
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 25 September 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1233125
 Yang Liu1†
Yang Liu1† Huimin Zhang2†
Huimin Zhang2† Zhi Wen1
Zhi Wen1 Yu Jiang3
Yu Jiang3 Jing Huang1
Jing Huang1 Chongjian Wang1
Chongjian Wang1 Caixia Chen1
Caixia Chen1 Jiahao Wang1
Jiahao Wang1 Erhao Bao1
Erhao Bao1 Xuesong Yang1*
Xuesong Yang1*Background: Panurothelial carcinoma is a rare and aggressive malignancy that requires effective treatment strategies to enhance patient outcomes.
Methods: We conducted a systematic search of English publications in databases including PubMed, Embase, Cochrane Library, and Web of Science up to May 2023. The quality of the literature was assessed using the Newcastle-Ottawa Scale (NOS) and the Methodological Quality and Synthesis of Case Series and Case Reports tool. Data statistics and analysis were performed using Stata 15.1 software (StataSE, USA).
Results: Six studies involving 339 patients were included in the analysis. Meta-analysis revealed that Simultaneous Radical Cystectomy and Nephroureterectomy had 2-year and 5-year overall survival rates of 68% (95% CI 60%-76%, I2 = 12.4%, P < 0.001) and 44% (95% CI 36%-53%, I2 = 0, P < 0.001), respectively. The 2-year and 5-year progression-free survival rates were 91% (95% CI 86%-95%, I2 = 95%, P < 0.001) and 65% (95% CI 58%-73%, I2 = 91.5%, P < 0.001), respectively. The 2-year and 5-year cancer-specific survival rates were 73% (95% CI 66%-81%, I2 = 16.7%, P < 0.001) and 57% (95% CI 49%-66%, I2 = 0, P < 0.001), respectively. Additionally, the incidence of minor complications was 19% (95% CI 15%-23%, P < 0.01), major complications was 49% (95% CI 34%-63%, P < 0.01), and the intraoperative blood transfusion rate was 53% (95% CI 44%-61%, P < 0.01).
Conclusions: Simultaneous radical cystectomy and nephroureterectomy represent feasible approaches for the treatment of Panurothelial carcinoma. Nonetheless, a comprehensive assessment of the surgical risks and benefits is imperative, and larger-scale prospective cohort studies are required to validate therapeutic efficacy.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42023426401.
Urothelial cell carcinoma (UCC) is emerging as the fourth most prevalent cancer in men, with 90%-95% of cases being bladder urothelial cancer and the majority of the remaining cases being upper tract urothelial carcinoma (UTUC) (1). The overall incidence rate of UTUC stands at 1.1 cases per 100,000 individuals, with a lower rate of 0.89 cases per 100,000 individuals for advanced-stage UTUC. Notably, in patients with metastatic UTUC, the overall one-year survival rate reaches 60.3% (2). Panurothelial carcinoma (Panuc) is a rare yet highly invasive malignancy (3). It represents the simultaneous occurrence of bladder urothelial cancer and UTUC (4, 5), accounting for approximately 11% of UTUC cases (6). Radical cystectomy (RC) is the established gold standard surgical procedure for treating invasive bladder urothelial cancer, while radical nephroureterectomy is the preferred treatment for invasive UTUC (7, 8). However, due to the rarity of Panurothelial carcinoma and the lack of original studies, there is currently no consensus on its optimal treatment strategy.
Previous small-scale case studies have reported on simultaneous radical cystectomy and nephroureterectomy (RCUN), suggesting that RCUN may reduce metastasis and disease recurrence. However, the safety and efficacy of RCUN in the treatment of Panuc patients remain unclear due to the limited sample sizes of these studies (9, 10), Recently, large-scale studies by Subiela et al. (11) and Britton et al. (12) have reported on the treatment of Panuc with RCUN. The study by Zein et al. (13) provided a comprehensive overview of perioperative outcomes in patients undergoing RCUN treatment for Panurothelial carcinoma. However, their included literature comprised numerous case reports and lacked statistical analyses of postoperative tumor survival data.
To better evaluate the effectiveness of RCUN in the treatment of Panuc, we conducted a systematic review and a single-arm meta-analysis. The aim of this study was to provide a comprehensive and scientifically grounded basis for the treatment of Panuc, offering valuable insights for clinical decision-making.
We conducted the present meta-analysis in accordance with the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (14) Furthermore, we have registered the study in PROSPERO (CRD42023426401) to ensure transparency and adherence to best practices in systematic reviews and meta-analyses.
We conducted a comprehensive search of the Embase, Web of Science, and Cochrane Library databases up until May 2023 to identify studies that met the inclusion criteria. Our search strategy involved combining relevant terms related to the intervention and the patient population: ((Radical Cystectomy OR Radical Cystectomies OR Radical Cystectom*) AND (Nephroureterectomy OR Nephroureterectomies OR Nephroureterectom*) AND (Upper tract urothelial carcinoma OR UTUC OR panurothelial carcinoma OR PanUC)). To ensure the comprehensiveness of our search, we also manually reviewed the references of relevant articles. Only studies reported in English were included.
The inclusion criteria were defined using the PICOS approach. The eligible studies needed to meet the following criteria: (1) patients diagnosed with Panuc, (2) patients who underwent simultaneous treatment with both RC and RCUN, (3) one or more of the following outcomes: perioperative, oncologic, or survival outcomes, and (4) study designs including cohort studies, single-arm studies, or randomized controlled trials (RCTs). Studies were excluded if they met any of the following criteria: (1) unavailable data for analysis, (2) comments, reviews, case reports, abstracts, or editorials, (3) duplicate publications.
The data extraction process was carried out independently by two reviewers. The extracted data included the following information: (1) general information such as the first author, publication year, and country; (2) population characteristics including the number of patients, age, body mass index (BMI), median follow-up time, pathological stage, and outcomes; (3) perioperative outcomes such as operative time, estimated blood loss, transfusion rates, and length of stay; (4) assessment of minor complications (defined as Clavien grade 1-2) and major complications (defined as Clavien grade ≥3); (5) survival data including overall survival (OS), recurrence-free survival (RFS), and cancer-specific survival (CSS). Any discrepancies that arose during the data extraction process were resolved through consensus or consultation with a third reviewer. Only studies reported in English were included in the reference list.
The quality of the included publications was assessed using the Newcastle-Ottawa Scale (NOS) (https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp) (15). The NOS was used to evaluate the case selection, comparability, and outcome reporting of each study. Two authors independently conducted the quality assessment, and any disagreements were resolved through discussion and consensus among all authors. For case series and case reports, the methodological quality and synthesis were evaluated using the “Methodological quality and synthesis of case series and case reports” tool (16).
We utilized STATA 15.1 software (StataSE, USA) to perform a meta-analysis combining overall survival (OS), recurrence-free survival (RFS), cancer-specific survival (CSS), and perioperative outcomes in patients who underwent simultaneous radical cystectomy and nephroureterectomy (RCUN) for Panuc. KM curve data was extracted and estimated using Digitizer 4.1 and Adobe Photoshop (17). All pooled results were presented with 95% confidence intervals (CIs). Inter-study heterogeneity was assessed using the I2 statistic and the Cochran Q test (18). For studies with high heterogeneity (I2 ≥ 50%), a random-effects model was employed, while studies with low heterogeneity (I2 ≤ 50%) were analyzed using a fixed-effects model. Statistical significance was considered at a P-value < 0.05. Sensitivity analysis was conducted by excluding each study individually to assess the robustness of our findings. However, due to the limited number of studies (three or fewer), sensitivity analysis could not be performed.As the number of included studies was ten or fewer, the power of the tests for publication bias assessment was inadequate (19, 20). Hence, we were unable to conduct a thorough evaluation of publication bias.
A total of 648 studies were retrieved. After initial screening, we identified 7 potentially eligible studies, of which 6 studies met the inclusion criteria and were included in our analysis (9–12, 21, 22) (Figure 1).
The included studies originated from the USA, China, and Spain. The sample sizes ranged from 8 to 170 patients, totaling 339 patients who underwent simultaneous radical cystectomy and nephroureterectomy for Panurothelial Carcinoma. Open surgery accounted for 64% of the total cases, while minimally invasive procedures constituted 36% of the total cohort. Table 1 summarizes the age, BMI, gender, date of follow-up, and other baseline information of the included patients. Table 2 provides an overview of the tumor stage, history of previous abdominal surgery, history of previous chemotherapy, and other relevant data for the included patients. Table 3 presents the quality evaluation of the case series.
The results of the single-arm meta-analysis showed that the operative time for simultaneous radical cystectomy and nephroureterectomy was 378.34 minutes (95% CI 368.95, 387.74, P < 0.001) (Figure 2A), the length of hospital stay was 8.04 days (95% CI 7.77, 8.31) (Figure 2B), and the blood loss was 711.36 ml (95% CI 638.85, 783.87, P < 0.001) (Figure 2C). Additionally, the incidence of postoperative minor complications (Clavien grade 1-2) was 19% (95% CI 15%-23%) (Figure 3A), the incidence of postoperative major complications (Clavien grade ≥ 3) was 49% (95% CI 34%-63%) (Figure 3B), and the intraoperative blood transfusion rate was 53% (95% CI 44%-61%) (Figure 3C). The median survival time was 42.21 months (95% CI 38.18, 46.23) (Figure 4A), and the tumor metastasis rate was 34% (95% CI 26%, 42%) (Figure 4B).
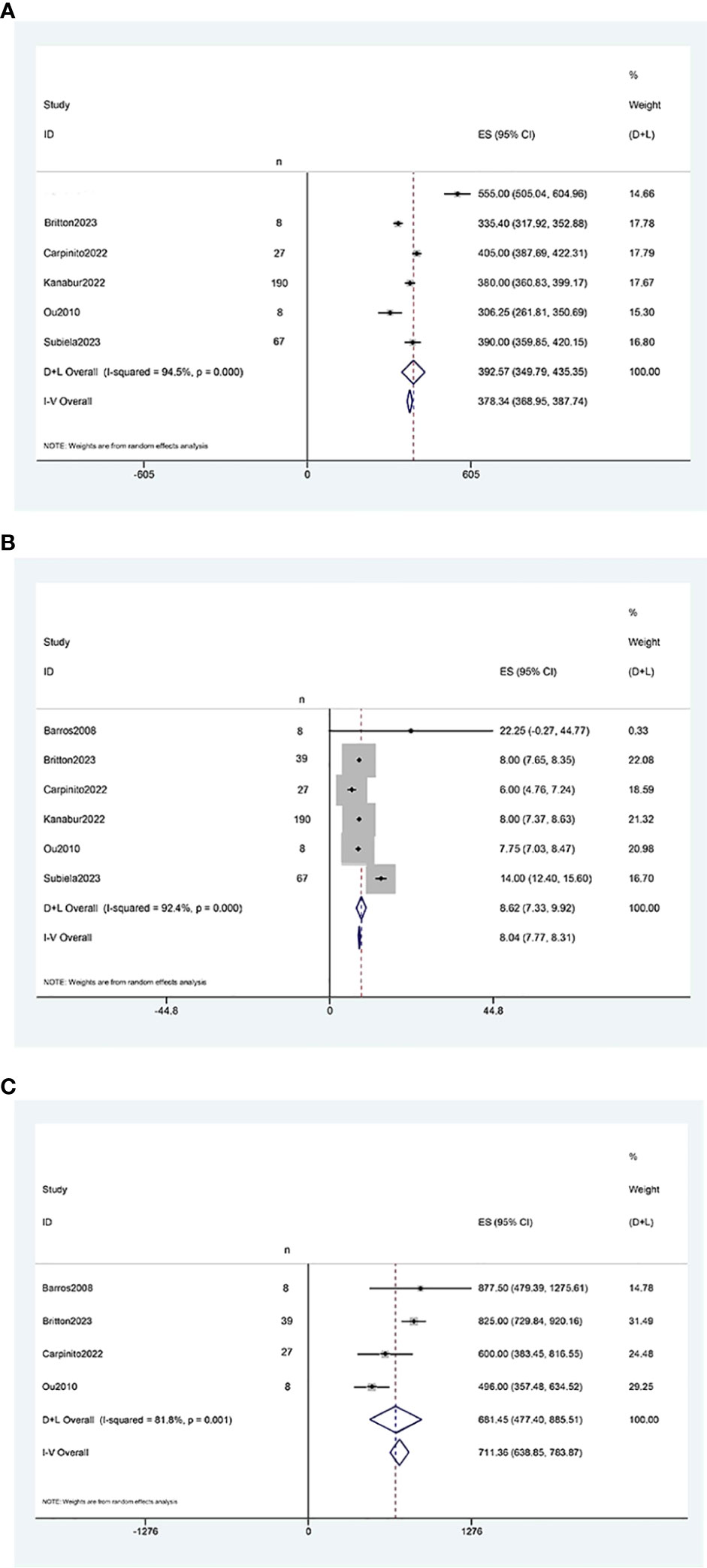
Figure 2 Forest plots of Perioperative outcome: (A) operation time was 378.34 minutes (95% CI 368.95, 387.74); (B) length of hospital stay was 8.04 days (95% CI 7.77, 8.31); (C) blood loss was 711.36 ml (95% CI 638.85, 783.87).
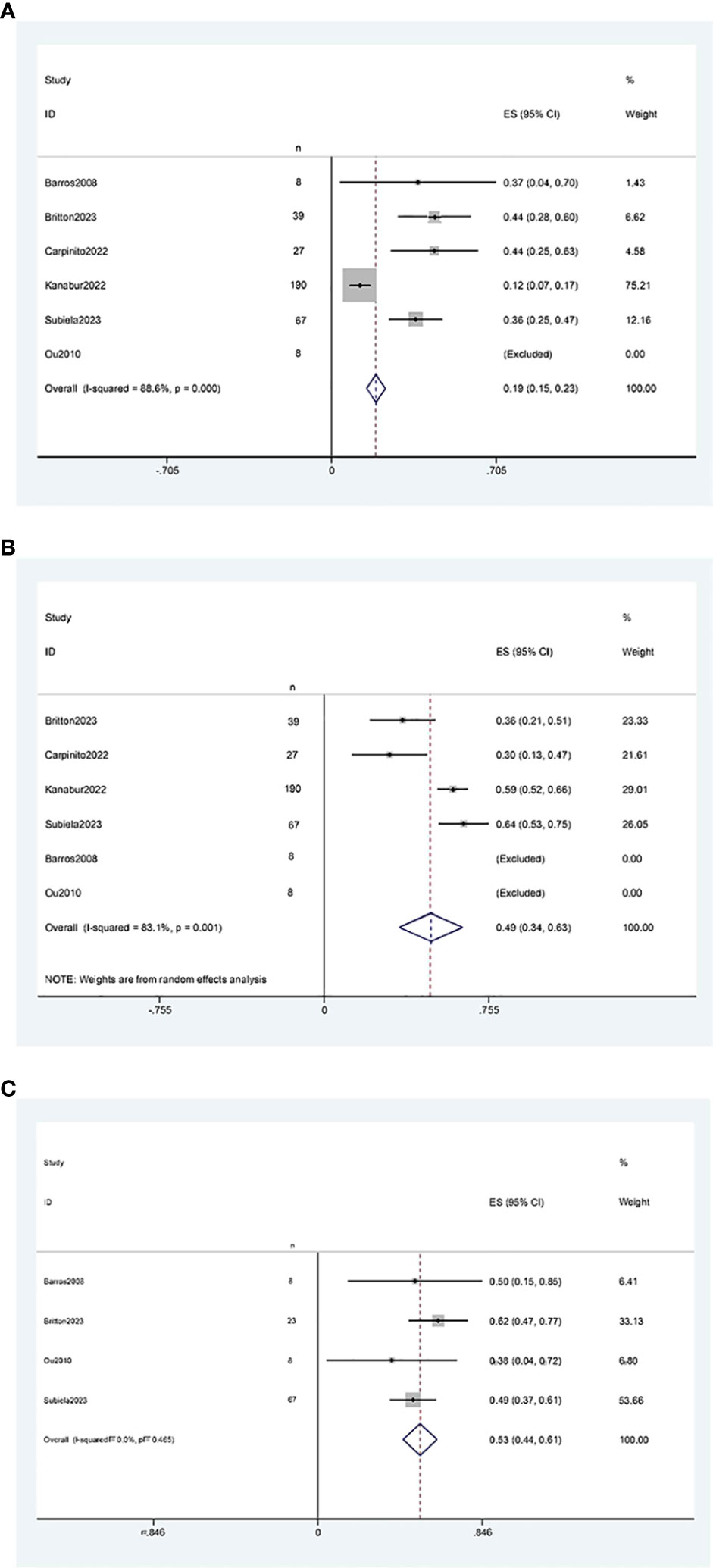
Figure 3 Forest plots of Perioperative outcome: (A) minor complications rate was 19% (95% CI 15%, 23%); (B) major complications rate was 49% (95% CI 34%, 63%); (C) blood transfusion rate was 53% (95% CI 44%, 61%).
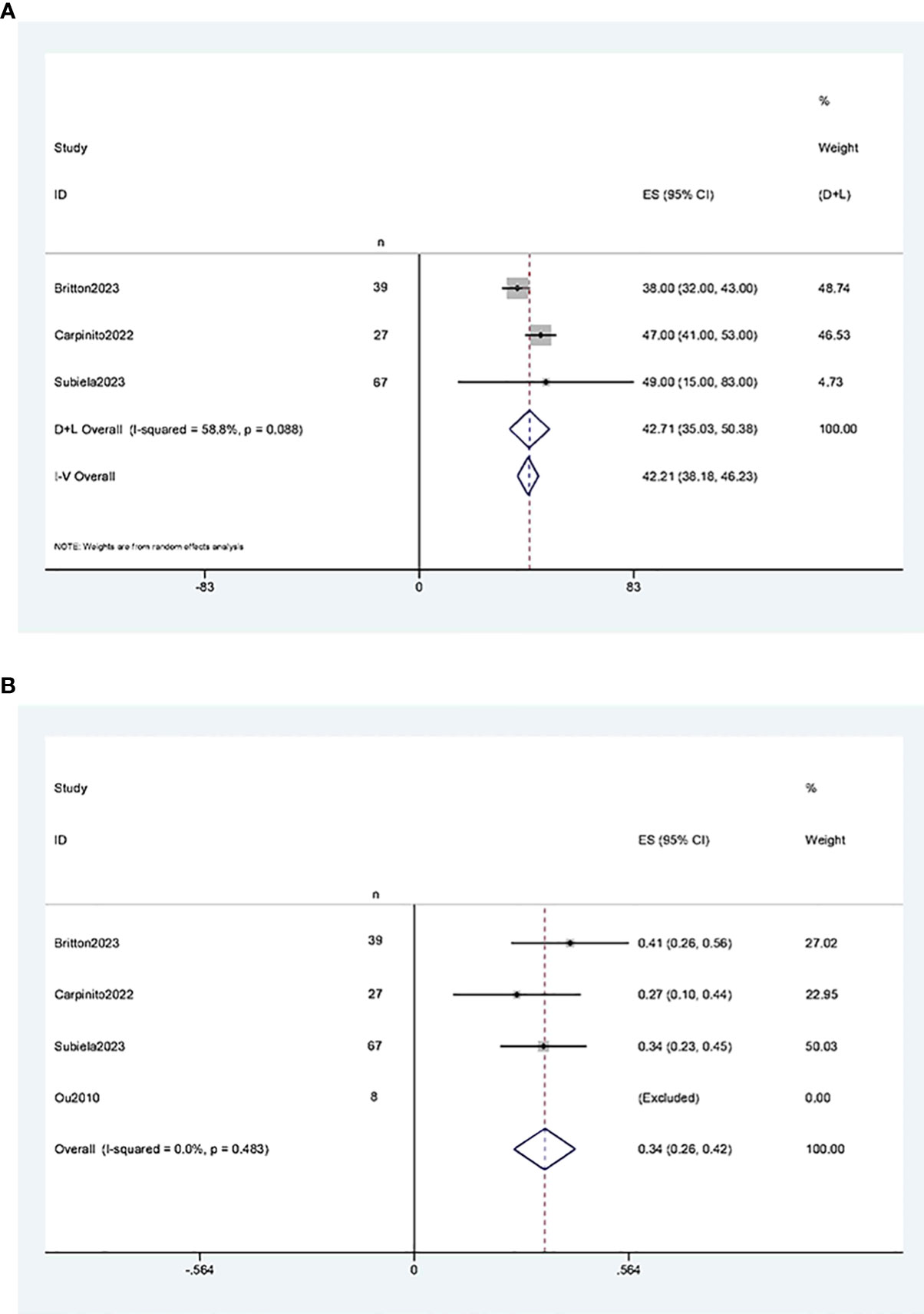
Figure 4 Forest plots of Oncologic Outcomes: (A) median survival time was 42.21 months (95% CI 38.18, 46.23); (B) tumor metastasis rate was 34% (95% CI 26%, 42%).
Three studies (11, 12, 21) reported two-year and five-year overall survival rates after RCUN. Meta-analysis demonstrated that the overall survival rate was 68% (95% CI 60%, 76%, I2 = 12.4%, P < 0.001) at 2 years (Figure 5A), and 44% (95% CI 36%, 53%, I2 = 0, P < 0.001) at 5 years at 5 years (Figure 5B).
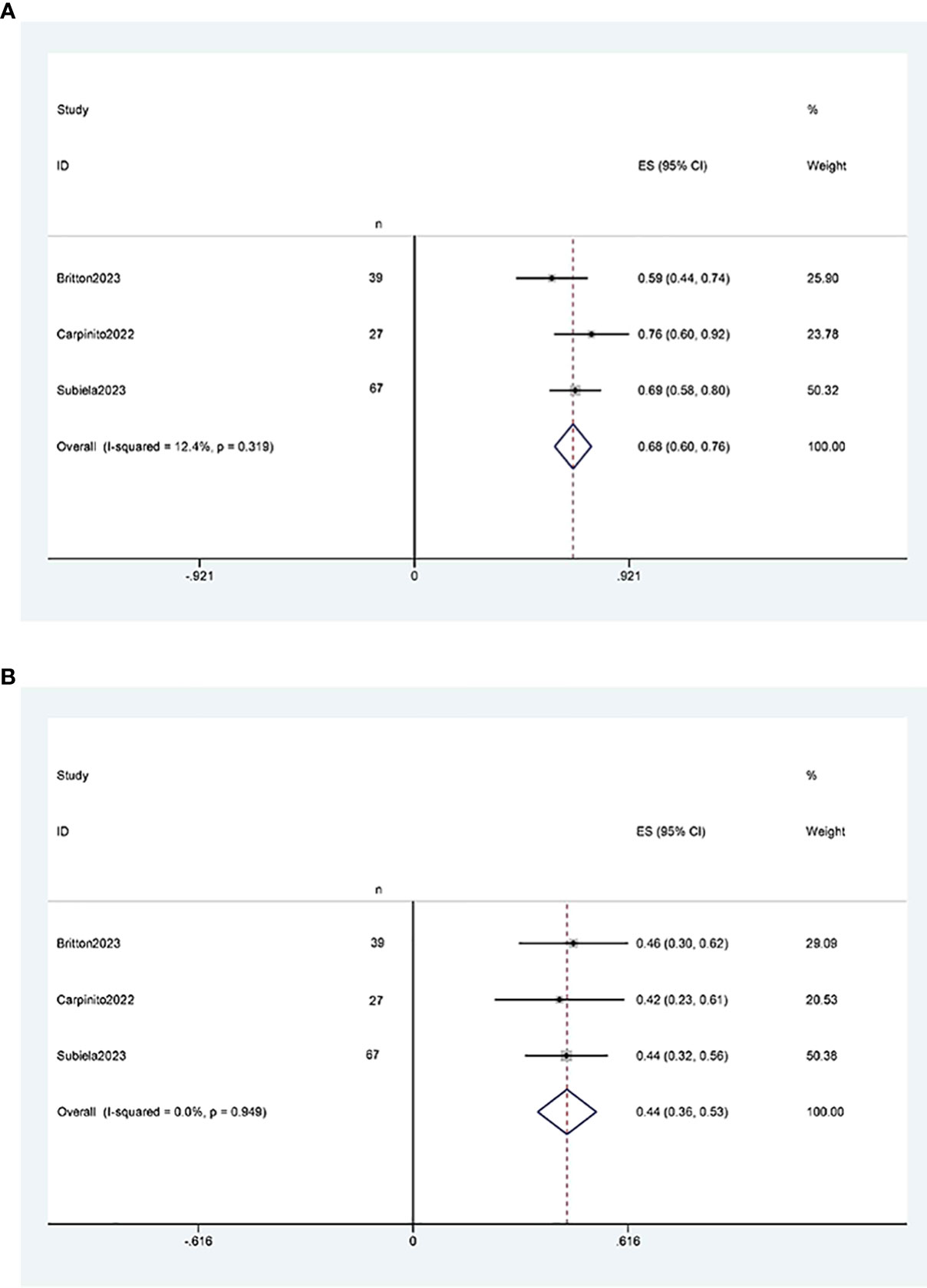
Figure 5 Forest plots of overall survival: (A) two years survival rate was 68% (95% CI 60%, 76%); (B) five years survival rate was 44% (95% CI 36%, 53%).
Three studies (11, 12, 21) reported two-year and five-year recurrence-free survival rates after RCUN. Meta-analysis showed that the recurrence-free survival rate was 91% (95% CI 86%, 95%, I2 = 95%, P < 0.001) at 2 years (Figure 6A), and 65% (95% CI 58%, 73%, I2 = 91.5%, P < 0.001) at 5 years (Figure 6B).
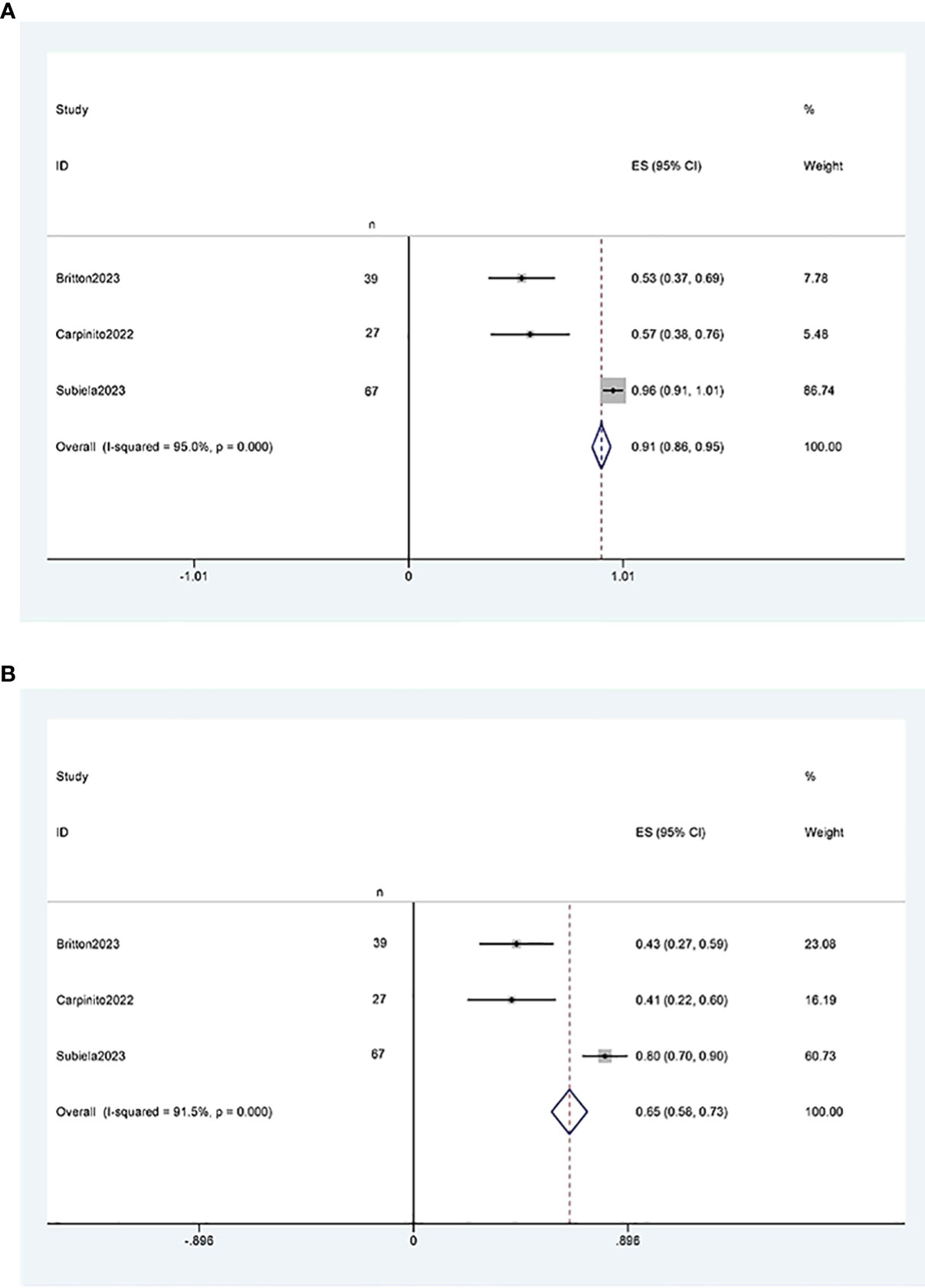
Figure 6 Funnel plot of recurrence-free survival: (A) two years survival rate was 91% (95% CI 86%, 95%); (B) five years survival rate was 65% (95% CI 58%, 73%).
Three studies (11, 12, 21) reported two-year and five-year cancer-specific survival rates after RCUN. Meta-analysis revealed that the cancer-specific survival rate was 73% (95% CI 66%, 81%, I2 = 16.7%, P < 0.001) at 2 years (Figure 7A), and 57%(95%CI 49%, 66%, I2 = 0, P<0.001) at 5 years (Figure 7B).
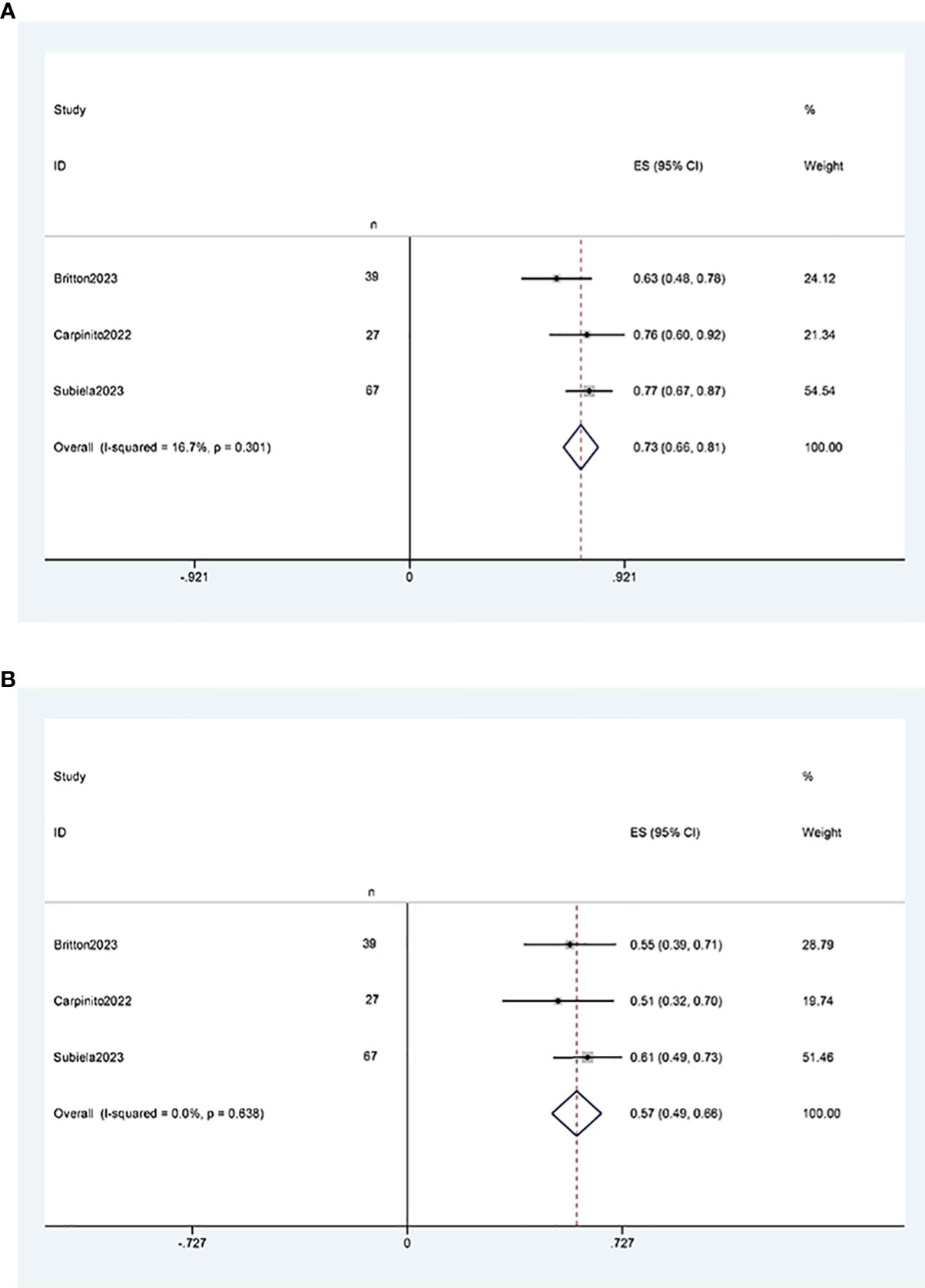
Figure 7 Funnel plot of cancer-specific survival: (A) two years survival rate was 73% (95% CI 66%, 81%); (B) five years survival rate was 57%(95%CI 49%, 66%).
This study represents a systematic review and single-arm meta-analysis investigating the efficacy of simultaneous radical cystectomy and nephroureterectomy (RCUN) in the treatment of Panuc. By conducting a comprehensive evaluation of the relevant literature, we have derived several important conclusions.
First and foremost, our research findings have revealed a disheartening efficacy of RCUN in the treatment of Panuc. Meta-analysis demonstrates a remarkable two-year recurrence-free survival rate exceeding 90%. This outcome can possibly be attributed to the effective eradication of all tumor lesions through simultaneous resection of the bladder and renal ureter, consequently reducing the risks of residual lesions and metastasis. However, the two-year overall survival rate and cancer-specific survival rate both fail to surpass 75%, suggesting that the surgical outcomes may not have met the expected efficacy. It is noteworthy that previous studies have reported a 4-6% urethral recurrence rate in patients undergoing RC (4), and tumor size can nearly directly predict the staging and grading of UTUC (23). Populations from different regions and residing environments may exhibit varying cancer-specific mortality risks in UTUC (24, 25). Furthermore, tumor liver metastasis independently predicts poorer survival rates (26).Risk factors such as smoking, a history of upper tract urothelial carcinoma (UTUC), and chronic kidney disease have also been suggested as potential explanations for malignancy recurrence (27–30). Nonetheless, our survival results indicate that Panuc exhibits a more aggressive behavior compared to primary epithelial UC. Therefore, we propose that RCUN may represent a viable treatment option, particularly for high-risk patients and complex cases involving panurothelial carcinoma.
Secondly, our study also observed a higher risk of surgical complexity and postoperative complications associated with RCUN. This outcome is anticipated as simultaneous excision increases the surgical difficulty and trauma (31–33), potentially resulting in longer operative times and an increased incidence of postoperative complications. Patients diagnosed with preoperative uremia exhibit an elevated risk of intraoperative immune system dysfunction, cardiovascular complications, and anesthesia-related adverse events. Perioperative complications, including bleeding, infection, atelectasis, and cardiovascular or cerebrovascular events, are more likely to occur (34–36). Hence, multidisciplinary collaboration involving anesthesiology, critical care medicine, nephrology, and urology is indispensable for the management of such patients. Consequently, when considering the feasibility of simultaneous surgery, physicians need to carefully evaluate the potential benefits against the surgical risks and the patient’s postoperative rehabilitation requirements. Cutaneous ureterostomy(CU) stands out as a crucial approach for managing early complications, offering advantages such as shorter urinary diversion surgical times, reduced blood loss, lower transfusion rates, and shorter hospital stays (37, 38). When compared to patients undergoing ileal conduit diversion, it may also exhibit a potentially lower occurrence of intraoperative and postoperative complications (39, 40). Current experience with robot-assisted radical cystectomy (RC) patients suggests that there appears to be no significant difference in the incidence of complications between intracorporeal and extracorporeal ileal conduit diversion (41). Given the diverse surgical approaches included in the studies, there is presently an insufficient quantity of data to adequately compare open and minimally invasive treatments for RCUN. However, in the context of radical cystectomy, minimally invasive procedures appear to exhibit a lower transfusion rate in comparison to open surgery (42, 43). Whether this outcome is applicable to RCUN remains subject to verification in subsequent research.
Moreover, given the high risk of recurrent residual urothelium in Panuc (27, 28), regular postoperative testing for residual urothelium should be performed in patients, and prophylactic urethrectomy should even be considered. These findings underscore the importance of meticulous urinary tract management during the perioperative and follow-up periods. Early and long-term follow-up is crucial to timely detect any recurrence. During the perioperative period, patients should receive specialized care and monitoring to ensure proper wound healing. Additionally, appropriate rehabilitation measures, including physical therapy and rehabilitation training, should be implemented to facilitate the recovery of urinary function. These measures should be widely implemented and optimized to provide better treatment options and enhance both survival and quality of life for patients with panurothelial carcinoma.
Furthermore, we recommend comparing our results with alternative treatment modalities such as radiation therapy, chemotherapy, or immunotherapy. Such comparisons will help determine the status and comparative advantages of RCUN in the treatment of panurothelial carcinoma. Unfortunately, the reduced kidney function observed in all RCUN patients in our study makes subsequent treatments, such as chemotherapy, virtually impossible (5, 44, 45). Moreover, integrating study findings with clinical practice guidelines can assist clinicians in formulating more specific treatment plans and decisions tailored to individual patient management.
Finally, we strongly encourage further collaborative and multicenter studies to validate our findings. Multicenter studies can increase sample sizes, enhance the statistical power of the research, and improve the reliability and external validity of the results. Additionally, international collaborative studies can facilitate comparisons between patient populations from diverse regions and ethnicities, enabling a deeper understanding of the effects of RCUN in different demographic groups.
Regarding future research directions, we propose further investigation into the optimal criteria for patient selection in RCUN. While our current study included a diverse range of patients, including high-risk individuals and complex cases, the assessment of feasibility in other patient subgroups was limited. Therefore, future studies should focus on evaluating the efficacy and safety of this surgical approach in specific patient subsets.
Furthermore, an important area of research lies in the technical refinement of simultaneous surgery. The introduction of novel surgical techniques and instruments holds promise for enhancing surgical precision and safety. For instance, the utilization of robot-assisted surgery and microscopy can improve procedural accuracy, visualization, and minimize damage to normal tissue. Comparative studies exploring the effectiveness of different techniques and instruments in simultaneous surgery should be conducted to optimize surgical outcomes (46, 47).
It is important to acknowledge the limitations of our study. The inclusion of a limited number of studies, along with the partial absence of research data and the potential for selection and publication biases, may have influenced our results. Furthermore, our study is based on retrospective data analysis, which only provides a lower level of evidence. It is crucial to carefully weigh the potential benefits against the surgical risks and prioritize postoperative urinary tract management. Future investigations should focus on refining patient selection criteria, technical advancements, and obtaining higher levels of evidence.
Panurothelial carcinoma represents a rare and highly invasive malignancy. Given the substantial postoperative complications associated with the RCUN approach, a meticulous evaluation of the surgical risks and benefits becomes imperative. Further research and collaborative efforts are warranted to validate our findings, enhance therapeutic strategies, and ultimately ameliorate the prognosis of patients afflicted by upper urinary tract urothelial carcinoma.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
YL: Protocol development, data collection and management, data analysis and manuscript writing. HZ: Protocol development, data collection and management, data analysis and manuscript writing. XY: Protocol development, data collection and management, data analysis and manuscript writing. ZW: Protocol development, data management, data analysis and manuscript writing. YJ: Data management, data analysis and manuscript writing. JH: Data management, data analysis and manuscript writing. CC: Data management and manuscript writing. CW: Data management and manuscript writing. JW: Data management and manuscript writing. EB: Data management and manuscript writing. All authors contributed to the article and approved the submitted version.
We would like to thank the researchers and study participants for their contributions. Thanks to Ms. Yu Jiang for her encouragement and support to YL in his medical career.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Colla Ruvolo C, Nocera L, Stolzenbach LF, Wenzel M, Cucchiara V, Tian Z, et al. Incidence and survival rates of contemporary patients with invasive upper tract urothelial carcinoma. Eur Urol Oncol (2021) 4(5):792–801. doi: 10.1016/j.euo.2020.11.005
3. Fang D, Liu P, Li X, Xiong G, Zhang L, Singla N, et al. Characteristics and treatment outcomes of pan-urothelial cell carcinom a: a descriptive analysis of 45 patients. Sci Rep (2015) 5:18014. doi: 10.1038/srep18014
4. Gakis G, Black PC, Bochner BH, Boorjian SA, Stenzl A, Thalmann GN, et al. Systematic review on the fate of the remnant urothelium after radical cystectomy. Eur Urol (2017) 71(4):545–57. doi: 10.1016/j.eururo.2016.09.035
5. Li Q, Assel M, Benfante N, Pietzak E, Bagrodia A, Cha E, et al. Clinical Outcomes in Patients with Panurothelial Carcinoma Treated wit h Radical Nephroureterectomy Following Cystectomy for Metachronous Recurrence. J Urol (2017) 198(3):546–51. doi: 10.1016/j.juro.2017.03.120
6. Cosentino M, Palou J, Gaya JM, Breda A, Rodriguez-Faba O, Villavicencio-Mavrich H. Upper urinary tract urothelial cell carcinoma: location as a predictiv e factor for concomitant bladder carcinoma. World J Urol (2013) 31(1):141–5. doi: 10.1007/s00345-012-0877-2
7. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European association of urology guidelines on upper urinary tract urot helial carcinoma: 2020 update. Eur Urol (2021) 79(1):62–79. doi: 10.1016/j.eururo.2020.05.042
8. Chang SS, Bochner BH, Chou R, Dreicer R, Kamat AM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/A STRO/SUO guideline. J Urol (2017) 198(3):552–9. doi: 10.1016/j.juro.2017.04.086
9. Barros R, Frota R, Stein RJ, Turna B, Gill IS, Desai MM. Simultaneous laparoscopic nephroureterectomy and cystectomy: a preliminary report. Int Braz J urol (2008) 34(4):413–21. doi: 10.1590/s1677-55382008000400003. discussion 421.
10. Ou YC, Yang CR, Yang CK, Cheng CL, Hemal AK. Simultaneous robot-assisted nephroureterectomy and cystectomy in patients with uremia and multifocal urothelial carcinoma. J endourol (2011) 25(6):979–84. doi: 10.1089/end.2010.0602
11. Subiela JD, González-Padilla DA, Huguet J, Aumatell J, Rodríguez-Faba O, Krajewski W, et al. Oncological and renal function outcomes in patients who underwent simultaneous radical cystectomy and nephroureterectomy for synchronous or metachronous panurothelial carcinoma. Urology (2023) 172:157–64. doi: 10.1016/j.urology.2022.08.064
12. Britton CJ, Gottlich HC, Tarrell RF, Thapa P, Joyce DD, Shah PH, et al. Perioperative and oncologic outcomes associated with simultaneous radical cystectomy and nephroureterectomy. Urology (2023) 172:149–56. doi: 10.1016/j.urology.2022.09.039
13. Zein M, Nasrallah AA, Abou Heidar NF, Najdi J, Hneiny L, El Hajj A. Concurrent radical cystectomy and nephroureterectomy indications and outcomes: a systematic review and comparative analysis. Ther Adv Urol (2023) 15:17562872231171757. doi: 10.1177/17562872231171757
14. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis prot ocols (PRISMA-P) 2015: elaboration and explanation. BMJ (2015) 350:g7647. doi: 10.1136/bmj.g7647
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment o f the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
16. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evidence-Based Med (2018) 23(2):60–3. doi: 10.1136/bmjebm-2017-110853
17. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into me ta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
18. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
19. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical te sts and prevalence in the literature. J Clin Epidemiol (2000) 53(11):1119–29. doi: 10.1016/s0895-4356(00)00242-0
20. Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ (2006) 333(7568):597–600. doi: 10.1136/bmj.333.7568.597
21. Carpinito GP, Cook GS, Tverye AN, Gold SA, Lotan Y, Margulis V, et al. Outcomes of patients undergoing concurrent radical cystectomy and nephroureterectomy: A single-institution series. Can Urol Assoc J = J l’Association Des urologues du Canada. (2022) 16(7):E363–e369. doi: 10.5489/cuaj.7612
22. Kanabur P, Patel S, Godoy G. Perioperative outcomes of patients undergoing concurrent nephroureterectomy and cystectomy for urothelial cancer: an nsqip database analysis. J Urol (2022) 207(5):E978–8. doi: 10.1097/JU.0000000000002639.15
23. Colla Ruvolo C, Nocera L, Stolzenbach LF, Wenzel M, Califano G, Tian Z, et al. Tumor size predicts muscle-invasive and non-organ-confined disease in upper tract urothelial carcinoma at radical nephroureterectomy. Eur Urol focus (2022) 8(2):498–505. doi: 10.1016/j.euf.2021.03.003
24. Colla Ruvolo C, Stolzenbach LF, Nocera L, Deuker M, Wenzel M, Tian Z, et al. Higher cancer mortality in rural upper urinary tract urothelial carcinoma patients. Urol internationalis (2021) 105(7-8):624–30. doi: 10.1159/000513361
25. Collà Ruvolo C, Wenzel M, Nocera L, Würnschimmel C, Tian Z, Shariat SF, et al. The effect of race on stage at presentation and survival in upper tract urothelial carcinoma. Urol Oncol (2021) 39(11):788.e7–788.e13. doi: 10.1016/j.urolonc.2021.07.001
26. Colla Ruvolo C, Deuker M, Wenzel M, Nocera L, Wurnschimmel C, Califano G, et al. Impact of the primary tumor location on secondary sites and overall mortality in patients with metastatic upper tract urothelial carcinoma. Urol Oncol (2022) 40(9):411 e1–8. doi: 10.1016/j.urolonc.2022.06.009
27. Fahmy O, Khairul-Asri MG, Schubert T, Renninger M, Kübler H, Stenzl A, et al. Urethral recurrence after radical cystectomy for urothelial carcinoma: A systematic review and meta-analysis. Urol Oncol (2018) 36(2):54–9. doi: 10.1016/j.urolonc.2017.11.007
28. Khanna A, Zganjar A, Lyon T, Shah P, Tollefson MK, Karnes RJ, et al. A contemporary analysis of urethral recurrence following radical cystectomy. J urol (2021) 206(4):970–7. doi: 10.1097/ju.0000000000001842
29. Boorjian SA, Kim SP, Weight CJ, Cheville JC, Thapa P, Frank I. Risk factors and outcomes of urethral recurrence following radical cystectomy. Eur urol (2011) 60(6):1266–72. doi: 10.1016/j.eururo.2011.08.030
30. Huguet J, Monllau V, Sabaté S, Rodriguez-Faba O, Algaba F, Palou J, et al. Diagnosis, risk factors, and outcome of urethral recurrences following radical cystectomy for bladder cancer in 729 male patients. Eur Urol (2008) 53(4):785–92. doi: 10.1016/j.eururo.2007.06.045. discussion 792-3.
31. Quek ML, Stein JP, Daneshmand S, Miranda G, Thangathurai D, Roffey P, et al. A critical analysis of perioperative mortality from radical cystectomy. J Urol (2006) 175(3 Pt 1):886–9. doi: 10.1016/s0022-5347(05)00421-0. discussion 889-90.
32. Novara G, Catto JW, Wilson T, Annerstedt M, Chan K, Murphy DG, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur urol (2015) 67(3):376–401. doi: 10.1016/j.eururo.2014.12.007
33. Bream MJ, Maurice MJ, Altschuler J, Zhu H, Abouassaly R. Increased use of cystectomy in patients 75 and older: A contemporary analysis of survival and perioperative outcomes from the national cancer database. Urology (2017) 100:72–8. doi: 10.1016/j.urology.2016.08.054
34. Kang CH, Chen CH, Chiang PH. Primary urothelial carcinoma of the upper urinary tract in dialysis patients with 5-year follow-up. Japan J Clin Oncol (2010) 40(3):241–6. doi: 10.1093/jjco/hyp143
35. Wu CF, Shee JJ, Ho DR, Chen WC, Chen CS. Different treatment strategies for end stage renal disease in patients with transitional cell carcinoma. J urol (2004) 171(1):126–9. doi: 10.1097/01.ju.0000101758.41635.28
36. Tai HC, Lai MK, Chung SD, Huang KH, Chueh SC, Yu HJ. Intermediate-term oncological outcomes of hand-assisted laparoscopic versus open bilateral nephroureterectomy for dialysis and kidney transplant patients with upper urinary tract urothelial carcinoma. J endourol (2009) 23(7):1139–44. doi: 10.1089/end.2008.0162
37. Deliveliotis C, Papatsoris A, Chrisofos M, Dellis A, Liakouras C, Skolarikos A. Urinary diversion in high-risk elderly patients: modified cutaneous ureterostomy or ileal conduit? Urology (2005) 66(2):299–304. doi: 10.1016/j.urology.2005.03.031
38. Korkes F, Fernandes E, Gushiken FA, Glina FPA, Baccaglini W, Timóteo F, et al. Bricker ileal conduit vs. Cutaneous ureterostomy after radical cystectomy for bladder cancer: a systematic review. Int Braz J urol: Off J Braz Soc Urol (2022) 48(1):18–30. doi: 10.1590/s1677-5538.Ibju.2020.0892
39. Arman T, Mher B, Varujan S, Sergey F, Ashot T. Health-related quality of life in patients undergoing radical cystectomy with modified single stoma cutaneous ureterostomy, bilateral cutaneous ureterostomy and ileal conduit. Int Urol nephrol (2020) 52(9):1683–9. doi: 10.1007/s11255-020-02470-6
40. Longo N, Imbimbo C, Fusco F, Ficarra V, Mangiapia F, Di Lorenzo G, et al. Complications and quality of life in elderly patients with several comorbidities undergoing cutaneous ureterostomy with single stoma or ileal conduit after radical cystectomy. BJU Int (2016) 118(4):521–6. doi: 10.1111/bju.13462
41. Mistretta FA, Musi G, Colla Ruvolo C, Conti A, Luzzago S, Catellani M, et al. Robot-assisted radical cystectomy for nonmetastatic urothelial carcinoma of urinary bladder: A comparison between intracorporeal versus extracorporeal orthotopic ileal neobladder. J endourol (2021) 35(2):151–8. doi: 10.1089/end.2020.0622
42. Mastroianni R, Ferriero M, Tuderti G, Anceschi U, Bove AM, Brassetti A, et al. Open radical cystectomy versus robot-assisted radical cystectomy with intracorporeal urinary diversion: early outcomes of a single-center randomized controlled trial. J urol (2022) 207(5):982–92. doi: 10.1097/ju.0000000000002422
43. Fontanet S, Basile G, Baboudjian M, Gallioli A, Huguet J, Territo A, et al. Robot-assisted vs. open radical cystectomy: systematic review and meta-analysis of randomized controlled trials. Actas urologicas espanolas (2023) 47(5):261–70. doi: 10.1016/j.acuroe.2023.01.003
44. Rodríguez Faba O, Palou J, Breda A, Maroto P, Fernández Gómez JM, Wong A, et al. Predictive factors for impaired renal function following nephroureterectomy in upper urinary tract urothelial cell carcinoma. Urol internationalis (2014) 92(2):169–73. doi: 10.1159/000353652
45. Vejlgaard M, Maibom SL, Stroomberg HV, Poulsen AM, Thind PO, Røder MA, et al. Long-term renal function following radical cystectomy for bladder cancer. Urology (2022) 160:147–53. doi: 10.1016/j.urology.2021.11.015
46. Mikhail D, Sarcona J, Mekhail M, Richstone L. Urologic robotic surgery. Surg Clinics North America (2020) 100(2):361–78. doi: 10.1016/j.suc.2019.12.003
Keywords: radical cystectomy, nephroureterectomy, panurothelial carcinoma, UTUC, meta-analysis
Citation: Liu Y, Zhang H, Wen Z, Jiang Y, Huang J, Wang C, Chen C, Wang J, Bao E and Yang X (2023) Simultaneous radical cystectomy and nephroureterectomy in the treatment of panurothelial carcinoma: a systematic review and single-arm meta-analysis. Front. Oncol. 13:1233125. doi: 10.3389/fonc.2023.1233125
Received: 01 June 2023; Accepted: 11 September 2023;
Published: 25 September 2023.
Edited by:
Clemens Mathias Rosenbaum, Asklepios Hospital Barmbek, GermanyReviewed by:
Simone Morra, University of Naples Federico II, ItalyCopyright © 2023 Liu, Zhang, Wen, Jiang, Huang, Wang, Chen, Wang, Bao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuesong Yang, eHVlc29uZ3lhbmcyMDIyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.