- 1Department of Radiation Oncology, Georgetown University Hospital, Washington, DC, United States
- 2Department of Urology, Einstein Healthcare Network, Philadelphia, PA, United States
- 3Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, United States
- 4School of Medicine, Georgetown University Hospital, Washington, DC, United States
- 5Department of Radiation Oncology, Beth Israel Deaconess Medical Center, Boston, MA, United States
- 6Department of Radiation Oncology, Tampa General Hospital, University of South Florida, Tampa, FL, United States
- 7Biomedical Research Institute, North Carolina Central State, Durham, NC, United States
- 8Department of Urology, Georgetown University Hospital, Washington, DC, United States
- 9Department of Medical Oncology, Georgetown University Hospital, Washington, DC, United States
Purpose: Intensity-modulated radiation therapy (IMRT) with brachytherapy boost for unfavorable prostate cancer has been shown to improve biochemical relapse-free survival compared to IMRT alone. Stereotactic body radiation therapy (SBRT) is a less-invasive alternative to brachytherapy. Early outcomes utilizing SBRT boost suggest low rates of high-grade toxicity with a maintained patient-reported quality of life. Here, we report the 5-year progression-free survival (PFS) and prostate cancer-specific survival (PCSS) of patients treated with IMRT plus SBRT boost.
Materials and methods: Between 2008 and 2020, 255 patients with unfavorable prostate cancer were treated with robotic SBRT (19.5 Gy in three fractions) followed by fiducial-guided IMRT (45–50.4 Gy) according to an institutional protocol. For the first year, the patient’s PSA level was monitored every 3 months, biannually for 2 years, and annually thereafter. Failure was defined as nadir + 2 ng/mL or a rising PSA with imaging suggestive of recurrence. Detection of recurrence also included digital rectal examination and imaging studies, such as MRI, CT, PET/CT, and/or bone scans. PFS and PCSS were calculated using the Kaplan–Meier method.
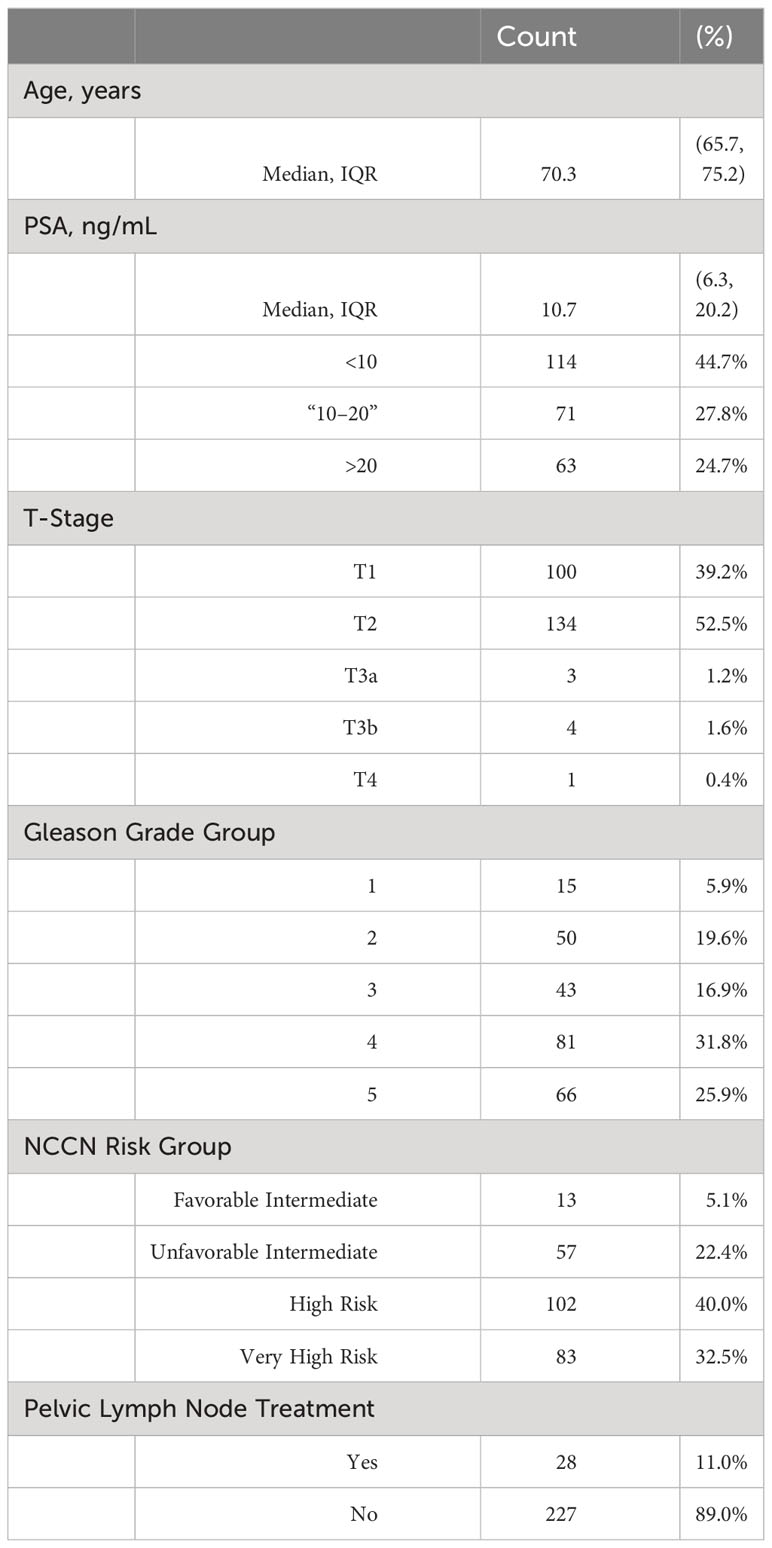
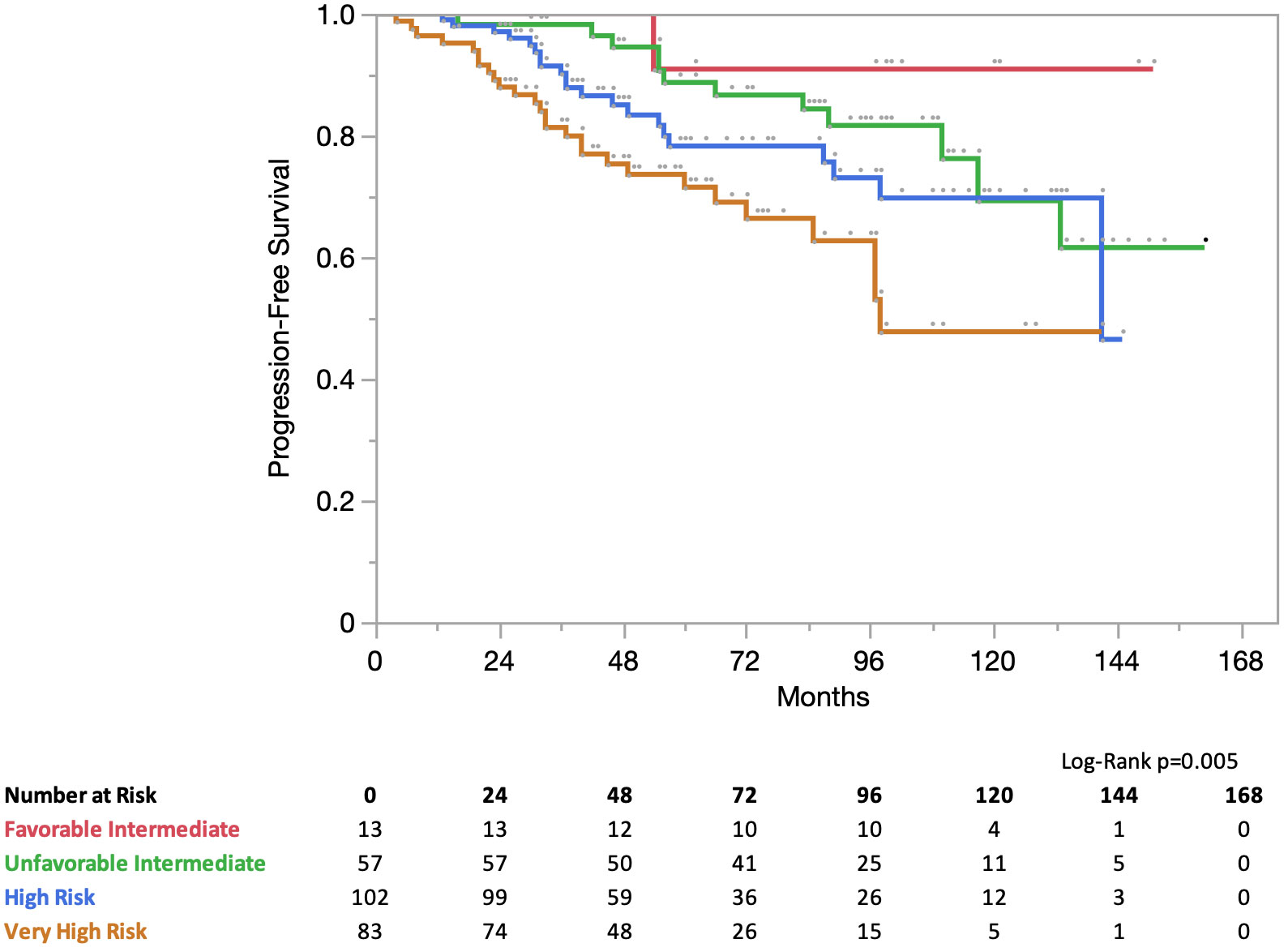
Results: The median follow-up period was 71 months. According to the NCCN risk classification, 5% (13/255) of the patients had favorable intermediate-risk disease, 23% (57/255) had unfavorable intermediate-risk disease, 40% (102/255) had high-risk disease, and 32% (83/255) had very high-risk disease. Androgen deprivation therapy was administered to 80% (204/255) of the patients. Elective pelvic lymph node IMRT was performed in 28 (10%) patients. The PFS for all patients at 5 years was 81% (favorable intermediate risk, 91%; unfavorable intermediate risk, 89%; high-risk, 78%; and very-high risk, 72%). The PCSS for all patients at 5 years was 97% (favorable intermediate risk, 100%; unfavorable intermediate risk, 100%; high risk, 100%; and very high risk, 89%).
Conclusion: The incidence of failure following IMRT plus SBRT for unfavorable prostate cancer remains low at 5 years.
1 Introduction
In total, 248,500 men were diagnosed with prostate cancer in 2022, making it the most prevalent malignancy among men in the United States (1). Approximately 20% of patients newly diagnosed with prostate cancer present with high-risk disease, with an expected increase in the proportion due to decreased PSA screening (2). Radiotherapy is the first-line treatment for patients with prostate cancer. Several randomized prospective trials have demonstrated that dose-escalated radiotherapy results in improved biochemical free survival in patients with intermediate- and high-risk diseases (3–5). The development and improvement of image-guided radiation therapy (IGRT) (6) and low-dose-rate brachytherapy boost (7, 8) have further improved outcomes in these patients. SBRT offers the potential for better results.
Large radiation fraction sizes have been shown to likely confer a radiobiologic advantage in the treatment of prostate adenocarcinoma (9), supporting the use of high-dose rate (HDR) brachytherapy as a boost to external beam radiation therapy (EBRT) for intermediate- and high-risk patients. Several retrospective reviews have reported 5-year biochemical control rates of 89%–93% (10–12) and 69%–83% (10–13) for intermediate- and high-risk prostate cancer, respectively. These results have also been shown prospectively (14–16). Not unexpectedly, these improved outcomes are achieved with an increased risk of significant long-term genitourinary toxicities including urethral stricture and incontinence (16–18).
SBRT efficiently delivers high doses of radiation without invasive procedures. We have examined the use of stereotactic body radiation therapy (SBRT) as a boost to image-guided intensity-modulated radiation therapy (IMRT) for the treatment of patients with unfavorable risk prostate cancer to maximize the benefits of administering high doses per fraction, and to minimize the short- and long-term consequences. Previously, we have reported early outcomes of this treatment modality, including: 3-year biochemical recurrence free survival, acute toxicity, 3-year toxicity and quality of life (18–20). Our reports suggest that this treatment approach has a minimal impact on long-term quality of life and provides excellent early disease outcomes (19, 20). These results have been demonstrated in several other studies (21–24). Here, we report the five-year progression-free survival (PFS) and prostate cancer-specific survival (PCSS) in a cohort of patients treated at Georgetown University Medical Center with this treatment approach.
2 Methods
2.1 Patient selection
Patients with histologically confirmed adenocarcinoma of the prostate and intermediate, high, or very high-risk prostate cancer according to the National Comprehensive Cancer Network (NCCN) risk grouping were included in the study. Several patients (~5%) were categorized as having favorable intermediate risk according to the NCCN criteria, as they were treated according to this institutional protocol prior to the establishment of the criteria. All patients underwent a bone scan and pelvic imaging (pelvic CT and/or MRI) as clinically indicated according to the national guidelines. The exclusion criteria were clinically involved lymph nodes, bone metastases or prior pelvic radiotherapy. Androgen deprivation therapy was considered for all unfavorable intermediate, high, and very high-risk patients and was ultimately administered at the discretion of the treating physicians. Institutional IRB approval was obtained for this study (IRB 09-510).
2.2 SBRT treatment planning and delivery
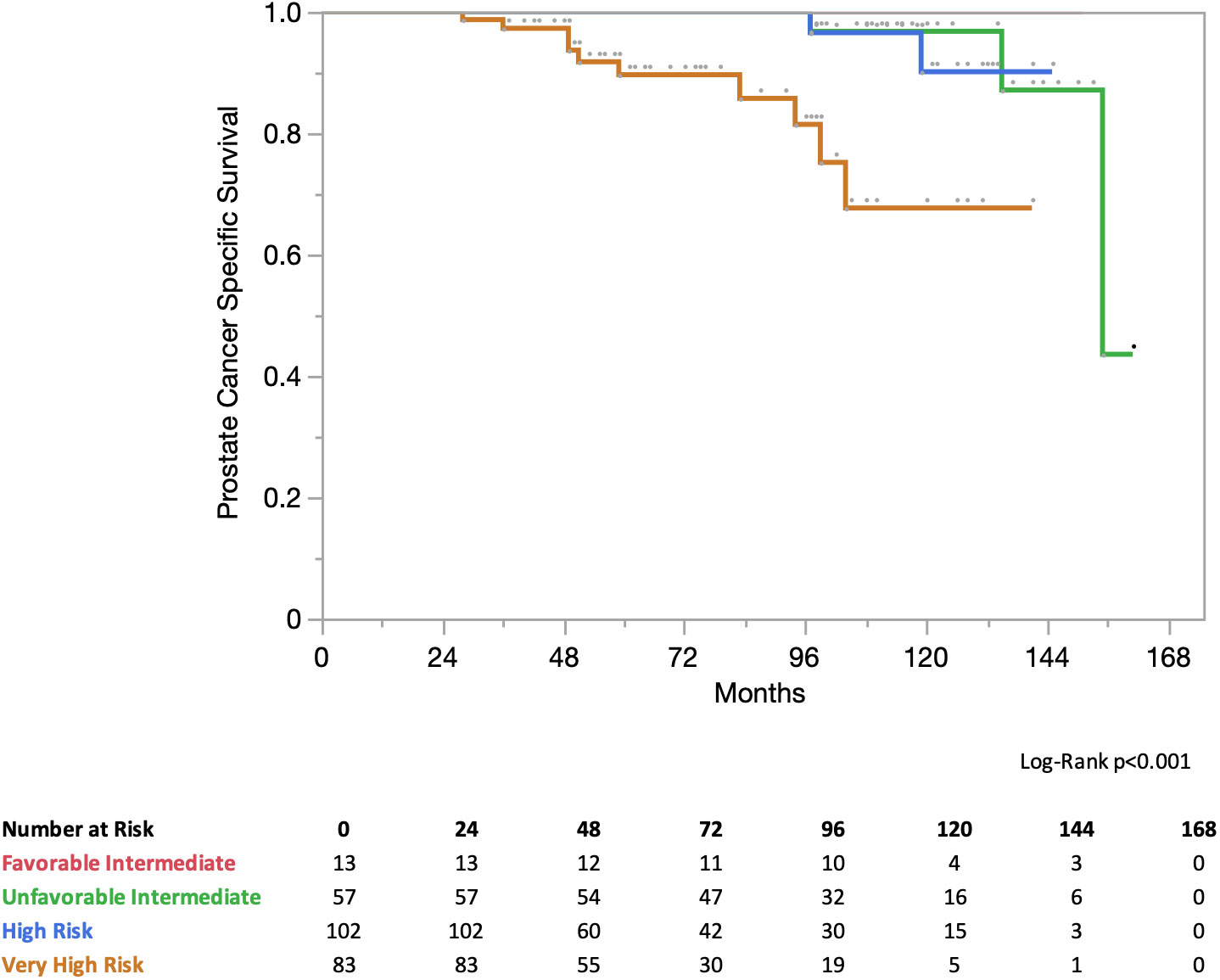
Treatment planning and delivery have been previously reported (20). Briefly, all patients received fiducials placed in the prostate prior to treatment planning. The patients then underwent MRI and thin-cut (1.25 mm) CT scan of the pelvis with an empty bladder. If a patient had a contraindication to MRI, a CT urethrogram at the time of CT simulation was employed as an alternative imaging approach to identify the location of the prostatic apex (25). Patients were advised to adhere to a low-gas, low-motility diet starting at least five days prior to all treatment planning imaging and treatment delivery. An enema was administered 1 h–2 h prior to imaging and SBRT. The CT and MR images were fused for treatment planning. The clinical target volume (CTV1) included the prostate, areas of radiographic extracapsular extension and seminal vesicles proximal to the point of separation. The SBRT planning target volume (PTV1) was equal to the CTV1 expanded 3 mm posteriorly and 5 mm in all other dimensions (Figure 1A). The prescription dose was 19.5 Gy to PTV1 delivered in three fractions of 6.5 Gy over 3–5 days. The prescription isodose line was limited to ≥75%, which limited the maximum prostatic urethral dose to 133% of the prescription dose. The rectum, bladder, penile bulb, and membranous urethra were contoured and evaluated using dose-volume histogram analysis during treatment planning using Multiplan (Accuray Inc., Sunnyvale, CA, USA) inverse treatment planning.
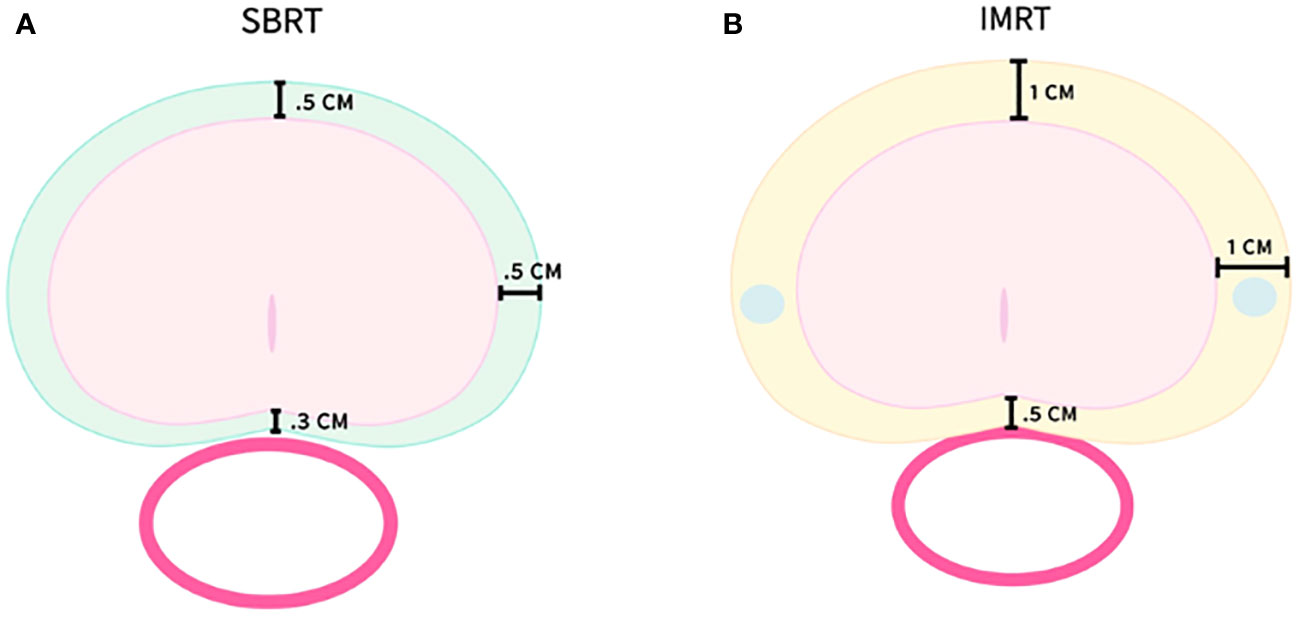
Figure 1 SBRT and IMRT volumes with PTV expansions. (A) PTV1–SBRT treatment volume. (B) PTV2–IMRT treatment volume.
Following SBRT, IMRT treatment was initiated the following week. Most patients (89%) were treated to the prostate alone with a more generous PTV2 including a margin of 1.0 cm around CTV1, except at the rectal interface where a margin of 0.5 cm was added (Figure 1B). A minority of patients (11%) were treated in the prostate and pelvic lymph node basins with PTV3 encompassing the previously noted expansion (PTV2) with the addition of the RTOG consensus lymph node basins (26). Daily doses of 1.8 Gy were delivered to PTV2/PTV3 5 days a week to a total dose of 45 Gy–50.4 Gy in 25–28 fractions. One hundred percent of PTV2/PTV3 received at least 95% of the prescription dose, and 5% of the volume received no more than 105% of the prescription dose. Dose and volume constraints as well as the process of combining the IMRT and SBRT plans into a radiobiologically equivalent dose-volume histogram (DVH) have been previously described (19, 20, 27).
2.3 Linear-quadratic transformation of a sample combined physical IMRT plus SBRT boost dose-volume histogram to a radiobiologically equivalent DVH
A radiobiologically equivalent dose of DVH was generated by adding doses in 2 Gy equivalents for IMRT and SBRT plans from a sample patient (28). Cumulative DVHs were extracted from the treatment planning software and converted to radiobiologically equivalent DVHs using MIM software (MIMvista Corporation). An α/β ratio of 1.5 was utilized to transform the target volume doses (GTV and PTV), and an α/β ratio of 3 was used to transform doses for all other organs at risk (OAR).
2.4 Follow-up
Patients were assessed at the start of and one month after therapy, every 3 months for the first year, and every 6 months thereafter. The patient’s PSA level was monitored every 3 months during the first year, biannually for 2 years, and annually thereafter. Failure was defined using the nadir + 2 ng/mL definition or a rising PSA level after long-term nadir with imaging suggestive of disease recurrence. Detection of recurrence included digital rectal examination, imaging studies such as MRI, CT, PET/CT (sodium-F, PSMA, and Axumin), and/or bone scan. PFS and PCSS were calculated using the Kaplan–Meier method.
3 Results
Between 2008 and 2020, 255 patients with intermediate- and high-risk prostate cancer were treated using an institutional IMRT plus SBRT boost protocol. The median follow-up was 5.9 years (range, 2–12 years). The patient characteristics are shown in Table 1. The median patient age was 70 years (IQ range, 65–75 years). The median pretreatment prostate-specific antigen (PSA) was 10.7 ng/ml. (IQ range, 6.3 ng/ml–20.2 ng/ml). A total of 29 (11%) patients had a PSA level >40 ng/ml prior to treatment. According to the NCCN Risk Classification, 5% were diagnosed with favorable intermediate-, 23% with unfavorable intermediate-, 40% with high-risk disease and 32% with very high-risk disease, respectively. Approximately 10% of the patients received prophylactic radiation to the RTOG consensus pelvic lymph node basins. Approximately 80% of patients received androgen deprivation therapy (ADT).
At 5 years, progression-free survival (PFS) for all patients was 81%. In the NCCN risk group the PFS was 5 years for favorable intermediate risk: 91% for unfavorable intermediate risk, 89% for high-risk, 78% for high-risk, and 72% for very high-risk (Figure 2). At 5 years, the prostate cancer-specific survival (PCSS) for all patients was 97%. In the NCCN risk group, the PCSS at 5 years for favorable intermediate risk was 100%, unfavorable intermediate risk was 100%, high-risk was 100%, and very high-risk was 89% (Figure 3).
4 Discussion
This study aimed to assess progression-free survival and prostate cancer-specific survival outcomes in patients with prostate cancer patients receiving IMRT plus SBRT boost. ASCENDE-RT examined men with intermediate- and high-risk prostate cancer randomized to receive pelvic irradiation followed by dose-escalated EBRT boost or LDR brachytherapy boost. In this trial, bPFS was significantly improved in the LDR brachytherapy arm compared to the EBRT arm (83% vs. 62% 9-year bPFS). Patients in the EBRT arm were twice as likely to experience biochemical failure. No significant difference was detected in the overall survival difference between the treatment arms. Importantly, the ASCENDE-RT trial reported a cumulative late grade ≥3 toxicity of 18.4% at 5-years compared with 5.2% in the EBRT arm. Specifically, LDR increases the risk of needing temporary catheterization and/or incontinence pads because of urethral strictures, urinary retention, or incontinence. SBRT boost was chosen for this study due to the potential radiobiological benefits of hypofractionation in addition to being a less invasive and toxic alternative to brachytherapy boost (18–21).
In our study, the incidence of failure following IMRT plus SBRT boost was low at 5-years. PFS and PCSS were 81% and 97% at 5-years in all patients. Unsurprisingly, PFS decreased in the high-risk groups: 91%, 89%, 78%, and 72% for favorable intermediate-risk, unfavorable intermediate-risk, high-risk, and very high-risk groups, respectively. The PCSS was 100% in favorable intermediate risk, unfavorable intermediate risk, and high-risk disease, but decreased to 89% in our very high-risk cohort. These results appear similar to those reported for brachytherapy boost, despite our very high-risk cohort, robust surveillance, and lack of prophylactic pelvic nodal irradiation.
The Phoenix definition (nadir PSA + 2 ng/mL) after radiation therapy was used to classify biochemical failure. However, the Phoenix criteria were developed for low-dose, conventionally fractionated EBRT. Compared with EBRT, PSA nadirs are lower with brachytherapy and SBRT (29, 30). An alternative criterion for classifying biochemical failure in SBRT patients has been proposed (31). As a result, our practice includes imaging before meeting the Phoenix criteria for failure. Ultimately, this could lead to lower metastasis-free survival and bRFS.
The prophylactic treatment of pelvic lymph nodes with RT has been a source of ongoing debate. The rationale has been to eradicate nodal micrometastases, with the goal of improving regional control. In GETUG-01, there was no observed benefit in event-free or OS with pelvic irradiation, although post-hoc analysis favored pelvic RT in patients with a <15% Roach nodal risk. More recently, POP-RT previously randomized prophylactic whole pelvic nodal RT to prostate-only radiation in 224 high-risk prostate cancer patients, with a median Roach nodal risk of 37.8%. In that study, WPRT demonstrated a higher 5-year bFFS (95% vs. 81.2%) and PFS (89.5% vs. 77.2%) than PORT. However, there was no significant difference in the 5-year overall survival (92.5% vs. 90.8%). Our study included 10% of patients who received elective nodal IMRT, many of whom had PSA >40 ng/mL, had T3+ disease, and were classified as very-high risk of NCCN. Similarly, POP-RT also included 162 patients with T3+ disease, 60 patients with >50 PSA levels, and 116 patients with high-risk disease. However, notably, the POP-RT trial utilized PSMA PET for staging in 80% of the patients, excluding patients with occult metastases. In our population study, PMSA PET was not used for staging.
Our study has several limitations. Our study was retrospective in nature and inherently limited. Our patients did not undergo fluciclovine/prostate specific membrane antigen (PSMA) PET scans for initial staging due to a lack of availability in the United States; as a result, many of the very high-risk patients in our study had occult metastases prior to treatment. Approximately 90% of patients did not receive prophylactic pelvic lymph node irradiation. Since the adoption of VMAT at our center and with improved outcomes shown in the POP-RT study, it is our current practice to treat high-risk patients with prophylactic pelvic lymph node irradiation. Our treatment approach may be unduly burdensome because of the extended (5 weeks) course of pelvic radiation used in this study. Future studies utilizing short-course pelvic radiation and directly comparing brachytherapy boost with SBRT boost are required.
5 Conclusion
IMRT with SBRT boost is a promising treatment option for men with unfavorable prostate cancer. The incidence of failure following IMRT plus SBRT for unfavorable prostate cancer remains low at 5 years. Future studies directly comparing brachytherapy boost with SBRT boost are warranted, with endpoints including disease control and patient-reported quality of life.
Data availability statement
The datasets presented in this article are not readily available because of patient confidentiality. Requests to access the datasets should be directed to MC, bWljaGFlbC5hLmNhcnJhc3F1aWxsYUBndW5ldC5nZW9yZ2V0b3duLmVkdQ==.
Ethics statement
The studies involving humans were approved by Georgetown University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to the treatment of patients, conceptualization of the study, drafting and editing the manuscript and/or follow up of patients and acquisition of continued follow up data over 5 years. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the Radiation Oncology Committee of the Alliance for Clinical Trials in Oncology for their helpful discussions.
Conflict of interest
We would like to disclose that SC, the principal investigator for this study is a clinical consultant for Accuray.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Agrawal V, Ma X, Hu JC, Barbieri CE, Nagar H. Trends in diagnosis and disparities in initial management of high-risk prostate cancer in the US. JAMA Netw Open (2020) 3(8):e2014674. doi: 10.1001/jamanetworkopen.2020.14674
3. Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. (2005) 294(10):1233–9. doi: 10.1001/jama.294.10.1233
4. Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys (2008) 70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054
5. Peeters STH, Heemsbergen WD, Koper PCM, Putten van WLJ, Slot A, Dielwart MFH, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol Off J Am Soc Clin Oncol (2006) 24(13):1990–6. doi: 10.1200/JCO.2005.05.2530
6. Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys (2012) 84(1):125–9. doi: 10.1016/j.ijrobp.2011.11.047
7. Shilkrut M, Merrick GS, McLaughlin PW, Stenmark MH, Abu-Isa E, Vance SM, et al. The addition of low-dose-rate brachytherapy and androgen-deprivation therapy decreases biochemical failure and prostate cancer death compared with dose-escalated external-beam radiation therapy for high-risk prostate cancer. Cancer. (2013) 119(3):681–90. doi: 10.1002/cncr.27784
8. Morris WJ, Tyldesley S, Pai HH, Keyes M, Halperin R, Pai H, et al. ASCENDE-RT*: A multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRT-B) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable-risk localized prostate cancer. J Clin Oncol (2015) 33(7_suppl):3–3. doi: 10.1200/jco.2015.33.7_suppl.3
9. Phan TP, Syed AMN, Puthawala A, Sharma A, Khan F. High dose rate brachytherapy as a boost for the treatment of localized prostate cancer. J Urol (2007) 177(1):123–127; discussion 127. doi: 10.1016/j.juro.2006.08.109
10. Demanes DJ, Rodriguez RR, Schour L, Brandt D, Altieri G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy’s 10-year results. Int J Radiat Oncol Biol Phys (2005) 61(5):1306–16. doi: 10.1016/j.ijrobp.2004.08.014
11. Galalae RM, Martinez A, Mate T, Mitchell C, Edmundson G, Nuernberg N, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys (2004) 58(4):1048–55. doi: 10.1016/j.ijrobp.2003.08.003
12. Martinez AA, Gustafson G, Gonzalez J, Armour E, Mitchell C, Edmundson G, et al. Dose escalation using conformal high-dose-rate brachytherapy improves outcome in unfavorable prostate cancer. Int J Radiat Oncol Biol Phys (2002) 53(2):316–27. doi: 10.1016/s0360-3016(02)02733-5
13. Sathya JR, Davis IR, Julian JA, Guo Q, Daya D, Dayes IS, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol Off J Am Soc Clin Oncol (2005) 23(6):1192–9. doi: 10.1200/JCO.2005.06.154
14. Hoskin PJ, Rojas AM, Bownes PJ, Lowe GJ, Ostler PJ, Bryant L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol (2012) 103(2):217–22. doi: 10.1016/j.radonc.2012.01.007
15. Khor R, Duchesne G, Tai KH, Foroudi F, Chander S, Van Dyk S, et al. Direct 2-arm comparison shows benefit of high-dose-rate brachytherapy boost vs external beam radiation therapy alone for prostate cancer. Int J Radiat Oncol Biol Phys (2013) 85(3):679–85. doi: 10.1016/j.ijrobp.2012.07.006
16. Hsu IC, Bae K, Shinohara K, Pouliot J, Purdy J, Ibbott G, et al. Phase II trial of combined high-dose-rate brachytherapy and external beam radiotherapy for adenocarcinoma of the prostate: preliminary results of RTOG 0321. Int J Radiat Oncol Biol Phys (2010) 78(3):751–8. doi: 10.1016/j.ijrobp.2009.08.048
17. Sullivan L, Williams SG, Tai KH, Foroudi F, Cleeve L, Duchesne GM. Urethral stricture following high dose rate brachytherapy for prostate cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol (2009) 91(2):232–6. doi: 10.1016/j.radonc.2008.11.013
18. Rodda S, Tyldesley S, Morris WJ, Keyes M, Halperin R, Pai H, et al. ASCENDE-RT: an analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys (2017) 98(2):286–95. doi: 10.1016/j.ijrobp.2017.01.008
19. Mercado C, Kress MA, Cyr RA, Chen LN, Yung TM, Bullock EG, et al. Intensity-modulated radiation therapy with stereotactic body radiation therapy boost for unfavorable prostate cancer: the georgetown university experience. Front Oncol (2016) 6:114. doi: 10.3389/fonc.2016.00114
20. Paydar I, Pepin A, Cyr RA, King J, Yung TM, Bullock EG, et al. Intensity-modulated radiation therapy with stereotactic body radiation therapy boost for unfavorable prostate cancer: A report on 3-year toxicity. Front Oncol (2017) 7:5. doi: 10.3389/fonc.2017.00005
21. Lin YW, Lin LC, Lin KL. The early result of whole pelvic radiotherapy and stereotactic body radiotherapy boost for high-risk localized prostate cancer. Front Oncol (2014) 4:278. doi: 10.3389/fonc.2014.00278
22. Katz A, Kang J. Stereotactic body radiotherapy with or without external beam radiation as treatment for organ confined high-risk prostate carcinoma: a six year study. Radiat Oncol (2014) 9(1):1. doi: 10.1186/1748-717X-9-1
23. Katz AJ, Santoro M, Ashley R, Diblasio F, Witten M. Stereotactic body radiotherapy as boost for organ-confined prostate cancer. Technol Cancer Res Treat (2010) 9(6):575–82. doi: 10.1177/153303461000900605
24. Anwar M, Weinberg V, Seymour Z, Hsu IJ, Roach M, Gottschalk AR. Outcomes of hypofractionated stereotactic body radiotherapy boost for intermediate and high-risk prostate cancer. Radiat Oncol Lond Engl (2016) 11:8. doi: 10.1186/s13014-016-0585-y
25. Paydar I, Kim BS, Cyr RA, Rashid H, Anjum A, Yung TM, et al. Urethrogram-directed stereotactic body radiation therapy for clinically localized prostate cancer in patients with contraindications to magnetic resonance imaging. Front Oncol (2015) 5:194. doi: 10.3389/fonc.2015.00194
26. Hall WA, Paulson E, Davis BJ, Spratt DE, Morgan TM, Dearnaley D, et al. NRG oncology updated international consensus atlas on pelvic lymph node volumes for intact and postoperative prostate cancer. Int J Radiat Oncol Biol Phys (2021) 109(1):174–85. doi: 10.1016/j.ijrobp.2020.08.034
27. Oermann EK, Slack RS, Hanscom HN, Lei S, Suy S, Park HU, et al. A pilot study of intensity modulated radiation therapy with hypofractionated stereotactic body radiation therapy (SBRT) boost in the treatment of intermediate- to high-risk prostate cancer. Technol Cancer Res Treat (2010) 9(5):453–62. doi: 10.1177/153303461000900503
28. Wheldon TE, Deehan C, Wheldon EG, Barrett A. The linear-quadratic transformation of dose-volume histograms in fractionated radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol (1998) 46(3):285–95. doi: 10.1016/s0167-8140(97)00162-x
29. Jiang NY, Dang AT, Yuan Y, Chu FI, Shabsovich D, King CR, et al. Multi-institutional analysis of prostate-specific antigen kinetics after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys (2019) 105(3):628–36. doi: 10.1016/j.ijrobp.2019.06.2539
30. Hegde AM, Cherry CR, Stroud CRG, He A, Ge W, Xu X, et al. Outcomes of immunomodulatory radiation strategies in combination with nivolumab compared with single agent nivolumab in lung cancer patients. J Clin Oncol (2018) 36(15_suppl):e21134. doi: 10.1200/JCO.2018.36.15_suppl.e21134
31. Ma TM, Sun Y, Malone S, Roach M, Dearnaley D, Pisansky TM, et al. Sequencing of androgen-deprivation therapy of short duration with radiotherapy for nonmetastatic prostate cancer (SANDSTORM): A pooled analysis of 12 randomized trials. J Clin Oncol Off J Am Soc Clin Oncol (2023) 41(4):881–92. doi: 10.1200/JCO.22.00970
Keywords: prostate cancer, SBRT, IMRT, CyberKnife, SBRT boost
Citation: Carrasquilla M, Sholklapper T, Pepin AN, Hodgins N, Lei S, Rashid A, Danner M, Zwart A, Bolanos G, Ayoob M, Yung T, Aghdam N, Collins B, Suy S, Kumar D, Hankins R, Kowalczyk K, Dawson N and Collins S (2023) Intensity modulated radiation therapy with stereotactic body radiation therapy boost for unfavorable prostate cancer: five-year outcomes. Front. Oncol. 13:1240939. doi: 10.3389/fonc.2023.1240939
Received: 15 June 2023; Accepted: 23 October 2023;
Published: 23 November 2023.
Edited by:
Xinglei Shen, University of Kansas Medical Center, United StatesReviewed by:
Shih-Chang Wang, Chi Mei Medical Center, TaiwanWee Loon Ong, The Alfred Hospital, Australia
Copyright © 2023 Carrasquilla, Sholklapper, Pepin, Hodgins, Lei, Rashid, Danner, Zwart, Bolanos, Ayoob, Yung, Aghdam, Collins, Suy, Kumar, Hankins, Kowalczyk, Dawson and Collins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Carrasquilla, TWljaGFlbC5hLmNhcnJhc3F1aWxsYUBndW5ldC5nZW9yZ2V0b3duLmVkdQ==
 Michael Carrasquilla
Michael Carrasquilla Tamir Sholklapper
Tamir Sholklapper Abigail N. Pepin
Abigail N. Pepin Nicole Hodgins
Nicole Hodgins Siyuan Lei1
Siyuan Lei1 Abdul Rashid
Abdul Rashid Malika Danner
Malika Danner Nima Aghdam
Nima Aghdam Deepak Kumar
Deepak Kumar Ryan Hankins
Ryan Hankins Sean Collins
Sean Collins