before chemotherapy to 83% at the end of the treatment; Xiao et al. (60) showed that sarcopenia increased by 26.1% after treatment. Given the wide use of CT scan at the time of diagnosis of HM, we suggested that sarcopenia should be incorporated into the existing prognostic system and given appropriate weight to guide the therapeutic strategy. Also, our findings highlight the importance of treating sarcopenia, in order to minimize adverse consequences. Available evidence suggests that nutrition may improve muscle mass (61), short-chain fatty acids (SCFAs) in particular, which are considered to be involved in the change of muscle biology (62). On the other hand, immune nutrition with some special nutrients has been widely used in patients with cancers, showing different supporting functions (63, 64). Therefore, adding nutrients like SCFAs to the formula of nutrition may help to treat both diseases.
Our meta-analysis has several limitations: 1) The cut-off values of SMI used to assess sarcopenia were not uniform. For example, the standards adopted in some studies were based on values given by Prado et al (65) or Martin et al (66), while values adopted in others were calculated from ROC curve based on their own samples. This may affect the conclusion, as demonstrated by Zilioli et al (18) with different results resulted from the same population. Thus, future studies are warranted to determine optimal cut-off levels when assessing sarcopenia with CT, specific for gender and ethnicity. 2) As the physical performance was not available due to the retrospective nature of the included studies, our meta-analysis cannot investigate the impact of severe sarcopenia on survival and efficacy. 3) Studies were pooled with different patient characteristics such as age, BMI, adipose tissue, etc. Due to this limitation, heterogeneity was severe for the majority of outcomes. We minimized the influence of heterogeneity by subgroup analyses and no significant difference between HRs was found for each variable. Sensitivity analyses were also performed in response to the severe heterogeneity. Both approaches provided concordant results. 4) As with any meta-analysis, our dataset was founded on each included study, and hence several missing variables with the prognostic value such as advanced stage at diagnosis, serum albumin and C-reactive protein may not be evaluated. 5) For some outcomes, the number of studies included was limited, which could increase uncertainty of the results. 6) As this meta-analysis was carried out in patients with HM, our findings cannot be used as a reference for improving the prognosis of non-hematological solid tumors.
Conclusions
In conclusion, the prevalence of pre-treatment sarcopenia is found to be very high in patients with HM, and even higher in HM patients older than 60 years. The presence of sarcopenia is independently associated with poor survival and treatment response throughout treatment period. Our meta-analysis suggests that HM and sarcopenia can aggravate each other. In future clinical work, sarcopenia screening prior to treating HM will contribute to guide patient stratification and therapeutic strategy, particularly for the elderly.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JX, KC and QC contributed to the conception and design this study. WH and MH carried out the development of the methodology. KC, MH and YW analyzed and interpreted the data. JX, KC and QC wrote the manuscript and approved the final submission of the study. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1249353/full#supplementary-material
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer (2019) 144(8):1941–53. doi: 10.1002/ijc.31937
2. Bron D, Ades L, Fulop T, Goede V, Stauder R. Aging and blood disorders: new perspectives, new challenges. Haematologica (2015) 100(4):415–7. doi: 10.3324/haematol.2015.126771
3. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol (2016) 34(20):2333–40. doi: 10.1200/JCO.2015.64.8899
4. Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia (2016) 30(5):1044–54. doi: 10.1038/leu.2016.5
5. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
6. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J cachexia sarcopenia Muscle (2016) 7(1):28–36. doi: 10.1002/jcsm.12048
7. Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chronic Respir Dis (2017) 14(1):85–99. doi: 10.1177/1479972316679664
8. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association between sarcopenia and cognitive impairment: A systematic review and meta-analysis. J Am Med Directors Assoc (2016) 17(12):1164.e7–.e15. doi: 10.1016/j.jamda.2016.09.013
9. Beaudart C, Biver E, Reginster JY, Rizzoli R, Rolland Y, Bautmans I, et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. J cachexia sarcopenia Muscle (2017) 8(2):238–44. doi: 10.1002/jcsm.12149
10. Peterson SJ, Mozer M. Differentiating sarcopenia and cachexia among patients with cancer. Nutr Clin Pract (2017) 32(1):30–9. doi: 10.1177/0884533616680354
11. Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer: A systematic review and meta-analysis. Chest (2019) 156(1):101–11. doi: 10.1016/j.chest.2019.04.115
12. Meyer HJ, Wienke A, Surov A. Sarcopenia as a prognostic marker for survival in gastric cancer patients undergoing palliative chemotherapy. A systematic review and meta analysis. Nutr Cancer (2022) 74(10):3518–26. doi: 10.1080/01635581.2022.2077387
13. Chen F, Chi J, Zhao B, Mei F, Gao Q, Zhao L, et al. Impact of preoperative sarcopenia on postoperative complications and survival outcomes of patients with esophageal cancer: a meta-analysis of cohort studies. Dis esophagus (2022) 35(9):doab100. doi: 10.1093/dote/doab100
14. Nandakumar B, Baffour F, Abdallah NH, Kumar SK, Dispenzieri A, Buadi FK, et al. Sarcopenia identified by computed tomography imaging using a deep learning-based segmentation approach impacts survival in patients with newly diagnosed multiple myeloma. Cancer (2022) 129(3):385–92. doi: 10.1002/cncr.34545
15. Ferraro V, Thormann M, Hinnerichs M, Pech M, Wolleschak D, Mougiakakos D, et al. Sarcopenia does not predict outcome in patients with CNS lymphoma undergoing systemic therapy. Oncol Lett (2022) 24(4):355. doi: 10.3892/ol.2022.13475
16. Albano D, Pasinetti N, Dondi F, Giubbini R, Tucci A, Bertagna F. Prognostic role of pre-treatment metabolic parameters and sarcopenia derived by 2-[(18)F]-FDG PET/CT in elderly mantle cell lymphoma. J Clin Med (2022) 11(5):1210. doi: 10.3390/jcm11051210
17. Albano D, Dondi F, Treglia G, Tucci A, Ravanelli M, Farina D, et al. Longitudinal Body Composition Changes Detected by [(18)F]FDG PET/CT during and after Chemotherapy and Their Prognostic Role in Elderly Hodgkin Lymphoma. Cancers (2022) 14(20):5147. doi: 10.3390/cancers14205147
18. Zilioli VR, Albano D, Arcari A, Merli F, Coppola A, Besutti G, et al. Clinical and prognostic role of sarcopenia in elderly patients with classical Hodgkin lymphoma: a multicentre experience. J cachexia sarcopenia Muscle (2021) 12(4):1042–55. doi: 10.1002/jcsm.12736
19. Williams A, Baruah D, Patel J, Szabo A, Chhabra S, Dhakal B, et al. Prevalence and significance of sarcopenia in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Bone marrow Transplant (2021) 56(1):225–31. doi: 10.1038/s41409-020-01008-9
20. Leone R, Sferruzza G, Calimeri T, Steffanoni S, Conte GM, De Cobelli F, et al. Quantitative muscle mass biomarkers are independent prognosis factors in primary central nervous system lymphoma: The role of L3-skeletal muscle index and temporal muscle thickness. Eur J Radiol (2021) 143:109945. doi: 10.1016/j.ejrad.2021.109945
21. Koyuncu MB, Köseci T, Güler E, Ten B, Apaydin FD, Nayir E, et al. The psoas muscle index and retrospective clinical frailty scale score predict overall survival in patients with multiple myeloma. Turk Geriatri Dergisi (2021) 24(4):518–25. doi: 10.31086/tjgeri.2021.249
22. Jung J, Lee E, Shim H, Park JH, Eom HS, Lee H. Prediction of clinical outcomes through assessment of sarcopenia and adipopenia using computed tomography in adult patients with acute myeloid leukemia. Int J Hematol (2021) 114(1):44–52. doi: 10.1007/s12185-021-03122-w
23. Jullien M, Tessoulin B, Ghesquières H, Oberic L, Morschhauser F, Tilly H, et al. Deep-learning assessed muscular hypodensity independently predicts mortality in DLBCL patients younger than 60 years. Cancers (2021) 13(18):4503. doi: 10.3390/cancers13184503
24. Iltar U, Sözel H, Sözel YK, Ataş Ü, Yücel OK, Salim O, et al. Prognostic impact of the psoas muscle index, a parameter of sarcopenia, in patients with diffuse large B-cell lymphoma treated with rituximab-based chemoimmunotherapy. Leuk Lymphoma (2021) 62(5):1098–106. doi: 10.1080/10428194.2020.1856833
25. Guo J, Cai P, Li P, Cao C, Zhou J, Dong L, et al. Body composition as a predictor of toxicity and prognosis in patients with diffuse large B-cell lymphoma receiving R-CHOP immunochemotherapy. Curr Oncol (Toronto Ont) (2021) 28(2):1325–37. doi: 10.3390/curroncol28020126
26. da Cunha ADJ, Silveira MN, Takahashi MES, de Souza EM, Mosci C, Ramos CD, et al. Adipose tissue radiodensity: A new prognostic biomarker in people with multiple myeloma. Nutr (Burbank Los Angeles County Calif) (2021) 86:111141. doi: 10.1016/j.nut.2021.111141
27. Besutti G, Massaro F, Bonelli E, Braglia L, Casali M, Versari A, et al. Prognostic impact of muscle quantity and quality and fat distribution in diffuse large B-cell lymphoma patients. Front Nutr (2021) 8:620696. doi: 10.3389/fnut.2021.620696
28. Rier HN, Kharagjitsing H, van Rosmalen J, van Vugt J, Westerweel PE, de Jongh E, et al. Prognostic impact of low muscle mass and low muscle density in patients with diffuse large B-cell lymphoma. Leuk Lymphoma (2020) 61(7):1618–26. doi: 10.1080/10428194.2020.1737686
29. Lin RJ, Michaud L, Lobaugh SM, Nakajima R, Mauguen A, Elko TA, et al. The geriatric syndrome of sarcopenia impacts allogeneic hematopoietic cell transplantation outcomes in older lymphoma patients. Leuk Lymphoma (2020) 61(8):1833–41. doi: 10.1080/10428194.2020.1742909
30. Go SI, Kim HG, Kang MH, Park S, Lee GW. Prognostic model based on the geriatric nutritional risk index and sarcopenia in patients with diffuse large B-cell lymphoma. BMC Cancer (2020) 20(1):439. doi: 10.1186/s12885-020-06921-2
31. Armenian SH, Iukuridze A, Teh JB, Mascarenhas K, Herrera A, McCune JS, et al. Abnormal body composition is a predictor of adverse outcomes after autologous haematopoietic cell transplantation. J cachexia sarcopenia Muscle (2020) 11(4):962–72. doi: 10.1002/jcsm.12570
32. Ando T, Fujisawa S, Teshigawara H, Matsumura A, Sakuma T, Suzuki T, et al. Computed tomography-defined sarcopenia: prognostic predictor of nonrelapse mortality after allogeneic hematopoietic stem cell transplantation: a multicenter retrospective study. Int J Hematol (2020) 112(1):46–56. doi: 10.1007/s12185-020-02870-5
33. Nakamura N, Ninomiya S, Matsumoto T, Nakamura H, Kitagawa J, Shiraki M, et al. Prognostic impact of skeletal muscle assessed by computed tomography in patients with acute myeloid leukemia. Ann Hematol (2019) 98(2):351–9. doi: 10.1007/s00277-018-3508-1
34. Armenian SH, Xiao M, Berano Teh J, Lee B, Chang HA, Mascarenhas K, et al. Impact of sarcopenia on adverse outcomes after allogeneic hematopoietic cell transplantation. J Natl Cancer Inst (2019) 111(8):837–44. doi: 10.1093/jnci/djy231
35. Chu MP, Lieffers J, Ghosh S, Belch A, Chua NS, Fontaine A, et al. Skeletal muscle density is an independent predictor of diffuse large B-cell lymphoma outcomes treated with rituximab-based chemoimmunotherapy. J cachexia sarcopenia Muscle (2017) 8(2):298–304. doi: 10.1002/jcsm.12161
36. Takeoka Y, Sakatoku K, Miura A, Yamamura R, Araki T, Seura H, et al. Prognostic effect of low subcutaneous adipose tissue on survival outcome in patients with multiple myeloma. Clin Lymphoma Myeloma Leuk (2016) 16(8):434–41. doi: 10.1016/j.clml.2016.04.010
37. Nakamura N, Hara T, Shibata Y, Matsumoto T, Nakamura H, Ninomiya S, et al. Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Ann Hematol (2015) 94(12):2043–53. doi: 10.1007/s00277-015-2499-4
38. Chu MP, Lieffers J, Ghosh S, Belch AR, Chua NS, Fontaine A, et al. Skeletal muscle radio-density is an independent predictor of response and outcomes in follicular lymphoma treated with chemoimmunotherapy. PloS One (2015) 10(6):e0127589. doi: 10.1371/journal.pone.0127589
39. Lanic H, Kraut-Tauzia J, Modzelewski R, Clatot F, Mareschal S, Picquenot JM, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma (2014) 55(4):817–23. doi: 10.3109/10428194.2013.816421
40. Camus V, Lanic H, Kraut J, Modzelewski R, Clatot F, Picquenot JM, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Eur J Haematol (2014) 93(1):9–18. doi: 10.1111/ejh.12285
41. Surov A, Wienke A. Sarcopenia predicts overall survival in patients with Malignant hematological diseases: A meta-analysis. Clin Nutr (Edinburgh Scotland) (2021) 40(3):1155–60. doi: 10.1016/j.clnu.2020.07.023
42. Xu XT, He DL, Tian MX, Wu HJ, Jin X. Prognostic value of sarcopenia in patients with diffuse large B-cell lymphoma treated with R-CHOP: A systematic review and meta-analysis. Front Nutr (2022) 9:816883. doi: 10.3389/fnut.2022.816883
43. Go SI, Park MJ, Song HN, Kim HG, Kang MH, Lee HR, et al. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J cachexia sarcopenia Muscle (2016) 7(5):567–76. doi: 10.1002/jcsm.12115
44. Go SI, Park MJ, Song HN, Kim HG, Kang MH, Kang JH, et al. A comparison of pectoralis versus lumbar skeletal muscle indices for defining sarcopenia in diffuse large B-cell lymphoma - two are better than one. Oncotarget (2017) 8(29):47007–19. doi: 10.18632/oncotarget.16552
45. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol (2009) 62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006
46. Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr (2016) 16(1):170. doi: 10.1186/s12877-016-0349-4
47. Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J cachexia sarcopenia Muscle (2022) 13(1):86–99. doi: 10.1002/jcsm.12783
48. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell (2012) 21(3):309–22. doi: 10.1016/j.ccr.2012.02.022
49. Tisdale MJ. Cancer cachexia. Curr Opin Gastroenterol (2010) 26(2):146–51. doi: 10.1097/MOG.0b013e3283347e77
50. Yamashita M, Kamiya K, Matsunaga A, Kitamura T, Hamazaki N, Matsuzawa R, et al. Prognostic value of psoas muscle area and density in patients who undergo cardiovascular surgery. Can J Cardiol (2017) 33(12):1652–9. doi: 10.1016/j.cjca.2017.10.009
51. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet (London England) (2019) 393(10191):2636–46. doi: 10.1016/S0140-6736(19)31138-9
52. Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J cachexia sarcopenia Muscle (2021) 12(5):1122–35. doi: 10.1002/jcsm.12755
53. Hu X, Liao DW, Yang ZQ, Yang WX, Xiong SC, Li X. Sarcopenia predicts prognosis of patients with renal cell carcinoma: A systematic review and meta-analysis. Int Braz J urol (2020) 46(5):705–15. doi: 10.1590/s1677-5538.ibju.2019.0636
54. Xiao DY, Luo S, O'Brian K, Ganti A, Riedell P, Sanfilippo KM, et al. Impact of sarcopenia on treatment tolerance in United States veterans with diffuse large B-cell lymphoma treated with CHOP-based chemotherapy. Am J Hematol (2016) 91(10):1002–7. doi: 10.1002/ajh.24465
55. Prado CM, Lima IS, Baracos VE, Bies RR, McCargar LJ, Reiman T, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer chemother Pharmacol (2011) 67(1):93–101. doi: 10.1007/s00280-010-1288-y
56. Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr (Edinburgh Scotland) (2018) 37(4):1101–13. doi: 10.1016/j.clnu.2017.07.010
57. Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab (2018) 27(1):10–21. doi: 10.1016/j.cmet.2017.09.015
58. Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care (2015) 18(6):535–51. doi: 10.1097/MCO.0000000000000216
59. Naderi N, Kleine CE, Park C, Hsiung JT, Soohoo M, Tantisattamo E, et al. Obesity paradox in advanced kidney disease: from bedside to the bench. Prog Cardiovasc Dis (2018) 61(2):168–81. doi: 10.1016/j.pcad.2018.07.001
60. Xiao DY, Luo S, O'Brian K, Sanfilippo KM, Ganti A, Riedell P, et al. Longitudinal body composition changes in diffuse large B-cell lymphoma survivors: A retrospective cohort study of United States veterans. J Natl Cancer Inst (2016) 108(11):djw145. doi: 10.1093/jnci/djw145
61. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Directors Assoc (2020) 21(3):300–7 e2. doi: 10.1016/j.jamda.2019.12.012
62. Zhang T, Cheng JK, Hu YM. Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res Rev (2022) 81:101739. doi: 10.1016/j.arr.2022.101739
63. Liu H, Chen J, Shao W, Yan S, Ding S. Efficacy and safety of Omega-3 polyunsaturated fatty acids in adjuvant treatments for colorectal cancer: A meta-analysis of randomized controlled trials. Front Pharmacol (2023) 14:1004465. doi: 10.3389/fphar.2023.1004465
64. Ambrosio MR, Spagnoli L, Perotti B, Petrelli F, Caini S, Saieva C, et al. Paving the path for immune enhancing nutrition in colon cancer: modulation of tumor microenvironment and optimization of outcomes and costs. Cancers (2023) 15(2):437. doi: 10.3390/cancers15020437
65. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol (2008) 9(7):629–35. doi: 10.1016/S1470-2045(08)70153-0
Keywords: hematological malignancies, sarcopenia, prevalence, survival, meta-analysis
Citation: Xiong J, Chen K, Huang W, Huang M, Cao F, Wang Y and Chen Q (2023) Prevalence and effect on survival of pre-treatment sarcopenia in patients with hematological malignancies: a meta-analysis. Front. Oncol. 13:1249353. doi: 10.3389/fonc.2023.1249353
Received: 28 June 2023; Accepted: 11 September 2023;
Published: 06 October 2023.
Edited by:
Monica Balzarotti, Humanitas Research Hospital, ItalyReviewed by:
Maria Raffaella Ambrosio, University of Siena, ItalyGayatri Vishwakarma, Indian Spinal Injuries Centre, India
Copyright © 2023 Xiong, Chen, Huang, Huang, Cao, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qifeng Chen, NTIzNDI3NzQxQHFxLmNvbQ==
 Jianzhu Xiong1
Jianzhu Xiong1 Kangkang Chen
Kangkang Chen Feiyan Cao
Feiyan Cao Qifeng Chen
Qifeng Chen