- 1Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 2Division of Pathology, Exploratory Oncology Research and Clinical Trial Center, National Cancer Center Hospital East, Kashiwa, Japan
Combined hepatocellular cholangiocarcinoma (cHCC-CCA) is a rare subtype of primary liver cancers. Therapeutic strategies for patients with cHCC-CCA are limited, and no standard systemic treatment has been established for unresectable cHCC-CCA. Here, we present six cases of cHCC-CCA treated with atezolizumab plus bevacizumab. We observed three partial responses and one stable disease as the best responses; two of these patients were still being treated with atezolizumab plus bevacizumab at the time of reporting (at least five months of treatment), whereas the remaining two patients were unable to continue treatment owing to adverse events. Atezolizumab plus bevacizumab may be an effective treatment for unresectable cHCC-CCA.
1 Introduction
Primary liver cancer, comprising chiefly hepatocellular carcinoma and cholangiocarcinoma, is the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death worldwide, with an increasing incidence in most Western countries (1, 2). It is a heterogeneous group of tumors with distinct risk factors, clinical outcomes, and histological and molecular features. Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare subtype comprising unequivocal and intimately mixed histopathological features of both hepatocellular carcinoma and cholangiocarcinoma within the same tumor (3). Its clinical outcome is considered poorer than that of hepatocellular carcinoma and equal to or worse than that of cholangiocarcinoma (4–7). Therapeutic strategies for patients with cHCC-CCA are limited; surgical resection is the only standard of care (8, 9). However, no standard systemic treatment has been established for patients with recurrent and/or advanced disease. Therefore, treatment regimens for hepatocellular carcinoma or cholangiocarcinoma have often been used as systemic therapies for cHCC-CCA. Recently, atezolizumab plus bevacizumab, a combination therapy of anti-programmed death ligand-1 (PD-L1) and anti-vascular endothelial growth factor (VEGF), was shown to be superior to sorafenib in the improvement both overall survival (OS) and progression-free survival (PFS) among patients with advanced hepatocellular carcinoma (10). However, few reports on the efficacy and safety of atezolizumab plus bevacizumab among patients with cHCC-CCA have been made. Herein, we report a series of six cases of unresectable cHCC-CCAs treated with atezolizumab plus bevacizumab. This study was approved by the Ethics Committee of the National Cancer Center in Japan (Approval No. 2021-477). Approval for review of the hospital records was obtained from the Institutional Review Board of the National Cancer Center, and informed consent was obtained from all patients.
2 Case descriptions
Characteristics of all the cases are summarized in Table 1.
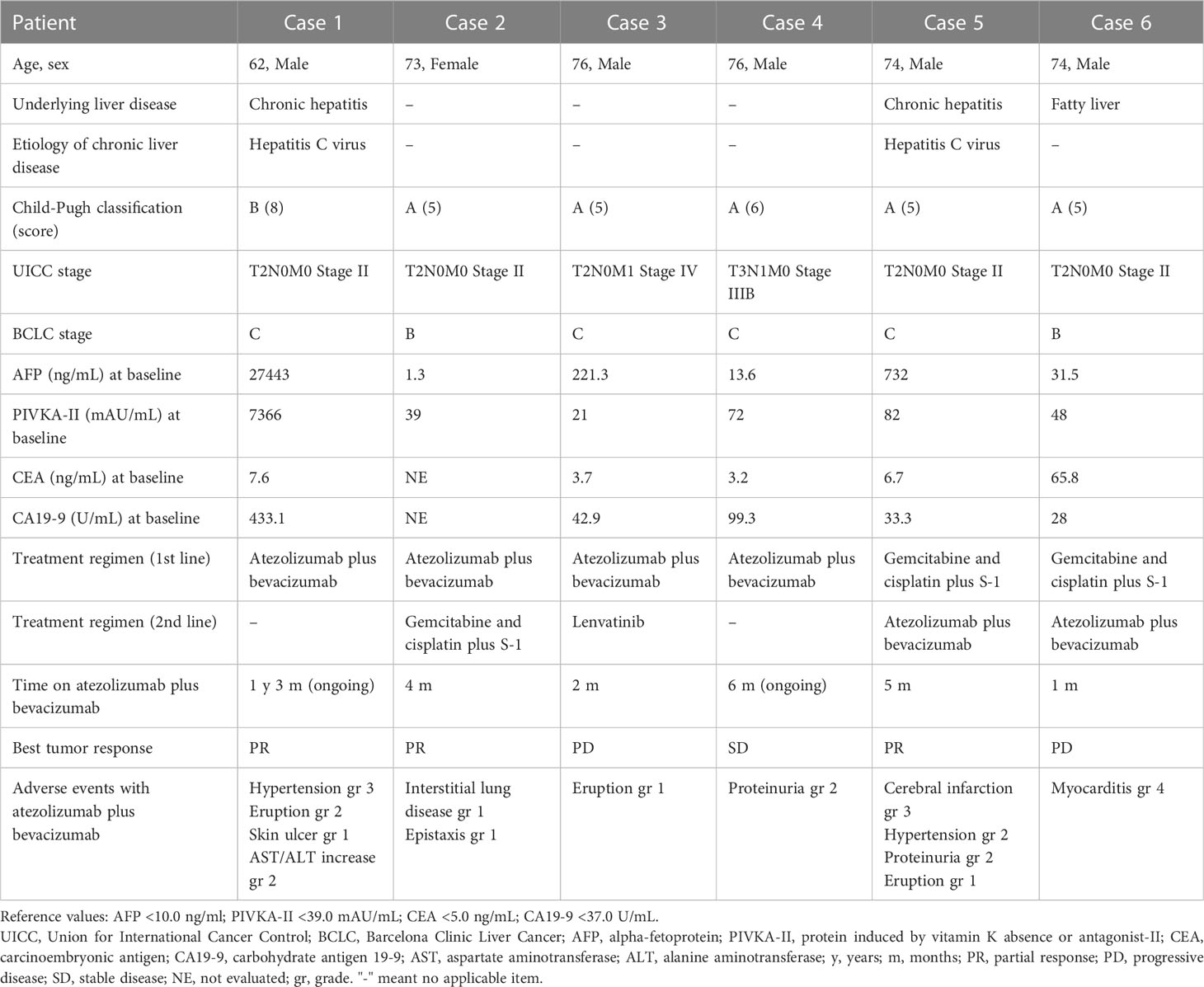
Table 1 Characteristics of patients with combined hepatocellular-cholangiocarcinoma treated with atezolizumab plus bevacizumab.
2.1 Case 1
A 62-year-old man presented to our hospital with a liver tumor due to cholangitis via computed tomography (CT). He was diagnosed with liver cancer containing an adenocarcinoma component via a bile duct biopsy obtained during endoscopic biliary drainage. An additional liver biopsy was performed, and immunohistochemistry revealed atypical cells with bidirectional hepatocellular differentiation, with the expression of hepatocytes, and ductal differentiation, with CK19 expression, leading to a diagnosis of cHCC-CCA (Figure 1). The liver tumor was unresectable because it had extensively invaded the main portal vein. At the start of treatment, the patient had Child-Pugh class B liver function, but his cHCC-CCA was Barcelona Clinic Liver Cancer stage C. We decided that systemic therapy was preferable to transcatheter arterial chemoembolization or transcatheter arterial infusion, and first-line therapy with atezolizumab plus bevacizumab was initiated. Regarding the therapeutic effect, the levels of tumor markers, such as alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II), had decreased one month after the start of treatment, and a partial response (PR; based on the Response Evaluation Criteria in Solid Tumors version 1.1) was observed four months after the start of treatment (Figures 2A, B). Regarding adverse events, grade 3 hypertension was observed immediately after the start of treatment but was well controlled with the addition of antihypertensive drugs. In addition, eruptions appeared in the second cycle, but atezolizumab plus bevacizumab was continued while treating the eruption with topical steroids. However, the eruption did not improve sufficiently, and oral steroid treatment was required from the fourth cycle onward. At the same time, skin ulcers and acneiform eruptions, thought to be caused by bevacizumab, were observed. As mild liver dysfunction was also observed, atezolizumab plus bevacizumab was discontinued. Oral steroids and the addition of minocycline reduced the acneiform eruptions, enabling gradual tapering of these oral medications. The eruption became controllable with topical drugs alone, and after two weeks without it, atezolizumab plus bevacizumab was resumed. The tumor continued to shrink and the patient was still receiving atezolizumab plus bevacizumab one year and three months after the start of treatment.
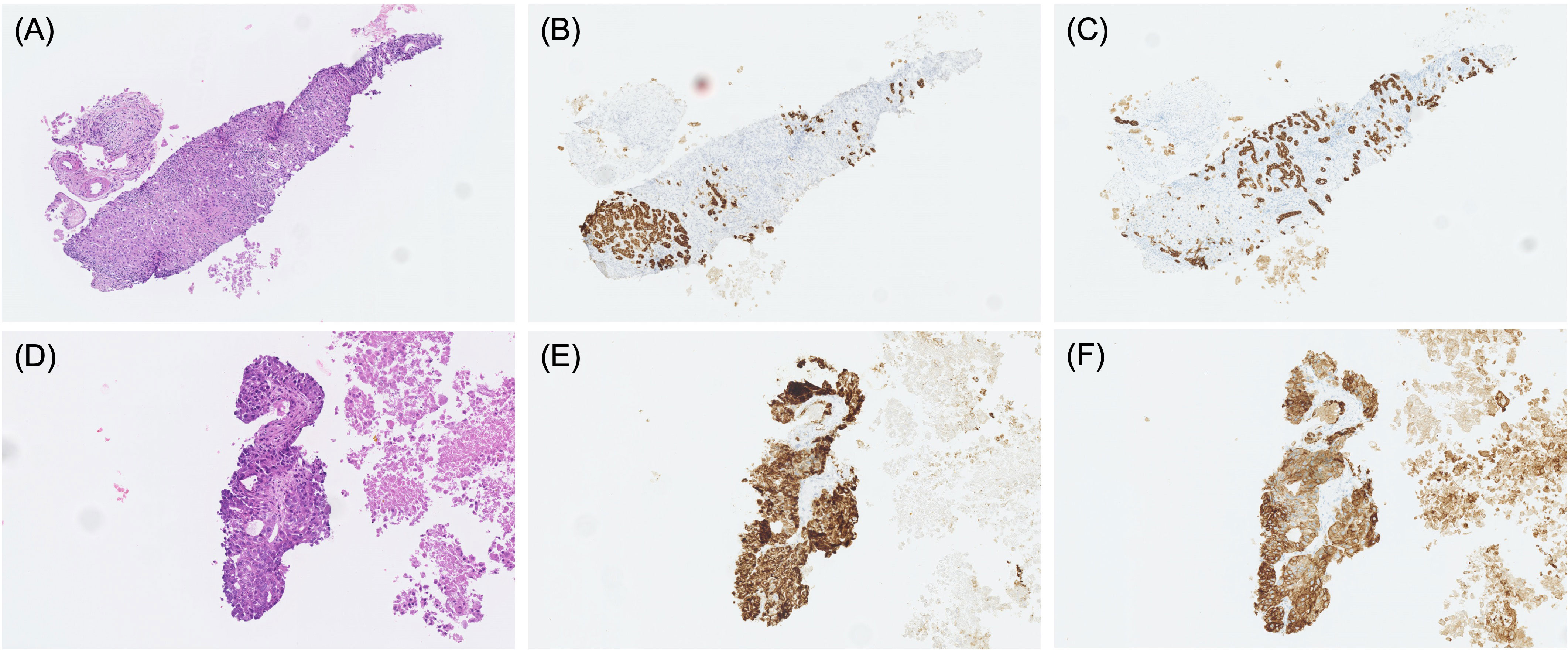
Figure 1 Pathological findings of specimens obtained by percutaneous liver biopsy in cases 1 (A–C) and case 4 (D–F). HE staining (A, D), hepatocyte staining (B, E), and CK19 staining (C, F).
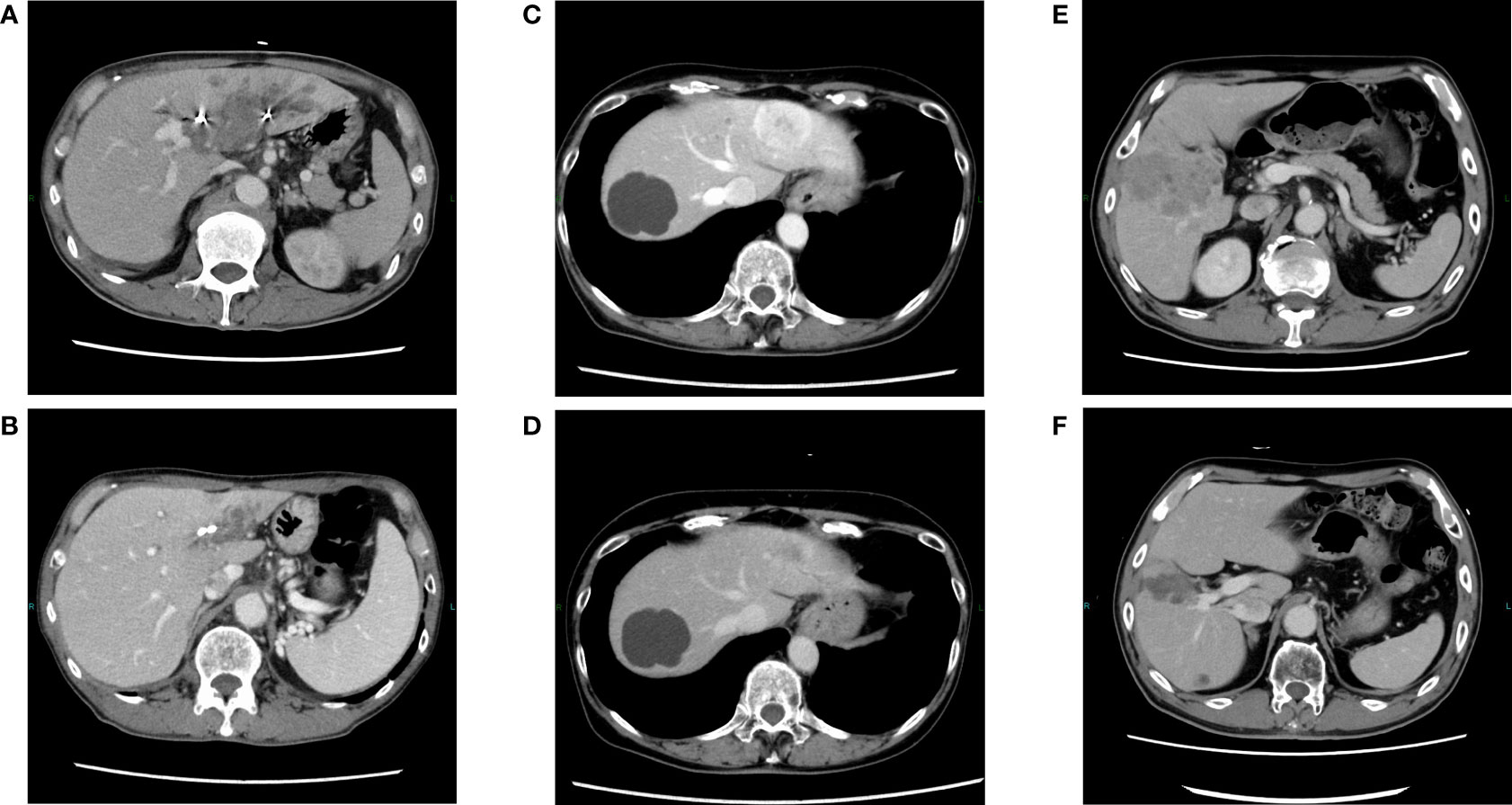
Figure 2 Changes in contrast-enhanced CT findings in responders during treatment. Pretreatment with atezolizumab plus bevacizumab in cases 1 (A), 2 (C), and 5 (E). After treatment with atezolizumab plus bevacizumab in cases 1 (B), 2 (D), and 5 (F), in which a partial response was observed.
2.2 Case 2
A 73-year-old woman was referred to our hospital because of liver tumors identified upon abdominal ultrasonography during a physical examination. Multiple hypervascular liver tumors occurred in both lobes of the liver. At the time, these tumors were judged to be unresectable hepatocellular carcinoma based on radiographic imaging features. First-line therapy with atezolizumab plus bevacizumab was initiated. Regarding the treatment effect, a PR was observed upon CT two months after the start of treatment (Figures 2C, D). As for adverse events, pneumonitis was discovered upon CT four months after the start of treatment. As the pneumonitis might have been drug-induced, the atezolizumab plus bevacizumab was discontinued and bronchoscopy was performed. During the bronchoscopy, a biopsy specimen was obtained, which revealed no tumor content. The patient recovered from the pneumonitis one month after treatment discontinuation. A diagnosis of atezolizumab-induced interstitial lung disease was made, and a liver biopsy was performed to examine the change from that treatment to the next. Immunohistochemistry confirmed adenocarcinoma components, including ductal differentiation, with CK7 expression, and hepatocellular differentiation, with the expression of hepatocytes, leading to a pathological diagnosis of cHCC-CCA. Considering the risk of recurrence of interstitial lung disease when resuming treatment with atezolizumab, we decided to switch to second-line therapy with gemcitabine plus cisplatin and S-1 (GCS). The disease was well controlled after initiation of GCS therapy, and gradual tumor shrinkage was observed. The tumor continued to shrink, and the patient was still alive two years and three months after starting atezolizumab plus bevacizumab treatment, receiving GCS therapy.
2.3 Case 3
A 76-year-old man was diagnosed with prostate cancer upon discovery of an elevated prostate-specific antigen concentration, and a CT scan revealed a liver tumor. A liver biopsy was performed, and immunohistochemistry revealed atypical cells with bidirectional hepatocellular differentiation, with the expression of hepatocytes, and ductal differentiation, with CK19 expression, leading to a diagnosis of cHCC-CCA. The liver tumor was unresectable, with portal vein invasion and bone metastasis, and first-line therapy with atezolizumab plus bevacizumab was initiated. Initially, the only adverse events were grade 1 skin eruptions, and treatment was continued. However, two months after the start of treatment, the patient developed progressive disease (PD), and atezolizumab plus bevacizumab was discontinued. Lenvatinib was started as second-line therapy, but PD was observed 2 months later, and lenvatinib was discontinued. In addition, thoracic aortic dissection was confirmed at that time, following which systemic treatment for cHCC-CCA was discontinued. Finally, best supportive care was provided in another hospital.
2.4 Case 4
A 76-year-old man was diagnosed with bladder cancer because of hematuria, and a CT scan at the time revealed liver tumors. Endoscopic ultrasound-guided tissue acquisition was performed, and immunohistochemistry revealed atypical cells with bidirectional hepatocellular differentiation, with the expression of hepatocytes, and ductal differentiation, with CK7/CK19 expression, leading to a diagnosis of cHCC-CCA (Figure 1). The patient had multiple liver tumors that were unresectable owing to multiple lymph node metastases, and first-line therapy with atezolizumab plus bevacizumab was initiated. The only adverse event was grade 2 proteinuria, and the treatment was continued. Two months after treatment initiation, the levels of AFP and PIVKA-II had decreased to normal levels, and stable disease (SD) with minor shrinkage was observed. At six months since the start of treatment, atezolizumab plus bevacizumab treatment was ongoing.
2.5 Case 5
A 74-year-old man with a history of proton therapy for hepatocellular carcinoma was regularly followed up. Four years and seven months after the proton therapy, multiple liver tumors were observed. Liver biopsy revealed poorly differentiated atypical cells that required differentiation between hepatocellular carcinoma and intrahepatic cholangiocarcinoma, and immunohistochemistry was positive for CK7/CK19; therefore, the patient was diagnosed with carcinomas mainly composed of adenocarcinoma. As multiple tumors were detected in each lobe and were unresectable, first-line treatment with GCS therapy was initiated. Two months after the start of treatment, PD was observed, and GCS was discontinued. Another liver biopsy revealed that the tumor was mainly composed of atypical cells with hepatocellular differentiation, with the expression of hepatocytes. Combined with the previous biopsy results, the tumor was diagnosed as cHCC-CCA. Atezolizumab plus bevacizumab was initiated as the second-line therapy. Two months later, PR was observed, and the levels of AFP and PIVKA-II had also decreased (Figures 2E, F). Subsequently, the treatment was continued while shrinkage was maintained; however, cerebral infarction developed before the seventh cycle of treatment. Owing to the aftereffects of cerebral infarction, it was difficult to continue systemic treatment for cHCC-CCA thereafter, and best supportive care was provided in another hospital.
2.6 Case 6
A 74-year-old man had previously been treated for follicular lymphoma and was followed-up regularly. Four years after partial remission of the lymphoma, multiple liver tumors were observed. Liver biopsy revealed that the main component was adenocarcinoma, including ductal differentiation with a slight mixture of atypical cells with hepatocellular differentiation, with the expression of hepatocytes, leading to a diagnosis of cHCC-CCA. Multiple unresectable tumors were discovered in both lobes of the liver, and first-line treatment with GCS therapy was initiated. Two months after the start of treatment, PD was observed, and GCS was discontinued. When liver biopsy was performed again, immunohistochemistry results were positive for hepatocytes, with a mixture of atypical cells exhibiting hepatocyte differentiation, consistent with the diagnosis of cHCC-CCA. Atezolizumab plus bevacizumab was initiated as second-line therapy. Three weeks after the start of administration, malaise and anorexia appeared, and liver and cardiac enzyme concentrations also increased. Electrocardiography revealed ST-segment changes, and coronary angiography revealed no evidence of ischemic heart disease. Myocardial biopsy revealed inflammatory cell infiltration, including of lymphocytes, leading to a diagnosis of immune-related acute lymphocytic myocarditis. Administration of 1000 mg of methylprednisolone was initiated for 3 days, and prednisolone (1 mg/kg) was continued thereafter. Although no marked deterioration in cardiac function was observed, the patient’s malaise and anorexia gradually worsened, even after the start of steroid treatment. CT revealed rapid tumor progression. The patient developed ascites, rapidly progressed to liver failure, and died two weeks after the onset of myocarditis.
3 Discussion
Combined hepatocellular-cholangiocarcinoma is a rare type of primary liver cancer; its reported incidence among primary liver cancers varies from 0.4% to 14.2% (11–15). This large incidence range is probably related to the evolving definition of cHCC-CCA over time in highly heterogeneous studies. Based on the National Cancer Registry, we previously reported that 0.53% of all primary liver cancers in Japan are cHCC-CCA (11). However, its true incidence is likely underestimated, as many patients have not undergone surgical resection. In such cases, cHCC-CCA may be misdiagnosed as hepatocellular carcinoma or cholangiocarcinoma by clinical diagnosis using imaging studies (12). Combined hepatocellular-cholangiocarcinoma is more common among men and individuals with cirrhosis or chronic liver disease caused by hepatitis B or C viral infections, and these features are more similar to hepatocellular carcinoma than to cholangiocarcinoma; therefore, certain cHCC-CCAs may be diagnosed as hepatocellular carcinoma if no biopsy is performed (16–19). In our cases, all patients underwent liver biopsy, and pathological evaluation, including immunostaining, led to a diagnosis of cHCC-CCA. In case 2, the patient was diagnosed with hepatocellular carcinoma based solely on radiographic imaging features without biopsy, but cHCC-CCA could be diagnosed via subsequent liver biopsy to confirm the pathology. In cases 5 and 6, cHCC-CCA was diagnosed from the beginning by liver biopsy, but the degree of mixture of hepatocellular carcinoma and cholangiocarcinoma components was different from that of the first biopsy in the pathological results of the second liver biopsy. These cases illustrate the complexity of accurately diagnosing cHCC-CCA using radiographic imaging or biopsy specimens, due to its heterogeneity.
Owing to its rarity, very little evidence and no guidelines are available for systemic treatment for cHCC-CCA, which is an unmet need. In many cases, cHCC-CCA does not have so-called “actionable” genetic alterations; thus, molecularly targeted therapy is considered only in select cases (20, 21). On the other hand, in recent years, development of treatments for hepatocellular carcinoma and cholangiocarcinoma using immune checkpoint inhibitors has progressed. In hepatocellular carcinoma, a randomized controlled trial revealed that atezolizumab plus bevacizumab significantly improved overall survival to a greater extent than sorafenib; accordingly, it is now widely used as the standard of care (10). In cholangiocarcinoma, a randomized controlled trial revealed that durvalumab plus gemcitabine and cisplatin significantly improved overall survival to a greater extent than gemcitabine and cisplatin, also becoming the standard of care (22). In addition, approximately 60% of cHCC-CCA cases belong to a particular subclass characterized by substantial immune infiltration, with activation of various pathways related to both innate and adaptive immunity and enrichment of several gene signatures associated with the response to immunotherapy in patients with hepatocellular carcinoma (23). Considering these results, we hypothesized that atezolizumab plus bevacizumab would yield therapeutic effects in cHCC-CCA; however, except for two case reports, no literature on immunotherapy for cHCC-CCA is available (24, 25).
Herein, we report the largest case series of unresectable cHCC-CCAs treated with atezolizumab plus bevacizumab. In this series, the best responses were PRs (three cases) and SD (one case), with only two patients exhibiting no improvement with atezolizumab plus bevacizumab. Positive responses to this treatment were observed when used as either first- or second-line therapy. These outcomes seem better than those demonstrated in previous reports of cHCC-CCA treatment (26–29). In a French multicenter retrospective study of 30 patients treated with gemcitabine combined with cisplatin or oxaliplatin, a PR was observed in 28.6% of patients, and the median PFS and OS were 9.0 and 16.2 months, respectively (26). In an American single-center retrospective analysis of 68 patients receiving systemic therapies, PRs to gemcitabine plus platinum-containing chemotherapy, gemcitabine plus fluorouracil, and sorafenib were observed in 24.3%, 15.4%, and 0% of patients, respectively (27). The median OS for these treatments was 11.5, 11.7, and 9.6 months, respectively and the median PFS was 8.0, 6.6, and 4.8 months, respectively. In a Japanese multicenter retrospective study of 36 patients, a PR or better was observed in 5.6% of the patients (28). The median OS in patients treated with gemcitabine plus cisplatin, fluorouracil plus cisplatin, and sorafenib was 10.2, 11.9, and 3.5 months, respectively and the median PFS was 3.0, 3.8, and 1.6 months, respectively. Multivariate analysis revealed that the OS of patients treated with sorafenib was inferior to that of those treated with platinum-containing therapy. In a recent single-center retrospective analysis from Korea, including 99 patients treated with sorafenib or cytotoxic chemotherapy, such as gemcitabine plus cisplatin and fluorouracil plus cisplatin, a PR or better was observed in 9.7% and 21.6% of patients, respectively, when used as first-line therapy (29). The median OS was 10.7 and 10.6 months, respectively and the median PFS was 4.2 and 2.9 months, respectively. When cytotoxic chemotherapy was divided into platinum-containing and non-platinum-containing chemotherapy, the response rate did not differ significantly. Taken together, all the available evidence suggests that platinum-containing chemotherapy is more effective than sorafenib as a treatment for unresectable cHCC-CCA. However, although our case series is limited by the small number of cases, our results indicate that atezolizumab plus bevacizumab may be even more effective than existing treatments for unresectable cHCC-CCA. As immunotherapy is effective against cHCC-CCA, it would be interesting to determine whether durvalumab plus gemcitabine and cisplatin, which is useful as a cholangiocarcinoma treatment, also has a therapeutic effect against cHCC-CCA. Further prospective studies are warranted to confirm these results in the absence of prospective trials of these agents.
Three patients in our case series had to discontinue treatment owing to adverse events. Interstitial lung disease and myocarditis were considered immune-related adverse events, and the possibility that the one case of cerebral infarction was also triggered by atezolizumab plus bevacizumab cannot be ruled out. All of these are known but rare adverse events of such treatment. Either multiple rare adverse events were observed by chance in our case series, or immune-related adverse events due to atezolizumab plus bevacizumab are more likely to occur in patients with cHCC-CCA than in those with hepatocellular carcinoma. Hence, further evaluation is required not only for the efficacy of this regimen but also for its safety.
4 Conclusion
Here, we presented a case series of patients with cHCC-CCA treated with atezolizumab plus bevacizumab. Our data showed promising effects of this treatment. This may be considered a reference case series for treatment selection for patients with this malignant disease, as no effective, evidence-based treatment is currently available. Further prospective investigations or studies with large numbers of cases are warranted to investigate the efficacy and safety of atezolizumab plus bevacizumab for unresectable cHCC-CCA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
All authors were involved in the preparation of this manuscript. TSa examined patients, collected the data, and prepared the original draft. TSh MS, and HI examined patients, collected the data, and reviewed and edited the manuscript. KW, SM, MK, and MI reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors wish to thank Kayo Takei and Yuriko Sato (National Cancer Center Hospital East) for secretarial support.
Conflict of interest
MI has received research funding from AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Chugai, Chiome Bioscience, Delta-Fly Pharma, Eisai, Eli Lilly Japan, Invitae, MSD, J-Pharma, Merck Biopharma, Merus N.V., Novartis, Nihon Servier, Ono, Pfizer, and Syneos Health; has consulted for AstraZeneca, Chugai, MSD, Nihon Servier, and Novartis; and received speaker honoraria from AbbVie, AstraZeneca, Chugai, Eisai, Eli Lilly Japan, Fujifilm Toyama Chemical, Guardant Health Japan, Incyte Biosciences Japan, MSD, Nihon Servier, Novartis, Nippon Kayaku, Ono, Taisho Pharmaceutical, Teijin, Takeda, Taiho, and Yakult. SM has received research funding from Chugai, Astellas, Toray, Ajinomoto, and Pfizer; and received speaker honoraria from Ono, Toray, and Otsuka. HI has received research funding from Ono, and Novartis; has consulted for Nihon Servier; and received speaker honoraria from Yakult, AstraZeneca, Nihon Servier, Kaneka Medix, and SB-Kawasumi Laboratories.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO Classification of Tumours Editorial Board. International agency for research on cancer WHO classification of tumours of the digestive system. 5th ed. Lyon: International Agency for Research on Cancer (2019).
2. The Global Cancer Observatory. Liver (2023). Available at: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf.
3. Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, et al. cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology (2018) 68:113–26. doi: 10.1002/hep.29789
4. Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, et al. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today (2006) 36:892–7. doi: 10.1007/s00595-006-3276-8
5. Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, et al. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol (2011) 45:69–75. doi: 10.1097/MCG.0b013e3181ce5dfa
6. Tang D, Nagano H, Nakamura M, Wada H, Marubashi S, Miyamoto A, et al. Clinical and pathological features of allen’s type c classification of resected combined hepatocellular and cholangiocarcinoma: a comparative study with hepatocellular carcinoma and cholangiocellular carcinoma. J Gastrointest Surg (2006) 10:987–98. doi: 10.1016/j.gassur.2006.01.018
7. Spolverato G, Bagante F, Tsilimigras D, Ejaz A, Cloyd J, Pawlik TM. Management and outcomes among patients with mixed hepatocholangiocellular carcinoma: a population-based analysis. J Surg Oncol (2019) 119:278–87. doi: 10.1002/jso.25331
8. Wang J, Li E, Yang H, Wu J, Lu HC, Yi C, et al. Combined hepatocellular-cholangiocarcinoma: a population level analysis of incidence and mortality trends. World J Surg Oncol (2019) 17:43. doi: 10.1186/s12957-019-1586-8
9. Fowler K, Saad NE, Brunt E, Doyle MB, Amin M, Vachharajani N, et al. Biphenotypic primary liver carcinomas: assessing outcomes of hepatic directed therapy. Ann Surg Oncol (2015) 22:4130–7. doi: 10.1245/s10434-015-4774-y
10. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
11. Satake T, Morizane C, Rikitake R, Higashi T, Okusaka T, Kawai A. The epidemiology of rare types of hepatobiliary and pancreatic cancer from national cancer registry. J Gastroenterol (2022) 57:890–901. doi: 10.1007/s00535-022-01920-5
12. Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, et al. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl (2014) 20:952–9. doi: 10.1002/lt.23897
13. Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, et al. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer (2002) 94:2040–6. doi: 10.1002/cncr.10392
15. Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, et al. Report of the 22nd nationwide follow-up survey of primary liver cancer in Japan (2012–2013). Hepatol Res (2022) 52:5–66. doi: 10.1111/hepr.13675
16. Yin X, Zhang BH, Qiu SJ, Ren ZG, Zhou J, Chen XH, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol (2012) 19:2869–76. doi: 10.1245/s10434-012-2328-0
17. Zhou YM, Zhang XF, Wu LP, Sui CJ, Yang JM. Risk factors for combined hepatocellular-cholangiocarcinoma: a hospital-based case-control study. World J Gastroenterol (2014) 20:12615–20. doi: 10.3748/wjg.v20.i35.12615
18. Okuda K. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J Gastroenterol Hepatol (2002) 17:401–5. doi: 10.1046/j.1440-1746.2002.02734.x
19. Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, et al. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol (2003) 33:283–7. doi: 10.1093/jjco/hyg056
20. Xue R, Chen L, Zhang C, Fujita M, Li R, Yan SM, et al. Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell (2019) 35:932–947.e8. doi: 10.1016/j.ccell.2019.04.007
21. Joseph NM, Tsokos CG, Umetsu SE, Shain AH, Kelley RK, Onodera C, et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol (2019) 248:164–78. doi: 10.1002/path.5243
22. Oh DY, Ruth He AR, Qin S, Chen L, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid (2022) 1:EVIDoa2200015. doi: 10.1056/EVIDoa2200015
23. Nguyen CT, Caruso S, Maille P, Beaufrère A, Augustin J, Favre L, et al. Immune profiling of combined hepatocellular- cholangiocarcinoma reveals distinct subtypes and activation of gene signatures predictive of response to immunotherapy. Clin Cancer Res (2022) 28:540–51. doi: 10.1158/1078-0432.CCR-21-1219
24. Rizell M, Åberg F, Perman M, Ny L, Stén L, Hashimi F, et al. Checkpoint inhibition causing complete remission of metastatic combined hepatocellular-cholangiocarcinoma after hepatic resection. Case Rep Oncol (2020) 13:478–84. doi: 10.1159/000507320
25. Saito N, Hatanaka T, Nakano S, Hazama Y, Yoshida S, Hachisu Y, et al. A case of unresectable combined hepatocellular and cholangiocarcinoma treated with atezolizumab plus bevacizumab. Clin Case Rep (2022) 10:e6129. doi: 10.1002/ccr3.6129
26. Salimon M, Prieux-Klotz C, Tougeron D, Hautefeuille V, Caulet M, Gournay J, et al. Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer (2018) 118:325–30. doi: 10.1038/bjc.2017.413
27. Trikalinos NA, Zhou A, Doyle MBM, Fowler KJ, Morton A, Vachharajani N, et al. Systemic therapy for combined hepatocellular-cholangiocarcinoma: a single-institution experience. J Natl Compr Canc Netw (2018) 16:1193–9. doi: 10.6004/jnccn.2018.7053
28. Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci (2018) 109:2549–57. doi: 10.1111/cas.13656
Keywords: combined hepatocellular-cholangiocarcinoma, atezolizumab, bevacizumab, immunotherapy, case series
Citation: Satake T, Shibuki T, Watanabe K, Sasaki M, Imaoka H, Mitsunaga S, Kojima M and Ikeda M (2023) Case Report: Atezolizumab plus bevacizumab for combined hepatocellular-cholangiocarcinoma. Front. Oncol. 13:1234113. doi: 10.3389/fonc.2023.1234113
Received: 03 June 2023; Accepted: 27 June 2023;
Published: 21 July 2023.
Edited by:
Takahiro Kodama, Osaka University, JapanReviewed by:
Satoshi Tanaka, National Hospital Organization Osaka National Hospital, JapanTetsu Tomonari, Tokushima University, Japan
Copyright © 2023 Satake, Shibuki, Watanabe, Sasaki, Imaoka, Mitsunaga, Kojima and Ikeda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomoyuki Satake, dG9zYXRha2VAZWFzdC5uY2MuZ28uanA=
 Tomoyuki Satake
Tomoyuki Satake Taro Shibuki1
Taro Shibuki1 Shuichi Mitsunaga
Shuichi Mitsunaga Motohiro Kojima
Motohiro Kojima