
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 26 September 2023
Sec. Cancer Immunity and Immunotherapy
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1240284
This article is part of the Research TopicCircadian Rhythms and Cancer Hallmarks: Toward Advances in Immune-Based Therapeutics, and OutcomesView all 12 articles
Circadian rhythms can be demonstrated in several biomarkers and behavioural activities, with rhythmical patterns occurring roughly over a 24-h period. Circadian disorders occur in patients with cancer and may be associated with poor clinical outcomes. This scoping review aimed to identify circadian rhythm research and reporting practices, circadian rhythm patterns, circadian rhythm disorders, and relevant associations of circadian rhythm disorders in patients with advanced cancer. Studies involved adult patients with locally advanced or metastatic cancer and used objective measures of circadian rhythmicity. Two independent authors completed initial screening of title and abstracts, full text reviews, data extraction, and data checking. A total of 98 articles were highlighted in the scoping review, which utilised physical activity measures (actigraphy and polysomnography), biomarkers (cortisol and melatonin), or a combination. Several circadian rhythms are commonly disordered amongst patients with advanced cancer and have significant implications for symptom burden, quality of life, and survival. It remains unclear which patients are most at risk of a circadian rhythm disorder. Significant heterogeneity exists in research and reporting practices. Standardising this approach may address discrepancies in the current literature and allow for research to focus on the most relevant parameters and approaches to improving circadian rhythmicity.
Circadian rhythms (CRs), repeating patterns approximately every 24 h, can be observed throughout the human body in behavioural activities, such as sleeping and feeding, and biochemical and hormonal changes, such as cortisol and melatonin secretion (1). CRs are coordinated by a central “pacemaker” or “clock” situated in the suprachiasmatic nuclei within the hypothalamus that attempts to synchronise internal body clocks with the 24-h light–dark cycle (1, 2). Additionally, areas within the brain and peripherally, such as endocrine organs, contain self-sustained secondary clocks (2).
In health, two well-established endocrine biomarkers of CRs are melatonin and cortisol, with levels being measurable in several samples, including serum, saliva, and urine (2–4). Serum melatonin begins to rise from around 22:00, peaking at around 04:00, before falling towards a baseline by 10:00, which persists throughout the day. Cortisol levels peak in the early morning, around 08:00, before falling during the day to a baseline at around 00:00 (2).
Physical activity demonstrates a circadian rhythm, with peak physical activity occurring around 14:00 and the most restful period centred around 03:00, although variability does exist between individuals (5). Circadian sleep and physical activity are primarily assessed using polysomnography and actigraphy (6). Although polysomnography and actigraphy are comparable, actigraphy can be applied in various settings and allows for prolonged periods of monitoring (6). Actigraphy utilises a wrist-worn device to detect physical movement during sleep and wake periods, with analysis of data producing several measures of circadian rhythmicity (6). Actigraphy is often accompanied by patient diaries, an approach supported by the American Academy of Sleep Medicine when investigating circadian rhythm sleep disorders (7). Diaries, however, can be burdensome, inaccurately completed, and subject to bias. Adult actigraphy research has focused on sleep–wake activity, particularly sleep onset–offset and the timing of activity phases. Various measures are used within research to describe the robustness of circadian rhythmicity or the timing and relationship of events over 24-h periods (see Table 1).
Circadian rhythmicity can alter during an individual’s lifespan and impact on health and disease. With advancing age, activity levels decline, peak activity occurs earlier, sleep becomes shorter and more fragmented, and daytime napping increases (33, 34). Circadian rhythm disorders (CRDs), where normal rhythmicity is altered, can perpetuate cancer and metabolic, neurodegenerative, psychological, and cardiovascular disease (35). CRDs are common amongst cancer patients, affecting up to 75%, and are associated with increased symptom burden, poorer quality of life, and shorter survival (36, 37). Interestingly, even misalignment between preferred and actual bedtimes is associated with cancer progression (38).
This review will broadly consider circadian rhythms of cortisol, melatonin, and physical activity in advanced cancer patients, with the aim of:
1. Identifying investigative approaches and reported parameters
2. Identifying circadian rhythm and disordered rhythm patterns
3. Identifying associations with circadian rhythm disorders, focusing on symptoms, quality of life, and survival.
A literature search was performed using PubMed, Embase, Web of Science, Ebsco host (CINAHL, Psychinfo, and Psycharticles), Scopus, and Cochrane on 20/04/2022. The search was updated on 05/05/2023. Keywords were restricted to title and abstract. No other limitations were placed.
An example search strategy within PubMed is as follows: (“circadian”[Title/Abstract] OR “sleep wake”[Title/Abstract] OR “rest activity”[Title/Abstract] OR “chrono*”[Title/Abstract] OR “clock”[Title/Abstract] OR “Chronobiology Disorders”[MeSH Terms]) AND ((“advanced”[Title/Abstract] OR “progressive”[Title/Abstract] OR “palliat*”[Title/Abstract] OR “terminal”[Title/Abstract] OR “metast*”[Title/Abstract] OR “end of life”[Title/Abstract]) AND (“cancer*”[Title/Abstract] OR “malig*”[Title/Abstract] OR “tumo*”[Title/Abstract] OR “neop*”[Title/Abstract] OR “oncol*”[Title/Abstract] OR “Neoplasms”[MeSH Terms])).
Studies were eligible for inclusion if the patients were ≥18 years old with a diagnosis of advanced cancer (locally advanced or metastatic). “Locally advanced” differed between cancer histology and several studies included, rather than focused solely on, patients with advanced cancer. Eligible studies also had to consider objective measures of four markers of circadian rhythm disorders (sleep–wake cycles, rest–activity cycles, cortisol levels, and melatonin levels) and be fully translated into English.
Two authors (CG and JP) independently screened the title and abstract for potential full-text review. Review papers identified in the initial search were also screened for additional articles. Full-text articles were reviewed independently by two authors (CG and JP). The reference lists of included articles were searched for additional articles. Where full-text copies were not immediately available, the leading author or associated research centre was contacted, and if no full-text made available, the article was excluded. Data were extracted by a single author (CG) and confirmed independently by a second author (JP). The data extraction tool was then coded into main themes including circadian measures, circadian rhythm patterns, and the association of circadian measures with symptoms, quality of life, survival and other relevant factors. The review is presented according to the PRISMA-ScR checklist.
The scoping review highlighted 98 articles, which were mainly observational in nature. The review process can be seen in Figure 1, and the results from individual studies are detailed in Tables 2A–D.
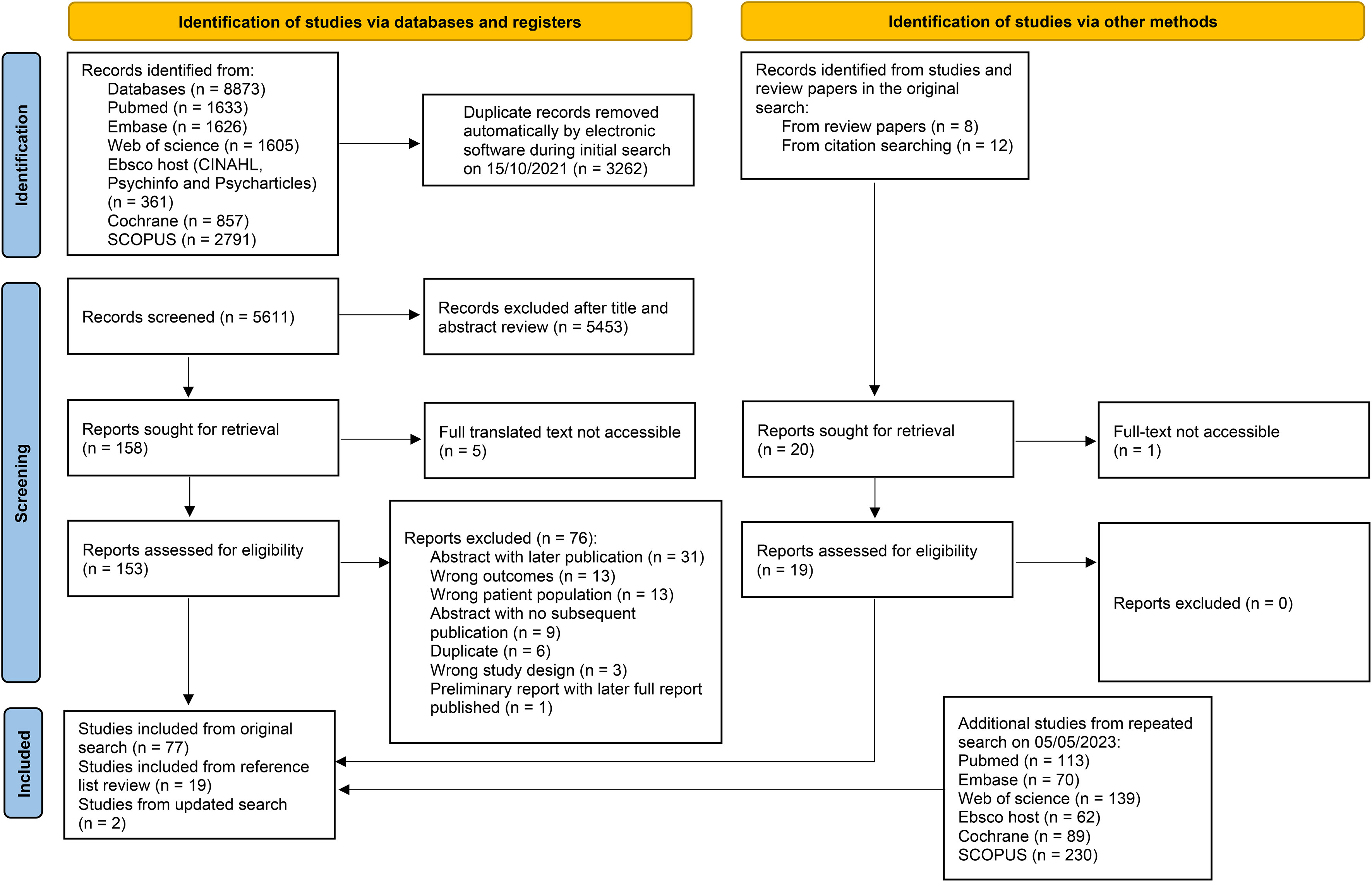
Figure 1 A flow chart of article identification, screening, and exclusion. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/.
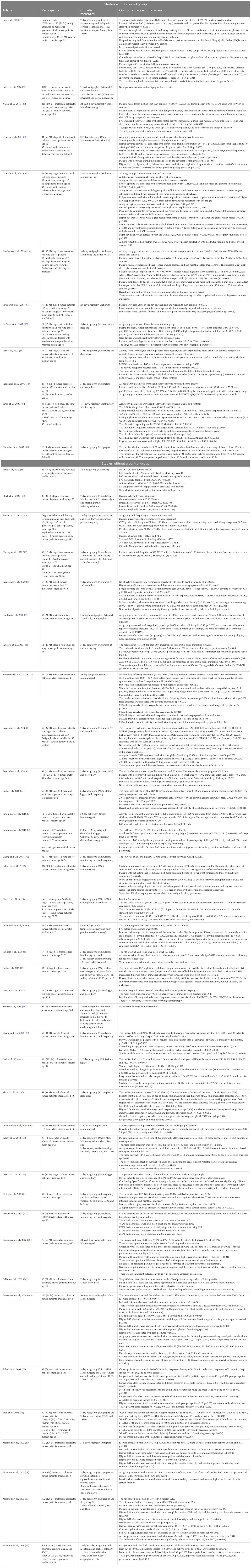
Table 2C Actigraphy-related circadian rhythms and their associations in patients with advanced cancer.
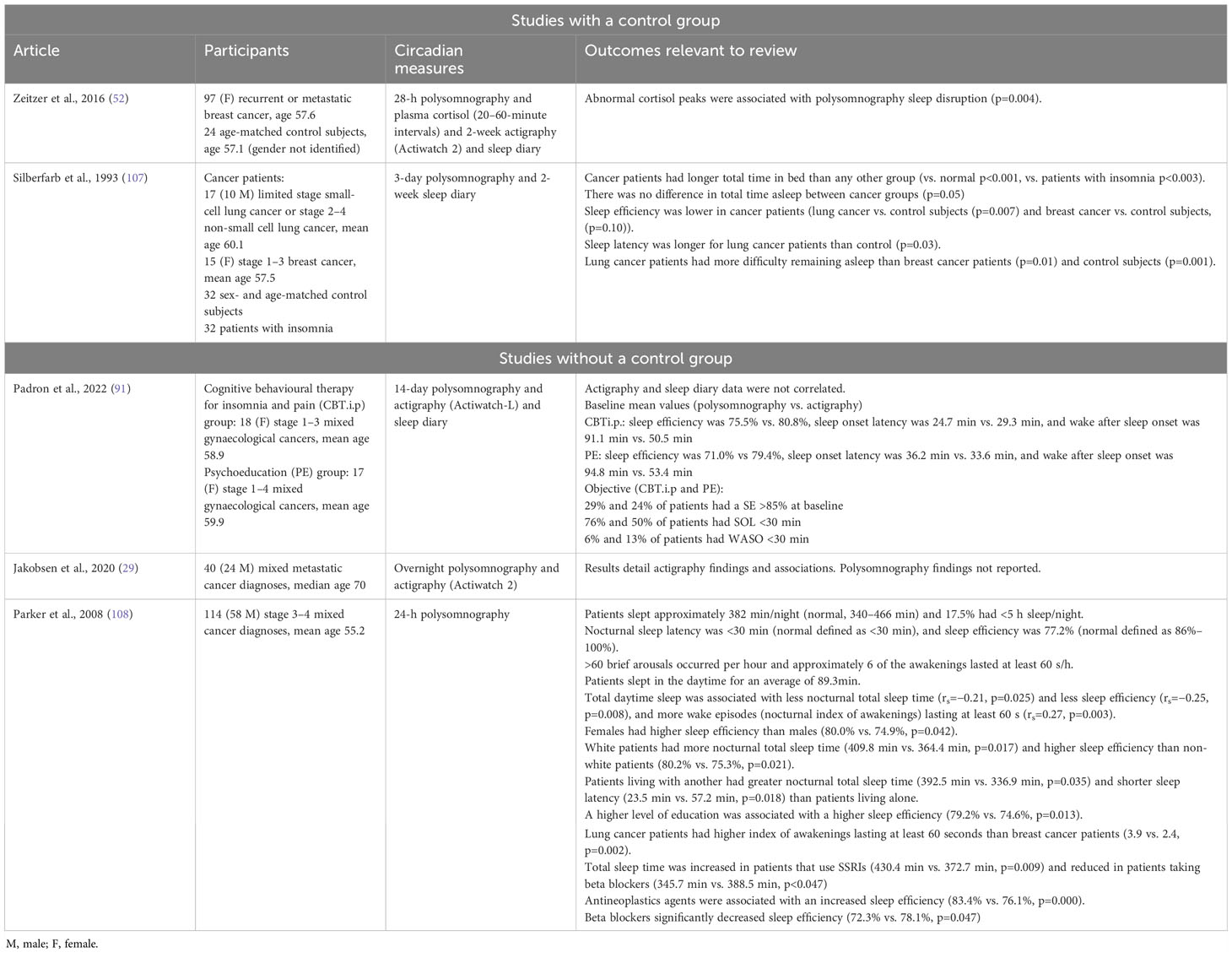
Table 2D Polysomnography-related circadian rhythms and their associations in patients with advanced cancer.
Authors utilised actigraphy (n=34), cortisol (n=33), combined assessment methods (n=18), melatonin (n=11), and polysomnography (n=2) in their investigations.
Articles focused on different cancer diagnoses, including breast (n=24), gastrointestinal (n=22), mixed cancer diagnoses (n=22), lung (n=20), gynaecological (n=7), head and neck (n=2), and renal (n=1). All studies included patients with advanced or metastatic cancer; 40 studies focused solely on advanced or metastatic cancer patients.
Heterogeneity was seen in the investigational approach and the reported measures of circadian rhythm. Studies assessing melatonin used between 20 and 190 patients, sampled melatonin at 1–16-h intervals, and used between 2 and 10 different time points. Studies assessing cortisol used between 13 and 210 patients and sampled at 20-min to 12-h intervals. Sampling included fixed times, time slots, and/or were reported in relation to waking and bedtime. Melatonin and cortisol studies lasted between 24 h and 3 days for most studies.
Variation was also seen in the samples utilised. As an example, articles focusing on cortisol measures (n=30) used serum (n=14), saliva (n=16), serum and saliva (n=3), or urine (n=1) samples. Reported measures included descriptive statistics, mean levels (MESOR), variation between peak and trough levels at several time points (amplitude, double amplitude, 12- and 24-h amplitude), timing of peak level (acrophase), the area under the curve, the shape of changing levels between peak and trough levels (diurnal slope, phase angles), cortisol variability, and the change in cortisol levels on waking (cortisol awakening response, CAR).
Actigraphy studies included between 11 and 436 patients, with a duration of monitoring varying from an overnight study to 30 days. The number of reported actigraphy parameters were much larger, with overlapping characteristics. Across sleep and wake periods, the timing and duration of activities, the relative proportions between different activities, and the variability both within and between 24-h periods were considered. At least 50 different actigraphy parameters were reported. When normal actigraphy values were stated, they differed between studies. The dichotomy index (I<O), for example, which measures proportional in-bed activity to out of bed activity, was reported in 20 studies. Unfavourable, or disordered, I<O values ranged from <89.5% to ≤97.5% (17, 24, 28, 94, 95, 98, 99, 101, 104). Favourable, or regular, I<O values varied between ≥89.5% and ≥99% (8, 17, 22, 24, 28, 49, 94, 95, 98–101, 104).
Finally, polysomnography studies included between 35 and 121 patients, with the duration of monitoring varying from an overnight study to a 14-day study.
Many articles did not describe normal circadian patterns or values.
Significant interindividual variation in melatonin circadian rhythms was noted (9, 17). 24-h melatonin rhythms, with a peak-to-trough pattern, were noted in non-small cell lung, gastrointestinal, mixed gynaecological, and mixed cancer cohorts (9, 11, 17, 39, 40, 42, 43, 45, 46). Abnormal 24-h rhythms were noted in two studies and affected 17% of the patients with metastatic colorectal cancer and 100% of patients with small cell lung cancer (49, 51). Detailed abnormalities included a smaller evening melatonin rise and earlier dim-light melatonin onset (DLMO) for patients with a gastrointestinal cancer who demonstrate more in-bed to out-of-bed physical activity (lower I<O) (17). Smaller evening melatonin rises were also noted in patients with non-small cell lung, gastrointestinal, and cervical cancers, particularly in advanced stages (40, 41, 43, 46). Advanced breast and lung cancer patients had higher mean melatonin levels compared to early-stage disease or controls (47).
No statistically significant associations were reported between the melatonin circadian rhythm parameters and symptoms, quality of life, or survival in any of the included studies.
Normal and abnormal rhythms were noted across cancer cohorts to varying degrees. Ten studies reported abnormal cortisol circadian rhythms in cancer patients (9, 36, 49, 60, 61, 72, 76, 79, 82, 106). This included mixed cancer cohorts (100%), ovarian cancer (75%), breast cancer (60%), and colorectal cancer (28%–56%) (36, 49, 50, 72, 76, 79, 83). Four studies noted normal cortisol circadian rhythms, including in patients with gastrointestinal (“most”), non-small cell lung (100%), nasopharyngeal cancer, and breast cancer patients (17, 39, 52, 55). Where present, abnormalities included a flattened cortisol slope, or reduced diurnal variation, coinciding with a lower morning cortisol rise and/or an increased evening cortisol (9, 12, 13, 16, 42, 54, 55, 58, 61–64, 72, 76, 77). These changes, along with the area under the curve, were more pronounced with later stage, higher grade or metastatic disease, and poorer performance status (9, 12, 13, 18, 54, 61, 64, 66, 69, 74, 76, 82). Cortisol levels at separate time points were not significantly different between cancer stages, or when compared with controls, but the overall mean 24-h cortisol was higher in cancer patients (39, 42, 52, 74). The timing of peak cortisol level (acrophase) ranged from 04:38 to 09:30 (55, 59, 82).
The change from peak to trough (cortisol slope) was unrelated to education level, marital status, age, time since recurrence, PR status, and metastatic sites in a breast cancer cohort (67). Patients with progesterone-receptor-positive breast cancer did, however, have a smaller cortisol awakening response (77). A flatter cortisol slope was associated with being male (69).
Salivary and serum cortisol were positively correlated, particularly when a strong circadian rhythm was present (16, 80).
Eight studies reported on survival, of which six report prognostic relevance of cortisol circadian rhythms. Patients with gynaecological cancer and either elevated night-time cortisol (HR, 1.802; p<0.001) or a flatter cortisol slope (HR, 1.633; p=0.001) had shorter survival (18). Conversely, patients with gynaecological cancer and more cortisol variability across the day had longer survival (HR, 0.644; p<0.001) (18). A lack of 24-h rhythmicity was prognostic in patients with lung cancer (HR, 68,052.8; p=0.009) and non-significantly in breast cancer (HR, 1.03; p=0.85) (68, 69). A flatter cortisol slope was prognostic in breast (HR, 464.9; p=0.0036) and renal cell cancer (HR, 1.88; p=0.002) (70, 81). Mean cortisol levels were prognostic in colorectal cancer (78). The early morning cortisol rise (CAR), area under the curve, and cortisol level at 00:00 were not prognostic in patients with lung cancer (61, 69). Altered cortisol rhythms in a study of patients with colorectal cancer were unrelated to survival (80).
Furthermore, a study of patients with breast cancer highlighted abnormal cortisol peaks, rather than the diurnal rhythm, to be associated with a shorter disease-free interval (r= −0.30, p=0.004) (52).
A smaller cortisol awakening response, flatter cortisol slope, and less diurnal variability were associated with increased total symptom scores, individual scores for fatigue, and interference with general activity, work, and walking (54, 64).
Within lung, ovarian, and mixed cancer cohorts, reduced diurnal cortisol variation, elevated evening cortisol, elevated morning cortisol, and higher area under the curve were associated with depression (19, 53, 54, 73, 74). Patients with steeper cortisol slopes, and therefore healthier rhythms, expressed less negative affect during psychological therapy and demonstrated more post-traumatic psychological growth following diagnosis, and those with flatter cortisol slopes were found to repress emotions (14, 67, 79). Abnormalities, including higher waking cortisol and lower cortisol awakening response, were associated with antidepressant use in patients with breast cancer (77). Some studies reported no significant correlations between cortisol levels, or cortisol slope, and psychological measures (12, 70, 73).
Flattened cortisol slopes, less cortisol variability, and elevated evening cortisol were associated with reduced physical well-being in patients with ovarian cancer (18, 54). Conversely, cortisol rhythms were also not correlated with quality-of-life measures for patients with breast cancer (65).
Abnormal cortisol peaks during sleep, coinciding with waking episodes, were reported in a subset of metastatic breast cancer patients (52). More frequent and longer lasting wake episodes and a progressive later waking time were also seen with flatter cortisol slopes (66, 75, 81).
The cortisol slope was correlated with the CAR, rather than waking level, and flatter slopes were associated with higher evening cortisol levels and an escape from cortisol suppression (63, 77).
Of the studies comparing patients with cancers to controls, 90% found abnormal actigraphy activity parameters in patients with cancer (17, 20, 23, 28, 30, 32, 85–88). Due to several reported parameters, the dichotomy index (I<O), 24-h autocorrelation coefficient (r24), and sleep efficiency (SE) are noted as examples.
Between 30%-95% of patients had a disrupted dichotomy index (I<O), suggesting proportionally more in-bed to out-of-bed activity, with average group values of 79%–98% (individual values of 49%–100%) (17, 21, 22, 24, 28, 37, 78, 87, 89, 94–96, 98–100, 104–106). Lower values suggest proportionally higher in-bed to out-of-bed activity. I<O values were lower in men, those with metastatic disease, and a poorer performance status (89, 99, 106). The I<O was reported to be the most discriminative parameter for cancer patients, although a large inter-subject variability was noted (17, 28).
Approximately 26%–28% of patients had a disordered 24-h autocorrelation coefficient (r24), representing the dissimilarity of rest–activity rhythms (RARs) between days, with average group values of 0.19–0.57 (individual values of −0.06–0.77) (15, 24, 32, 37, 49, 65, 71, 78, 88, 100, 105, 106). A r24 approaching 1 represents a prominent RAR (105). r24 values were lower in those with poorer performance status and in African-American women (37, 65, 105, 106).
Approximately 12%–88% of patients had disordered sleep efficiency, which measures sleep during time in bed, with average group values of 71%–92% (individual values, 20.2%–100%) (10, 15, 20, 22–27, 29, 30, 32, 68, 75, 85, 87, 91, 94, 97, 100, 102, 103). Higher values suggest that more time in bed has been spent sleeping, and lower values suggest that more time in bed has been spent awake.
Some studies reported circadian actigraphy parameters were unrelated to performance status, whilst others reported that time awake spent immobile (TASI), I<O, r24, and mean activity were significantly correlated with performance status (8, 17, 31, 37, 50, 78, 89, 99, 105, 106).
A total of 12 studies commented on survival and all linked circadian disruption to survival. Stronger RARs evidence by improved dichotomy index (I<O), 24-h autocorrelation coefficient (r24), physical activity amplitude and MESOR, nighttime restfulness, sleep efficiency, and time awake spent immobile were associated with longer survival in patients with colorectal, breast, head and neck, non-small cell lung, and mixed cancer diagnoses (8, 9, 31, 37, 68, 78, 89, 93, 98, 99, 104, 105). In a mixed cancer cohort, disordered I<O was not prognostic; however, r24 and sleep efficiency were prognostic (89).
Examples of prognostic relevance include colorectal and mixed cancer cohort patients with an I<O <97.5%, or below median I<O, having a reduced overall survival (OS) of between 2.1 and 9.7 months, and reduced progression-free survival (PFS) of 4.2 months (98, 99, 104). Similarly, patients with colorectal cancer and an I<O ≥99.2% had 11.5 months longer survival than those with an I<O <92.4% (105). The I<O was an independent prognostic factor when accounting for factors including age, gender, performance status, cancer diagnosis and stage, previous chemotherapy, and surgery (98, 99, 104). Similarly, sleep efficiency was an independent risk factor for patients with breast cancer whereby those with a sleep efficiency >85% had over a double survival compared to those with poor SE (68). However, I<O, r24, mean activity, and sleep activity parameters were reported also reported to not be significantly correlated with overall survival or progression-free survival (68, 89, 92, 104, 105).
Abnormal circadian activity rhythms were associated with pain, fatigue, drowsiness, nausea, vomiting, anorexia, and weight loss (20, 27, 37, 50, 78, 101, 106). Higher I<O values were specifically associated with less pain, fatigue, anorexia, sleep disturbance, constipation, and dyspnoea, and improved sleep quality (95, 100, 105, 106). Higher r24 values were specifically associated with less insomnia, daytime dysfunction, fatigue, anorexia, pain, and dyspnoea (20, 30, 100, 105). Higher sleep efficiency was associated with less pain (92). Greater time to sleep once in bed (sleep onset latency, SOL), wake after sleep onset (WASO), and time in bed (TIB) were associated with gastrointestinal symptoms in a mixed cancer cohort (92). Increase time spent napping was associated with increased pain, fatigue, and daytime sleepiness (92). No association between circadian activity parameters and pain or fatigue was found in a mixed cancer cohort (8).
Lower sleep efficiency, I<O, r24, and mean activity along with increased time spent napping or in bed were all associated with increased depression (37, 50, 75, 92, 93, 106). A lower r24 and more daytime inactivity were associated with intrusive thoughts and avoidant coping in patients with breast cancer (71). Studies also reported that anxiety and depression were not associated with sleep–activity rhythms, including the I<O (8, 17, 23).
Circadian disruption was associated with interference with activity, work, relations, and enjoyment of life for patients with colorectal cancer (95). Improved r24, I<O, and meanAct were associated with improved global QoL, along with health, physical, social, and functioning subscores (20, 50, 78, 95, 96, 105, 106). A lower amplitude and MESOR and a later acrophase were associated with worse global QoL in a mixed cancer cohort (8). The strongest correlation between an actigraphy parameter and quality of life measure in a mixed cancer cohort was the 24-h correlation coefficient (32). Studies also noted that circadian parameters were not associated with the fatigue, emotional, or cognitive subscales of quality-of-life measures (105, 106). One study of patients with breast cancer noted that WASO and r24 were unrelated to global QoL (65).
There were mixed reports regarding chemotherapy response and circadian rhythmicity in patients with colorectal cancer. One study noted that disordered rhythmicity during chemotherapy was associated with earlier death but not to objective response or toxicity, while another study noted objective response to be influenced by r24 and I<O (37, 104). Patients receiving chemotherapy who also had evidence of circadian disruption were more likely to experience weight loss and fatigue (101).
I<O appeared to correlate with circadian temperature rhythms, self-reported physical activity, and chronotype (17). More robust circadian rhythms were associated with greater light exposure (8).
Subjective and objective measures differed for physical activity but were closely correlated for sleep (27, 102). Subjective sleep disruption and circadian disruption can occur together or independently (22). Although total sleep time (TST), SE, and WASO were associated with subjective sleep quality, physical activity measures were also not significantly different between those who report their sleep as good or poor (26, 29, 37, 85, 102).
Subjective scores of pain and physical function correlated with objective physical activity, and those using analgesia had more abnormal circadian activity rhythms (84, 105). Daytime sleep, or inactivity, was related to sleep medication use, night-time sleep disturbance, daytime dysfunction, night-time sleep, and sleep quality (30, 38, 108). Sleep efficiency was reported to be correlated with chest metastases, hormone use, and radiotherapy (75). Patients with a higher r24 had less daytime dysfunction and less insomnia (20, 30). Circadian disruption was associated with tumour progression markers (15).
Patients with cancer spent more time in bed were noted to have multiple nocturnal awakenings and had an average sleep efficiency of up to 77.2% (91, 107, 108). Increased daytime sleep was associated with less night-time sleep and more nocturnal awakenings in a mixed cancer cohort (108). Medications were also found to impact on sleep. Anticancer therapies were associated with increased sleep efficiency, whereas beta blocker use was associated with reduced sleep efficiency (108). Sleep efficiency was higher in women, white patients, and those with a higher education level (108).
Increased diurnal physical activity variability was associated with increased diurnal melatonin and cortisol variability along with an earlier DLMO (17). Salivary cortisol levels appeared unrelated to I<O, cortisol rhythmicity positively was correlated with r24, and more robust actigraphy rhythms were associated with a steeper cortisol slope (17, 37, 71).
The dichotomy index was correlated with r24, mean activity, sleep motor activity, sleep efficiency, and WASO (28, 89). r24, I<O, and mean activity were also correlated (106).
Higher I<O and r24 were associated with improved sleep efficiency (100).
Polysomnography-derived values for sleep efficiency were lower, and wake after sleep onset higher, than actigraphy-derived values (91).
This review supports, expands upon, and updates several previous reviews of circadian rhythmicity in patients with advanced cancer. It highlights that, for several patients with advanced cancer, disordered cortisol, melatonin, and physical activity circadian rhythms are associated with increased symptom burden, poorer quality of life, and shortened survival. Other important associations with CRDs include poorer performance status and raised biomarkers of tumour progression.
A review of rest–activity rhythms in advanced cancer patients found that CRDs are particularly evident amongst men, those undergoing chemotherapy, and those who were symptomatic (109). Additionally, circadian disruption may be seen across the cancer trajectory, with worse biopsychosocial outcomes reported in cancer survivors who have disordered cortisol rhythms (110). This review highlights that circadian rhythms may be maintained in some patients with cancer and that wide inter-individual variation exists. Future research aimed at identifying patients that are at risk of circadian rhythm disorders, and impacted by their associations, is important, particularly when considering interventional studies to improve circadian rhythms and patient outcomes.
Articles were predominantly observational in nature, and many studies lacked a control group. Causality is difficult to establish, particularly due to the bi-directional relationship between cancers and circadian rhythm disorders, and the influence from external factors. CRDs impact on several neuroendocrine-immune functions, including inflammatory responses and hormonal secretion, and predispose individuals to developing cancer (111). Cancer in turn generates a pro-inflammatory state, and increased circulating cytokines levels can disrupt circadian rhythms (111). Rest–activity patterns are influenced by age, sex, race, education, and voluntary behaviour (6, 112). Cortisol values are influenced by sex, age, body mass index, menstrual cycle, sleep disturbances, renal disease, and acute illness, for example (4). The review highlights studies of patients prior to, during, and after anticancer therapies, and within the inpatient and community setting. Limited information on previous and current therapeutic regimes, and location of metastatic disease, limits the ability to synthesise findings. Potential modifying factors of circadian rhythmicity should be reported and taken into account when reviewing findings (113).
CRDs and their impact are not solely seen in cancer patients. Circadian disruption has been reported in patients with neurodegenerative conditions including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease (114). Despite conflicting findings, evidence highlights altered rest–activity, body temperature, melatonin, and cortisol rhythms within this population and associations with physical and psychological well-being, and quality of life (114). At present, the similarities in circadian disruption between clinical conditions are not clear.
Furthermore, the review highlights heterogeneity in the investigation and reporting of circadian rhythms in cancer patients and a lack of threshold values to identify circadian parameter abnormalities. Several reporting measures, overlapping definitions, and an absence of clear definitions were found in the investigation of circadian rhythms, particularly when using actigraphy. Heterogeneity in actigraphy research is not limited to cancer populations. A review of 126 actigraphy studies of children highlighted a lack of standardisation in actigraphy practice, including the reporting of epoch length, artefact detection, and definition of variables (115). Additionally, within cohorts of bipolar disorder patients, over 30 possible actigraphy parametric and non-parametric measures were reported (116). In this review, only four actigraphy parameters (I<O, r24, mean activity, and SE) were associated with at least three of the areas of interest (physical symptoms, psychological symptoms, quality of life measures, and survival). Although a wealth of information can be obtained using actigraphy, the reporting parameters should be aligned with the overall study objectives to allow a clear message in the literature. Analysis of actigraphy data takes many forms and lacks standardisation (6, 112). Similarly, variable sampling protocols, analysis, and reporting practices has been seen in cortisol and melatonin studies (3, 110). When faced with such heterogeneity in approaches, it is challenging to make firm conclusions, and standardisation may improve research practice. The development of recommendations to identify, and subsequently report, optimal sampling processes, particularly the frequency and timing of samples, and the calculation of circadian parameters are required.
Actigraphy data can report the timing of events, duration of events, or relationship between events. Although studies may focus on “sleep–wake” or “rest–activity” periods, there is significant overlap. Diagnostic criteria have been formulated for circadian rhythm sleep–wake disorders by American Academy of Sleep Medicine (117). The diagnosis considers the timing of sleep onset and offset, and the presence of jet lag or shift work, to categorise patients into seven different diagnoses. Many studies of advanced cancer patients reported actigraphy measures across the 24-h period rather than focusing on this timing of sleep onset–offset. The circadian activity rhythm disorders in cancer patients are likely separate to intrinsic circadian sleep–wake rhythm disorders. Recent international consensus recommendations have been developed for the assessment and diagnosis of circadian rest–activity rhythm disorders (CARDs) (118). The recommendations outline key modifiers of circadian rhythmicity, areas to consider within a clinical history, patient sleep and activity diary, and accelerometery during assessment, and criteria to diagnose a CARD. Diagnostic criteria of other forms of CRDs do not currently exist.
The scoping review was strengthened by using independent authors at multiple stages of the review process. Additional evidence was actively sought through hand searching review papers and reference lists. The scoping review is inclusive of available evidence and placed minimal limitations in the search strategy. It made no attempt to critically analyse the quality of evidence. Although the review aimed to focus on advanced cancer patients, several studies included non-advanced cancer patients. This approach may dampen associations, but it was felt to be more inclusive and to provide a broader insight of the topic. Furthermore, the review did not exclude several confounding factors in selected articles, such as medications and chemotherapy. This information was not available in several studies, and through exclusion, it would have limited the generalisability of the findings. Studies reporting on circadian rhythmicity in patients with cancer would benefit from detailed information on recent and existing modifiers of circadian rhythmicity, and the presence and location of metastatic disease.
Box 1:
Gaps in the current literature.
■ What are the risk factors for a patient with cancer to develop a circadian rhythm disorder?
■ How do circadian rhythm disorders differ between malignant and non-malignant clinical conditions?
■ How do circadian rhythm disorders different between malignant subgroups?
■ What are the optimal measurement and analytical approaches when assessing cortisol, melatonin, and rest–activity circadian rhythms?
■ What are the abnormal threshold values for cortisol, melatonin, and rest–activity parameters when diagnosing a circadian rhythm disorder?
■ Do current investigative approaches translate into the clinical setting, considering the ease and acceptability for patients and clinicians?
Cancer patients, particularly those with advanced disease, are at risk of circadian rhythm disorders and significant associated complications. It remains unclear which subset of patients are most susceptible. Conflicting results within the review highlight the need for further studies to identify patient populations that are most impacted by circadian rhythm disorders. Current investigative approaches require a multiple sampling approach (blood, urine, and saliva) or a prolonged period of activity monitoring. In the clinical setting, and advanced cancer population, this may require an alternative approach. Current gaps in the literature are highlighted in Box 1. There needs to be an attempt to standardise research approaches and reporting practice within circadian rhythm research and to develop criteria to identify circadian rhythm disorders. Research standardisation and targeted approaches may help in future research aimed at developing management approaches to circadian rhythm disorders.
CG was responsible for the conceptualisation of the review and development of the search strategy. CG and JP conducted the scoping review, data extraction, and data checking. CG wrote the initial draft of the manuscript with editorial input from JP and AD. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Skene DJ, Arendt J. Physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem (2006) 43:344–53.
2. Oster H, Challet E, Ott V, Arvat E, de Kloet R, Dijk D-J, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocrine Rev (2017) 38:3–45.
3. Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, et al. Measuring melatonin in humans. J Clin Sleep Med (2008) 4:66–9.
4. El-Farhan N, Rees DA CE. Measuring cortisol in serum, urine and saliva - are our assays good enough? Ann Clin Biochem (2017) 54(3):308–22.
5. Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, et al. Actigraphy-derived daily rest-activity patterns and body mass index in community-dwelling adults. Sleep (2017) 40(12):zsx168.
6. Ancoli-Israeli S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythm. Sleep. (2003) 26(3):342–92. doi: 10.1093/sleep/26.3.342
7. Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora N, Boehlecke B, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. Sleep. (2015) 30(11):1445–59.
8. Bernatchez M, Savard J, Ivers H. Disruptions in sleep-wake cycles in community-dwelling cancer patients receiving palliative care and their correlates. Chronobiology Int (2018) 35:49–62. doi: 10.1080/07420528.2017.1381615
9. Mormont M, Hecquet B, Bogdan A, Benavides M, Touitou Y, Levi F. Non-invasive estimation of circadian rhythm in serum cortisol in patients with ovarian or colorectal cancer. Int J Cancer. (1998) 78(4):421–4. doi: 10.1002/(SICI)1097-0215(19981109)78:4<421::AID-IJC5>3.0.CO;2-W
10. Cheung D, Takemura N, Lam T, Ho J, Deng W, Smith R, et al. Feasibility of aerobic exercise and tai-chi interventions in advanced lung cancer patients: A randomized controlled trial. Integrated Cancer Therapies. (2021) 20:15347354211033352. doi: 10.1177/15347354211033352
11. Baranowski M, Muc-Wierzgon M, Madej K, Braczkowski R, Wierzgon J. Circadian fluctuations of melatonin and tumour necrosis factor-α in the circulation of patients with advanced cancer. Cent Eur J Immunol (1999) 24:30–5.
12. Abercrombie H, Giese-Davis J, Sephton S, Epel E, Turner-Cobb J, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. (2004) 29:1082–92. doi: 10.1016/j.psyneuen.2003.11.003
13. Kim K, Kim Y, Oh I, Kim S, Choi J, Ahn R. Association of worse prognosis with an aberrant diurnal cortisol rhythm in patients with advanced lung cancer. Chronobiology Int (2012) 29(8):1109–20. doi: 10.3109/07420528.2012.706767
14. Giese-Davis J, DiMiceli S, Sephton S, Spiegel D. Emotional expression and diurnal cortisol slope in women with metastatic breast cancer in supportive-expressive group therapy: a preliminary study. Biol Psychol (2006) 73(2):190–8. doi: 10.1016/j.biopsycho.2006.04.003
15. Cash E, Sephton S, Chapgar AB, Spiegel D, Rebholz W, Zimmaro L, et al. Circadian disruption and biomarkers of tumour progression in breast cancer patients awaiting surgery. Brain Behaviour Immunity. (2015) 48:102–14. doi: 10.1016/j.bbi.2015.02.017
16. Zeitzer J, Nouriani B, Neri E, Spiegel D. Correspondance of plasma and salivary cortisol patterns in women with breast cancer. Neuroendocrinology. (2015) 100:153–61. doi: 10.1159/000367925
17. Lévi F, Komarzynski S, Huang Q, Young T, Ang Y, Fuller C, et al. Tele-monitoring of cancer patients’ Rhythms during daily life identifies actionable determinants of circadian and sleep disruption. Cancers (2020) 12(7):1938.
18. Schrepf A, Thaker P, Goodheart M, Bender D, Slavich G, Dahmoush L, et al. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology (2015) 53:256–67. doi: 10.1016/j.psyneuen.2015.01.010
19. Jehn C, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer A, et al. Biomarkers of depression in cancer patients. Cancer. (2006) 107(11):2723–9. doi: 10.1002/cncr.22294
20. Grutsch J, Ferrans C, Wood P, Du-Quiton J, Quiton D, Reynolds J, et al. The association of quality of life with potentially remediable disruptions of circadian sleep/activity rhythms in patients with advanced lung cancer. BMC Cancer. (2011) 11:193. doi: 10.1186/1471-2407-11-193
21. Ortiz-Tudela E, Innominato P, Rol M, Lévi F, Madrid J. Relevance of internal time and circadian robustness for cancer patients. BMC Cancer. (2016) 16:285. doi: 10.1186/s12885-016-2319-9
22. Palesh O, Haitz K, Lévi F, Bjarnson G, Deguzman C, Alizeh I, et al. Relationship between subjective and actigraphy-measured sleep in 237 patients with metastatic colorectal cancer. Qual Life Res (2017) 26(10):2783–91. doi: 10.1007/s11136-017-1617-2
23. Du-Quiton J, Wood P, Burch J, Grutsch J, Gupta D, Tyer K, et al. Actigraphic assessment of daily sleep-activity pattern abnorMalities reflects self-assessed depression and anxiety in outpatients with advanced non-small cell lung cancer. Psychooncology. (2010) 19(2):180–9. doi: 10.1002/pon.1539
24. Chen H, Tsai C, Wu Y, Lin K, Lin C. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: A randomised controlled trial. Br J Cancer. (2016) 115(11):1304–12. doi: 10.1038/bjc.2016.356
25. Bernatchez M, Savard J, Savard M, Aubin M, Ivers H. Sleep-wake difficulties in community-dwelling cancer patients receiving palliative care: subjective and objective assessment. Palliative Supportive Care (2018) 16(6):756–66. doi: 10.1017/S1478951517000815
26. Gibbins J, McCoubrie R, Kendrick A, Senior-Smith G, Davies A, Hanks G. Sleep-wake disturbances in patients with advanced cancer and their family carers. J Pain Symptom Management. (2009) 38(6):860–70. doi: 10.1016/j.jpainsymman.2009.04.025
27. Komarzynski S, Huang Q, Lévi F, Palesh O, Ulusakarya A, Bouchahda M, et al. The day after: correlates of patient-reported outcomes with actigraphy-assessed sleep in cancer patients at home (inCASA project). Sleep. (2019) 42(10):1–12. doi: 10.1093/sleep/zsz146
28. Natale V, Innominato P, Boreggiani M, Tonetti L, Filardi M, Parganiha A, et al. The difference between in bed and out of bed activity as a behavioral marker of cancer patients: A comparative actigraphic study. Chronobiology Int (2015) 32(7):925–33. doi: 10.3109/07420528.2015.1053909
29. Jakobsen G, Engstrøm M, Thronæs M, Løhre E, Kassa S, Fayers P, et al. Sleep quality in hospitalized patients with advanced cancer: an observational study using self-reports of sleep and actigraphy. Supportive Care Cancer. (2020) 28(4):2015–23. doi: 10.1007/s00520-019-04998-5
30. Grutsch J, Wood P, Du-Quiton J, Reynolds J, Lis C, Levin R, et al. Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. J Circadian Rhythms. (2011) 9:4. doi: 10.1186/1740-3391-9-4
31. Fujisawa D, Temel J, Greer J, El-Jawahri A, Traeger L, Jacobs J, et al. Actigraphy as an assessment of performance status in patients with advanced lung cancer. Palliative Supportive Care (2019) 17(5):574–8. doi: 10.1017/S1478951518001074
32. Fernandes R, Stone P, Andrews P, Morgan R, Sharma S. Comparison between fatigue, sleep disturbance, and circadian rhythm in cancer inpatients and healthy volunteers: evaluation of diagnostic criteria for cancer-related fatigue. J Pain Symptom Management. (2006) 32(3):245–54. doi: 10.1016/j.jpainsymman.2006.03.014
33. Li J, Somers VK, Lopez-Jimenez F, Di J, Covassin N. Demographic characteristics associated with circadian rest-activity rhythm patterns: a cross-sectional study. Int J Behav Nutr Phys Activity. (2021) 18:107. doi: 10.1186/s12966-021-01174-z
34. Huang YL, Liu RY, Wang QS, Van Someren EJW, Xu H, Zhou J. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behavior. (2002) 76:597–603. doi: 10.1016/S0031-9384(02)00733-3
35. Ruan W, Yuan X, Eltzschig HK. Circadian rhythm as a therapeutic target. Nat Rev (2021) 20:287–307. doi: 10.1038/s41573-020-00109-w
36. Touitou Y, Bogdan A, Lévi F, Benavides M, Auzeby A. Disruption of the circadian patterns of serum cortisol in breast and ovarian cancer patients: relationships with tumour marker antigens. Br J Cancer. (1996) 74:1248–52. doi: 10.1038/bjc.1996.524
37. Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res (2000) 6(8):3038–45.
38. Hahm BJ, Jo B, Dhabhar FS, Palesh O, Aldridge-Gerry A, Bajestan SN, et al. Bedtime misalignment and progression of breast cancer. Chronobiology Int (2014) 31(2):214–21. doi: 10.3109/07420528.2013.842575
39. Mazzoccoli G, Sothern R, Francavilla M, Giuliani F, Carughi S, Muscarella L, et al. Hormone and cytokine circadian alteration in non-small cell lung cancer patients. Int J Immunopathology Pharmacol (2012) 25(3):691–702. doi: 10.1177/039463201202500315
40. Hu S, Shen G, Yin S, Xu W, Hu B. Melatonin and tryptophan circadian profiles in patients with advanced non-small cell lung cancer. Adv Ther (2009) 26(9):886–92. doi: 10.1007/s12325-009-0068-8
41. Karasek M, Kowalski A, Suzin J, Zylinska K, Swietoslawski J. Serum melatonin circadian profiles in women suffering from cervical cancer. J Pineal Res (2005) 39:73–6. doi: 10.1111/j.1600-079X.2005.00221.x
42. Mazzoccoli S, Carughi S, De Cata A, La Viola M, Vendemiale G. Melatonin and cortisol serum levels in lung cancer patients at different stages of disease. Med Sci Monitor (2005) 11(6):284–8.
43. Muc-Wierzgon M, Nowakowska-Zajdel E, Zubelewicz B, Wierzgon J, Kokot T, Klakla K, et al. Circadian fluctuations of melatonin, tumour necrosis factor-alpha and its soluble receptors in the circulation of patients with advanced gastrointestinal cancer. J Exp Clin Cancer Res (2003) 22(2):171–8.
44. Ermachenkov M, Gulyaev A, Arutyunyan A, Milyutina Y, Anisimov V. Age-related changes in 6-hydroxymelatonin sulfate excretion in patients with gastric and colorectal cancer. Adv Gerontology. (2013) 3(2):148–53. doi: 10.1134/S2079057013020069
45. Karasek M, Kowalski A, Zylinska K. Serum melatonin circadian profile in women suffering from the genital tract cancers. Neuroendocrinol Letters. (2000) 21(2):109–13.
46. Tarquini B, Cornelissen G, Tarquini R, Perfetto F, Halberg F. General and unspecific damping by Malignancy of the circadian amplitude of circulating human melatonin? Neuroendocrinol Lett (1999) 20:25–8.
47. Dogliotti L, Berruti A, Buniva T, Torta M, Bottini A, Tampellini M, et al. Melatonin and human cancer. J Steroid Biochem (1990) 37(6):983–7. doi: 10.1016/0960-0760(90)90454-S
48. Bartsch C, Bartsch H, Jain A, Laumas K, Wetterberg L. Urinary melatonin levels in human breast cancer patients. J Neural Transmission. (1981) 52:281–94. doi: 10.1007/BF01256753
49. Mormont MC, Langouët AM, Claustrat B, Bogdan A, Marion S, Waterhouse J, et al. Marker rhythms of circadian system function: a study of patients with metastatic colorectal cancer and good performance status. Chronobiology Int (2002) 19:141–55. doi: 10.1081/CBI-120002593
50. Mormont M, Claustrat B, Waterhouse J, Touitou Y, Levi F. Biological clocks: Mechanisms and applications. Amsterdam: Excerpta Medica (1998) p. 497–505.
51. Viviani S, Bidoli P, Spinazze S, Rovelli F, Lissoni P. NorMalization of the light/dark rhythm of melatonin after prolonged subcutaneous administration of interleukin-2 in advanced small cell lung cancer patients. J Pineal Res (1992) 12(3):114–7. doi: 10.1111/j.1600-079X.1992.tb00037.x
52. Zeitzer J, Nouriani B, Rissling M, Sledge G, Kaplan K, Aasly L, et al. Aberrant nocturnal cortisol and disease progression in women with breast cancer. Breast Cancer Res Treat (2016) 158:43–50. doi: 10.1007/s10549-016-3864-2
53. Du Y, Zhang H, Li B, Wu X, Lv Y, Jin H, et al. Sputum interleukin-6, tumour necrosis factor-α and salivary cortisol as new biomarkers of depression in lung cancer patients. Prog Neuropsychopharmacol Biol Psychiatry (2013) 47:69–76. doi: 10.1016/j.pnpbp.2013.08.004
54. Weinrib A, Sephton S, DeGeest K, Penedo F, Bender D, Zimmerman B, et al. Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer. (2010) 116(18):4410–9. doi: 10.1002/cncr.25299
55. Wu M, Zeng Z, Li S, Guo L, Zhang J, Qiu F, et al. Circadian variation of plasma cortisol and whole blood reduced glutathione levels in nasopharyngeal carcinoma patients. Ai Zheng. (2008) 27(3):237–42.
56. Baranowski M, Muc-Wierzgon M, Madej K, Wierzgon J, Zubelewicz B. The estimation of endogenous tumour necrosis factor alpha and cortisol levels in serum in advanced neoplasm. J Exp Clin Cancer Res (1999) 18(2):241–5.
57. Singh R, Singh R, Mahdi A, Misra S, Rai S, Singh D, et al. Studies on circadian periodicity of urinary corticoids in carcinoma of the breast. In Vivo. (1998) 12:69–73.
58. Payer J, Huorka M, Duris I, Ondrejka P, Kratochvilova H, Ilkova M, et al. Circadian rhythmicity of plasma somatostatin, gastrin and cortisol in colon cancer patients. Hepatogastroenterology. (1997) 44(13):72–7.
59. Singh R, Mahdi A, Singh D, Rai S, Cornelissen G. Studies on circadian periodicity of plasma 17-hydroxycorticosteroids (17-OHCS) in carcinoma of the breast. In Vivo. (1995) 9(4):279–82.
60. Singh R, Singh S, Razdan J. Circadian periodicity of plasma 17-hydroxycorticosteroids in advanced breast cancer. Prog Clin Biol Res (1987) 227:335–42.
61. DeMeester T, Golomb H, Dudek P, Hunter R, Fang V. The relationship between immune reactivity, serum cortisol, and stage of disease in patients with non-oat-cell bronchogenic carcinoma. Surgery. (1979) 86:130–7.
62. Bishop M, Ross E. Adrenocortical activity in disseminated Malignant disease in relation to prognosis. Br J Cancer. (1970) 24(4):719–25. doi: 10.1038/bjc.1970.86
63. Allende S, Medina JL, Spiegel D, Zeitzer JM. Evening salivary cortisol as a single stress marker in women with metastatic breast cancer. Psychoneuroendocrinology. (2020) 115:104648. doi: 10.1016/j.psyneuen.2020.104648
64. Oh I, Kim K, Kim Y, Park J, Yoo K, Do S, et al. Altered hypothalamus-pituitary-adrenal axis function: A potential underlying biological pathway for multiple concurrent symptoms in patients with advanced lung cancer. Psychosomatic Med (2019) 81:41–50. doi: 10.1097/PSY.0000000000000648
65. Rebholz W, Cash E, Zimmaro L, Bayley-Veloso R, Phillips K, Siwik C, et al. Distress and quality of life in an ethnically diverse sample awaiting breast cancer surgery. J Healh Psychol (2018) 23(11):1428–51. doi: 10.1177/1359105316659916
66. Hsiao F, Kuo W, Jow G, Chang K, Yang P, Lam H, et al. Habitual sleep-wake behaviors and lifestyle as predictors of diurnal cortisol patterns in young breast cancer survivors: a longitudinal study. Psychoneuroendocrinology. (2015) 53:60–8. doi: 10.1016/j.psyneuen.2014.12.014
67. Diaz M, Aldridge-Gerry A, Spiegel D. Posttraumatic growth and diurnal cortisol slope among woomen with metastatic breast cancer. Psychoneuroendocrinology. (2014) 44:83–7. doi: 10.1016/j.psyneuen.2014.03.001
68. Palesh O, Aldridge-Gerry A, Zeitzer J, Koopman C, Neri E, Giese-Davis J, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. (2014) 37(5):837–42. doi: 10.5665/sleep.3642
69. Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behaviour Immun (2013) 30:S163–70. doi: 10.1016/j.bbi.2012.07.019
70. Cohen L, Cole S, Sood A, Prinsloo S, Kirschbaum C, Arevalo J, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PloS One (2012) 7(8):e42324. doi: 10.1371/journal.pone.0042324
71. Dedert E, Lush E, Chapgar A, Dhabhar F, Segerstrom S, Spiegel D, et al. Stress, coping, and circadian disruption among women awaiting breast cancer surgery. Ann Behav Med (2012) 44:10–20. doi: 10.1007/s12160-012-9352-y
72. Brivio F, Fumagalli L, Fumagalli G, Pescia S, Brivio R, Di Fede G, et al. Synchronisation of cortisol circadian rhythm by the pineal hormone melatonin in untreatable metastatic solid tumor patients and its possible prognostic significance on tumor progression. In Vivo. (2010) 24(2):239–41.
73. Sephton S, Dhabhar F, Keuroghlian A, Giese-Davis J, McEwen B, Ionan A, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behaviour Immunity. (2009) 23(8):1148–55. doi: 10.1016/j.bbi.2009.07.007
74. Lutgendorf S, Weinrib A, Penedo F, Russell D, DeGeest K, Costanzo E, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol (2008) 26(29):4820–7. doi: 10.1200/JCO.2007.14.1978
75. Palesh O, Zeitzer J, Conrad A, Giese-Davis J, Mustian K, Popek V, et al. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med (2008) 4(5):441–9. doi: 10.5664/jcsm.27280
76. Mussi C, Crippa S, Bonardi C, Fontana A, Caprotti R, Uggeri F. Endocrine and immunological alterations during cancer processes. Int Surgery. (2006) 91(2):68–71.
77. Spiegel D, Giese-Davis J, Taylor B, Kraemer HC. Stress sensitivity in metastatic breast cancer: Analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. (2006) 31(10):1231–44. doi: 10.1016/j.psyneuen.2006.09.004
78. Rich T, Innominato P, Boerner J, Mormont M, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res (2005) 11(5):1757–64. doi: 10.1158/1078-0432.CCR-04-2000
79. Giese-Davis J, Abercrombie HC, Sephton SE, Duran REF. Repression and high anxiety are associated with aberrant diurnal cortisol rhythms in women with metastatic breast cancer. Health Psychol (2004) 23(6):645–50. doi: 10.1037/0278-6133.23.6.645
80. Mormont M, Bogdan A, Cormont S, Touitou Y, Levi F. Cortisol diurnal variation in blood and saliva of patients with metastatic colorectal cancer: relevance for clinical outcome. Anticancer Res (2002) 22(2b):1243–9.
81. Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Institute (2000) 92(12):994–1000. doi: 10.1093/jnci/92.12.994
82. Touitou Y, Lévi F, Bogdan A, Benavides M, Bailleul F, Misset J. Rhythm alteration in patients with metastatic breast cancer and poor prognostic factors. J Cancer Res Clin Oncol (1995) 121(3):181–8. doi: 10.1007/BF01198101
83. Touitou Y, Lévi F, Bogdan A, Bruguerolle B. Abnormal patterns of plasma cortisol in breast cancer patients. Annu Rev Chronopharmacology. (1990) 7:245–8.
84. Fouladiun M, Korner U, Gunnebo L, Sixt-Ammilon P, Bosaeus I, Lundholm K. Daily physical-rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res (2007) 13(21):6379–85. doi: 10.1158/1078-0432.CCR-07-1147
85. Le Guen Y, Gagnadoux F, Hureaux J, Jeanfaivre T, Meslier N, Racineux J, et al. Sleep disturbances and impaired daytime functioning in outpatients with newly diagnosed lung cancer. Lung Cancer. (2007) 58:139–43. doi: 10.1016/j.lungcan.2007.05.021
86. Pati A, Parganiha A, Kar A, Soni R, Roy S, Choudhary V. Alterations of the characteristics of the circadian rest-activity rhythm of cancer in-patients. Chronobiology Int (2007) 24(6):1179–97. doi: 10.1080/07420520701800868
87. Levin RD, Daehler MA, Grutsch JF, Quiton J, Lis CG, Peterson C, et al. Circadian function in patients with advanced non-small-cell lung cancer. Br J Cancer. (2005) 93(11):1202–8. doi: 10.1038/sj.bjc.6602859
88. Chevalier V, Mormont M, Cure H, Chollet P. Assessment of circadian rhythms by actimetry in healthy subjects and patients with advanced colorectal cancer. Oncol Rep (2003) 10(3):733–7.
89. Patel SD, Davies A, Laing E, Wu H, Mendis J, Dijk D-J. Prognostication in advanced cancer by combining actigraphy-derived rest-activity and sleep parameters with routine clinical data: An exploratory machine learning study. Cancers. (2023) 15:503. doi: 10.3390/cancers15020503
90. Block HI, Gyllenhaal C, Grutsch JF, Block PB, Kazlausky T, Blask D, et al. Advanced cancer patients in a randomized clinical trial of night-simulating eyeglasses observed to have a normal 24-h circadian rhythm during chemotherapy. SAGE Open Med (2022) 10:20503121221100137. doi: 10.1177/20503121221100137
91. Padron A, McCrae CS, Robinson ME, Waxenberg LB, Antoni MH, Berry RB, et al. Impacts of cognitive behavioural therapy for insomnia and pain on sleep in women with gynecologic MALIgnancies: A randomised controlled trial. Behav Sleep Med (2022) 20(4):460–76. doi: 10.1080/15402002.2021.1932500
92. Bernatchez M, Savard J, Aubin M. Correlates of disrupted sleep-wake variables in patients with advanced cancer. BMJ Supportive Palliative Care (2020) 10:55–63. doi: 10.1136/bmjspcare-2018-001505
93. Cash E, Duck C, Brinkman C, Rebholz W, Albert C, Worthen M, et al. Depressive symptoms and actigraphic-measured circadian disruption predict head and neck cancer survival. Psycho-Oncology. (2018) 27:2500–7. doi: 10.1002/pon.4862
94. Innominato P, Komarzynski S, Karaboue A, Ulusakarya A, Bouchahda M, Haydar M, et al. Home-Based e-Health platform for multidimensional telemonitoring of symptoms, body weight, sleep, and circadian activity: Relevance for chronomodulated administration of irinotecan, fluorouracil-leucovorin, and oxaliplatin at home - results from a pilot study. JCO Clin Cancer Informatics. (2018) 2:1–15. doi: 10.1200/CCI.17.00125
95. Innominato P, Komarzynski S, Palesh O, Dallmann R, Bjarnson G, Giacchetti S, et al. Circadian rest-activity rhythm as an objective marker of patient-reported outcomes in patients with advanced cancer. Cancer Med (2018) 7(9):4396–405. doi: 10.1002/cam4.1711
96. Chang W, Lin C. Changes in sleep-wake rhythm, sleep quality, mood, and quality of life of patients receiving treatment for lung cancer: a longitudinal study. Chronobiology Int (2017) 34(4):451–61. doi: 10.1080/07420528.2017.1293678
97. Dean G, Sabdah E, Yingregreung S, Ziegler P, Chen H, Steinbrenner L, et al. Sleeping with the enemy. Sleep Qual Life patients Lung cancer. Cancer Nursing. (2015) 38:60–70. doi: 10.1097/NCC.0000000000000128
98. Chang W, Lin C. Correlation between rest-activity rhythm and survival in cancer patients experiencing pain. Chronobiology Int (2014) 31(8):926–34. doi: 10.3109/07420528.2014.931412
99. Lévi F, Dugué PA, Innominato P, Karaboué A, Dispersyn G, Parganiha A, et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiology Int (2014) 31(8):891–900. doi: 10.3109/07420528.2014.924523
100. Ma C, Chang W, Lin C. Rest/activity rhythm is related to the coexistence of pain and sleep disturbance among advanced cancer patients with pain. Supportive Care Cancer. (2014) 22:87–94. doi: 10.1007/s00520-013-1918-0
101. Ortiz-Tudela E, Iurisci I, Beau J, Karaboue A, Moreau T, Rol M, et al. The circadian rest-activity rhythm, a potential safety pharmacology endpoint of cancer chemotherapy. Int J Cancer. (2014) 134(11):2712–25. doi: 10.1002/ijc.28587
102. Dean G, Redeker N, Wang Y, Rogers A, Dickerson S, Steinbrenner L, et al. Sleep, mood, and quality of life in patients receiving treatment for lung cancer. Oncol Nurs Forum. (2013) 40(5):441–51. doi: 10.1188/13.ONF.441-451
103. Dhruva A, Paul S, Cooper B, Lee K, West C, Aouizerat B, et al. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Management. (2012) 44(2):215–28. doi: 10.1016/j.jpainsymman.2011.08.010
104. Innominato PF, Giacchetti S, Bjarnson GA, Focan C, Garufi C, Coudert B, et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer (2012) 131(11):2684–92. doi: 10.1002/ijc.27574
105. Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, et al. Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res (2009) 69(11):4700–7. doi: 10.1158/0008-5472.CAN-08-4747
106. Mormont M, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiology Int (2002) 19:312–23. doi: 10.1081/CBI-120002606
107. Silberfarb P, Hauri P, Oxman T, Schnurr P. Assessment of sleep in patients with lung cancer and breast cancer. J Clin Oncol (1993) 11(5):997–1004. doi: 10.1200/JCO.1993.11.5.997
108. Parker K, Bliwise D, Ribeiro M, Jain S, Vena C, Kohles-Baker M, et al. Sleep/Wake patterns of individuals with advanced cancer measured by ambulatory polysomnography. J Clin Oncol (2008) 26(15):2464–72. doi: 10.1200/JCO.2007.12.2135
109. Milanti A, Chan DNS, Li C, So WKW. Actigraphy-measured rest-activity circadian rhythm disruption in patients with advanced cancer: a scoping review. Supportive Care In Cancer. (2021) 29:7145–69. doi: 10.1007/s00520-021-06317-3
110. Hullet JM, Fessele KL, Clayton MF, Eaton LH. Rigor and reproducibility: a systematic review of salivary cortisol sampling and reporting parameters used in cancer survivorship research. Biol Res Nursing. (2019) 21(3):318–34. doi: 10.1177/1099800419835321
111. Zhou L, Zhang Z, Nice E, Huang C, Zhang W, Tang Y. Circadian rhythms and cancers: the intrinsic links and therapeutic potentials. J Haematology Oncol (2022) 15:21. doi: 10.1186/s13045-022-01238-y
112. Mitchell JA, Quante M, Godbole S, James P, Hipp JA, Marinac CR, et al. Variations in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiology Int (2017) 34(8):1042–56. doi: 10.1080/07420528.2017.1337032
113. Sultan A, Pati AK, Choudhary V, Parganiha A. Hospitalization-induced exacerbation of the ill effects of chemotherapy on rest-activity rhythm and quality of life of breast cancer patients: a prospective and comparative cross-sectional follow-up study. Chronobiology Int (2018) 35(11):1513–32. doi: 10.1080/07420528.2018.1493596
114. Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’ - circadian rhythms in neurodegenerative disorders. Nat Rev Neurology. (2014) 10(12):683–93. doi: 10.1038/nrneurol.2014.206
115. Schoch SF, Kurth S HW. Actigraphy in sleep research with infants and young children: Current practices and future benefits of standardizing reporting. J Sleep Res (2021) 30(3):e13134. doi: 10.1111/jsr.13134
116. Scott J, Colom F, Young A, Beliivier F, Etain B. An evidence map of actigraphy studies exploring longitudinal associations between rest-activity rhythms and course and outcome of bipolar disorders. Int J Bipolar Disord (2020) 8:37. doi: 10.1186/s40345-020-00200-6
117. American Academy of Sleep Medicine. International classification of sleep disorders. 3rd edition. Darien, IL: American Academy of Sleep Medicine (2014).
Keywords: cancer, circadian rhythms, symptoms, quality of life, survival
Citation: Gouldthorpe C, Power J and Davies A (2023) Circadian rhythm disorders in patients with advanced cancer: a scoping review. Front. Oncol. 13:1240284. doi: 10.3389/fonc.2023.1240284
Received: 14 June 2023; Accepted: 16 August 2023;
Published: 26 September 2023.
Edited by:
Sandra E. Sephton, Brigham Young University, United StatesReviewed by:
DeeDee Smart, National Cancer Institute (NIH), United StatesCopyright © 2023 Gouldthorpe, Power and Davies. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Craig Gouldthorpe, Z291bGR0aGNAdGNkLmll
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.