
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 29 November 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1240405
This article is part of the Research TopicAdvances in Immunotherapy and Combination Therapy for Biliary Tract CancersView all 6 articles
Primary pancreatic malignancies are mostly composed of the adenocarcinoma histological subtype. However, squamous cell carcinoma (SCC) accounts for approximately 0.5%–1% of all malignant pancreatic cancers. Because of the rarity of SCC of the pancreas, guideline-directed treatment is lacking, treatment response is difficult to access, and treatment options are poorly defined. Here, we report a case of a 65-year-old man diagnosed with pancreatic carcinoma with dominant squamous cell differentiation, who achieved complete pathologic response (CPR) after treatment with gemcitabine, cisplatin, and nab-paclitaxel every 14 days for six cycles and who continues to lead a high quality of life 7 months later. To our knowledge, this is the first case of CPR in a case of SCC of the pancreas. To highlight the ambiguity and the need for further studies, we also performed a narrative review analyzing recent cases and compared them to our case.
Pancreatic carcinoma accounts for about 3% of all cancers in the United States and has an average 5-year relative survival rate of 44% when localized. This rate decreases to 3% when distant metastasis is present (1). Squamous cell carcinoma (SCC) is an extremely rare finding and accounts for only approximately 0.5%–1% of all malignant pancreatic cancers (2, 3). Because of this infrequency, there are no established or standardized guidelines to treat SCC of the pancreas, and many different treatment regimens have been tried among the published case reports of pancreatic SCC. Here, we report a case of a 65-year-old Hispanic man who was diagnosed with pancreatic carcinoma with dominant squamous cell differentiation who achieved complete pathologic response (CPR) after treatment with gemcitabine, cisplatin, and nab-paclitaxel every 14 days for six cycles (Figure 1). This case is unique in that it is the first known CPR of a squamous cell pancreatic carcinoma published in the literature.
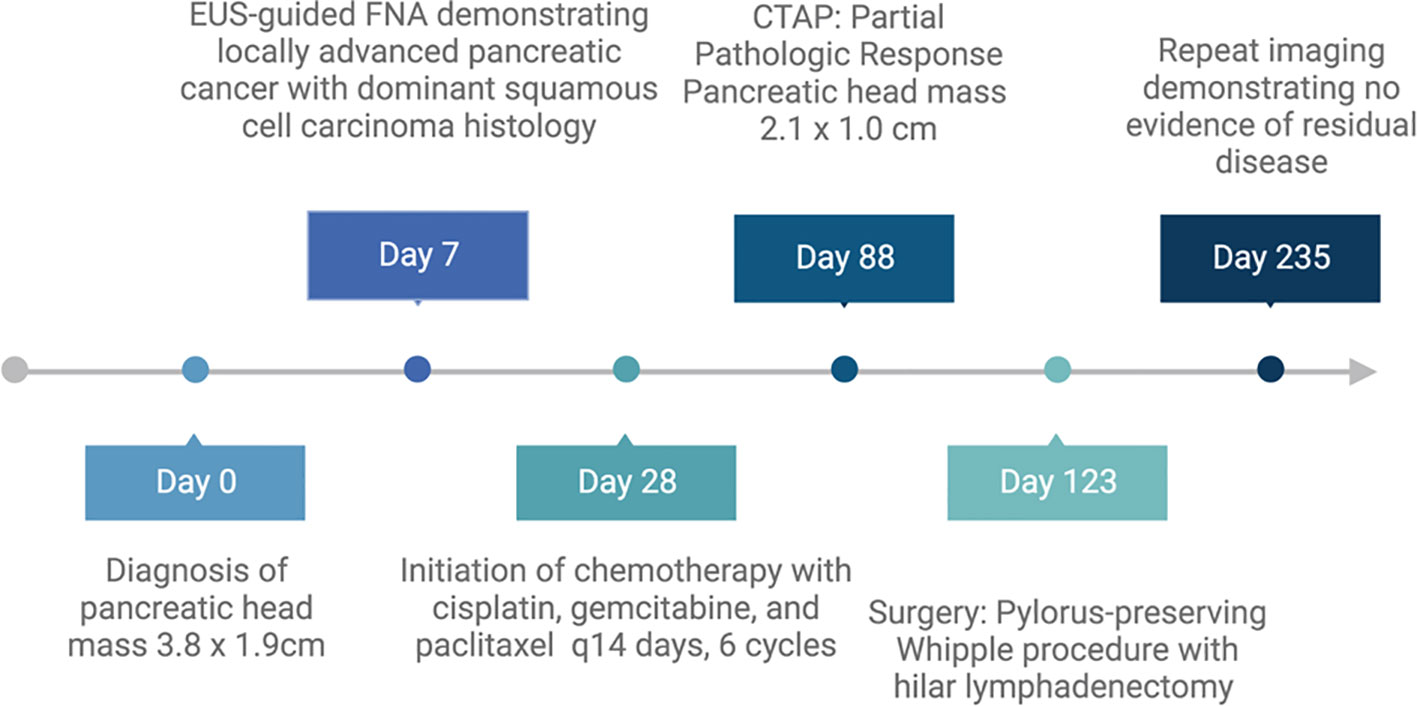
Figure 1 Timeline of patient’s course of treatment. Created with BioRender.com.
A 65-year-old man with a past medical history of type 2 diabetes on insulin and hyperlipidemia on atorvastatin presented to the emergency department with complaints of umbilical abdominal pain radiating to his back for a year prior. He had experienced constant, increasing pain over the past month that resulted in difficulty in sleeping due to overwhelming pain. He had some itching of his palms bilaterally and associated fatigue. He denied any fevers, chills, night sweats, headaches, yellowing of his eyes or skin, nausea, vomiting, diarrhea, or unintended weight loss. He also was able to eat a significant amount of food and denied early satiety. He took no medications. He had no smoking history and drank 2 beers a month. He had no family history of pancreatic or gastrointestinal cancers. His physical exam was notable for epigastric pain to light palpation without visible nodules or masses. He had no jaundice or scleral icterus.
His laboratory work was notable for a total bilirubin level of 0.3 mg/dL (reference, 0.4–2.0), aspartate aminotransferase of 18 U/L (reference, 15–37), alanine aminotransferase of 20 U/L (reference, 16–61), and alkaline phosphatase of 71 U/L (reference, 45–117). Computed tomography (CT) of the chest, abdomen, and pelvis was notable for a mass of 3.8 cm × 1.9 cm at the head of the pancreas concerning for a neoplasm with adjacent lymph nodes. Both the pancreatic mass and the lymph node were larger compared with that in an initial CT performed about 4 weeks earlier. The lungs were without metastatic foci (Figures 2A, B).
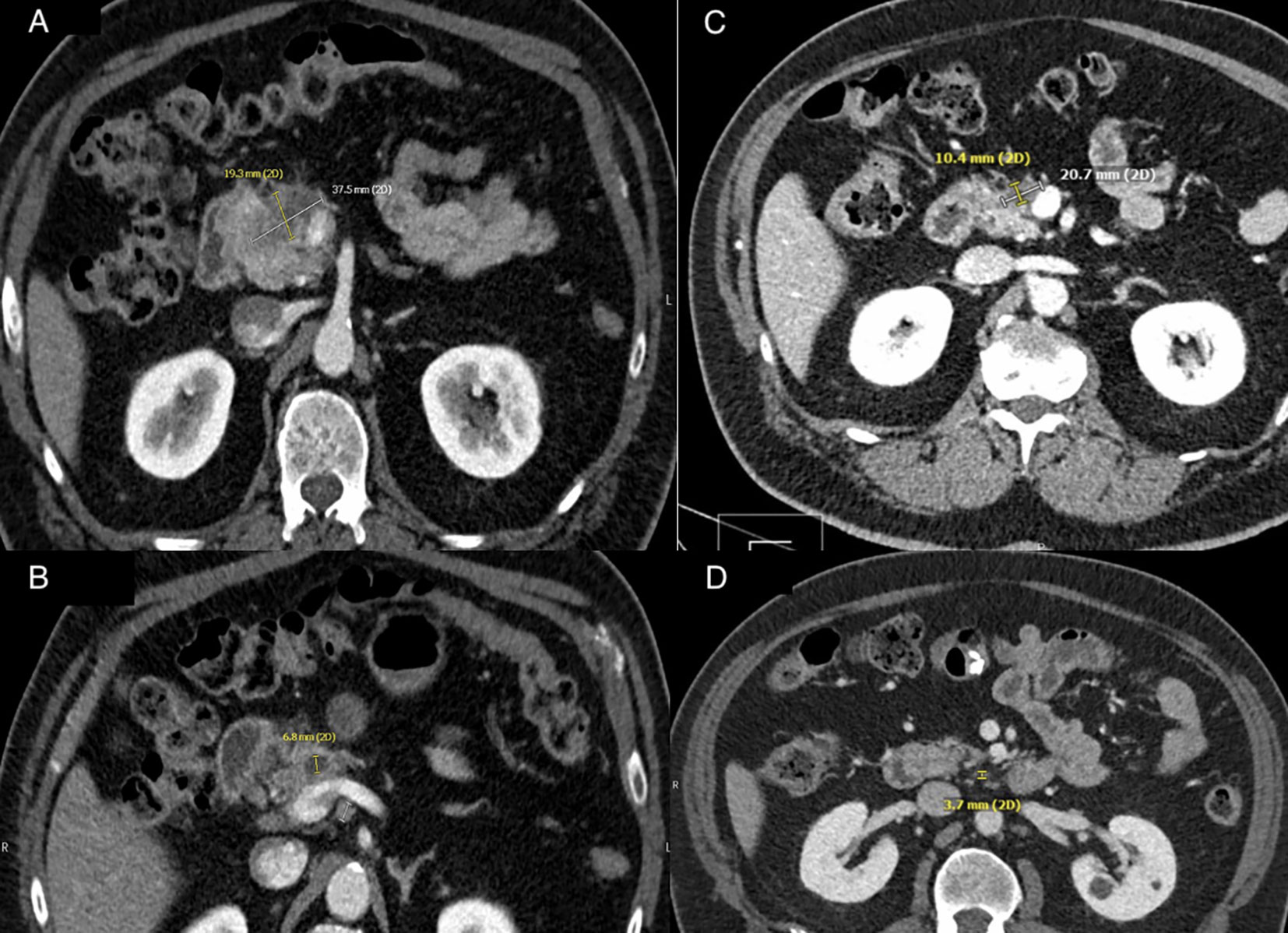
Figure 2 (A) CT abdominal imaging of pancreatic mass at time of diagnosis. A heterogeneous hypodense mass at anterior pancreatic head measuring 3.8 cm × 1.9 cm with associated pancreatic ductal obstruction. (B) CT abdominal imaging of lymphadenopathy at time of diagnosis. Lymph node posterior to the uncinate process measuring 10 mm on axial imaging. (C) CT abdominal imaging of pancreatic mass after five cycles of chemotherapy. Pancreatic mass with interval decrease in size to 2.1 cm × 1.0 cm. (D) CT abdominal imaging of lymphadenopathy after five cycles of chemotherapy. Interval decrease in size of lymph node posterior to the uncinate process measuring 3.7 mm on axial imaging. Created with BioRender.com.
He was referred to a tertiary care center for a biopsy and further care. A week later, he underwent an endoscopic ultrasound sonography (EUS) that showed a malignant-appearing pancreatic mass, 31 mm × 23 mm, at the uncinate process with upstream dilatation of the pancreatic duct and distal obstruction from the mass without dilation. There were no endosonographic abnormalities of the esophagus, stomach, and duodenum. The EUS-guided fine needle aspiration (FNA) demonstrated poorly differentiated malignant cells with both squamous and glandular differentiation (Figure 3). Direct communication with the pathologist rendering the diagnosis indicated that the squamous cell was the dominant feature. He had baseline tumor markers with a carcinoid embryonic antigen (CEA) of 2.4 ng/mL (reference, 0.0–3.0) and a carbohydrate antigen (CA) 19-9 of 93 U/mL (reference, <35). Next-generation sequencing of his tumor was notable for microsatellite stability, a programmed death ligand–1 percentage of 5%, with wild-type Neurotrophic tropomyosin receptor kinase 1/2/3/4, v-Raf murine sarcoma viral oncogene homolog B (BRAF), and Neuregulin 1 status. There was an insufficient quantity of malignant cells for whole-exome sequencing. We subsequently performed a positron electron tomography (PET)/CT that demonstrated a large f-fluorodeoxyglucose (FDG)–avid pancreatic mass compatible with the primary neoplasm and multiple avid lymph nodes consistent with nodal disease but no distant metastases.
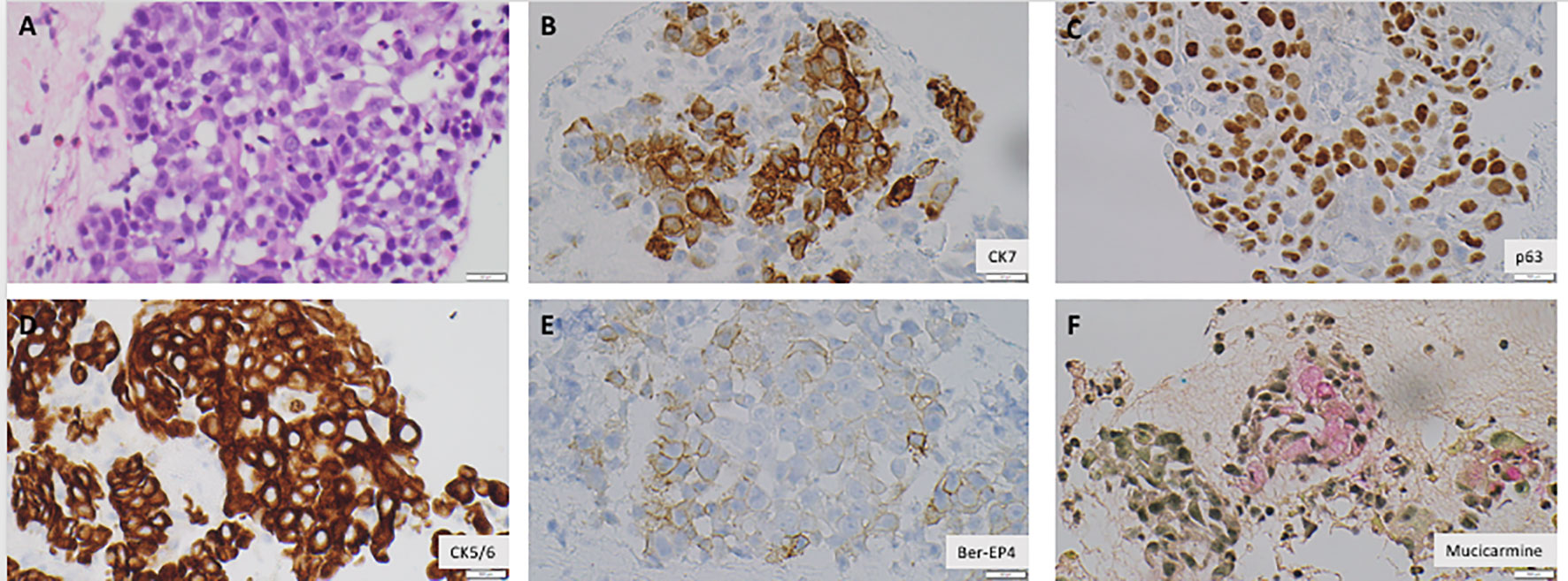
Figure 3 Malignant cells are positive for Cytokeratin 7, Cytokeratin 5/6, Epithelial Specific Antigen 4 (Ber-EP4) (B, D, E), consistent with carcinomatous origin. The cells are also positive for p63, a squamous cell marker, mucicarmine, an adenocarcinoma marker, consistent with poorly differentiated carcinoma with squamous and glandular differentiations (A, C, F). All images were obtained with a magnification of ×400. Created with BioRender.com.
Given the uniqueness of the tumor, the case was presented at a multidisciplinary tumor board meeting that recommended a combination regimen of gemcitabine (800 mg/m (2)), cisplatin (25 mg/m (2)), and nab-paclitaxel (100 mg/m (2)) every 14 days for six cycles. We planned to refer for surgical intervention if subsequent imaging confirmed improvement.
The first cycle of chemotherapy was administered 3 weeks after his FNA. After completing four cycles of chemotherapy, he reported a complete resolution of his abdominal pain. His CEA remained within normal range at 2.8 ng/mL, and his CA 19-9 decreased to normal range at 19 U/mL. After the fifth cycle of chemotherapy, we performed a response evaluation CT of the chest, abdomen, and pelvis, which demonstrated a decreased pancreatic head mass size (2.1 cm × 1.0 cm), decreased lymph node size, and no new lymphadenopathy or metastatic disease (Figures 2C, D). He completed his sixth cycle of chemotherapy, and, 2 weeks later, he underwent a pylorus-preserving Whipple procedure with a hilar lymphadenectomy and falciform ligament patch on the stump of the gastroduodenal artery with negative intraoperative frozen section margins. The pathology showed no residual carcinoma with resection margins free of malignancy or high-grade dysplasia, with no malignant cells identified in all 20 lymph nodes. He was staged as an ypT0N0 (0/20)Mx after surgery. At 3 months follow-up visit with repeat imaging, CT chest, abdomen, and pelvis, no evidence for residual or metastatic disease was demonstrated. He recovered well from surgery and continues to live with high quality of life.
SCC of the pancreas is uncommon, as adenocarcinoma is the dominant histology. There are several theories speculating the etiology of primary SCC development within the pancreas. One theory suggests that primitive cells can differentiate into either a squamous or glandular histology (4). Alternative theories propose that a pre-existing adenocarcinoma can undergo squamous differentiation or that squamous cells in the ductal epithelium are capable of malignant transformation (4). In our case, the pathology report demonstrated both squamous and glandular differentiation, leading us to hypothesize that our patient had adenocarcinoma with squamous transformation resulting in mixed histology.
Thorough evaluation of a primary pancreatic lesion with squamous cell differentiation requires appropriate radiographic imaging of the thorax, abdomen, and pelvis as well as endoscopic evaluation to rule out a primary lesion sourced from another location, as this drastically impacts the treatment plan (5). Given that several previous case reports utilized PET/CT scanning for excluding other potential primary locations and for staging of pancreatic SCC, we chose to utilize the same imaging modality for our patient and advocate for the utilization of PET/CT for these purposes (6).
Given the rarity of pancreatic SCC, there are no formal, standardized guidelines on the treatment of squamous cell pancreatic cancer. However, there are several case reports that have proposed various potential treatment options. Ntanasis-Stathopoulos et al. performed a systematic review and pooled survival analysis of SCC of the pancreas that consisted of 54 cases with a mean age of 61.9 years (7). It was found that the median overall survival (OS) was 7 months, utilizing a variety of treatment modalities such as chemotherapy, immunotherapy, and surgical resection (7). The authors found that ability to be resected and more recent publication year were statistically significant when being associated with better OS, as was low to intermediate tumor grade. The authors suggested that further collaborative studies are necessary to validate the results found.
We performed an updated narrative review of case reports published after the 2017 review by Ntanasis-Stathopoulos et al. We performed a literature review of the PubMed database by using the search terms “pancreatic,” “squamous cell carcinoma,” “treatment,” and “primary” (Table 1). Two cases were excluded because the patients did not receive treatment. Articles that are not in the English language were excluded.
In our review of recent literature, the average age ranged from 52 to 79 with a mean age of 62.2 years. Similar to the review by Ntanasis-Stathopoulos et al., we found no preference in gender when comparing the cases. The treatments varied from combined immunotherapy, chemotherapy, and/or surgical intervention. Three of the seven case reports had disease localized in the pancreas. In our narrative analysis of the recent cases since 2017, we found the progression-free survival to be denoted at 5.28 months. From the findings of our narrative review and the review by Ntanasis-Stathopoulos et al., our case is unique in that, to our knowledge, it is the only case of pancreatic SCC to achieve CPR utilizing only chemotherapy as a treatment modality. Our regimen was similar to that of Zhang et al., who documented a pancreatic lesion with lymph node metastasis treated with intensity-modulated radiation therapy with four cycles of paclitaxel, cisplatin, and gemcitabine (6). We determined this regimen on the phase II clinical trial by Shroff et al., in which patients received gemcitabine (800 mg/m (2)), cisplatin (25 mg/m (2)), and nab-paclitaxel (100 mg/m (2)), as they reduced the dosages due to hematological toxicities (8). In contrast to that in the work of Zhang et al., our patient was treated with an extended six-cycle course of treatment with chemotherapy, and we decided not to add radiation as part of pre-surgery treatment based on A021501 phase 2 randomized clinical trial showing no favorable benefit in OS in patients adding hypofractionated radiotherapy to FOLFIRINOX (9).
Immune checkpoint inhibitors have shown a great promise in a variety of SCC cancers (10–12). In particular, Yang et al. opted to treat SCC of the pancreas with 90% Programmed death-ligand 1–positive tumor cells using sintilimab (anti–PD-1) and gemcitabine, yielding a partial response (13). Because our patient had a PD-L1 percentage of only 5% and a lack of beneficial data for the use of check point inhibitor(s) in pancreatic adenocarcinoma, we decided against a combination of systemic chemotherapy with a check point inhibitor for our patient. Further research is needed to determine what the potential benefit immunotherapy could have in pancreatic cancer with squamous cell differentiation.
Huang et al. published a case report of a patient with stage IV pancreatic SCC with somatic Breast cancer 2 genes mutation, treated with cisplatin and nanoparticle albumin-bound paclitaxel (14). Similarly, the phase 1b/2 pilot clinical trial by Jameson et al. shows a high overall response rate in patients with advanced pancreatic cancer treated with a triple regimen of cisplatin and albumin-bound paclitaxel plus gemcitabine (15). Therefore, although the initial regimen planned for our patient was FOLFIRINOX, the regimen was changed to cisplatin, nab-paclitaxel, and gemcitabine after the pathology report for our patient showed dominant squamous cell differentiation. This was due to the reason that irinotecan did not show satisfying benefit in SCCs in general, such as in the study by Bai et al. who evaluated liposomal irinotecan and 5-fluorouracil in SCC of the esophagus and head/neck (16).
This case report is the first known case of a primary squamous cell pancreatic carcinoma utilizing systemic therapy to achieve a complete pathological remission. This case sheds light on potential duration and type of antineoplastic treatment needed to adequately treat pancreatic carcinoma with squamous cell differentiation. However, there are limitations in that this is a case report of a singular patient, and larger cohort studies are needed to further define the treatment algorithm for pancreatic SCC. This case and its accompanying narrative review highlights a need for guideline-directed treatment regimens to help improve the prognosis of patients with pancreatic SCC. There is a further need to determine the ideal treatment regimen for SCC of the pancreas.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CG: designed the study, provided clinical review, provided literature review, and drafted and reviewed all versions of manuscript. ZA: provided data interpretation, editing, oversight, and reviewed all versions of the manuscript. F-CL: conceptualized the study, provided direct patient care, data interpretation, editing, oversight, and reviewed all versions of the manuscript. TT: provided pathologic figures, data interpretation, editing, and reviewed all versions of the manuscript.
We would like to acknowledge Dr. Rony Kampalath and Dr. Gerald Lee for their impression of the radiographic findings.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CA 19-9, carbohydrate antigen 19-9; CEA, carcinoid embryonic antigen; CPR, complete pathologic response; CT, computed tomography; EUS, endoscopic ultrasound sonography; FDG, f-fluorodeoxyglucose; FNA, fine needle aspiration; OS, overall survival; PET, positron electron tomography; SCC, squamous cell carcinoma.
1. Survival Rates for Pancreatic Cancer. Available at: https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (Accessed March 27, 2023).
2. Brown HA, Dotto J, Robert M, Salem RR. Squamous cell carcinoma of the pancreas. J Clin Gastroenterol (2005) 39(10):915–9. doi: 10.1097/01.MCG.0000180636.74387.E6
3. Qin WX, Wu Y, Liu J, Qin BD, Liu K, Jiao X-D, et al. Primary squamous cell carcinoma of pancreas: a population-based study. Gland Surg (2021) 10(3):1029037–1021037. doi: 10.21037/GS-20-317
4. Al-Shehri A, Silverman S, King KM. Squamous cell carcinoma of the pancreas. Curr Oncol (2008) 15(6):293. doi: 10.3747/CO.V15I6.265
5. Mehta M, Sinha J, Ogawa M, Ganguly A, Xiang D, Poddar N. Unusual case of squamous cell carcinoma of pancreas with review of literature. J Gastrointest Cancer (2015) 46(4):426–9. doi: 10.1007/S12029-015-9712-5/
6. Zhang G, Cheng ZZ, Xu GH, Jiang X, Wang XX, Wang QF. Primary squamous cell carcinoma of the pancreas with effective comprehensive treatment: A case report and literature review. Medicine (2018) 97(41). doi: 10.1097/MD.0000000000012253
7. Ntanasis-Stathopoulos I, Tsilimigras DI, Georgiadou D, Kanavidis P, Riccioni O, Salla C, Psaltopoulou T, et al. Squamous cell carcinoma of the pancreas: A systematic review and pooled survival analysis. Eur J Cancer (2017) 79:193–204. doi: 10.1016/J.EJCA.2017.04.006
8. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial. JAMA Oncol (2019) 5(6):824–30. doi: 10.1001/JAMAONCOL.2019.0270
9. Katz MHG, Shi Q, Meyers J, Herman JM, Chuong M, Wolpin BM, et al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol (2022) 8(9):1263–70. doi: 10.1001/JAMAONCOL.2022.2319
10. Enomoto N, Yamada K, Terayama M, Kato D, Tagi S, Wake H, et al. Current status of immune checkpoint inhibitor therapy for advanced esophageal squamous cell carcinoma. Glob Health Med (2021) 3(6):378. doi: 10.35772/GHM.2020.01112
11. Yuan H, Liu J, Zhang J. The current landscape of immune checkpoint blockade in metastatic lung squamous cell carcinoma. Molecules (2021) 26:1392. doi: 10.3390/MOLECULES26051392
12. Kao HF, Lou PJ, Pei-Jen Lou C, Shan Rd CS. Immune checkpoint inhibitors for head and neck squamous cell carcinoma: Current landscape and future directions. Head Neck (2019) 41(S1):4–18. doi: 10.1002/HED.25930
13. Yang B, Ren H, Yu G. Case report: squamous cell carcinoma of pancreas with high PD-L1 expression: A rare presentation. Front Oncol (2021) 11. doi: 10.3389/FONC.2021.680398
14. Huang X, Wang C, Ma T, Huang Z, Zhou H, Xu L, et al. The efficacy of combined cisplatin and nanoparticle albumin-bound paclitaxel in a stage IV pancreatic squamous cell carcinoma patient with a somatic BRCA2 mutation: A case report. Front Oncol (2021) 11:585983/FULL. doi: 10.3389/FONC.2021.585983/FULL
15. Jameson GS, Borazanci E, Babiker HM, Poplin E, Niewiarowska AA, Gordon MS, et al. Response rate following albumin-bound paclitaxel plus gemcitabine plus cisplatin treatment among patients with advanced pancreatic cancer: A phase 1b/2 pilot clinical trial. JAMA Oncol (2020) 6(1):125–32. doi: 10.1001/JAMAONCOL.2019.3394
16. Bai LY, Yang MH, Chiang NJ, Wu SY, Lin CY, Lien MY, et al. A phase 2 study of liposomal irinotecan with 5-fluorouracil and leucovorin in squamous cell carcinoma of head and neck or esophagus after prior platinum-based chemotherapy or chemoradiotherapy. J Clin Oncol (2021) 39(15_suppl):6025–5. doi: 10.1200/JCO.2021.39.15_SUPPL.6025
Keywords: case report, pancreatic cancer, squamous cell carcinoma, chemotherapy, complete pathologic response
Citation: Grant CR, Arter ZL, Truc T and Lee F-C (2023) Case Report: A complete pathologic response in pancreatic cancer with squamous cell differentiation. Front. Oncol. 13:1240405. doi: 10.3389/fonc.2023.1240405
Received: 15 June 2023; Accepted: 11 September 2023;
Published: 29 November 2023.
Edited by:
Haitao Zhao, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Rong-Hua Tao, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2023 Grant, Arter, Truc and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher R. Grant, Y3JncmFudDFAaHMudWNpLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.