
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 22 September 2023
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1235679
This article is part of the Research Topic Treatment Strategies for Lung Cancer: A Progress Update and Future Perspectives View all 6 articles
Several cases of STRN-ALK fusion have been reported, and some anaplastic lymphoma kinase (ALK) inhibitors have been shown to be effective for treatment. Nevertheless, no cases of COVID-19 leading to heart failure and respiratory failure have been reported in people older than 70 years treated with ALK inhibitors. The present case report describes a 70-year-old patient with usual chronic obstructive pulmonary disease, diabetes, depression, and carotid plaque disease. Next-generation sequencing of tissue obtained by puncture biopsy revealed a STRN-ALK mutation accompanied by a TP53 mutation. The patient was treated with ensartinib and developed COVID-19 leading to heart failure and respiratory failure; nevertheless, he had a good clinical outcome and exhibited high treatment tolerability.
The overall incidence of non-small cell lung cancer (NSCLC) is high in patients of all ages (1).Approximately 1 to 8 million cases of lung cancer are newly diagnosed each year, and 1 to 6 million deaths are related to this disease. Lung cancer is the leading cause of cancer-related death globally (2). China has the highest lung cancer-related mortality rate, with NSCLC accounting for the majority of cases (3). In 3% to 7% of patients with NSCLC, chromosomal rearrangements involving anaplastic lymphoma kinase (ALK) have been identified as oncogenic factors (4, 5). The clinical outcomes and prognosis of patients treated with tyrosine kinase inhibitors (TKIs) are better than those of patients treated with conventional chemotherapy drugs (6).
With the recent development of diagnostic methods, next-generation sequencing (NGS) to detect ALK fusions has become more helpful in driving treatment decisions (7). Additionally, some rare ALK fusions have been detected in the clinical setting (8). This report describes a patient with STRN-ALK fusion who could not tolerate surgery and received ensartinib as first-line treatment. He thereafter developed COVID-19 (omicron variant) but nevertheless demonstrated good tolerability and clinical efficacy.
A 70-year-old man was admitted to our hospital because of an acute attack of chronic obstructive pulmonary disease (COPD) (recurrent cough and sputum for more than 5 years, aggravated by recurrence for 1 week). The patient had type 2 diabetes mellitusinternal carotid artery plaque and history of depression. The patient had smoked an average of 30 cigarettes a day for 50 years. Physical examination only found mild edema of both lower extremities.
Computed tomography (CT) of his chest on 29 September 2022 showed a mass (4.2 × 3.8 cm) in his right upper lung with cavity formation. Emphysema and microscopic nodules were present in the left upper lung and right lower lung (Figure 1).
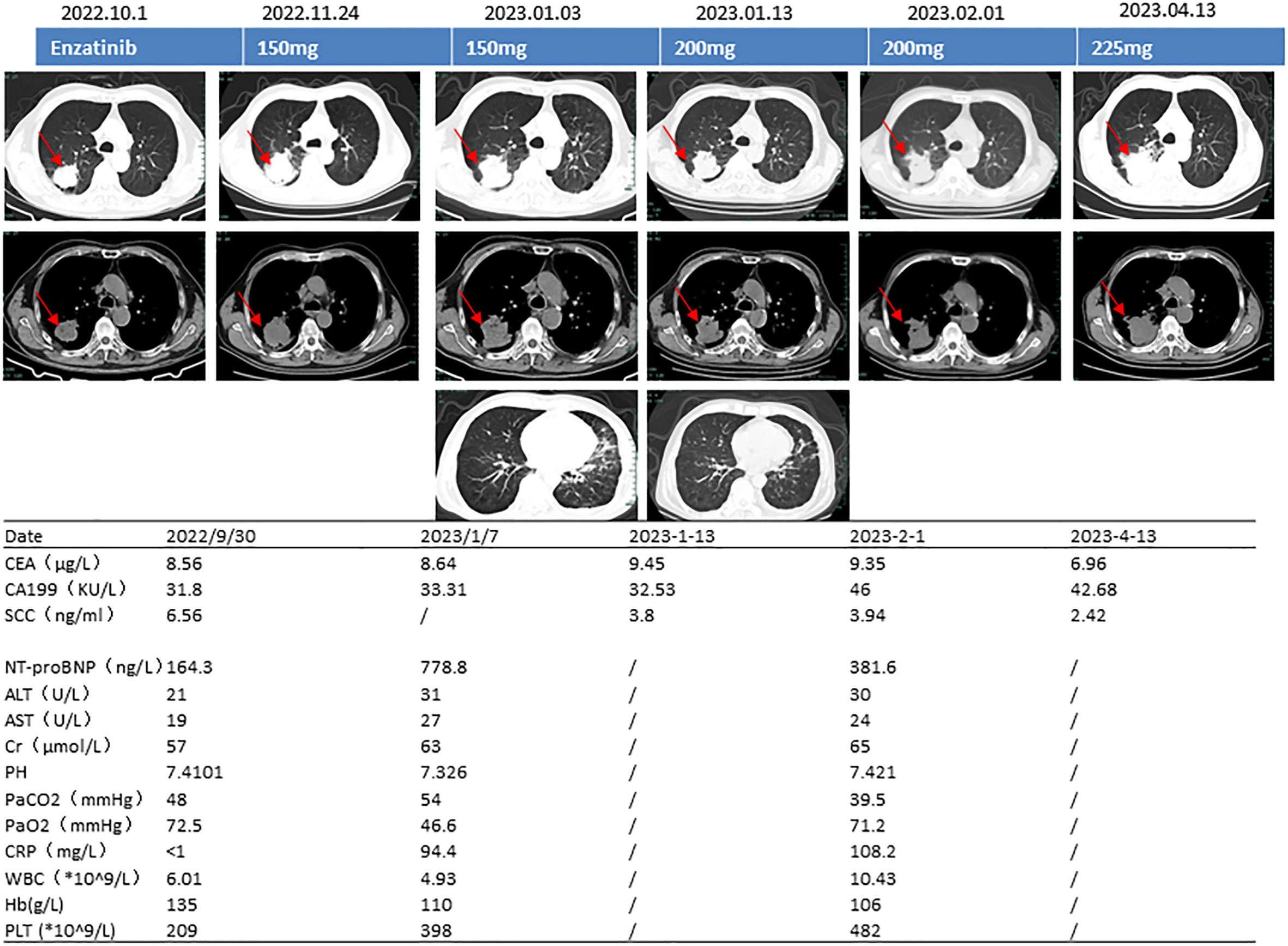
Figure 1 Computed tomography images, ensartinib dose, and blood test results. CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; SCC, squamous epithelial cell carcinoma antigen; NT-proBNP, N-terminal brain natriuretic peptide precursor; ALT, alanine transaminase; AST, aspartate transaminase; pH, potential of hydrogen; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; CRP, C-reactive protein; WBC, white blood cell count; Hb, hemoglobin; PLT, platelet count.
Routine blood testing on 29 September 2022 showed the following results: C-reactive protein, <1.0 mg/L; white blood cell count, 6.01 × 109/L; hemoglobin, 135 g/L; and platelet count, 209 × 109/L. Arterial blood gas analysis indicated a pH of 7.41, partial pressure of carbon dioxide (PaCO2) of 48.0 mmHg, and partial pressure of oxygen (PaO2) of 72.5 mmHg. The concentration of glycosylated hemoglobin was 9.70%. The D-dimer concentration was normal. The concentration of N-terminal natriuretic peptide precursor in his brain was 164.30 ng/L (reference range, 0–125 ng/L). The concentration of carcinoembryonic antigen (CEA) was 8.56 μg/L (reference range, 0–5 μg/L), and the concentration of squamous epithelial cell carcinoma antigen (SCC) was 6.560 ng/mL (reference range, 0–3 μg/L).
Pulmonary function testing revealed very severe mixed ventilation dysfunction, severely reduced diffusion function, and a negative bronchial diastolic test (FEV1 0.87L/S, FEV1% 32.1, FEV1/FVC 41.63%). CT-guided pulmonary puncture biopsy was performed on 3 October 2022. Enhanced magnetic resonance imaging on 5 October 2022 showed no tumor metastasis in brain (Figures 2K–M). 10 October 2022 (combination of hematoxylin–eosin staining and immunohistochemistry) showed non-small cell carcinoma. The immunohistochemical results were as follows: carcinoembryonic antigen (−), cytokeratin 5/6 (+), epidermal growth factor receptor (EGFR) (membrane +), cytokeratin 7 (+), Ki-67 (+, 60%), p40 (few +), p53 (3+), pan-cytokeratin (+), thyroid transcription factor-1 (few +), napsin A (few +), cytokeratin 20 (−), and GATA-3 (+) (Figures 2A–J). On 11 October 2022, kidney, ureter, and bladder ultrasound showed no abnormal findings. The patient had poor lung function and could not tolerate surgery, and he refused other tests and treatments. Therefore, we recommended pulmonary function exercise. A repeat CT examination of his chest 1.5 months later showed that the lung mass had grown (5.9 × 5.4 cm) and that the sub-rhomboid lymph nodes had become enlarged (Figure 1). The patient’s Eastern Cooperative Oncology Group performance status (PS) score was 2.

Figure 2 Pathology and magnetic resonance imaging. (A) Hematoxylin–eosin. (B) Cytokeratin 5/6 (+). (C) Epidermal growth factor receptor (+). (D) Cytokeratin 7 (+). (E) Ki67 (+, 60%). (F) p40 (few+). (G) p53 (+++). (H) Pan-cytokeratin (+). (I) Thyroid transcription factor-1 (few +). (J) Napsin A (few +). (K) T1-weighted imaging. (L) Fluid-attenuated inversion recovery. (M) T2-weighted imaging.
The patient underwent next-generation sequencing, which showed STRN-ALK (STRN_exon3-ALK_exon20) fusion mutation (1.70%) and TP53 NM_000546 exon5 c.473G>T p.R158L (14.10%).
Considering the patient’s underlying concomitant diseases and poor PS score, we prescribed ensartinib at a starting dosage of 150 mg/day. One month later, repeat CT of his chest showed that the tumor was smaller and that the treatment had resulted in stable disease. The patient experienced no intolerance even after increasing the dosage to 200 mg/day. Two months later, however, he became infected with the omicron variant of SARS-CoV-2, resulting in type 1 respiratory failure and heart failure (pH, 7.326; PaCO2, 54 mmHg; PaO2, 46.6 mmHg; NT-proBNP, 778.8 ng/L). We carried out targeted treatment and continued the ensartinib. At follow-up on 13 April 2023, the tumor was stable but had slightly increased in size (5.3 × 5.5 cm) (Figure 1). We thereafter recommended increasing the dosage of ensartinib to 225 mg/day, and no additional adverse effects were noted at the subsequent telephone follow-up.
ALK is a member of the insulin receptor family of receptor tyrosine kinases, which are expressed in different types of cancers (9). In normal cells, ALK sits on cell membranes and interacts with growth factors. EML4 is the most common ALK rearrangement in NSCLC. With advances in clinical genetic testing technology, more genes that are coexpressed with ALK have been identified (6, 10, 11). STRN-ALK fusion is a rare type of ALK rearrangement in NSCLC (12).
The STRN gene is located on chromosome 2 and encodes a protein with a coiled-coil domain that leads to constitutive activation of ALK via dimerization (8). Treatment options for patients who have NSCLC with rare ALK fusion often rely on past case reports. Many different ALK inhibitors have been reported to be effective for treating STRN-ALK, including crizotinib, ceritinib, ensartinib, and alectinib (8, 12–14).
We retrieved 11 reports describing cases of STRN-ALK in PubMed (Table 1). At the time of identification of the STRN-ALK mutation, gefitinib had previously been used in two cases, crizotinib in two cases, and alectinib in two cases. Of all 11 patients, 9 were male, at least 7 were Chinese, and 8 were nonsmokers. Five patients had right-sided lesions, five had left-sided lesions, and one was not described. Adenocarcinoma was present in 10 patients, and the pathologic type was not described in 1 patient. Pulmonary embolism occurred in two patients, and bone metastases were present in at least six. Most of the patients underwent NGS, mostly using tissue for testing. The STRN-ALK mutation rate ranged from 0.04% to 27.11% (15–18).
Various other mutations were present in combination with STRN-ALK, including EGFR exon 19 deletion, TP53, and PIK3CA. First-line crizotinib was used in 5 of the 11 patients, with maintenance of efficacy ranging from 5 to 18 months. First-line alectinib was used in four patients, with maintenance of efficacy ranging from 5 to 17 months. One patient treated with first-line ceritinib achieved maintenance of efficacy of 26 months, and one patient treated with chemotherapy showed no effect. Three patients were treated with second-line therapy: one treated with crizotinib and one treated with ensartinib achieved maintenance of 5 and 13 months, respectively, and the remaining patient treated with chemotherapy achieved no clinical benefit. Four patients had TP53 mutations, and the duration of first-line treatment maintenance ranged from 5 to 11 months (5, 6, 7, and 11 months, respectively) (13, 14, 19–21).
Ensartinib is a second-generation ALK-TKI. In the phase I/II eXalt2 trial, ensartinib demonstrated a high response rate in patients with ALK fusions resistant to prior crizotinib treatment (22). Use of ensartinib to treat NSCLC exhibiting STRN-ALK fusion has demonstrated good clinical efficacy in patients with crizotinib-resistant relapsed NSCLC (12).Our patient’s lung function was so poor that he could not tolerate surgical treatment; he also had many concomitant diseases and a low PS score.
Considering our patient’s advanced age and poor underlying condition, we used a dose creep of ensartinib and achieved a therapeutic response at the 150 mg dose. COVID-19 subsequently resulted in respiratory failure combined with heart failure, and ensartinib was continued at 200 mg/day with some clinical success. Notably, this is the oldest reported patient with COVID-19 who had STRN-ALK fusion and was treated by first-line ensartinib. Furthermore, the patient showed coalteration of TP53. A previous study reported that TP53 mutations disrupt the tumor suppressor function of the p53 protein and can be detected in almost all types of cancer, including ALK-positive NSCLC, in which TP53 mutations are the most common genomic alteration detected. Patients with this mutation generally have poor progression-free survival (23). In four previously reported cases of STRN-ALK treated with ALK-TKIs, the sustained response time ranged from 5 to 11 months (5, 6, 7, and 11 months, respectively) (13, 14, 19–21). After 13 April 2023, we increased the dose of ensartinib to 225 mg/day in our patient with the goal of achieving a good long-term clinical outcome.
This case provides valuable clinical evidence of the response to ensartinib as a first-line treatment for patients with NSCLC exhibiting rare STRN-ALK fusions. Our patient had a very poor underlying condition, advanced age, and type 1 respiratory failure and heart decompensation due to COVID-19; nevertheless, he was still able to tolerate treatment with ensartinib. The presence of the TP53 mutation may be associated with poor clinic outcomes.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The studies involving humans were approved by Changxing county hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
G-QS and Y-ZL have contributed equally to this work and share first authorship (G-QS write and polish this paper, Y-ZL do some check work), WK confirm the pathology and check the figures. G-QH was responsible for the design of the paper, the cost and the confirmed end edition, who can be considered as a corresponding author. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non–small cell lung cancer in the US. JAMA Oncol (2021) 7:1824. doi: 10.1001/jamaoncol.2021.4932
2. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu Y-L, et al. Lung cancer: current therapies and new targeted treatments. Lancet (2017) 389:299–311. doi: 10.1016/S0140-6736(16)30958-8
3. Cao W, Chen H-D, Yu Y-W, Li N, Chen W-Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
4. Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non–small-cell lung cancer who Harbor EML4-ALK. J Clin Oncol (2009) 27:4247–53. doi: 10.1200/JCO.2009.22.6993
5. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature (2007) 448:561–6. doi: 10.1038/nature05945
6. Soria J-C, Tan DSW, Chiari R, Wu Y-L, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK -rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet (2017) 389:917–29. doi: 10.1016/S0140-6736(17)30123-X
7. Lin C, Shi X, Yang S, Zhao J, He Q, Jin Y, et al. Comparison of ALK detection by FISH, IHC and NGS to predict benefit from crizotinib in advanced non-small-cell lung cancer. Lung Cancer (2019) 131:62–8. doi: 10.1016/j.lungcan.2019.03.018
8. Ren H, Hou X, Eiken PW, Zhang J, Pierson KE, Nair AA, et al. Identification and development of a lung adenocarcinoma PDX model with STRN-ALK fusion. Clin Lung Cancer (2019) 20:e142–7. doi: 10.1016/j.cllc.2018.11.002
9. Collier TL, Normandin MD, Stephenson NA, Livni E, Liang SH, Wooten DW, et al. Synthesis and preliminary PET imaging of 11C and 18F isotopologues of the ROS1/ALK inhibitor lorlatinib. Nat Commun (2017) 8:15761. doi: 10.1038/ncomms15761
10. Lasota J, Chłopek M, Wasąg B, Kowalik A, Christiansen J, Lamoureux J, et al. Colorectal adenocarcinomas harboring ALK fusion genes: A clinicopathologic and molecular genetic study of 12 cases and review of the literature. Am J Surg Pathol (2020) 44:1224–34. doi: 10.1097/PAS.0000000000001512
11. Tabbò F, Muscarella LA, Gobbini E, Trombetta D, Castellana S, Rigutto A, et al. Detection of ALK fusion variants by RNA-based NGS and clinical outcome correlation in NSCLC patients treated with ALK-TKI sequences. Eur J Cancer (2022) 174:200–11. doi: 10.1016/j.ejca.2022.07.026
12. Zhang L, Xiao P, Meng F, Zhong D. STRN-ALK fusion in lung adenocarcinoma with brain metastasis responded well to ensartinib: A case report. Curr Oncol (2022) 29:6749–53. doi: 10.3390/curroncol29100530
13. Sun K, Nie L, Nong L, Cheng Y. Primary resistance to alectinib in a patient with STRN-ALK -positive non-small cell lung cancer: A case report. Thorac Cancer (2021) 12:1927–30. doi: 10.1111/1759-7714.13983
14. Zeng H, Li Y, Wang Y, Huang M, Zhang Y, Tian P, et al. Case report: identification of two rare fusions, PDK1-ALK and STRN-ALK, that coexist in a lung adenocarcinoma patient and the response to alectinib. Front Oncol (2021) 11:722843. doi: 10.3389/fonc.2021.722843
15. Yang Y, Qin S-K, Zhu J, Wang R, Li Y-M, Xie Z-Y, et al. A rare STRN-ALK fusion in lung adenocarcinoma identified using next-generation sequencing–based circulating tumor DNA profiling exhibits excellent response to crizotinib. Mayo Clinic Proceedings: Innovations Qual Outcomes (2017) 1:111–6. doi: 10.1016/j.mayocpiqo.2017.04.003
16. Zhou C, Zeng L, Zhang Y, Yang N. Responder of gefitinib plus crizotinib in osimertinib failure EGFR-mutant NSCLC-resistant with newly identified STRN-ALK by next-generation sequencing. J Thorac Oncol (2019) 14:e143–4. doi: 10.1016/j.jtho.2019.02.014
17. Su C, Jiang Y, Jiang W, Wang H, Liu S, Shao Y, et al. STRN-ALK fusion in lung adenocarcinoma with excellent response upon alectinib treatment: A case report and literature review. OTT (2020) 13:12515–9. doi: 10.2147/OTT.S282933
18. Nagasaka M, Sarvadevabatla N, Iwata S, Ge Y, Sukari A, Klosowski C, et al. STRN-ALK, A novel in-frame fusion with response to alectinib. JTO Clin Res Rep (2021) 2:100125. doi: 10.1016/j.jtocrr.2020.100125
19. Nakanishi Y, Masuda S, Iida Y, Takahashi N, Hashimoto S. Case report of non–small cell lung cancer with STRN-ALK translocation: A nonresponder to alectinib. J Thorac Oncol (2017) 12:e202–4. doi: 10.1016/j.jtho.2017.08.009
20. Zeng Q, Gao H, Zhang L, Qin S, Gu Y, Chen Q. Coexistence of a secondary STRN–ALK, EML4–ALK double-fusion variant in a lung adenocarcinoma patient with EGFR mutation: a case report. Anti-Cancer Drugs (2021) 32:890–3. doi: 10.1097/CAD.0000000000001094
21. Li M, An Z, Tang Q, Ma Y, Yan J, Chen S, et al. Mixed responses to first-line alectinib in non-small cell lung cancer patients with rare ALK gene fusions: A case series and literature review. J Cell Mol Med (2021) 25:9476–81. doi: 10.1111/jcmm.16897
22. Horn L, Infante JR, Reckamp KL, Blumenschein GR, Leal TA, Waqar SN, et al. Ensartinib (X-396) in ALK-positive non–small cell lung cancer: results from a first-in-human phase I/II, multicenter study. Clin Cancer Res (2018) 24:2771–9. doi: 10.1158/1078-0432.CCR-17-2398
Keywords: STRN-ALK, NSCLC, ensartinib, case report, TKIs
Citation: Song G-q, Li Y-z, Kong W and Hu G-q (2023) Case Report: A rare case of non-small cell lung cancer with STRN-ALK fusion in a patient in very poor condition treated with first-line ensartinib. Front. Oncol. 13:1235679. doi: 10.3389/fonc.2023.1235679
Received: 15 June 2023; Accepted: 24 August 2023;
Published: 22 September 2023.
Edited by:
Baoming Wang, Chengdu University, ChinaReviewed by:
Usama Hussein, University of Texas Health Science Center at Houston, United StatesCopyright © 2023 Song, Li, Kong and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-qiang Song, d3p5eHlzZ3FAMTI2LmNvbQ==; Guo-qiang Hu, Y2hhbmd4aW5naGdxQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.